In their comments, Lacaze and Castro-Roman [1] seek to correct some statements that we made in our recent paper published in this journal in February 2020 [2] regarding the model for spheroidal graphite growth developed by Lacaze et al. [3]. We will take this opportunity to demonstrate that our statements were accurate.
Our first criticism addressed the choice of the range of the undercooling, ΔT. Specifically we stated that an “undercooling higher than about 50 K will be conducive to metastable structure in Fe-C-Si 57 alloys”. Lacaze and Castro-Roman [1] claim that we “misunderstood the undercooling as … evaluated with respect to the eutectic instead of with respect to the graphite liquidus”. To debate this issue, we need to put it into the larger context of the basic equations developed in the original paper by Lacaze et al. [3].
Their nucleation equation, based on the 2-D nucleation models developed for pure substances by Cahn et al. [4] and by Hillig [5], is (Equation (1) in ref. [3]):
where is Avogadro’s number, is the molar volume of the liquid, L is the melting enthalpy, is the melting temperature, is the current temperature, is the undercooling, is a correction for structural factors, D is the liquid diffusivity, is the atomic radius, is the excess energy needed for nucleus formation, and is Boltzmann’s constant. Lacaze et al. attempted to adapt this equation to the crystallization of graphite from liquid Fe-C binary alloys. After some manipulation, they produce their final equation for nucleation (Equation (11) in ref. [3]):
Here, ξ is the diffuseness parameter, and is the undercooling with respect to the liquidus. To arrive at this equation, Lacaze et al. introduce some simplifications and assumptions. We will discuss some of them and demonstrate that they invalidate the ensuing calculations.
First: from the analysis of Hillig’s paper we found that the term in Equation (1) is valid in the particular case of a diffuseness parameter . A more complex equation is suggested for ξ ≪ 1. Cahn et al. [4] also state that “diffuseness is of the order of 1 for sharp interfaces”. As graphite crystallizes with a sharp interface, should be used for this case. Thus, Equation (1) is only valid for a diffuseness of 1. However, diffuseness reappears in Equation (2). According to Lacaze et al., “ξ is interface diffuseness which is 1 at most for a sharp interface and can be much smaller in practice”. This statement is claimed to be supported by a quotation of the Cahn et al. paper [4]. There is no such statement in the quoted paper. In the Lacaze et al. paper, diffuseness values of 0.5 and 0.1 are unjustifiably used as adjustable parameters in the nucleation Equation (11) and in the growth Equations (17) and (29) in ref. [3], the three main equations of the paper.
Second: the calculation of in Equation (1) requires a value for the molar free enthalpy change upon the melting of the solid, . Lacaze et al. use the approximation from the work of Hillert and Subba-Rao [6], which is an analysis for eutectic cast iron. This was derived on the basis of 1415 K, the eutectic temperature, and for the eutectic composition. Thus, is the undercooling with respect to the eutectic temperature of the Fe-C alloy, and not with respect to the liquidus as is the case in the Lacaze paper. We now have three different definitions of undercooling in Equations (1) and (2): (Cahn, Hillig), (Hillert) and (Lacaze). We emphasize that calculations restricted to the eutectic composition cannot be extended to derive Equation (2) (Equation (11) in ref. [3]) and the following ones.
Third: calculations in the Lacaze paper are made for the eutectic composition, xC = 0.175 (see also Equation (2) in [3]) and . The selection of this particular temperature is not explained. They state “ is the undercooling with respect to the graphite liquidus”, but the calculation is for the eutectic composition, which explains our comment on undercooling [2]. is composition-dependent, yet the composition does not appear in Equation (2). Thus, ignoring the composition as in ref. [3], we can assume a slightly hypereutectic composition where . Then, an undercooling of 250 K with respect to produces a temperature of 1176 K, well down into the solid state. This validates our first criticism regarding inappropriate values and selection of undercooling.
