In Vitro and In Vivo Study of Titanium Grade IV and Titanium Grade V Implants with Different Surface Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Preparation of the Titanium Disc
2.1.2. Material Characterizations
2.1.3. In Vitro Cell Culture
2.2. In Vivo Study
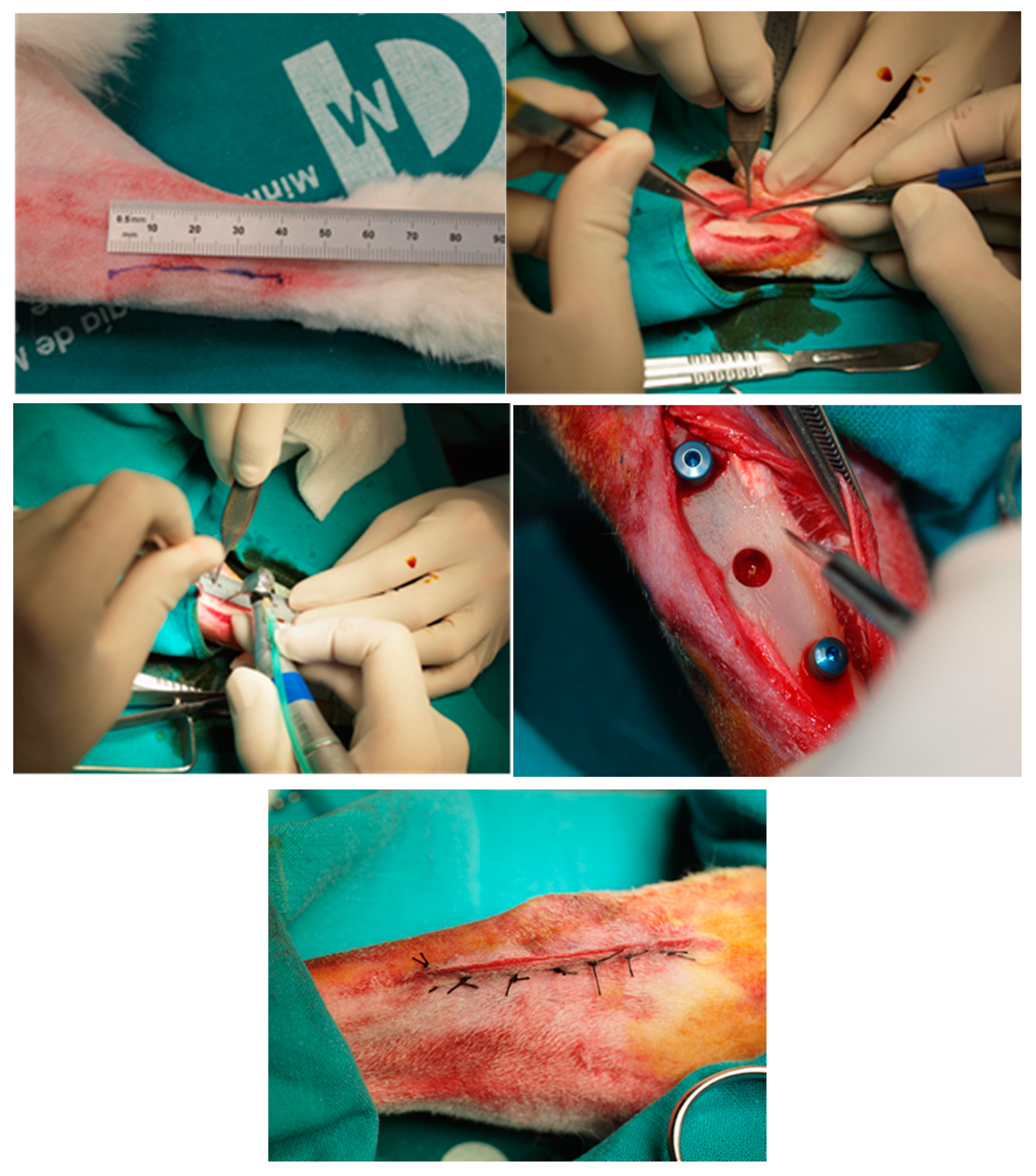
2.2.1. Animal Experimentation Specimens and Surgical Procedure
2.2.2. Bone Density (BoneJ) Comparison
- –
- Bone Surface Area (BS), which involves measuring the surface of a structure to characterize 3D objects such as trabecular bone and can be calculated by the construction of a triangular surface mesh using the marching cubes algorithm, thus computing the sum of the triangular areas making up the mesh to obtain the total bone surface area [29,30].
