Abstract
To manufacture TiO2, a high-purity synthetic rutile, the recovery of Ti was investigated using a hydro-metallurgical process. Using a feed solution containing 32050 mg/L Ti, 110 mg/L Si, 88 mg/L Nb, 2614 mg/L Fe, and 130 mg/L Zr, solvent-extraction experiments were conducted with alkyl phosphine oxide in conjunction with diluents such as kerosene and xylene. The results showed that the extraction mechanism of both diluents was very similar to slope analysis, which had a value of 1.9; however, the extraction equilibrium constant value of organic–metallic species in xylene as a diluent was lower than in kerosene as a diluent. This result affected the stripping efficiency of Ti in particular; therefore, xylene was selected as a diluent. To recover Ti ion from a leaching solution, a series of experiments was conducted, such as the McCabe–Thiele method and countercurrent simulation test for extraction and stripping of Ti. As a result, Ti and impurities such as Fe and Zr were extracted to 99.9% from Si and Nb under optimal conditions using countercurrent four-stage extraction, with 1 M Cyanex 923 at a ratio of organic phase/ aqueous phase=3. In the stripping test, Ti was selectively stripped to 90.1% from Fe and Zr in the organic phase by 1 M HCl. The obtained powder, which was hydrolyzed from an impurity-free solution, was analyzed to a purity of 99.9% by inductively coupled plasma. The TiO2, which has a spherical shape and a diameter of approximately 2 µm according to SEM, was evident by XRD.
1. Introduction
The titanium industry relies on Ti and TiO2, a synthetic rutile. The Ti alloy is used in the aerospace, automotive, and biomedical industries due to properties such as high strength and rigidity, low density, ability to withstand high temperatures, resistance to corrosion, and excellent biocompatibility. The other industrial source of Ti is TiO2, which is used in pigment, rubber, filter paper, catalysts, and the plastics industry. The major ore of Ti is natural TiO2 (rutile) and ilmenite (FeTiO3), which contain over 90% Ti and 30–65% Ti, respectively. The rutile contains the highest concentration of Ti among the ores of Ti; however, the reserved area is limited. On the other hand, ilmenite is reserved at 94% all over the world. Therefore, ilmenite is more often used as a source for industrial Ti than rutile because of the ready availability of the mineral [1,2,3,4,5].
From ilmenite, the commercial metallurgy processes of Ti are the slag process, the upgraded titanium slag (UGS) process, the Becher process, and the Benelite process, which can concentrate TiO2 up to 86%, 95%, 90%, and 95%, respectively. Concentrated Ti could be manufactured as sponge metallic Ti or TiO2 (synthetic rutile) by the Kroll process or chloride process. The commercial processes are mainly pyro-metallurgical. In the hydro-metallurgical process, the sulfuric acid process is well known as a first commercial hydro process of Ti, which removes impurities by directly leaching mineral from Ti [6,7,8,9,10,11]. Although the sulfuric acid process could only manufacture TiO2, the purity of the product is low. Thus, hydro-metallurgical processes are used to report research on how to upgrade method for concentration of TiO2 from ilmenite. The methods were reported by using direct leaching method, high pressure leaching process, or leaching process after preheat treatment method [12,13,14,15,16,17,18,19,20]. According to these processes, the Fe could be significantly removed and the TiO2 could be concentrated over 90%. However, to manufacture TiO2 with high purity, research on the separation and purification of Ti is required and researchers have reported using a leaching solution for Ti in the hydro-metallurgical process.
To extract Ti, acid extractants were applied, such as bis-(2-ethylhexyl) phosphoric acid (D2EHPA) and 2-ethylhexyl phosphonic acid mono-2-ethylhexyl phosphoric ester (EHEHPA).
Saji and Reddy [21] reported extraction efficiency of Ti by EHEHPA, depending on diluents and stripping efficiency of Ti from solution containing 0.01 M Ti based on HCl. In this experiment, kerosene was superior to other diluents, with 5.27 distribution ratio (D value), and the mixture solution of H2SO4 and H2O2 increased the stripping efficiency of Ti by up to 99.9% compared to 1 M HCl, with a stripping efficiency of 0.5% Ti. Borsalani et al. [22] reported a similar result. From a solution containing 0.01-0.02 M Ti, the Ti was extracted by EHEHPA. In the stripping process, a solution containing Na2CO3 and H2SO4 + H2O2 was applied to a stripping solution to increase the stripping efficiency of Ti. However, a third phase was observed during the extraction process. Therefore, they proposed restricting the concentration of Ti in the organic phase. In contrast, Saji et al. [13,23] have reported research on extraction of Ti by EHEHPA from a solution containing 0.001 M Ti. In this study, using only HCl, the stripping efficiency of Ti reached 90%.
