Microstructure and Mechanical Properties of Titanium–Equine Bone Biocomposites
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Characterizations
3. Results and Discussion
3.1. Microstructures
3.2. Mechanical Properties
4. Conclusions
- (1)
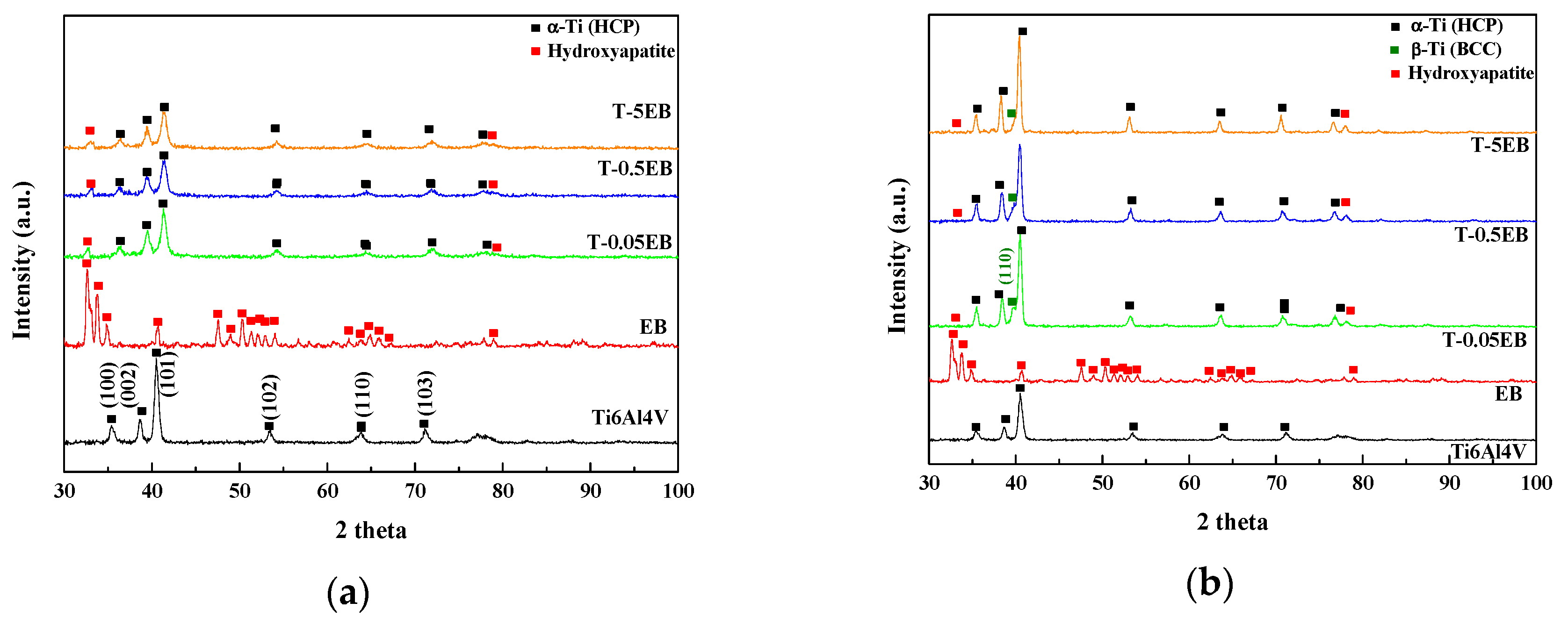
- The existence of hydroxyapatite, the major component of equine bones, in both Ti6Al4V-EB composite powder and sintered Ti6Al4V-EB composites, was confirmed by SEM-EDS and XRD analysis.
- (2)
- The hardness of the Ti6Al4V-EB composites increased as the EB contents increased owing to uniformly distribution of EB in the Ti6Al4V matrix. The composites SPSed at 1000 °C, which is the beta-phase transformation temperature, provided well-fabricated specimens and showed reasonable mechanical properties.