Our second criticism refers to calculations that Lacaze et al. performed with the growth equations that they have developed. In particular, they refer to our statement, “also notice some numerical incongruities: At a reasonable undercooling of 25 K (thin vertical line on the figure) it will take 3 hrs. for the formation of a 1 µm graphite spheroid.” which addresses the calculation in Figure 5 in ref. [3]. They claim that we “mixed up the plots” and that we used the wrong equation (Equation (17) in ref. [3] rather than Equation (29) in ref. [3]). Again, we will proceed by analyzing the growth equations proposed by Lacaze et al. by extending the 2-D poly-nucleation mechanism developed by Amini and Abbaschian [7] for plate-like graphite growing in Ni-C alloys to spheroidal graphite growing in Fe-C alloys. To address this issue, let us analyze the growth equations. As indicated in the Comment [1], the Lacaze et al. 2-D nucleation model expresses the overall growth rate of a nodule from the liquid as (Equation (14) in ref. [3]):
where a is the height of one atom layer, is the lateral spreading rate of the growth units, and other symbols are as before. The subscript PNG stands for poly-layer growth. As this equation includes , which as we demonstrated is calculated with the defective Equation (2), this and any other equation that includes is invalid. However, after using Equation (13) from Cahn et al. [4] and assigning numerical values as discussed before, they derive a first growth equation (Equation (17) in [3], Equation (2) in [1]):
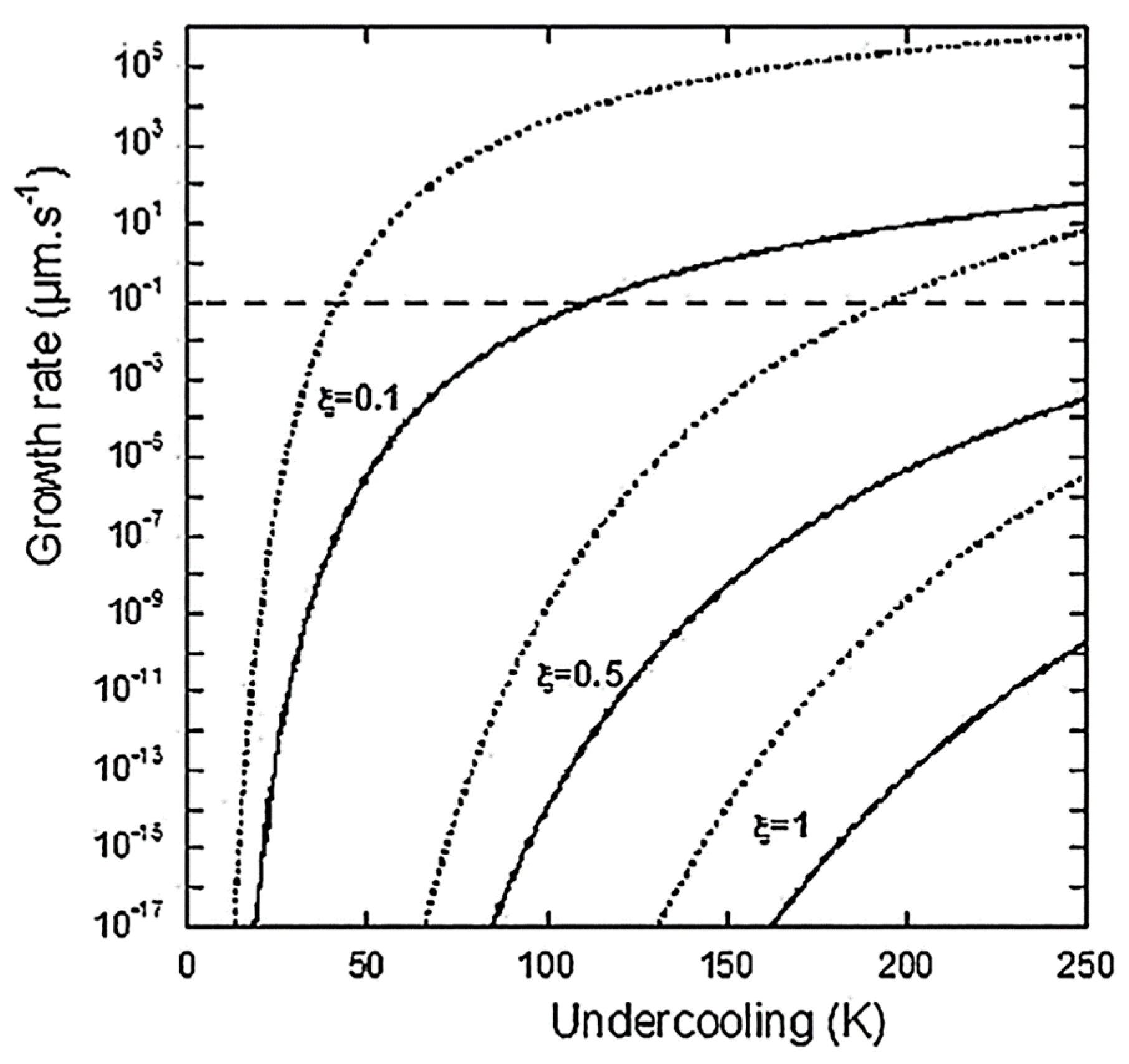