In the use of D2EHPA to extract Ti from HCl solution, the extraction mechanism was investigated but almost no research on the stripping of Ti has been reported [24,25]. In contrast, Singh and Dhadke [26] have reported that recovery of Ti was affected by diluents and stripping solution. The result showed that the use of toluene, xylene, and kerosene as diluents increased the extraction efficiency of Ti. In the stripping process, the Ti was stripped to lower than 5% using only HCl, whereas the mixed solution of H2SO4 and H2O2 completely stripped the Ti of the organic phase. However, these studies were conducted using a solution containing 0.01–0.1 M Ti.
The solvation extractants were used to extract TiCl4 or TiOCl2, TiOSO4 species, from the aqueous phase because of the excellent stripping efficiency of Ti.
Tri-alkyl phosphine oxide (Cyanex 923) has extracted TiCl4 in the xylene as a diluent by adding HCl into the base of a sulfate solution containing 0.001 M Ti. In this research, the stripping of Ti was reported to be 90% using only HCl [27]. Tri-octyl phosphine oxide (TOPO) was used to extract 0.0175 M Ti. Mao et al. [28] reported that the Ti can be selectively recovered from Fe by increasing the concentration of HCl in the stripping process. Allal et al. [29] compared extractants such as tributyl phosphate (TBP), decanol, and TOPO on extraction of Ti from base of HCl and CaCl2 solution containing 65 mg/L Ti. They have reported that mixed extractants of TBP and decanol recovered 95% Ti from a high concentration of 6 M HCl. However, research on the stripping process has not been reported. From the ilmenite leaching solution containing 475 mg/L Ti by HCl, the TBP was used to extract 97% Ti in kerosene. In the stripping process, 94.8% of Ti was recovered by 0.1 M HCl. On the other hand, TBP was used, in the base of a sulfate and nitrate solution containing 0.01–0.02 M Ti from a leaching solution of titaniferous magnetite, to extract Ti, as an extractant. In this study, the extraction of TiO2+ ion species was affected, depending on the concentration of H2SO4 and HNO3 in the aqueous phase. Also, under all experimental conditions, the third phase was observed. However, in the stripping process, a mixed solution of H2SO4 and H2O2, and a solution of Na2CO3, increased the stripping efficiency of Ti [30,31]. This could, therefore, imply that the occurrence of a third phase in the extraction behavior of Ti and the stripping efficiency by acid depends on what the base of the aqueous phase is, despite a similar use of extractant, such as TBP. However, this research was conducted using a solution containing 0.01–0.1 M Ti.
Based on an aqueous solution containing a high concentration of Ti, studies on TiO2 recovery have been reported. Zaki [32] manufactured TiO2 from ilmenite leaching solution containing 25,000 mg/L Ti based on HCl. To selectively extract Ti, the Fe3+ ion was reduced to Fe2+ by the addition of Fe powder to the leaching solution. Then, the Ti was selectively extracted from Fe by TOPO. In the stripping process, the Ti was stripped to 91.5% by five courses of stripping with 0.2 M HCl. In another study on recovery of TiO2, Middlemas et al. [33] reported manufacturing TiO2 from a raffinate solution in which impurities such as Fe were removed by a mixture of tri-(octyl/decyl) amine and tertiary amine (Alamine 336). Similarly, Wang et al. [34] recovered TiO2 using hydrolysis method from raffinate solution after preferential extraction of Fe by methyl iso-butyl ketone from hydrochloric acid leaching solution.
In the literature mentioned above, almost all research on recovery of Ti was based on a low concentration of Ti. Also, some research reported that a third phase was observed during the extraction process. To increase the stripping efficiency for organic-metallic Ti in the organic phase, the stripping solution mixture of H2O2 and H2SO4 was used, whereas other research reported that the stripping of Ti was successful using only HCl. This is because the recovery of Ti was affected by the concentration of Ti in the aqueous phase; aqueous bases, such as Cl−, SO42−, and NO3−; extractant type; kind of diluent; and stripping solution.
Therefore, in the current study, to manufacture TiO2 with high purity, the research focused on not only extraction behavior depending on diluents but also stripping behavior of Ti from ilmenite leaching solution containing a high concentration of Ti.
2. Materials and Methods
2.1. Reagents
Tri-alkyl phosphine oxide (molecular weight: 348 g/mol, density: 0.88 g/cm3, purity: 93% weight), which has the commercial name Cyanex 923, was supplied by Cytec Inc.(Woodland Park, NJ, USA) and was used without further purification.
The diluents such as kerosene (density: 0.80 g/cm3, purity: 95%) and xylene (purity: 83%) were supplied by Junsei, Japan. The HCl (density: 1.18 g/cm3, purity: 35%) and NaCl (density: 2.165 g/cm3, purity: 99.5%) were also supplied by Junsei, Japan. The ilmenite ore used for this study is from Pyeongtaek County in Korea.