- (3)
- The composites containing 0.5 wt.% EB exhibited Vickers hardness and elastic modulus of 540.6 HV and 130.5 GPa, which are high strength and reasonable stiffness values for biomedical implants. Slightly high elastic modulus values of the composites can cause stress shielding problems compared to Ti6Al4V (110 GPa).
- (4)
- Ca, P, and O constituting the hydroxyapatite were detected on the surface of all Ti6Al4V-EB composites, which is no change of surface components before and after sintering due to discharge plasma sintering. Therefore, this study can suggest that the Ti6Al4V-EB composites have high bioactivity by increasing the bonding strength between implant and bone.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wally, Z.J.; Van Grunsven, W.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Porous titanium for dental implant applications. Metals 2015, 5, 1902–1920. [Google Scholar] [CrossRef]
- Yemisci, I.; Mutlu, O.; Gulsoy, N.; Kunal, K.; Atre, S.; Gulsoy, H.O. Experimentation and analysis of powder injection molded Ti10Nb10Zr alloy: A promising candidate for electrochemical and biomedical application. J. Mater. Res. Technol. 2019, 8, 5233–5245. [Google Scholar] [CrossRef]
- Slokar, L.; Štrkalj, A.; Glavaš, Z. Synthesis of Ti-Zr alloy by powder metallurgy. Eng. Rev. 2019, 39, 115–123. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.; Guo, H.; Li, P.; Hu, T.; Chung, C.Y.; Chu, P.K. Surface nano-architectures and their effects on the mechanical properties and corrosion behavior of Ti-based orthopedic implants. Surf. Coat. Technol. 2013, 233, 13–26. [Google Scholar] [CrossRef]
- Hsieh, M.-F.; Perng, L.-H.; Chin, T.-S. Hydroxyapatite coating on Ti6Al4V alloy using a sol–gel derived precursor. Mater. Chem. Phys. 2002, 74, 245–250. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Sousa, S.R.; Barbosa, M.A. Effect of hydroxyapatite thickness on metal ion release from Ti6Al4V substrates. Biomaterials 1996, 17, 397–404. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayed hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Linez-Bataillon, P.; Monchau, F.; Bigerelle, M.; Hildebrand, H.F. In vitro MC3T3 osteoblast adhesion with respect to surface roughness of Ti6Al4V substrates. Biomol. Eng. 2002, 19, 133–141. [Google Scholar] [CrossRef]
