A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions
Abstract
1. Introduction
2. Experimental
2.1. Materials and Sample Preparation
2.2. Gravimetric Testing and Visual Assessment
2.3. DC Polarization Measurements
2.4. Electrochemical Impedance Spectroscopy
2.5. SEM and EDX Analysis and ATR-FTIR Spectrometry
3. Results and Discussion
3.1. Visual Assessment and Gravimetric Analysis
3.2. Open Circuit Potential Measurement (OCP)
3.3. Linear Polarization Measurements
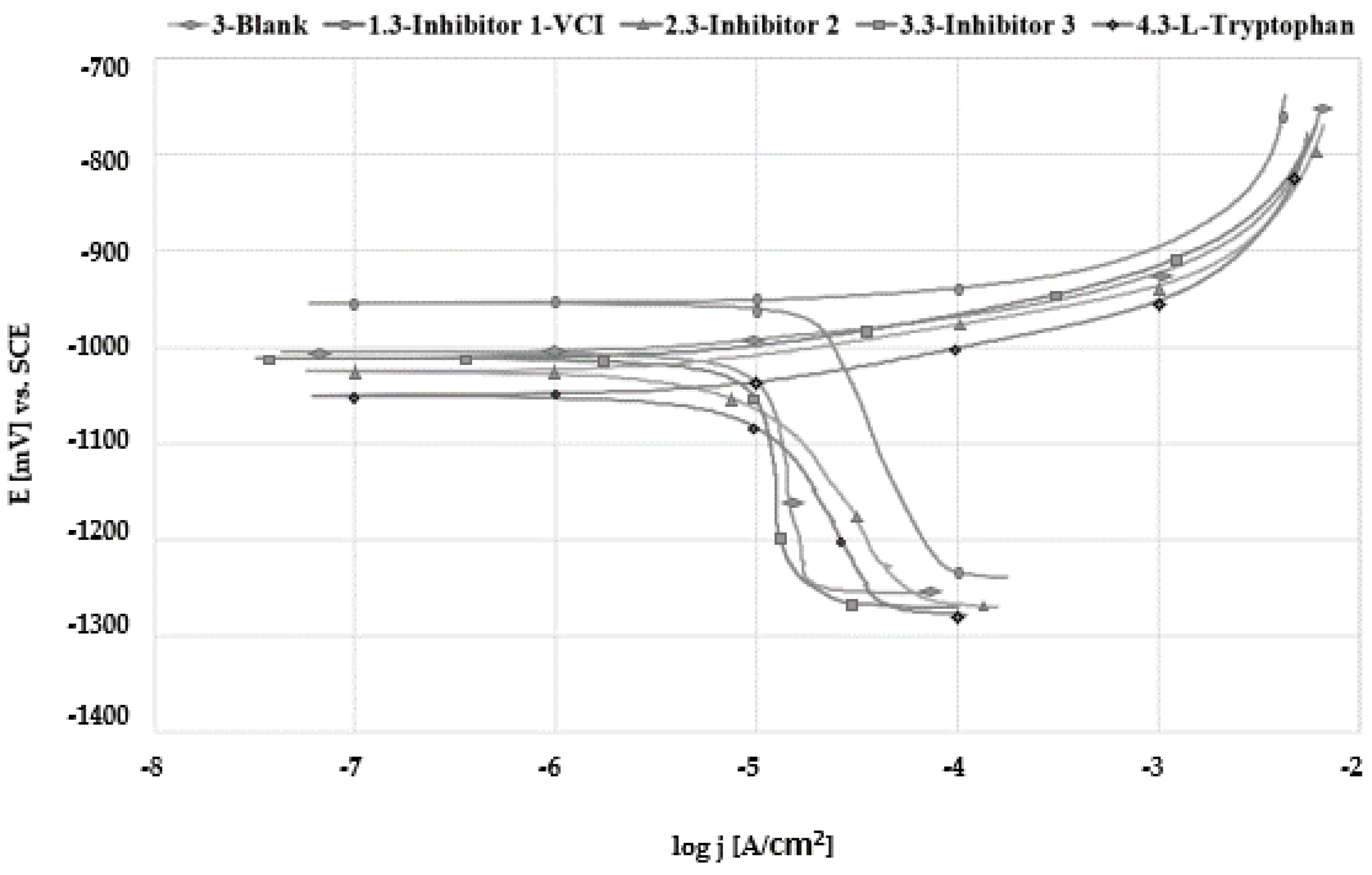
3.4. Tafel Extrapolation
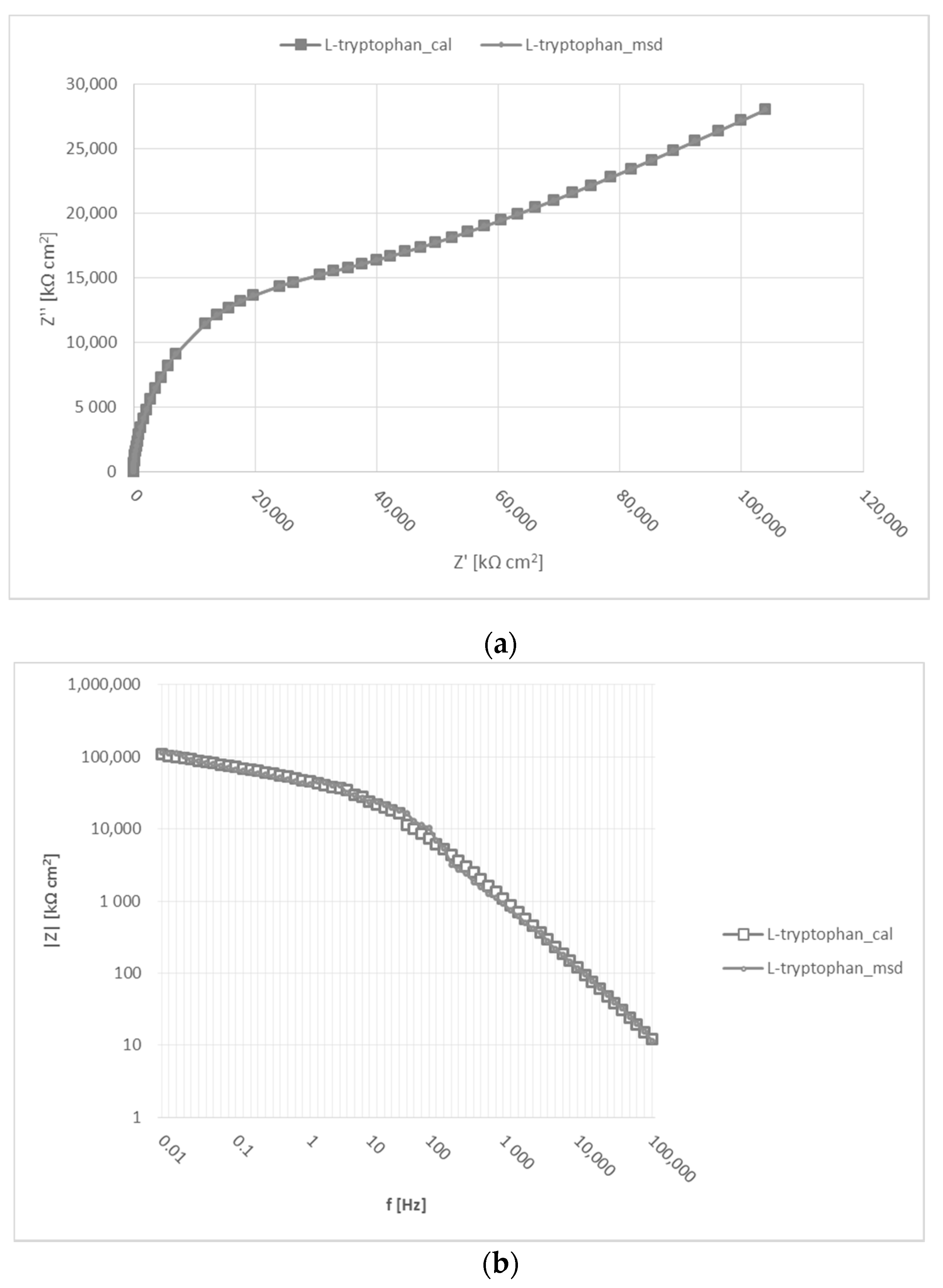
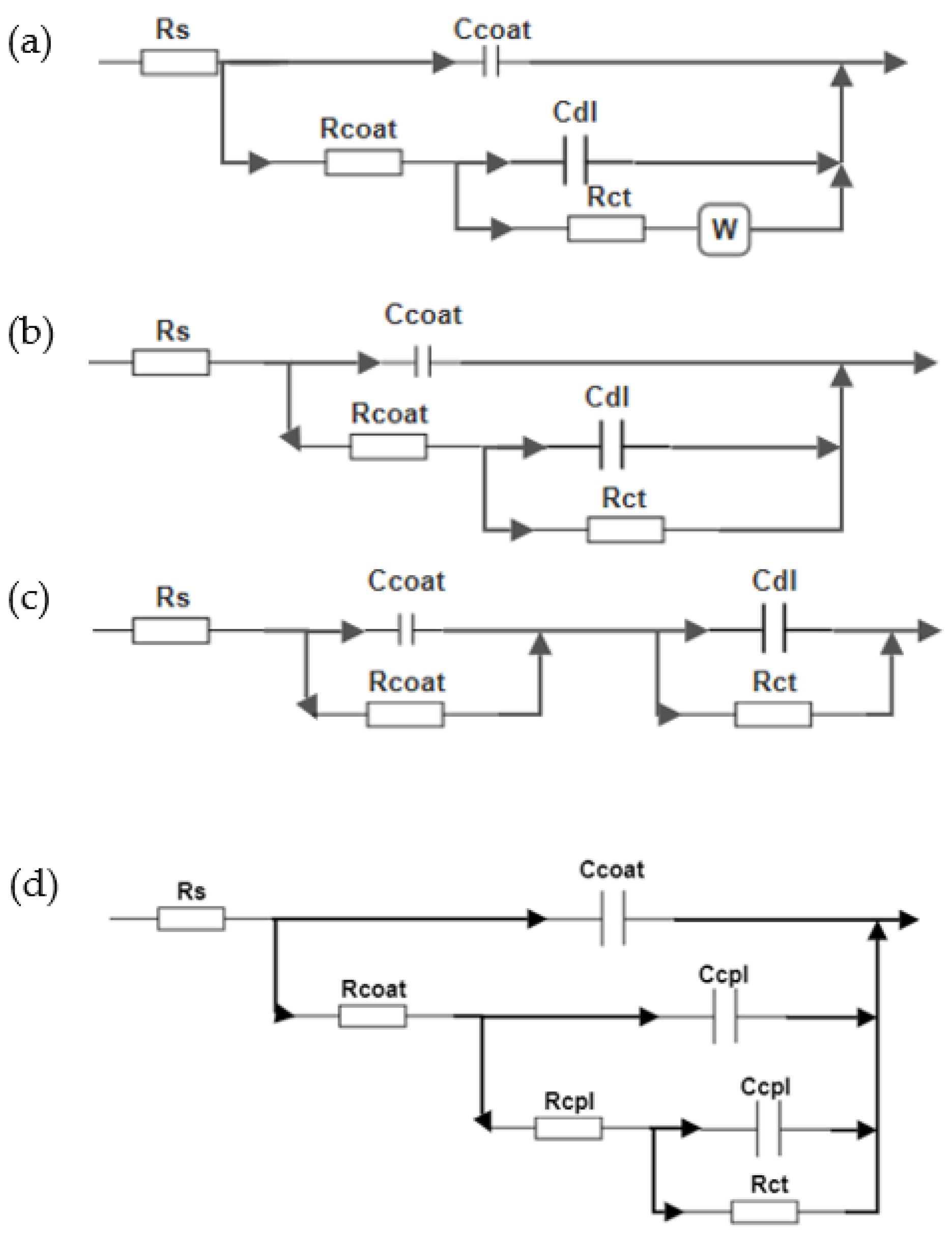
3.5. Electrochemical Impedance Spectroscopy (EIS)
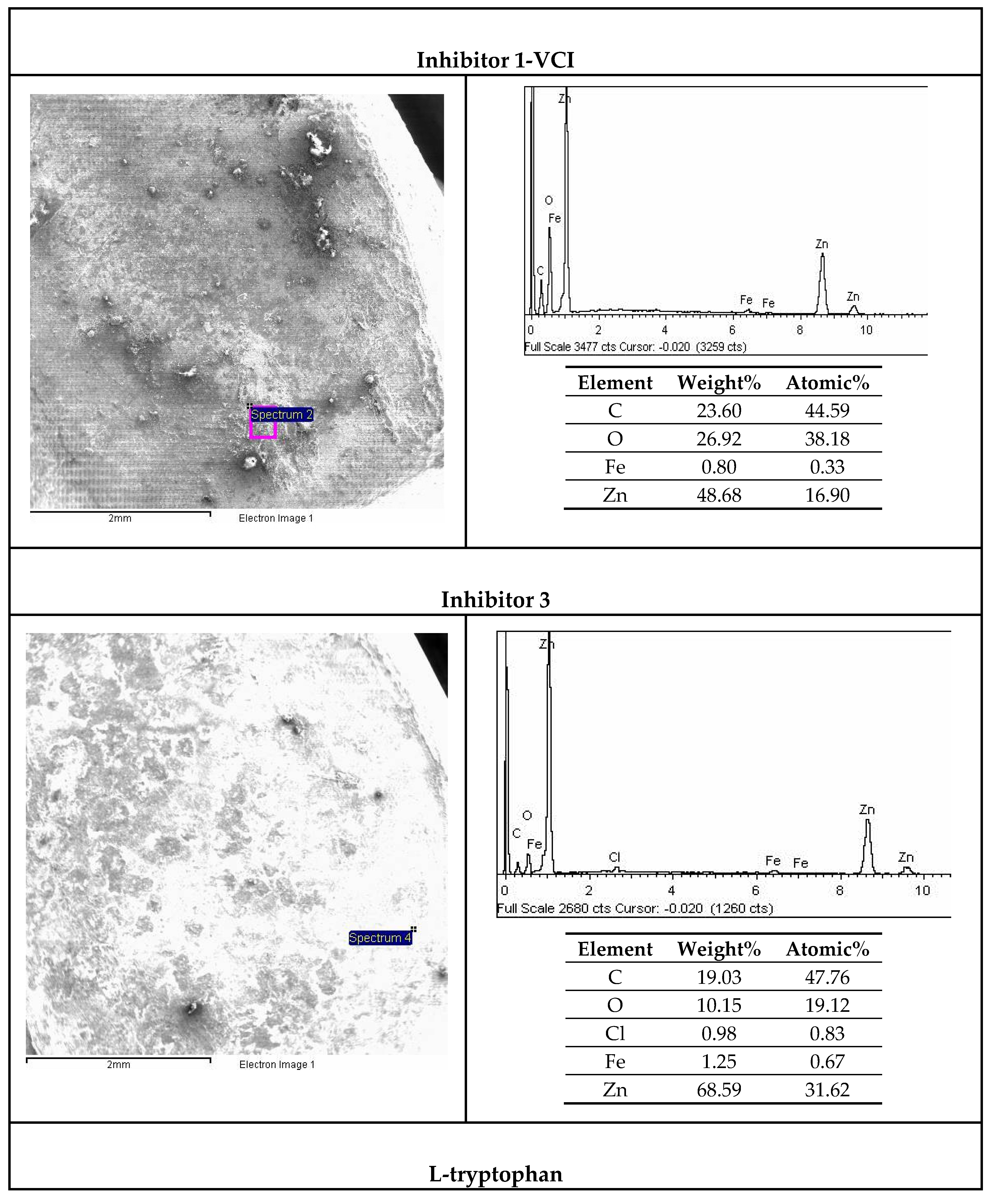
3.6. SEM and EDX Analysis
3.7. ATR-FTIR Spectrometry