2.2. Procedure
2.2.1. Preparation of Feed Solution for Solvent Extraction of Ti
The Fe was removed by selective chlorination [35] in ilmenite ore from Pyeongtaek in Korea, and was concentrated to approximately 90% TiO2. Then, a series of experiments [4] was conducted: Soda roasting at 600 °C, water washing, and leaching by HCl. The leaching solution contained 32,050 mg/L Ti, 110 mg/L Si, 88 mg/L Nb, 2614 mg/L Fe, and 130 mg/L Zr (Table 1).

Table 1.
The composition of valuable metals in leaching solution by 5 M HCl. unit: mg/L.
2.2.2. Solvent Extraction Experiment
The shake-out tests were conducted by shaking incubator (SI-600R, Jeio Tech, Korea), with an organic phase/aqueous phase ratio (O/A ratio) of 1:1, an agitation speed of 250 RPM, and at 25 °C for 30 min. After about 60 min of being stationary, in equilibrium, the phase was separated into organic and aqueous phases and divided using a separation funnel. The extraction efficiency was theoretically calculated with a base of metal ion in the aqueous phase to balance the weight of metal extracted (Equation (1)).
By applying the shake-out test method, the stripping experiments were conducted with various stripping solutions, such as distilled water, HCl, and NaCl at an O/A ratio of 2.
In order to determine the number of stages that are required at chosen volume phase ratio, the extraction and stripping isotherm was obtained at a different O/A phase ratios.
All analyses of collected aqueous samples were performed using an ICP-OES (inductively coupled plasma optical emission spectrometry, iCAP 6000 series, Thermo Fisher Scientific, Waltham, MA, USA).
2.2.3. Hydrolysis
After the solvent extraction process, the obtained stripping solution was subjected to a hydrolysis test which was conducted for 12 h at 90 °C. The obtained powder was washed with water until the pH was over 4.5. Then, it was sintered at 700 °C for 12 h in a box furnace (SF3281K, Thermo Fisher, Waltham, MA, USA). The obtained white powder was analyzed by XRD (D/Max-2200, Rigaku, Tokyo, Japan) and SEM (JSM-6380LA, JEOL, Tokyo, Japan) for observation of TiO2.
3. Results and Discussions
3.1. Effect of Diluents on Extraction of Ti by Cyanex 923
Figure 1 shows the extraction behavior of valuable metals with increasing concentrations of Cyanex 923 in kerosene and xylene as diluent. In both diluents, the Fe and Zr were extracted completely at a low concentration of Cyanex 923, 0.1 M, whereas the Si and Nb were hardly extracted despite an increase of concentration of Cyanex 923. In both diluents, the extraction efficiency of Ti was increased with an increase of concentration of Cyanex 923. Thus, the extraction order of metals by Cyanex 923 was followed by Fe > Zr > Ti> Si and Nb. When comparing the effect of diluents on extraction of Ti, there was only one difference: That Ti was not extracted at 0.1 M Cyanex 923 in xylene. The extraction behavior of valuable metals was similar. However, in the use of kerosene and xylene as diluents, the stripping efficiency was different from the loaded organic phase of organic–metallic species (Table 2). When using 1 M HCl as the stripping solution from the loaded organic phase at different concentrations of Cyanex 923, the stripping concentration of Ti in kerosene as diluent was lower than in xylene. The reason for the differences in stripping efficiency depending on the diluent is that the diluents affected KE (extraction equilibrium constant) in the extraction mechanism of a solvation extractant such as Cyanex 923 due to being the same in all the other conditions. In this study, it was assumed that the species of Ti ion is TiO2+ in the chloride medium aqueous phase [24,27,36,37].
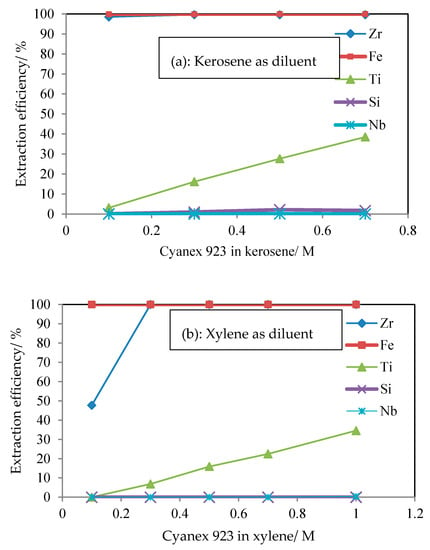
Figure 1.
The extraction efficiency of metal ion from HCl leaching solution by Cyanex 923 at different diluents (a) kerosene, (b) xylene as diluent.

Table 2.
The composition of Ti ion in stripping solution by 1 M HCl. unit: mg/L.
Then, an expected extraction reaction between Cyanex 923 and TiO2+ can be expressed as Equation (2), where L means solvation extractant, Cyanex 923.