- Meachim, G.; Williams, D. Changes in nonosseous tissue adjacent to titanium implants. J. Biomed. Mater. Res. 1973, 7, 555–572. [Google Scholar] [CrossRef]
- Fojt, J. Ti–6Al–4V alloy surface modification for medical applications. Appl. Surf. Sci. 2012, 262, 163–167. [Google Scholar] [CrossRef]
- Evans, S.L.; Gregson, P.J. The effect of a plasma-sprayed hydroxyapatite coating on the fatigue properties of Ti-6Al-4V. Mater. Lett. 1993, 16, 270–274. [Google Scholar] [CrossRef]
- Kweh, S.W.K.; Khor, K.A.; Cheang, P. Plasma-sprayed hydroxyapatite (HA) coatings with flame-spheroidized feedstock: Microstructure and mechanical properties. Biomaterials 2000, 21, 1223–1234. [Google Scholar] [CrossRef]
- Wen, C. Surface coating and modification of metallic biomaterials; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Sidane, D.; Chicot, D.; Yala, S.; Ziani, S.; Khireddine, H.; Iost, A.; Decoopman, X. Study of the mechanical behavior and corrosion resistance of hydroxyapatite sol–gel thin coatings on 316 L stainless steel pre-coated with titania film. Thin Solid Films 2015, 593, 71–80. [Google Scholar] [CrossRef]
- Pilliar, R.; Deporter, D.; Watson, P.; Pharoah, M.; Chipman, M.; Valiquette, N.; Carter, S.; De Groot, K. The effect of partial coating with hydroxyapatite on bone remodeling in relation to porous-coated titanium-alloy dental implants in the dog. J. Dent. Res. 1991, 70, 1338–1345. [Google Scholar] [CrossRef]
- Wiria, F.; Leong, K.; Chua, C.; Liu, Y. Poly-ε-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta. Biomater. 2007, 3, 1–12. [Google Scholar] [CrossRef]
- Guizzardi, S.; Montanari, C.; Migliaccio, S.; Strocchi, R.; Solmi, R.; Martini, D.; Ruggeri, A. Qualitative assessment of natural apatite in vitro and in vivo. J. Biomed. Mater. Res. 2000, 53, 227–234. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Knabe, C.; Berger, G.; Gildenhaar, R.; Klar, F.; Zreiqat, H. The modulation of osteogenesis in vitro by calcium titanium phosphate coatings. Biomaterials 2004, 25, 4911–4919. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Shao, Z.; Zhu, D.; Luo, Z. Research Progress Regarding Interfacial Characteristics and the Strengthening Mechanisms of Titanium Alloy/Hydroxyapatite Composites. Materials 2018, 11, 1391. [Google Scholar] [CrossRef]