- –
- Trabecular Thickness (TBTH) and Max. Trabecular thickness. Trabecular bone microarchitecture is calculated by a plugin based on Bob Dougherty’s Local Thickness Plugin, which assesses the mean and the standard deviation directly from the pixel values in the resulting thickness map. These parameters are used as predictors for the resistance to fracture and the healing process [31,32].
- –
- Bone Volume, Total Volume, and the Bone Volume Fraction (BV/TV). These parameters are obtained from a simple voxel-counting method or from volumetric marching cubes (VOMACs). This analysis may enhance our capacity to predict resistance to fracture due to the correlation with bone mechanical properties and quantity [33,34].
- –
- –
- Number of branches (branches), the number of (i) junctions (junctions), (ii) end-point voxels (voxels), (iii) junction voxels (voxels), average branch length (pixels), as well as the number of triple points (points), quadruple points and the maximum branch length (pixels).
2.2.3. Histological Sample Processing
2.3. Statistical Analysis
3. Results
3.1. Material Characterisations
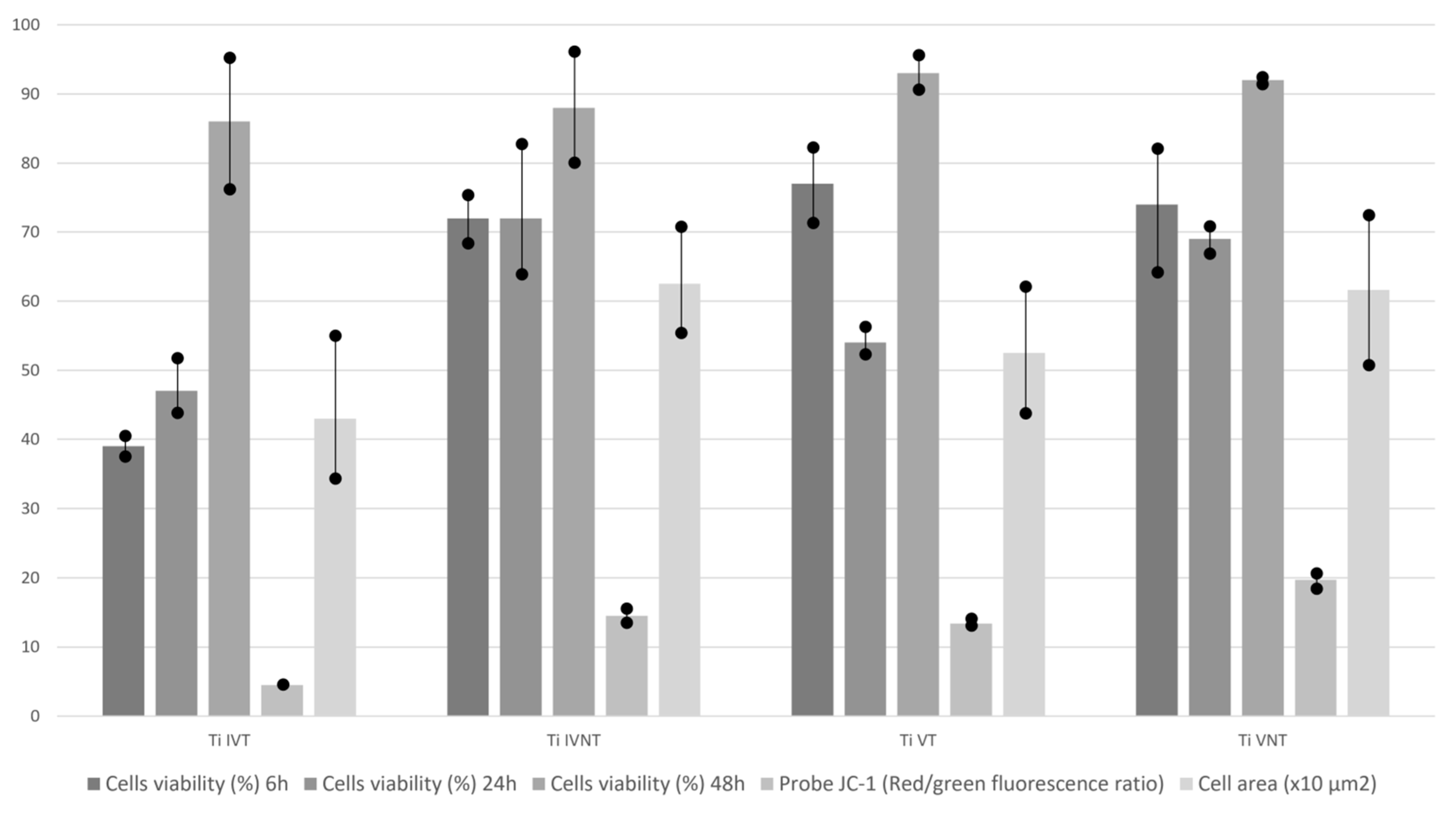
3.2. In Vitro Cell Culture
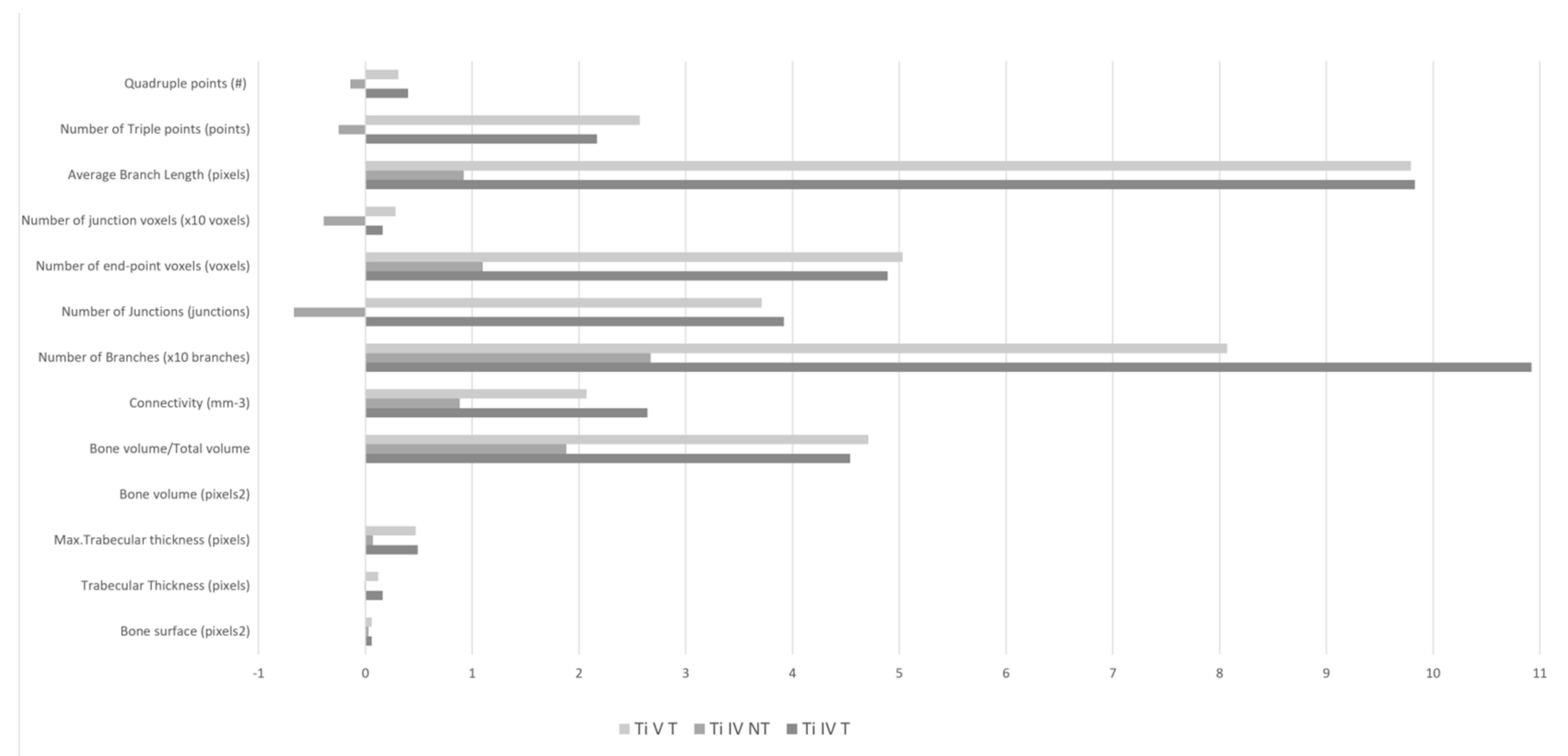
3.3. Comparison of Bone Density (BoneJ)
3.4. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niu, W.; Wang, P.; Zhu, S.; Liu, Z.; Ji, P. Marginal bone loss around dental implants with and without microthreads in the neck: A systematic review and meta-analysis. J. Prosthet. Dent. 2016, 117, 34–40. [Google Scholar] [CrossRef]
- Moraschini, V.; Velloso, G.; Luz, D.; Barboza, E.P. Implant survival rates, marginal bone level changes, and complications in full-mouth rehabilitation with flapless computer-guided surgery: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2015, 44, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Koodaryan, R.; Hafezeqoran, A. Evaluation of Implant Collar Surfaces for Marginal Bone Loss: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2016, 2016, 4987526. [Google Scholar] [CrossRef] [PubMed]
- Charyeva, O.; Altynbekov, K.; Zhartybaev, R.; Sabdanaliev, A. Long-term dental implant success and survival—A clinical study after an observation period up to 6 years. Swed. Dent. J. 2012, 36, 1–6. [Google Scholar] [PubMed]
- Van Velzen, F.J.J.; Ofec, R.; Schulten, E.A.J.M.; Bruggenkate, C.M. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: A prospective cohort study in 177 fully and partially edentulous patients. Clin. Oral Implant. Res. 2015, 26, 1121–1128. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000, 73, 22–40. [Google Scholar] [CrossRef]
- Ribeiro da Silva, J.; Castellano, A.; Malta Barbosa, J.P.; Gil, L.F.; Marin, C.; Granato, R.; Bonfante, E.A.; Tovar, N.; Janal, M.N.; Coelho, P.G. Histomorphological and Histomorphometric Analyses of Grade IV Commercially Pure Titanium and Grade V Ti-6Al-4V Titanium Alloy Implant Substrates: An In Vivo Study in Dogs. Implant. Dent. 2016, 25, 650–655. [Google Scholar] [CrossRef]
- Bothe, R.; Beaton, L.; Davenport, H. Reaction of bone to multiple metallic implants. Surg. Gynecol. Obstet. 1940, 71, 598–602. [Google Scholar]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- International a ASTM B265–13ae1, Standard Specification for Titanium and Titanium Alloy Strip, Sheet, and Plate; ASTM International: West Conshohocken, PA, USA, 2013.