TiO2+ + mCl− + nL = TiO2+·mCl−·nL
From Equation (2), we can assume that the value of m is 2 because the species of metal ion, which could be extracted in the solvent extraction, should be an electrically neutral species. Also, theoretically, we can assume that solvation extractants, such as Cyanex 923, did not substitute metal ions, such as the mechanism of cation exchanger, and did not associate ions, such as the mechanism of anion exchanger, to extract metal ion from the aqueous phase to the organic phase in the solvation extraction mechanism. For this reason, the extractable species ion of Ti in the aqueous phase should be TiOCl2. Although the concentration of chloride ion could affect the extraction mechanism of solvation for Ti, in the use of both diluents the extraction mechanism was not different in the concentration of chloride ion under the same conditions in this study. Also, the concentration of chloride ion was enough to extract the Ti. In other words, the concentration of chloride ion did not affect the comparison of equilibrium-constant values for both diluents. Therefore, the equilibrium constant can be expressed as shown in Equation (3) from Equation (2).
where the bar denotes organic phase and distribution ratio (D value) is defined as follows
Taking the logarithms of Equation (3), we can obtain Equation (5), by plotting log [D] versus log [L] at constant log [Cl−]. Further, this equation can be expressed as Equation (5).
Then, we should obtain the slope of a straight line which has n value and has an intercept equal to log KE + log [Cl−]. From Equation (5), we can compare the equilibrium constant values for both diluents because concentration of chloride ion is meaningless in this study. In this study, the n value was calculated until n value and slope value were equal value by trial and error method (Figure 2). As a result, the n values of both diluents were found to be 1.9, which of value is similar to other studies [29,38]. This means that two molecules of Cyanex 923 were required to extract one molecule of Ti. In other words, the extraction mechanisms of TiO2+·2Cl− are quite similar by Cyanex 923 when using kerosene and xylene as diluents. However, the intercept value of the slope in the use of xylene as a diluent was 0.17, and the intercept value of the slope when using kerosene as a diluent was 0.75, which differed from the intercept value of the slope containing log KE. The intercept value of slope of kerosene as diluent was 4.4 times higher than the intercept value of the slope of xylene. In this study, the calculating of accurate KE was difficult due to the effect of other impurities, such as Fe and Zr, and the concentration of chloride ion. However, it was sufficiently comparable for which KE value was higher because the extraction behavior of Cyanex 923 in both diluents was very similar and the concentration of chloride ion was enough to extract Ti. In other words, in the Cyanex 923 extraction reaction, the organic–metallic species, when using kerosene as a diluent, is more stable than xylene. This result is supported by other studies [27,38]. Therefore, the organic–metallic species, when using kerosene as a diluent, was hardly stripped, whereas the organic–metallic species, when using xylene, was relatively easy.
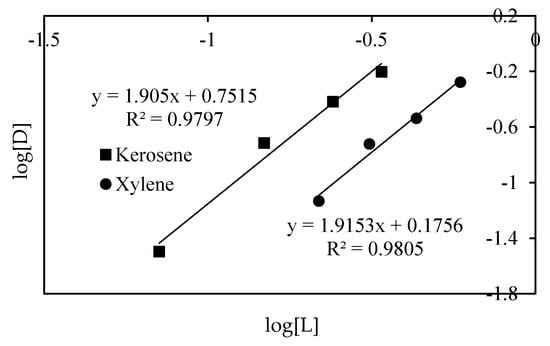
Figure 2.
Comparison of value of slope and intercept in log D vs. log[L] at different diluent systems by Cyanex 923.
3.2. Extraction of O/A Ratio
A McCabe–Thiele diagram was drawn according to the O/A ratio result in Figure 3. From Figure 3, at operating line 1, no Ti could be extracted because the operating line is outside of the McCabe–Thiele diagram. This means that the Ti could not be concentrated in the extraction mechanism by Cyanex 923 when using xylene as a diluent. To extract the Ti, the value of operating line shall be at least 3 in the extraction process. At operating line 3, a four-stage extraction stage is required for extraction of Ti by Cyanex 923 in xylene. To reduce the number of extraction stages, if the value of the operating line is increased to 5, only a two-stage extraction stage is required. To confirm the predicted result of the McCabe–Thiele diagram, countercurrent simulation extraction tests were conducted by 1 M Cyanex 923 in xylene with an O/A ratio of 3:5 (Table 3). At an operating line of 3 with a four-stage extraction, 130 mg/L Ti, which corresponds to 99.6% extraction efficiency of Ti, was left in the aqueous phase. On the other hand, a two-stage extraction at an operating line of 5, 420 mg/L Ti, which corresponds to 98.7% extraction efficiency of Ti, was left in the aqueous phase. Impurities such as Fe and Zr were completely extracted, whereas Si and Nb were hardly extracted regardless of the number of extraction stages and operating lines. Therefore, the optimum condition was decided for this study: A four-stage extraction at an operating line of 3 using 1 M Cyanex 923 in xylene as diluent for extraction of Ti.