- Hameed, P.; Gopal, V.; Bjorklund, S.; Ganvir, A.; Sen, D.; Markocsan, N.; Manivasagam, G. Axial Suspension Plasma Spraying: An ultimate technique to tailor Ti6Al4V surface with HAp for orthopaedic applications. Colloids Surf. B 2019, 173, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kawakami, M.; Kuribayashi, K.; Takenaka, T.; Minamide, A.; Tamaki, T. Effects of sintered bovine bone on cell proliferation, collagen synthesis, and osteoblastic expression in MC3T3-E1 osteoblast-like cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1999, 17, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Hamdi, M.; Ramesh, S. Properties of hydroxyapatite produced by annealing of bovine bone. Ceram. Int. 2007, 33, 1171–1177. [Google Scholar] [CrossRef]
- Jang, K.-J.; Cho, W.J.; Seonwoo, H.; Kim, J.; Lim, K.T.; Chung, P.-H.; Chung, J.H. Development and Characterization of Horse Bone-derived Natural Calcium Phosphate Powders. J. Biosyst. Eng. 2014, 39, 122–133. [Google Scholar] [CrossRef]
- Yan, W.-Q.; Nakamura, T.; Kawanabe, K.; Nishigochi, S.; Oka, M.; Kokubo, T. Apatite layer-coated titanium for use as bone bonding implants. Biomaterials 1997, 18, 1185–1190. [Google Scholar] [CrossRef]
- Belcarz, A.; Bieniaś, J.; Surowska, B.; Ginalska, G. Studies of bacterial adhesion on TiN, SiO2–TiO2 and hydroxyapatite thin layers deposited on titanium and Ti6Al4V alloy for medical applications. Thin Solid Films 2010, 519, 797–803. [Google Scholar] [CrossRef]
- Uezono, M.; Takakuda, K.; Kikuchi, M.; Suzuki, S.; Moriyama, K. Hydroxyapatite/collagen nanocomposite-coated titanium rod for achieving rapid osseointegration onto bone surface. J. Biomed. Mater. Res. B 2013, 101B, 1031–1038. [Google Scholar] [CrossRef]
- Niespodziana, K.; Jurczyk, K.; Jakubowicz, J.; Jurczyk, M. Fabrication and properties of titanium–hydroxyapatite nanocomposites. Mater. Chem. Phys. 2010, 123, 160–165. [Google Scholar] [CrossRef]