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jaffe, S.B.; Fleming, R.; Karles, M. Sunstainable Design Basics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 196–200. [Google Scholar]
- Juraga, I.; Alar, V.; Stojanović, I. Korozija i Zaštita Premazima; FSB: Zagreb, Croatia, 2014; pp. 52–57. [Google Scholar]
- Laque, F.L.; Copson, H.R. Corrosion Resistance of Metals and Alloys; Reinhold Publishing Corporation/Chapman & Hall Ltd.: New York, NY, USA, 1963; pp. 196–245. [Google Scholar]
- Hamadia, L.; Mansouria, S.; Oulmib, K.; Karechea, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Fu, J.J.; Li, S.N.; Cao, L.H.; Wang, Y.; Yan, L.H.; Lu, D. L-Tryptophan as green corrosion inhibitor for low carbon steel in hydrochloric acid solution. J. Mater Sci. 2010, 45, 979–986. [Google Scholar] [CrossRef]
- Wint, N.; Sullivan, J.H.; Penney, D.J. The Role of pH on the Inhibition of Aqueous Zinc Corrosion by L-tryptophan. J. Electrochem. Soc. 2017, 164, C356–C366. [Google Scholar] [CrossRef]
- Mikšić, B.A. VpCI Technology Handbook; Cortec Corporation: Saint Paul, MN, USA, 2014; pp. 242–258. [Google Scholar]
- Mobin, M.; Parveen, M.; Khan, M.A. Inhibition of Mild Steel Corrosion Using L-tryptophan and Synergistic Surfactant Additives. Port. Electrochim. Acta 2011, 29, 391–403. [Google Scholar] [CrossRef]
- Liu, L.F.; Yang, L.L.; Jin, K.Y.; Xu, D.Q.; Gao, C.J. Recovery of l-tryptophan from crystallization wastewater by combined membrane process. Sep. Purif. Technol. 2009, 66, 443–449. [Google Scholar] [CrossRef]
- Snyder, H.R.; Smith, C.W. A Convenient Synthesis of dl-Tryptophan. J. Am. Chem. Soc. 1944, 66, 350–351. [Google Scholar] [CrossRef]