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.; Bonfante, E.A.; Machado, L.S.; Tovar, N.; Coelho, P.G. Mechanical evaluation of two titanium alloy grades used in implant dentistry. Int. J. Oral Maxillofac. Implant. 2015, 30, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Gómez-Moreno, G.; Aguilar-Salvatierra, A.; Guardia, J.; Delgado-Ruiz, R.A.; Romanos, G.E. Marginal bone loss evaluation around immediate non-occlusal microthreaded implants placed in fresh extraction sockets in the maxilla: A 3-year study. Clin. Oral Implant. Res. 2015, 26, 761–767. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. Conjoint corrosion and wear in titanium alloys. Biomaterials 1999, 20, 765–772. [Google Scholar] [CrossRef]
- Saulacic, N.; Bosshardt, D.D.; Bornstein, M.M.; Berner, S.; Buser, D. Bone apposition to a titanium-zirconium alloy implant, as compared to two other titanium-containing implants. Eur. Cells Mater. 2012, 23, 273–286. [Google Scholar] [CrossRef]
- Johansson, C.B.; Han, C.H.; Wennerberg, A.; Albrektsson, T. A quantitative comparison of machined commercially pure titanium and titanium-aluminum vanadium implants in rabbit bone. Int. J. Oral Maxillofac. Implant. 1998, 13, 315–321. [Google Scholar]
- Han, C.H.; Johansson, C.B.; Wennerberg, A.; Albrektsson, T. Quantitative and qualitative investigations of surface enlarged titanium and titanium alloy implants. Clin. Oral Implant. Res. 1998, 9, 1–10. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.K.; Lee, H.R.; Lee, S.J.; Lee, D.; Um, S.H.; Lee, J.H.; Woo, Y.H.; Zhang, L.G.; Lee, D.W.; et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid Interface Sci. 2016, 469, 129–137. [Google Scholar] [CrossRef]
- Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.-A.; Torres-Lagares, D. Superficial Characteristics of Titanium after Treatment of Chorreated Surface, Passive Acid, and Decontamination with Argon Plasma. J. Funct. Biomater. 2018, 9, 71. [Google Scholar] [CrossRef]
- Di Toro, R.; Betti, V.; Spampinato, S. Biocompatibility and integrin-mediated adhesion of human osteoblasts to poly (dl–lactide–co–glycolide) copolymers. Eur. J. Pharm. Sci. 2004, 21, 161–169. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Lv, W.; Zhang, M.; Yang, S.; Yin, L.; Hong, J.; Han, D.; Chen, C.; Swarts, S.; et al. Interleukin 11 protects bone marrow mitochondria from radiation damage. Adv. Exp. Med. Biol. 2013, 789, 257–264. [Google Scholar]