- Benjamin, J.; Volin, T. The mechanism of mechanical alloying. Metall. Trans. 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- Semiatin, S.; Knisley, S.; Fagin, P.; Barker, D.; Zhang, F. Microstructure evolution during alpha-beta heat treatment of Ti-6Al-4V. Metall. Mater. Trans. A 2003, 34, 2377–2386. [Google Scholar] [CrossRef]
- Prendergast, P.; Taylor, D. Stress analysis of the proximo-medial femur after total hip replacement. J. Biomed. Eng. 1990, 12, 379–382. [Google Scholar] [CrossRef]
- Sumner, D.R.; Galante, J.O. Determinants of stress shielding. Clin. Orthop. Relat. Res. 1991, 274, 202–212. [Google Scholar] [CrossRef]
- Van Rietbergen, B.; Huiskes, R.; Weinans, H.; Sumner, D.; Turner, T.; Galante, J. The mechanism of bone remodeling and resorption around press-fitted THA stems. J. Biomech. 1993, 26, 369–382. [Google Scholar] [CrossRef]
- Schreurs, B.W.; Huiskes, R.; Buma, P.; Slooff, T.J.J.H. Biomechanical and histological evaluation of a hydroxyapatite-coated titanium femoral stem fixed with an intramedullary morsellized bone grafting technique: An animal experiment on goats. Biomaterials 1996, 17, 1177–1186. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Inter. J. Biomater. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Y. On the microstructure of biocomposites sintered from Ti, HA and bioactive glass. Biomaterials 2004, 25, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Dujovne, A.; Bobyn, J.; Krygier, J.; Miller, J.; Brooks, C. Mechanical compatibility of noncemented hip prostheses with the human femur. J. Arthroplasty 1993, 8, 7–22. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Ruys, A.; Wei, M.; Sorrell, C.; Dickson, M.; Brandwood, A.; Milthorpe, B. Sintering effects on the strength of hydroxyapatite. Biomaterials 1995, 16, 409–415. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Yang, J.; Zhang, W.; Wang, Y. Sintering Behavior and Mechanical Properties of Mullite Fibers/Hydroxyapatite Ceramic. Materials 2018, 11, 1859. [Google Scholar] [CrossRef]