- Pokorny, P.; Kolisko, J.; Balik, L.; Novak, P. Description of structure of Fe-Zn intermetallic compounds presents in HOT-DIP Galvanized Coatings on steel. Metalurgija 2015, 54, 707–710. [Google Scholar]
- Moretti, G.; Guidi, F. Tryptophan as copper corrosion inhibitor in 0.5 M aerated sulfuric acid. Corros. Sci. 2002, 44, 1995–2011. [Google Scholar] [CrossRef]
- Chen, Y.; Xing, W.; Wang, L.; Chen, L. Experimental and Electrochemical Research of an Efficient Corrosion and Scale Inhibitor. Materials 2019, 12, 1821. [Google Scholar] [CrossRef]
- Naqvi, I.; Saleemi, A.R.; Naveed, S. Cefixime: A drug as Efficient Corrosion Inhibitor for Mild Steel in Acidic Media. Int. J. Electrochem. Sci. 2011, 6, 146–161. [Google Scholar]
- Kurtela, M.; Šimunović, V.; Stojanović, I.; Alar, V. Effect of the cerium (III) chloride heptahydrate on the corrosion inhibition of aluminum alloy. Mater. Corros. 2020, 71, 125–147. [Google Scholar] [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L. EIS Studies of Coated Metals in Accelerated Exposure. Prog. Org. Coat. 2003, 46, 148–157. [Google Scholar] [CrossRef]
- Vikneshvaran, S.; Velmathi, S. Experimental, theoretical and surface studies. Surf. Interfaces 2017, 6, 134–142. [Google Scholar] [CrossRef]
- Sherif, E.S.M. A Comparative Study on the Electrochemical Corrosion Behavior of Iron and X-65 Steel in 4.0 wt% Sodium Chloride Solution after Different Exposure Intervals. Molecules 2014, 19, 9962–9974. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.S.; Rashwan, S.; Emam, A.; El-Morsy, F.E. Corrosion Inhibition of Zinc in Acid Medium using some Novel Organic Compounds. Int. J. Electrochem. Sci. 2018, 13, 3719–3744. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Ghasemi, Z.; Seifzadeh, D. The inhibition effect of some amino acids towards the corrosion of aluminum in 1 M HCl + 1 M H2SO4 solution. Appl. Surf. Sci. 2005, 249, 408–418. [Google Scholar] [CrossRef]
- Ehsani, A.; Shiri, H.M.; Mahjani, M.G.; Moshrefi, R.; Safari, R.; Mater, K. Inhibitory effect of new azole derivative in the corrosion of aluminum in acidic medium: Common electrochemical and electrochemical noise investigation. J. Colloid Interface Sci. 2016, 54, 233–239. [Google Scholar] [CrossRef]
- Vikneshvaran, S.; Velmathi, S. Impact of Halide-Substituted Chiral Schiff Bases on Corrosion Behaviour of Carbon Steel in Acidic Environment. J. Nanosci. Nanotechnol. 2019, 19, 4458–4464. [Google Scholar] [CrossRef]
- Kaşarcı, A.; Köse1, D.A.; Avcı, G.A.; Avcı. E. Synthesis and Characterization of CoII, NiII, CuII and ZnII Cation Complexes with Tyrptophan. Investigation of Their Biological Properties. Hacettepe J. Biol. Chem. 2013, 41, 167–177. [Google Scholar]
- Alsamuraee, A.M.A.; Jaafer, H.I.; Ameen, H.A.; Abdullah, A.Q. Electrochemical impedance spectroscopic evaluation of corrosion protection properties of polyurethane/polyvinyl chloride blend coatings on steel. Am. J. Sci. Ind. Res. 2011, 2, 2153–2649. [Google Scholar] [CrossRef]
- Choe, H.B.; Lee, H.S.; Ismail, M.A.; Hussin, M.W. Evaluation of Electrochemical Impedance Properties of Anticorrosion Films by Arc Thermal Metal Spraying Method. Int. J. Electrochem. Sci. 2015, 10, 9775–9789. [Google Scholar]
- Moretti, G.; Guidi, F.; Grion, G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 2004, 46, 387–403. [Google Scholar] [CrossRef]