- Waggoner, A.; DeBiasio, R.; Conrad, P.; Bright, G.R.; Ernst, L.; Ryan, K.; Nederlof, M.; Taylor, D. Multiple spectral parameter imaging. Methods Cell Biol. 1989, 30, 449–478. [Google Scholar] [PubMed]
- Kubista, M.; Aakerman, B.; Norden, B. Characterization of interaction between DNA and 40,6-diamidino-2-phenylindolebyopticalspectroscopy. Biochemistry 1987, 26, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Reichart, P.A.; Buser, D.; Bosshardt, D.D. Tissue response and wound healing after placement of two types of bioengineered grafts containing vital cells in submucosal maxillary pouches: An experimental pilot study in rabbits. Int. J. Oral Maxillofac. Implant. 2011, 26, 768–775. [Google Scholar]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.L.; Batista-Cruzado, A.; Lynch, C.D.; Sloan, A.J.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. Pre-prosthetic use of poly (lactic–co–glycolicacid) membranes treated with oxygen plasma and TiO2 nanocomposite particles for guided bone regeneration processes. J. Dent. 2016, 47, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.N.; Keane, D.T. X-ray microtomography. Mater. Charact. 2010, 61, 1305–1316. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordeliéres, F.; Dougherty, R.P.; Jackson, J.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Fan, Y.; Li, Z.; Lv, C.; Zhang, B. Bone surface mapping method. PLoS ONE 2012, 7, e32926. [Google Scholar] [CrossRef]
- Morgan, E.F.; Mason, Z.D.; Chien, K.B.; Pfeiffer, A.J.; Barnes, G.L.; Einhorn, T.A.; Gerstenfeld, L.C. Micro-computed tomography assessment of fracture healing: Relationships among callus structure, composition, and mechanical function. Bone 2009, 44, 335–344. [Google Scholar] [CrossRef]
- Bart, Z.R.; Wallace, J.M. Microcomputed tomography applications in bone and mineral research. Adv. Comput. Tomogr. 2013, 2, 121. [Google Scholar] [CrossRef]
- Casanova, M.; Schindeler, A.; Little, D.; Müller, R.; Schneider, P. Quantitative phenotyping of bone fracture repair: A review. BoneKEy Rep. 2014, 3, 550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Jean-Pierre, J.; René, C. Accelerating volume rendering with quantized voxels. In Proceedings of the 2000 IEEE Symposium on Volume Visualization; ACM: New York, NY, USA, 2000; pp. 63–70. [Google Scholar]
- Ginzburg, V.L.; Gören, Y. Iterated index and the mean Euler characteristic. J. Topol. Anal. 2015, 7, 453–481. [Google Scholar] [CrossRef]
- Odgaard, A.; Gundersen, H.J. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 1993, 14, 173–182. [Google Scholar] [CrossRef]