where is again the undercooling from the graphite liquidus. Lacaze et al. then state “… the PNG model is expected to predict too high growth rates”. This does not deter them from claiming, “Figure 5 shows with dotted lines the evolution of GPNG according to Equation (17) for β set to 1 and ξ set to 0.1, 0.5 and 1. The horizontal interrupted line corresponds to the experimental thickening rate of graphite plates along their c-direction as estimated by Amini and Abbaschian [7]. This value of 0.1 µm/s is obtained for an undercooling of 40 K at ξ = 0.1 and 200 K at ξ = 0.5, both values being well within the reported experimental range of undercooling”. The reader is referred to Figure 1 and Figure 2 in this reply for further revelations. Thus, while in the Comment [1] Lacaze objected to our use of their Equation (17) in ref. [3] for the calculation of the growth rate in their Figure 5 in ref. [3], they use it for validation of their theory on the same figure! How can they state that we were wrong when we criticized the numerical incongruities generated by this particular equation? Then, they proceed to the development of another growth equation, this time based on a solution by Bosze and Trivedi [8] (Equation (29) in ref. [3]; Equation (3) in ref. [1]):
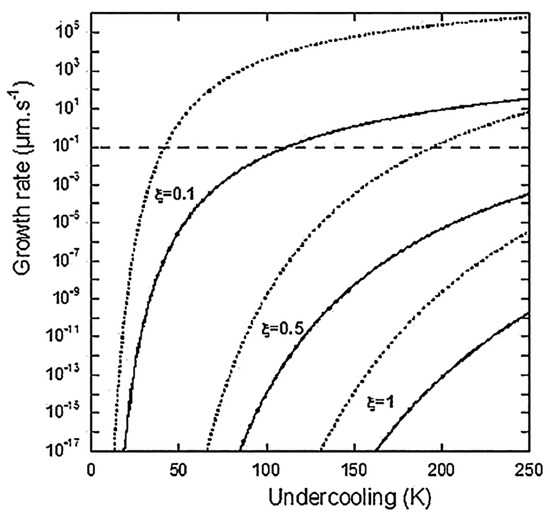
Figure 1.
Overall growth rate of a graphite spheroid according to the PNG model as a function of undercooling, for three values of . Dotted lines are according to Equation (17) in ref. [3] and solid lines are according to Equation (29) in ref. [3].