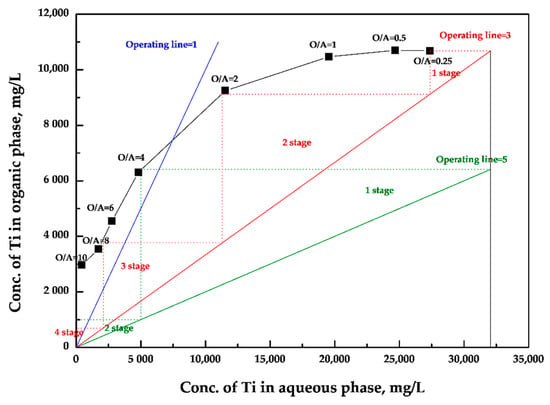
Figure 3.
McCabe–Thiele diagram for extraction of Ti by 1 M Cyanex 923 in xylene.

Table 3.
The result of countercurrent batch simulation test for extraction 1 M Cyanex 923 in xylene, unit: mg/L.
3.3. Effect of Stripping Solution for Selective Recovery of Ti
Table 4 shows the amount of valuable metals stripped by stripping solution and number of times from the loaded organic phase. For distilled water, the highest stripping amount of Ti was reached, 12,460 mg/L, corresponding to 58.7% stripping efficiency of Ti in the first stripping test. In the second stripping test, the amount of Ti was decreased to half, corresponding to 5100 mg/L Ti. Increasing the concentration of HCl decreased the amount of stripped Ti. Increasing the concentration of NaCl decreased the amount of stripped Ti. This means that the concentration of Cl− ion decreased the stripping efficiency of Ti. These results could be explained using Equation (2). However, when comparing HCl and NaCl under the same concentration of Cl− ion, the solution containing less acid was superior to stripping of Ti. Other impurities, such as Zr, Si, and Nb, were hardly stripped in all conditions except for Fe. In the first stripping test, a small amount of Fe was stripped lower than 5 mg/L Fe; however, because when the number of times increased on the second time, the amount of Fe stripped increased. In the second test using distilled water, the Fe was stripped to 240 mg/L and in the second test using 0.1 M HCl, Fe was stripped to 108 mg/L. In the case of NaCl, regardless of the number of stripping stages and concentration of NaCl, the amount of Fe stripped was constant at about 2.5 mg/L. This means that the stripping of Fe was affected by acid more than the concentration of Cl− when comparing HCl with NaCl, which contains the same concentration of Cl−. In particular, using 1 M HCl, the Fe was not stripped, even though the number of strips was increased. Therefore, the optimal stripping solution for high purity of Ti was selected as 1 M HCl, which stripped the least amount of Fe, depending on the results of the stripping experiments. This means that Ti can be selectively recovered from Fe.

Table 4.
Stripping behavior of metals from loaded organic phase of Cyanex 923 in xylene, O/A = 1, unit: mg/L.
3.4. Stripping of O/A Ratio
The McCabe–Thiele diagram was drawn for stripping of Ti according to the O/A ratio result in Figure 4. Figure 4 shows that the full amount of Ti could not be stripped and was left in the organic phase at approximately 4000 mg/L using 1 M HCl regardless of the operating line. Through the McCabe–Thiele diagram, it is possible to predict the amount of Ti which can be stripped from each stage. At an O/A ratio of 2, the Ti could be stripped to approximately 14,000 mg/L at the first stage, the Ti could be stripped to approximately 9000 mg/L at the second stage, the Ti could be stripped to approximately 6000 mg/L at the third stage, and the Ti could be stripped to approximately 2000 mg/L at the fourth stage. On the other hand, if the operating line is reduced to 1, the number of stripping stages could be reduced to 2, but the Ti could be recovered to approximately 7000 mg/L at the first stage. Therefore, an operating line of 2 was chosen for recovery of high concentration of Ti. This meant that four-stage stripping was required.
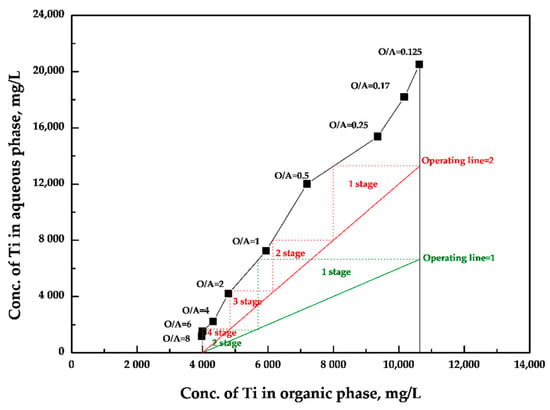
Figure 4.
McCabe–Thiele diagram for stripping by 1 M HCl from loaded organic phase by Cyanex 923 in xylene.