- Tjong, W.; Kazakia, G.J.; Burghardt, A.J.; Majumdar, S. The effect of voxel size on high-resolution peripheral computed tomography measurements of trabecular and cortical bone microstructure. Med. Phys. 2012, 39, 1893–1903. [Google Scholar] [CrossRef]
- Ito, M. Recent progress in bone imaging for osteoporosis research. J. Bone Min. Metab. 2011, 29, 131–140. [Google Scholar] [CrossRef]
- Lopez-Píriz, R.; Fernández, A.; Goyos-Ball, L.; Rivera, S.; Díaz, L.A.; Fernández-Domínguez, M.; Prado, C.; Moya, J.S.; Torrecillas, R. Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs. Materials 2017, 10, 614. [Google Scholar] [CrossRef]
- Hannigan, A.; Lynch, C.D. Statistical methodology in oral and dental research: Pitfalls and recommendations. J. Dent. 2013, 41, 385–392. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tanaka, A.; Murata, A.; Sakurada, T. Formulation for XPS spectral change of oxides by ion bombardment as a function of sputtering time. Surf. Sci. 2004, 556, 22–32. [Google Scholar] [CrossRef]
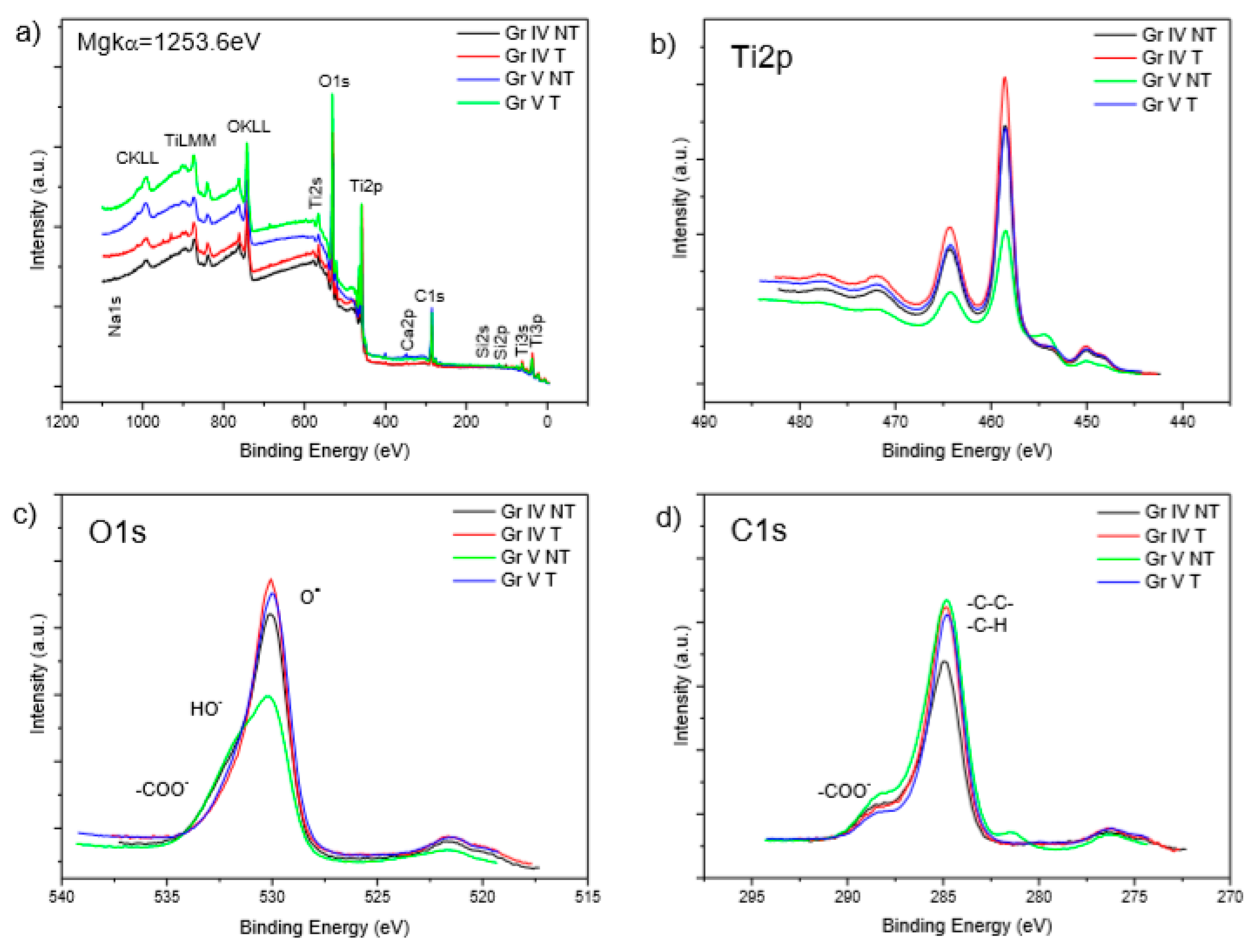
| Element | TiIVNT | TiIVT | TiVNT | TiVT |
|---|---|---|---|---|
| %Ti 2p | 16.9 | 21.5 | 11.4 | 17.8 |
| %O 1s | 46.2 | 47.0 | 37.4 | 44.7 |
| %C 1s | 31.6 | 31.4 | 43.5 | 33.5 |
| %Ca 2p | 0.2 | 0.3 | 0.7 | 0.1 |
| %Al 2p | - | - | 2.4 | 3.4 |
| %Si 2p | 5.0 | - | 1.0 | - |
| In Vitro Cell Culture Results | TiIVT | TiIVNT | TiVT | TiVNT | p | |
|---|---|---|---|---|---|---|
| Cell viability (%) | 6 h | 39 ± 3 | 72 ± 7 | 77 ± 11 | 74 ± 18 | NS |
| 24 h | 47 ± 8 | 72 ± 19 | 54 ± 4 | 69 ± 4 | NS | |
| 48 h | 86 ± 19 | 88 ± 16 | 93 ± 5 | 92 ± 1 | NS | |
| Probe JC-1 (Red/green fluorescence ratio) | 4.5 ± 0.6 | 14.5 ± 2 | 13.4 ± 1 | 19.7 ± 2.2 | NS | |
| Cell area (μm2) | 430 ± 208 | 625 ± 154 | 525 ± 184 | 616 ± 218 | NS | |
| Comparison of Bone Density (BoneJ) | TiIVT | TiIVNT | TiVT | N.S. |
|---|---|---|---|---|
| Bone surface (pixels 2) | 1.85 ± 5.55 | 0.19 ± 4.84 | 2.00 ± 5.59 | |
| Trabecular thickness (pixels) | 0.06 ± 0.19 | 0.03 ± 0.09 | 0.06 ± 0.19 | |
| Max. trabecular thickness (pixels) | 0.16 ± 0.28 | −0.01 ± 0.18 | 0.12 ± 0.28 | |
| Bone volume (pixels 2) | 0.49 ± 2.03 | 0.07 ± 1.44 | 0.47 ± 2.03 | |
| Bone volume/total volume | 0.00 ± 0.04 | 0.00 ± 0.03 | 0.00 ± 0.04 | |
| Connectivity (mm-3) | 4.54 ± 58.15 | 1.88 ± 19.93 | 4.71 ± 58.15 | |
| Number of branches (branches) | 26.42 ± 104.39 | 8.83 ± 47.87 | 20.71 ± 104.39 | |
| Number of junctions (junctions) | 10.92 ± 52.50 | 2.67 ± 26.26 | 8.07 ± 52.50 | |