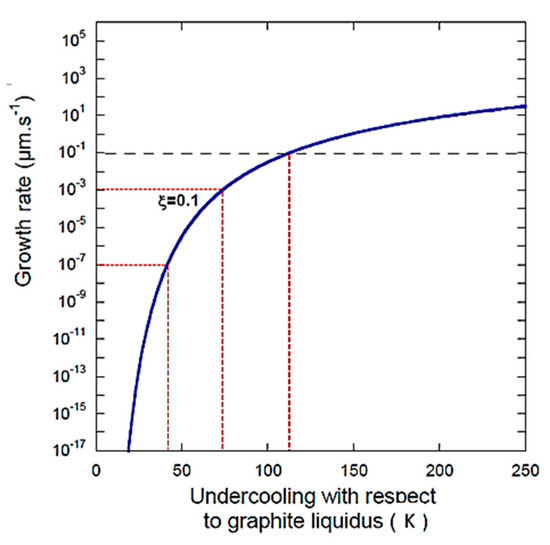
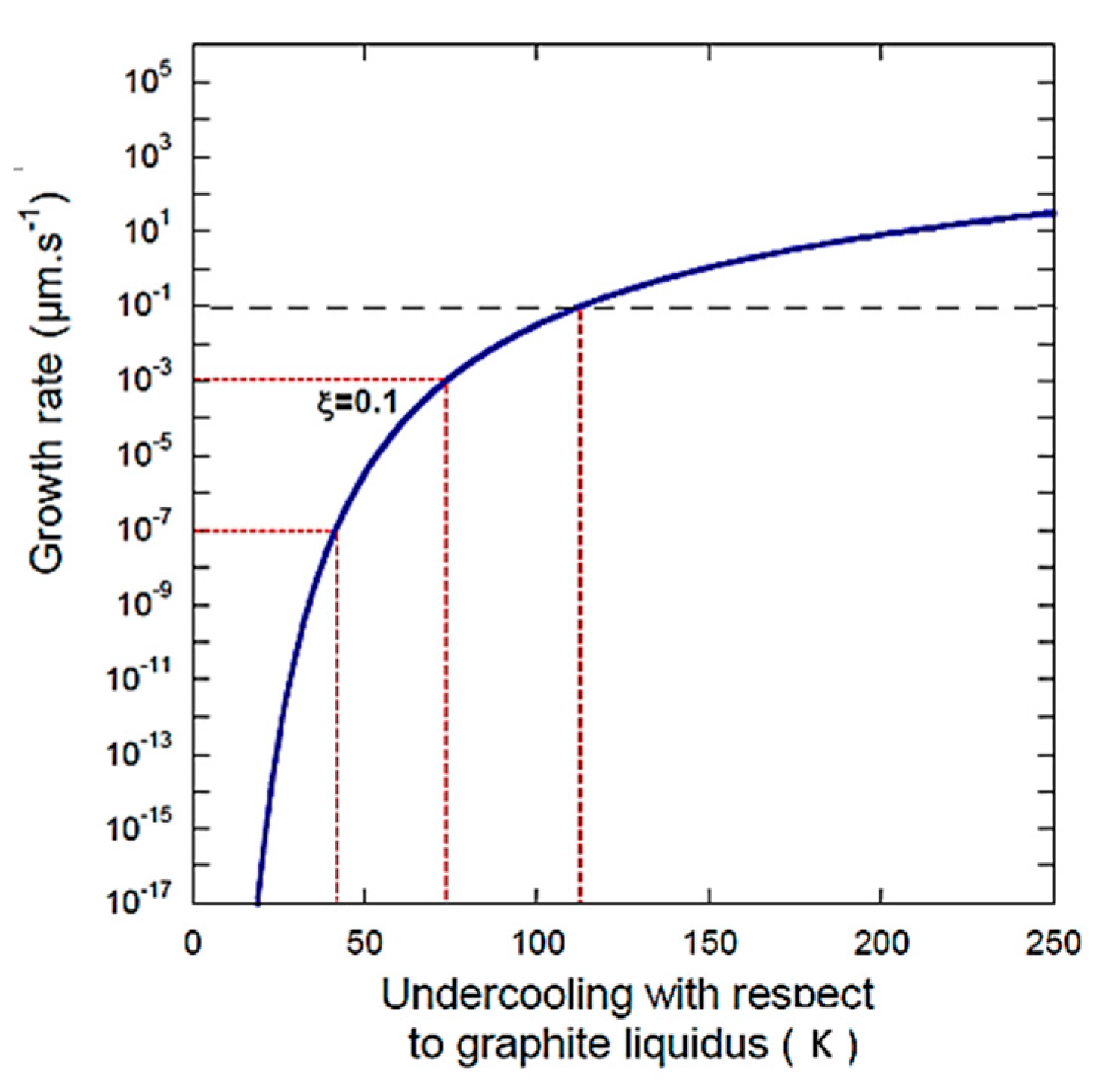
Figure 2.
Growth rate according to the PNG model of Equation (5), as a function of undercooling (Figure 1b in ref. [1], Figure 5 in ref. [3]) showing data selected for the calculation in Table 1.