- Kumari, R.; Scharnweber, T.; Pfleging, W.; Besser, H.; Majumdar, J.D. Laser surface textured titanium alloy (Ti–6Al–4V)–Part II–Studies on bio-compatibility. Appl. Surf. Sci. 2015, 357, 750–758. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J.V.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta. Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Woo, K.D.; Kim, S.H.; Kim, J.Y.; Park, S.H. Fabrication and Biomaterial Characteristics of HA added Ti-Nb-HA Composite Fabricated by Rapid Sintering. Korean J. Met. Mater. 2012, 50, 86–91. [Google Scholar] [CrossRef]
- Bovand, D.; Yousefpour, M.; Rasouli, S.; Bagherifard, S.; Bovand, N.; Tamayol, A. Characterization of Ti-HA composite fabricated by mechanical alloying. Mater. Des. (1980–2015) 2015, 65, 447–453. [Google Scholar] [CrossRef]

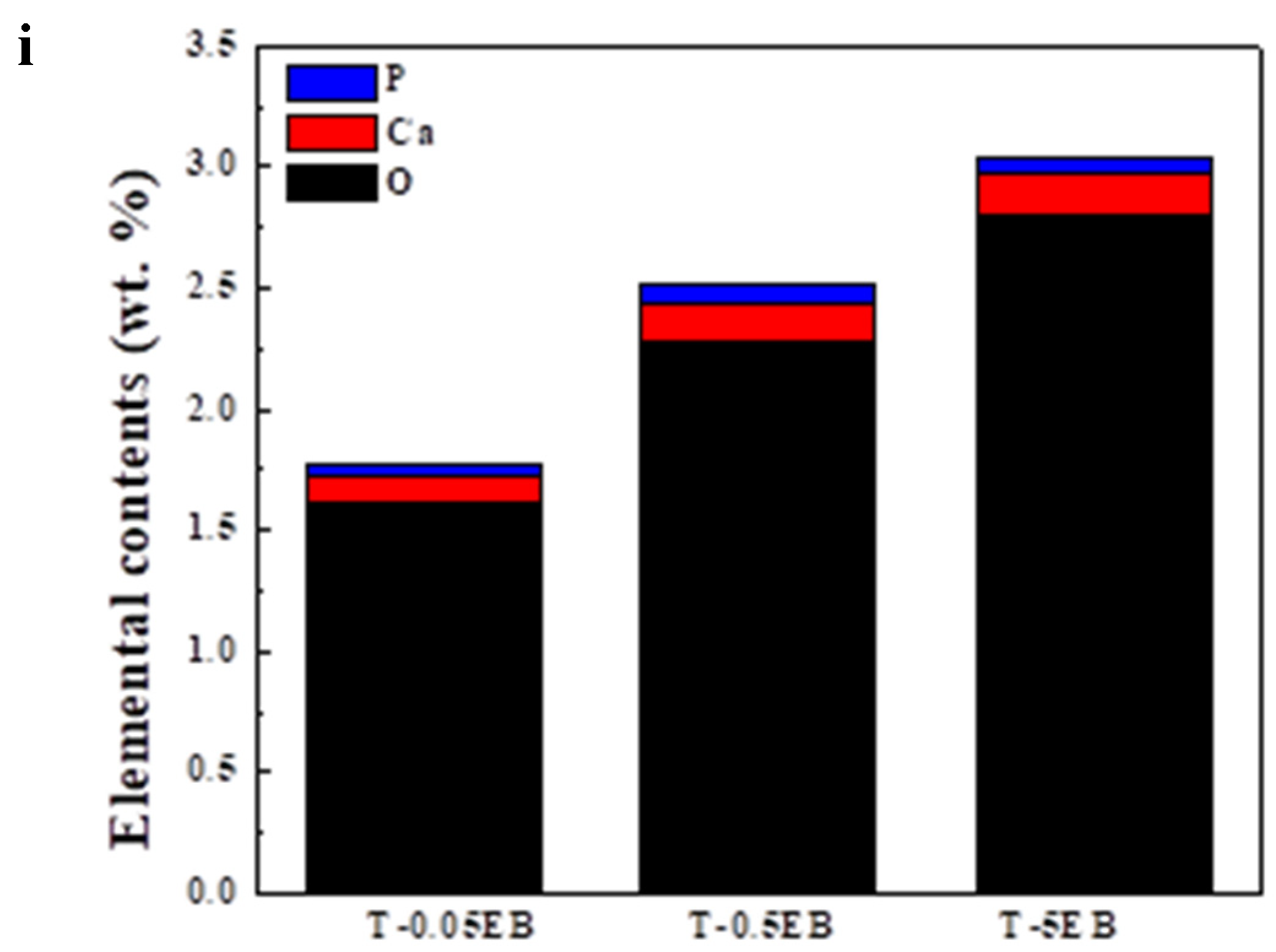
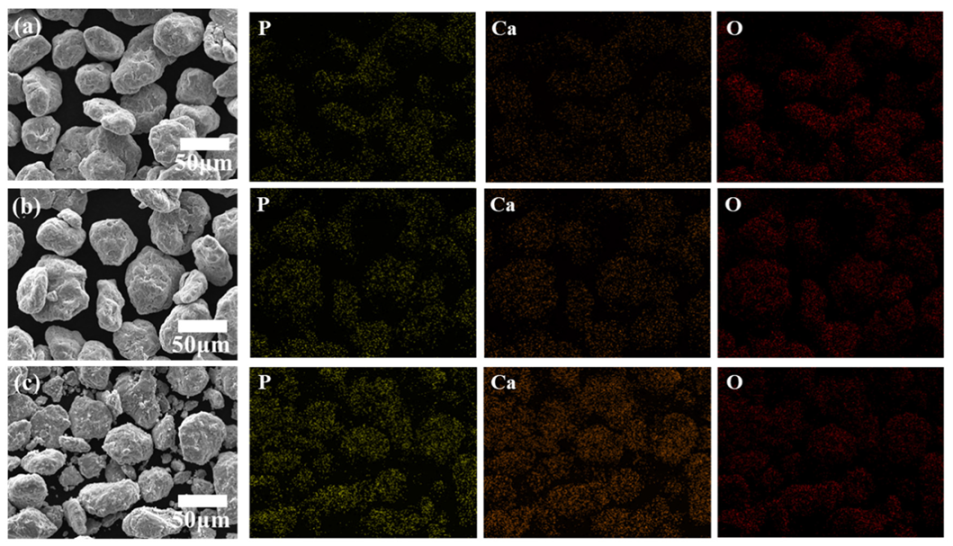
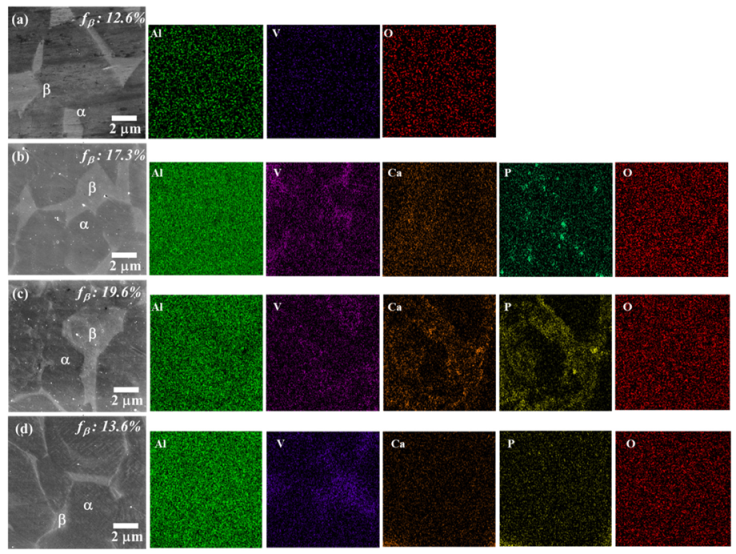

| Element | (a) Ti6Al4V | (b) T-0.05EB | (c) T-0.5EB | (d) T-5EB | ||||
|---|---|---|---|---|---|---|---|---|
| α-Phase | β-Phase | α-Phase | β-Phase | α-Phase | β-Phase | α-Phase | β-Phase | |
| Al | 5.94 | 5.21 | 6.90 | 5.93 | 6.58 | 5.55 | 6.40 | 6.65 |
| P | - | - | 0.04 | 0.05 | 0.00 | 0.09 | 0.03 | 0.05 |
| Ca | - | - | 0.00 | 0.03 | 0.30 | 0.05 | 0.18 | 0.19 |
| O | 0.18 | 0.15 | 1.29 | 1.51 | 1.97 | 2.54 | 2.3 | 4.2 |
| Ti | 90.12 | 89.37 | 88.69 | 84.26 | 88.61 | 83.54 | 88.08 | 79.35 |
| V | 3.76 | 5.28 | 3.08 | 8.22 | 2.54 | 8.23 | 3.01 | 5.37 |
| Totals | 100.00 | 100.00 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, W.; Shin, S.-E.; Choi, H. Microstructure and Mechanical Properties of Titanium–Equine Bone Biocomposites. Metals 2020, 10, 581. https://doi.org/10.3390/met10050581
Jeong W, Shin S-E, Choi H. Microstructure and Mechanical Properties of Titanium–Equine Bone Biocomposites. Metals. 2020; 10(5):581. https://doi.org/10.3390/met10050581
Chicago/Turabian StyleJeong, Wonki, Se-Eun Shin, and Hyunjoo Choi. 2020. "Microstructure and Mechanical Properties of Titanium–Equine Bone Biocomposites" Metals 10, no. 5: 581. https://doi.org/10.3390/met10050581
APA StyleJeong, W., Shin, S.-E., & Choi, H. (2020). Microstructure and Mechanical Properties of Titanium–Equine Bone Biocomposites. Metals, 10(5), 581. https://doi.org/10.3390/met10050581