To confirm the predicted result of the McCabe–Thiele diagram, countercurrent simulation stripping tests were conducted using 1 M HCl with an O/A ratio of 2 (Table 5). At an operating line of 2 with four-stage stripping, 13040 mg/L Ti, which corresponds to 61.2% stripping efficiency, was recovered. The remaining amount of Ti was calculated as 4120 mg/L based on a theoretical amount of Ti in the loaded organic phase minus the aqueous phase. To recover the remaining amount of Ti in the loaded organic phase, after conducting a countercurrent stripping test, cross-stripping tests were conducted twice at an O/A ratio of 1. The results showed that in the first cross-stripping test 2690 mg/L Ti was stripped and in the second cross-stripping test 490 mg/L Ti was stripped. Finally, from an initial concentration of 10640 mg/L Ti in the loaded organic phase, a total of 9700 mg/L Ti was theoretically recovered, which corresponds to 91.1% recovery efficiency of Ti.

Table 5.
The result of stripping of countercurrent batch simulation test and cross-stripping by 1 M HCl, unit: mg/L.
The obtained stripping solution was subjected to hydrolysis treatment for recovery of TiO2. After 12 h hydrolysis, the Ti was precipitated over 98.1%. The obtained precipitate powder was dried at 120 °C for 24 h. The color of the obtained powder was light ivory, not white, due to Cl− in the stripping solution. Therefore, the obtained precipitate powder was washed by distilled water until the pH no longer increased (pH 4.5) from an initial pH of 2.4. The color of the obtained powder was changed from light ivory to white. Then, the washed powder was sintered at a temperature of 700 °C. The recovered powder was analyzed by ICP, XRD, and SEM. Table 6 shows the purities of recovered powder, which contains only 0.03% SiO2 impurity by ICP. Also, the recovered powder is TiO2, which, of phases, was found to be rutile and brookite by XRD analysis (Figure 5), and the morphology of TiO2 had a spherical shape and its diameter was approximately 2 µm according to SEM (Figure 6).

Table 6.
The calculated composition of metals in manufactured TiO2 by base on ICP analysis. Unit: %.
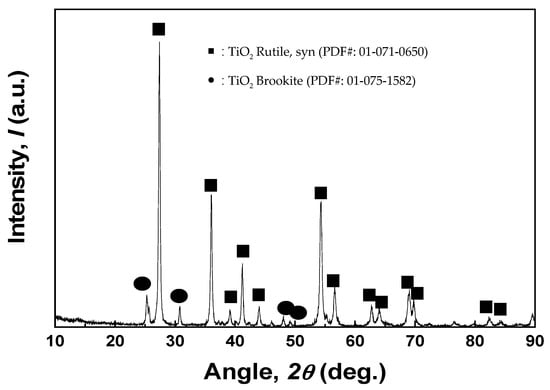
Figure 5.
XRD analysis of sintered powder at 700 °C after hydrolysis and water washing.

Figure 6.
SEM analysis of sintered powder at 700 °C after hydrolysis and water washing.
4. Conclusions
(1) Both diluents did not affect the extraction mechanism of Ti with Cyanex 923; however, the KE values were affected, depending on the diluents. When using xylene as a diluent, the KE value was lower than kerosene. Therefore, when using xylene, the stripping efficiency of Ti was higher than kerosene when using HCl. This means that the organic–metallic species was not stable in xylene compared to kerosene.
(2) In the extraction system using xylene as a diluent, we found the optimum extraction conditions to be countercurrent and four stages, using 1 M Cyanex 923 at O/A = 3. The Ti was extracted to 99.9% under these conditions.
(3) The concentration of acid affected the stripping of Fe more than the concentration of Cl− ion. The Fe was not stripped from the loaded organic phase by 1 M HCl. Therefore, the Ti could be selectively recovered from Fe.
(4) Through the McCabe–Thiele diagram for stripping of Ti, the full amount of Ti could not be recovered regardless of operating line. In the countercurrent stripping test, the stripping efficiency of Ti was reached at 68% using 1 M HCl. Then, through the cross-stripping test, the stripping efficiency of Ti was increased to 90.1%.
(5) A series of experiments were conducted, such as hydrolysis from Ti solution, washing, and sintering at 700 °C, to ensure that the TiO2 had a purity above 99.9%. The obtained phases of powder were found to be rutile and brookite by XRD analysis, and the morphology of TiO2 had a spherical shape and a diameter of approximately 2 µm according to SEM.
(6) This study will contribute to the manufacture of high-purity TiO2 via a hydro-metallurgical route and will need to improve the stripping efficiency of Ti, and additional processes will need to be used and studied in more detail in the future.