Unfortunately, as for their previous equation, the equation is plagued by the same erroneous deviation for the nucleation rate; Equations (2), (4) and (5) are used to generate the data in Figure 5 in ref. [3]. The caption of Figure 5 in ref. [3] reads “Overall growth rate of a graphite spheroid according to the PNG model as function of undercooling …” Thus, the times to grow graphite particles to given sizes can be calculated for different undercoolings. As requested by Lacaze and Castro-Roman, using data from Figure 1b in the Comment [1] calculated from Equation (5), we present some new calculations in Table 1, together with Figure 2 that plots the data in Table 1 on the original Figure 5 in ref. [3]. It is seen that the growth of 1 µm of graphite requires 1.08 h at 73 K undercooling, and an incredible 3.59·108 h at 27 K undercooling. This validates our second criticism regarding unreasonable values for the growth rate of graphite.

Table 1.
Calculation of time to grow 1 µm of graphite based on Equation (5) (Equation (29) in ref. [3])
We further note that from Figure 5 from ref. [3], reproduced here as Figure 1, we can obtain a wide, unreasonable range of values, depending on the equation selection and the use of the adjustable parameter . Taking only the best-case scenario shown in Figure 2, as suggested by Lacaze et al., an increase in undercooling from 27 to 113 K produces a change in the growth rate from 7.74 × 10−13 µm/s to 2.91 × 10−2, an incredible and unrealistic 11 orders of magnitude.
The third and final objection in the Comment [1] refers to the use of adjustable parameters. Lacaze and Castro-Roman state that we “wrongly accused to use a high number of fitting parameters, while there was only a single one, the interface diffuseness”. This is only correct if the figures are concerned. As we have demonstrated that in the case of graphite must be used, and as we showed the extent to which the calculations produce unrealistic results, even this should be enough. However, setting β to 1 (while calculations by Cahn from experimental data conclude that for “all symmetrical molecules appears to be of the order of 10”) and setting a to two different constant values (“a is the height of one single atom layer” and then later “ m is the distance between the basal planes”), can also be construed as an exercise in fitting.
To conclude, the attempt by Lacaze et al. [3] to extend the Cahn/Hillig model for the 2-D nucleation of pure substances to the crystallization of spheroidal graphite from Fe-C alloys failed because of misinterpretations of conditions in the derivation of the main equations, and because of several ill-defined parameters. This failed attempt at analytical modelling supports our statement in ref. [2]: “analytical models cannot describe the complexities associated with crystallization of spheroidal graphite”, may be with the caveat that carefully developed models, but certainly not the one discussed here, could provide some general trends.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lacaze, J.; Castro-Roman, M.J. Comment on Stefanescu, D.M.; Alonso, G.; Suarez, R. Recent Developments in Understanding Nucleation and Crystallization of Spheroidal Graphite in Iron-Carbon-Silicon Alloys. Metals 2020, 10, 221. Metals 2020, 10, 471. [Google Scholar] [CrossRef]
- Stefanescu, D.M.; Alonso, G.; Suarez, R. Recent developments in understanding nucleation and crystallization of spheroidal graphite in Iron-Carbon-Silicon alloys. Metals 2020, 10, 221. [Google Scholar] [CrossRef]
- Lacaze, J.; Bourdie, J.; Castro-Roman, M.J. A 2-D nucleation-growth model of spheroidal graphite. Acta Mater. 2017, 134, 230–235. [Google Scholar] [CrossRef]
- Cahn, J.W.; Hillig, W.B.; Sears, G.W. The molecular mechanism of solidification. Acta Metall. 1964, 12, 1421–1439. [Google Scholar] [CrossRef]
- Hillig, W.B. A derivation of classical two-dimensional nucleation kinetics and associated crystal growth laws. Acta Metall. 1966, 14, 1868–1869. [Google Scholar] [CrossRef]
- Hillert, M.; Subba-Rao, V.V. Grey and White Solidification of Cast Iron. In The Solidification of Metals; The Inst. of Metals: London, UK, 1968; pp. 204–212. [Google Scholar]
- Amini, S.; Abbaschian, R. Nucleation and growth kinetics of graphene layers from a molten phase. Carbon 2013, 51, 110–123. [Google Scholar] [CrossRef]
- Bosze, W.P.; Trivedi, R. On the kinetic expression for the growth of precipitate plates. Metall. Trans. 1974, 5, 511–512. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).