| Number of end-point voxels (voxels) | 3.92 ± 20.67 | −0.67 ± 7.45 | 3.71 ± 20.67 | |
| Number of junction voxels (voxels) | 48.92 ± 171.31 | 11.00 ± 65.29 | 50.29 ± 171.31 | |
| Average branch length (pixels) | 0.16 ± 0.13 | −0.39 ± 1.22 | 0.28 ± 0.13 | <0.05 |
| Number of triple points (points) | 9.83 ± 33.38 | 0.92 ± 19.39 | 9.79 ± 33.38 | |
| Quadruple points | 217 ± 10.42 | −0.25 ± 5.80 | 2.57 ± 10.42 | |
| Maximum branch length (pixels) | 0.40 ± 0.83 | −0.14 ± 1.54 | 0.31 ± 0.83 |
| Histological Analysis | TiIVT | TiIVNT | TiVT | N.S. |
|---|---|---|---|---|
| Bone height (µm) | 4759.83 ± 1928.98 | 3724.68 ± 1900.63 | 4825.76 ± 1928.98 | |
| Bone height (%) | 39.66 ± 16.07 | 31.04 ± 15.84 | 40.21 ± 16.07 | |
| Mature bone height (µm) | 3082.37 ± 1355.53 | 2522.13 ± 1332.88 | 3178.36 ± 1355.53 | |
| Mature bone height (%) | 25.69 ± 11.30 | 21.02 ± 11.11 | 26.49 ± 11.30 | |
| Osteoid (µm) | 1677.46 ± 1326.66 | 1202.55 ± 862.96 | 1647.40 ± 1326.66 | |
| Osteoid (%) | 13.98 ± 11.06 | 10.02 ± 7.19 | 13.73 ± 11.06 | |
| Mean height of bone (µm) | 246.30 ± 139.33 | 157.00 ± 100.72 | 248.10 ± 139.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Sanchez, R.-M.; de-Paz-Carrion, A.; Serrera-Figallo, M.-A.; Torres-Lagares, D.; Barranco, A.; León-Ramos, J.-R.; Gutierrez-Perez, J.-L. In Vitro and In Vivo Study of Titanium Grade IV and Titanium Grade V Implants with Different Surface Treatments. Metals 2020, 10, 449. https://doi.org/10.3390/met10040449
Diaz-Sanchez R-M, de-Paz-Carrion A, Serrera-Figallo M-A, Torres-Lagares D, Barranco A, León-Ramos J-R, Gutierrez-Perez J-L. In Vitro and In Vivo Study of Titanium Grade IV and Titanium Grade V Implants with Different Surface Treatments. Metals. 2020; 10(4):449. https://doi.org/10.3390/met10040449
Chicago/Turabian StyleDiaz-Sanchez, Rosa-Maria, Alvaro de-Paz-Carrion, Maria-Angeles Serrera-Figallo, Daniel Torres-Lagares, Angel Barranco, Juan-Rey León-Ramos, and Jose-Luis Gutierrez-Perez. 2020. "In Vitro and In Vivo Study of Titanium Grade IV and Titanium Grade V Implants with Different Surface Treatments" Metals 10, no. 4: 449. https://doi.org/10.3390/met10040449
APA StyleDiaz-Sanchez, R.-M., de-Paz-Carrion, A., Serrera-Figallo, M.-A., Torres-Lagares, D., Barranco, A., León-Ramos, J.-R., & Gutierrez-Perez, J.-L. (2020). In Vitro and In Vivo Study of Titanium Grade IV and Titanium Grade V Implants with Different Surface Treatments. Metals, 10(4), 449. https://doi.org/10.3390/met10040449