Author Contributions
Conceptualization, S.-H.J. and S.M.S.; Data curation, S.M.S.; Formal analysis, S.-H.J., D.L. and J.-T.P.; Investigation, S.-H.J., D.J.S., D.L., J.K., M.-s.K., H.J. and S.M.S.; Methodology, S.-H.J., D.J.S., D.L., J.K., M.-s.K., H.J., J.-T.P. and S.M.S.; Project administration, M.-s.K. and H.J.; Resources, D.J.S. and J.K.; Software, D.L.; Writing—original draft, S.-H.J.; Writing—review & editing, S.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Research Project (20-3212-1, GP2020-013) of the Korea Institute of Geoscience and Mineral Resources (KIGAM) funded by the Ministry of Science, ICT, and Future Planning of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.P.; Wang, H.Z.; Ruan, G.L.; Wang, S.H.; Xiao, Y.X.; Jiang, L.D. Applications and prospects of titanium and its alloys in seawater desalination industry. Mater. Sci. Eng. 2019, 688, 033036. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 14–34. [Google Scholar]
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar]
- Shin, D.J.; Joo, S.-H.; Lee, D.S.; Kang, J.S.; Kim, M.-S.; Park, J.-T.; Min, D.J.; Shin, S.M. Leaching Behavior of Titanium from Na2TiO3. Mater. Trans. 2020, 61, 150–155. [Google Scholar]
- Bedinger, G.M. Mineral Commodity Summaries: Titanium Mineral Concentrates; U.S. Geological Survey: Washington, DC, USA, 2013; p. 174175. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/titanium/mcs-2013-timin.pdf (accessed on 1 February 2020).
- Becher, R.G.; Canning, R.G.; Goodheart, B.A.; Uusna, S. A new process for upgrading Hmenitic mineral sands. Proc. Aust. Inst. Min. Metall. 1965, 21, 21–44. [Google Scholar]
- Hoecker, W. Process for the Production of Synthetic Rutile. European Patent EP0612854, 31 August 1994. [Google Scholar]
- Guéguin, M.; Cardarelli, F. Chemistry and mineralogy of titania-rich slags. Part 1—Hemo-ilmenite, sulphate, and upgraded titania slags. Extr. Miner. Process. Metall. Rev. 2007, 28, 1–58. [Google Scholar]
- Akhtar, M.K.; Vemury, S.; Pratsinis, S.E. Competition between TiCl4 hydrolysis and oxidation and its effect on product TiO2 powder. AIChE J. 1994, 40, 1183–1192. [Google Scholar]
- Kroll, W. The Production of Ductile Titanium. Trans. Electrochem. Soc. 1940, 78, 35–47. [Google Scholar] [CrossRef]
- Kang, J.; Moon, H.; Kim, M.-S.; Okabe, T.H. Production of High-grade Titanium Dioxide from Titanium Ore Using Titanium Scrap and Iron Chloride Waste. Met. Mater. Int. 2019, 25, 257–267. [Google Scholar] [CrossRef]
- Habashi, F. Ilmenite for pigment and metal production. Interdiscip. J. Chem. 2016, 1, 28–33. [Google Scholar]
- Habashi, F.; Kamaleddine, F.; Bourricaudy, E. A New Process to Upgrade Ilmenite to Synthetic Rutile Proceedings Conference of Metallurgists, Canadian Institute of Mining, Metallurgy, and Petroleum, Montreal. Metall 2015, 69, 27–30. [Google Scholar]
- Zhang, J.; Zhu, Q.; Xie, Z.; Li, H. Influence of redox pretreatment on the pulverization of panzhihua ilmenite during hydrochloric acid leaching. Hydrometallurgy 2015, 157, 226–233. [Google Scholar] [CrossRef]
- Guo, T.; Liu, S.; Jiang, T.; Qiu, G.; Chen, F. A process for producing synthetic rutile from Panzhihua titanium slag. Hydrometallurgy 2014, 147–148, 134–141. [Google Scholar] [CrossRef]
- Jia, L.; Liang, B.; Lü, L.; Yuan, S.; Zheng, L.; Wang, X.; Li, C. Beneficiation of titania by sulfuric acid pressure leaching of Panzhihua ilmenite. Hydrometallurgy 2014, 59, 92–98. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Qi, T.; Chen, D.; Zhao, H.; Liu, Y. A novel method to extract iron, titanium, vanadium, and chromium from high-chromium vanadium-bearing titanomagnetite concentrates. Hydrometallurgy 2014, 149, 106–109. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, J.; Li, H. Influence of phase and microstructure on the rate of hydrochloric acid leaching in pretreated Panzhihua ilmenite. Particuology 2014, 14, 83–90. [Google Scholar]
- Janssen, A.; Putnis, A. Processes of oxidation and HCl-leaching of Tellnes ilmenite. Hydrometallurgy 2011, 109, 194–201. [Google Scholar]
- Zhang, L.; Hu, H.; Liao, Z.; Chen, Q.; Tan, J. Hydrochloric acid leaching behavior of different treated Panxi ilmenite concentrations. Hydrometallurgy 2011, 107, 40–47. [Google Scholar] [CrossRef]
- Saji, J.; Reddy, M.L.P. Selective Extraction and Separation of Titanium(IV) from Multivalent Metal Chloride Solutions Using 2-Ethylhexyl Phosphonic Acid Mono-2-ethylhexyl Ester. Sep. Sci. Technol. 2003, 38, 427–441. [Google Scholar]
- Borsalani, A.; Ghahremani, H.; Seyfi, S.; Abdi, M. Solvent Extraction of tetravalent titanium from chloride and nitratesolutions by 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester(EHEHPA) in Kerosene. Chem. Sin. 2011, 2, 204–211. [Google Scholar]
- Saji John, K.; Rao, T.P.; Ramamohan, T.R.; Reddy, M.L.P. Solvent extraction of tetravalent titanium from acidic chloride solutions by 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Hydrometallurgy 1999, 53, 245–253. [Google Scholar] [CrossRef]
- Biswas, R.K.; Begum, D.A. Solvent extraction of tetravalent titanium from chloride solution by di-2-ethylhexyl phosphoric acid in kerosene. Hydrometallurgy 1998, 49, 263–274. [Google Scholar] [CrossRef]
- Awwada, N.S.; Ibrahium, H.A. Kinetic extraction of titanium (IV) from chloride solution containing Fe(III), Cr(III) and V(V) using the single drop technique. J. Environ. Chem. Eng. 2013, 1, 65–72. [Google Scholar]
- SINGH, R.K.; DHADKE, P.M. Extraction and separation of titanium(IV) with D2EHPA and PC-88A from aqueous perchloric acid solutions. J. Serb. Chem. Soc. 2002, 67, 507–521. [Google Scholar]
- John, K.S.; Saji, J.; Reddy, M.L.P.; Ramamohan, T.R.; Rao, T.P. Solvent extraction of titanium(IV) from acidic chloride solutions by Cyanex 923. Hydrometallurgy 1999, 51, 9–18. [Google Scholar] [CrossRef]
- MAO, X.H.; LIU, D.J. Solvent Extraction Separation of Titanium(IV) and Iron(III) from Acid Chloride Solutions by Trioctylphosphine Oxide. Asian J. Chem. 2013, 25, 4753–4756. [Google Scholar]
- Allal, K.M.; Hauchard, D.; Stambouli, M.; Pareau, D.; Durand, G. Solvent extraction of titanium by tributylphosphate, trioctylphosphine oxide and decanol from chloride media. Hydrometallurgy 1997, 45, 113–128. [Google Scholar] [CrossRef]
- Seyfi, S.; Abdi, M. Extraction of titanium (IV) from acidic media by tri-n-butyl phosphate in kerosene. Miner. Eng. 2009, 22, 116–118. [Google Scholar]
- Baba, A.A.; Adekola, F.A.; Arodola, O.A.; Ibrahim, L.; Bale, R.B.; Ghosh, M.K.; Sheik, A.R. Simultaneous recovery of total iron and titanium from ilmenite ore by hydrometallurgical processing. Metall. Mater. Eng. 2012, 18, 67–78. [Google Scholar]
- Zaki, S.A. Preparation of pigment grade TiO2 from Rosetta ilmenite via selective TOPO extraction of titanium from its hydrochloric acid solution. Inorg. Chem. 2014, 9, 085–094. [Google Scholar]
- Middlemas, S.; Fang, Z.Z.; Fan, P. A new method for production of titanium dioxide pigment. Hydrometallurgy 2013, 131–132, 107–113. [Google Scholar]
- Wang, X.; Liang, B.; Lü, L.; Wu, P.; Li, C.; Ma, J. Simultaneous oxidation and extraction of iron from simulated ilmenite hydrochloric acid leachate. Hydrometallurgy 2012, 129–130, 105–110. [Google Scholar]
- Kang, J.S.; Okabe, T.H. Removal of Iron from Titanium Ore through Selective Chlorination Using Magnesium Chloride. Mater. Trans. 2013, 54, 1444–1453. [Google Scholar]
- Sato, T. Liquid-Liquid Extraction of Titanium (IV) from Hydrochloric Acid Solution by Di-(2-Ethylhexyl)-Phosphoric Acid. J. Min. Mater. Process.Inst. Jpn. 2003, 119, 175–181. [Google Scholar]
- Saji, J.; John, K.S.; Reddy, M.L.P. Liquid-liquid extraction of tetravalent titanium from acidic chloride solutions by bis(2,4,4-trimethylpentyl)phosphinicacid. Solvent Extrac. Ion Exchan. 2000, 18, 877–894. [Google Scholar]
- Remya, P.N.; Reddy, M.L.P. Solvent extraction separation of titanium(IV), vanadium(V) and iron(III) from simulated waste chloride liquors of titanium minerals processing industry by the trialkylphosphine oxide Cyanex 923. J. Chem. Technol. Biotechnol. 2004, 79, 734–741. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).