- Mo, M.; Zhao, W.; Chen, Z.; Liu, E.; Xue, Q. Corrosion inhibition of functional graphene reinforced polyurethane nanocomposite coatings with regular textures. RSC Adv. 2016, 6, 7780–7790. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, F.; Jin, F.; Zhang, R.; Luo, B.; Zhang, T. The Evaluation of Coating Performance by Analyzing the Intersection of Bode Plots. Int. J. Electrochem. Sci. 2015, 9, 5116–5125. [Google Scholar]
- Prasad Yadav, A. Electrochemical Impedance Response of Zn and Galvanized Steel Corroding under Marine Atmospheric Environments. J. Nepal Chem. Soc. 2009, 23, 33–42. [Google Scholar] [CrossRef]
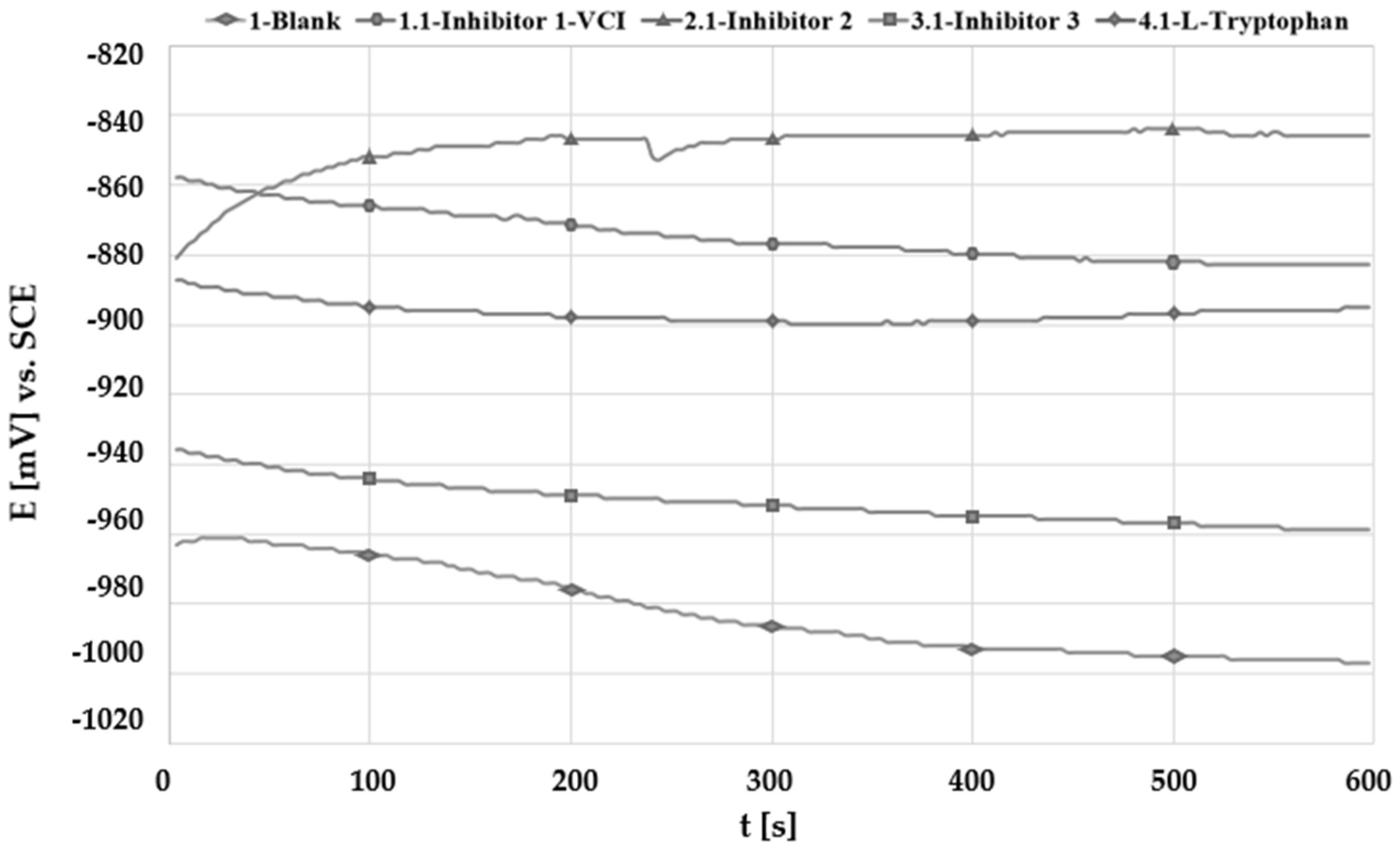
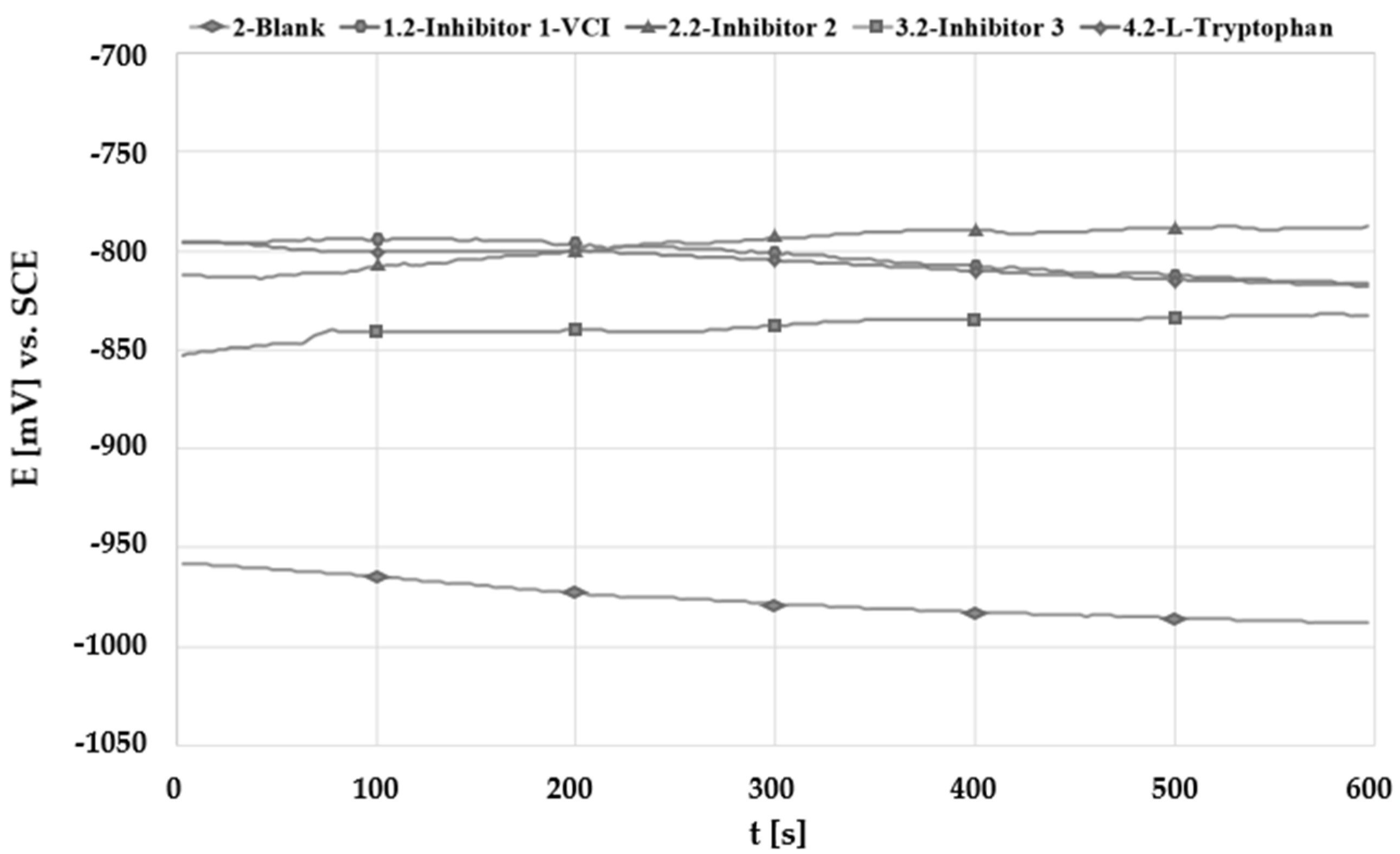
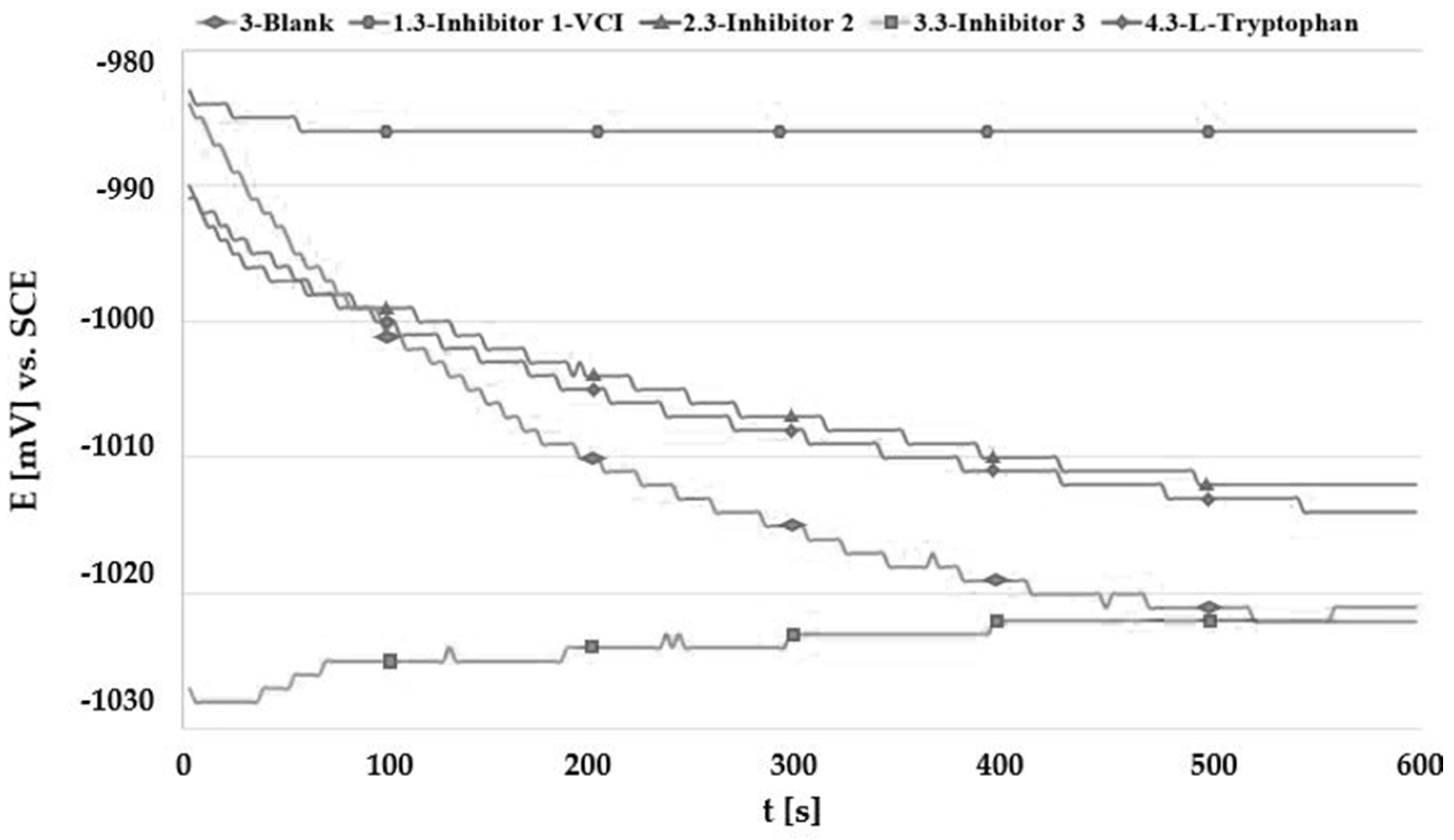

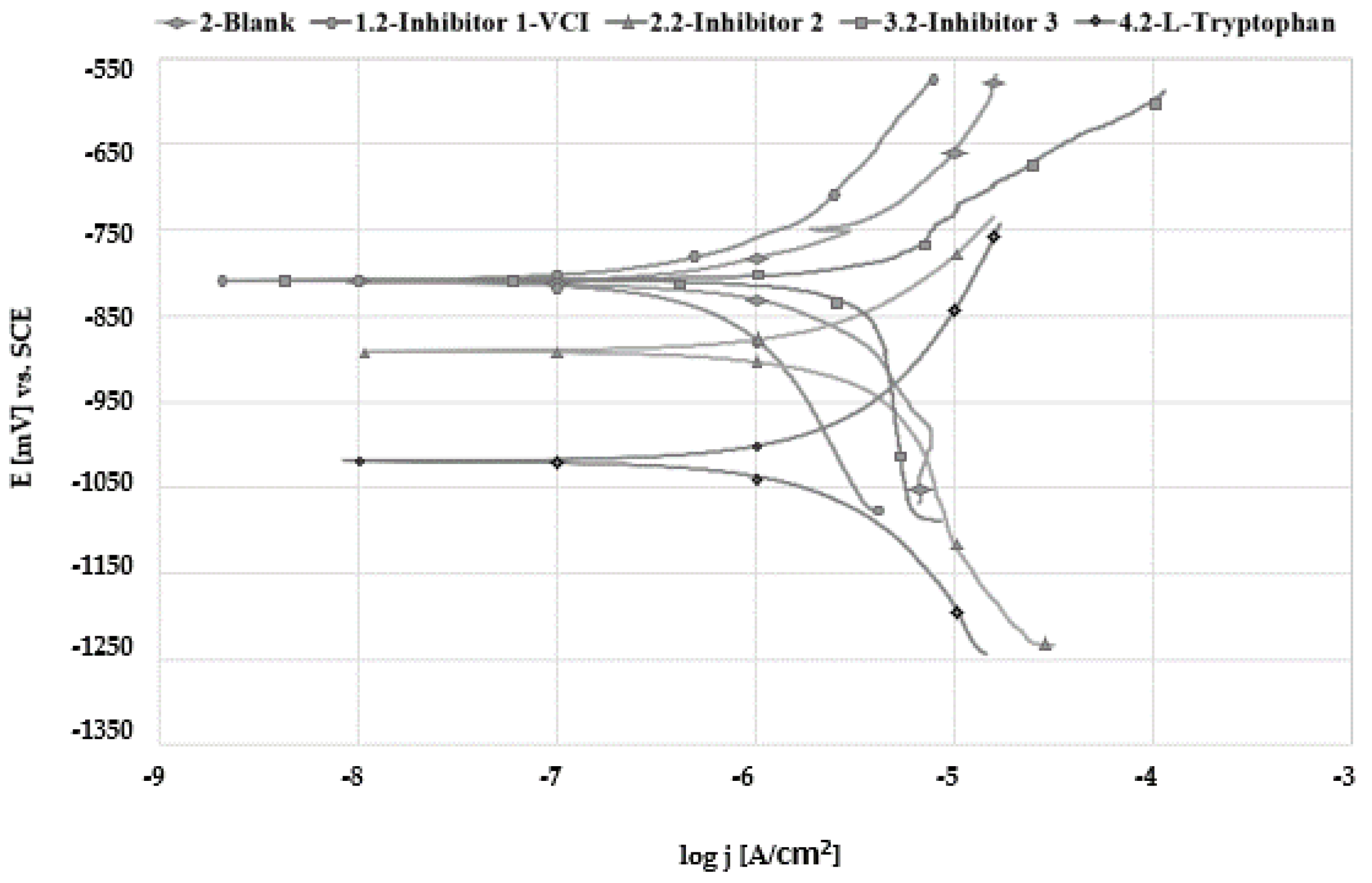


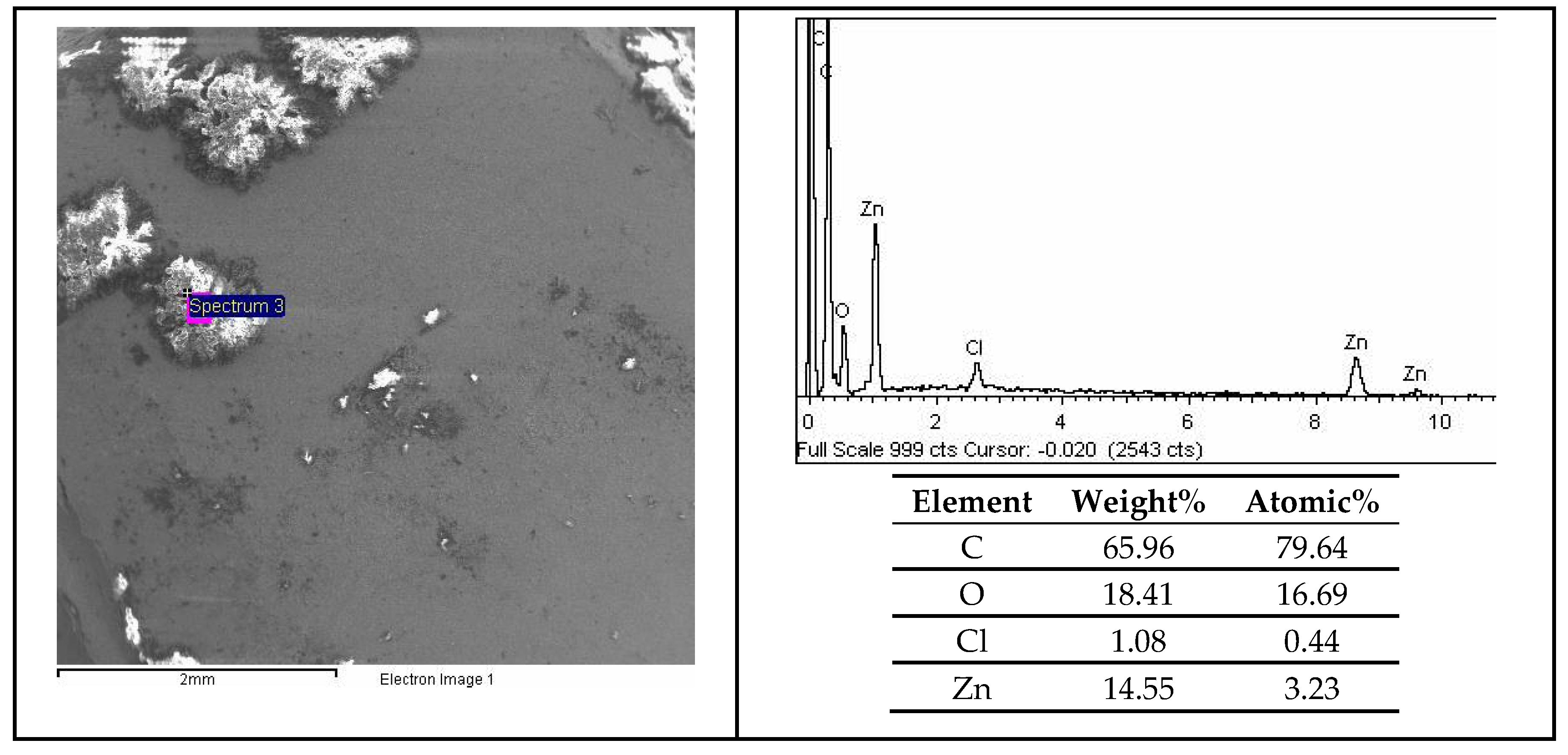
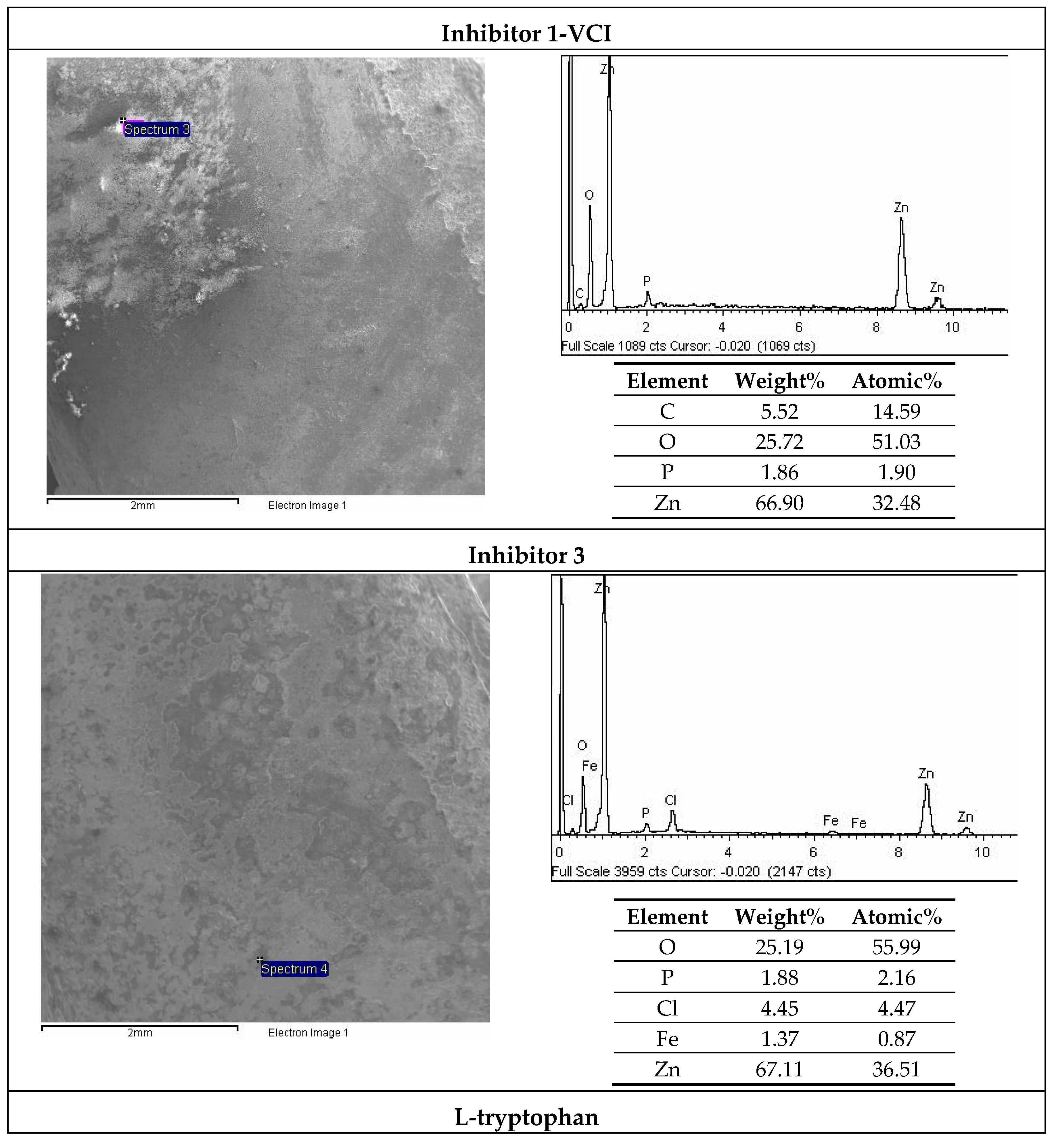

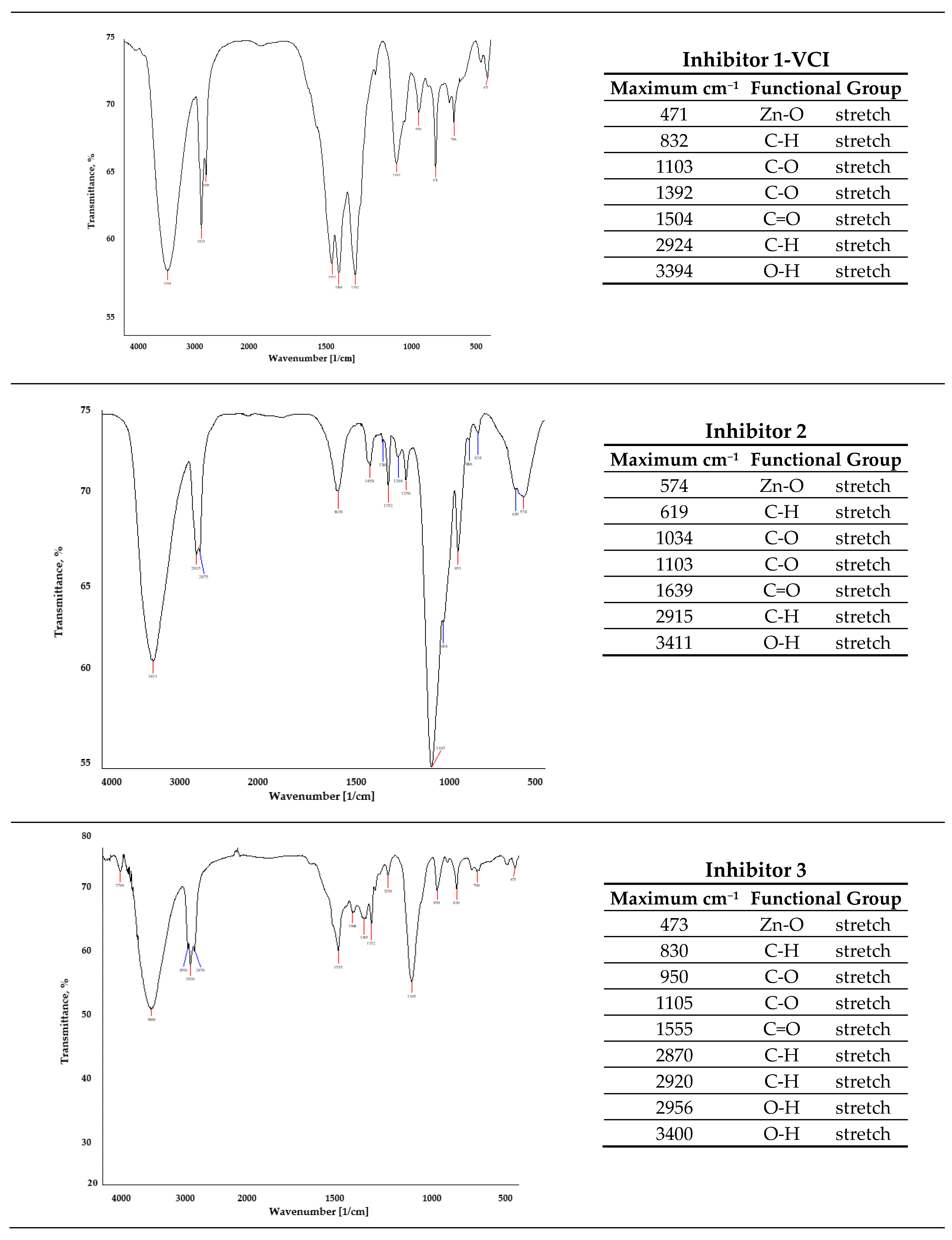
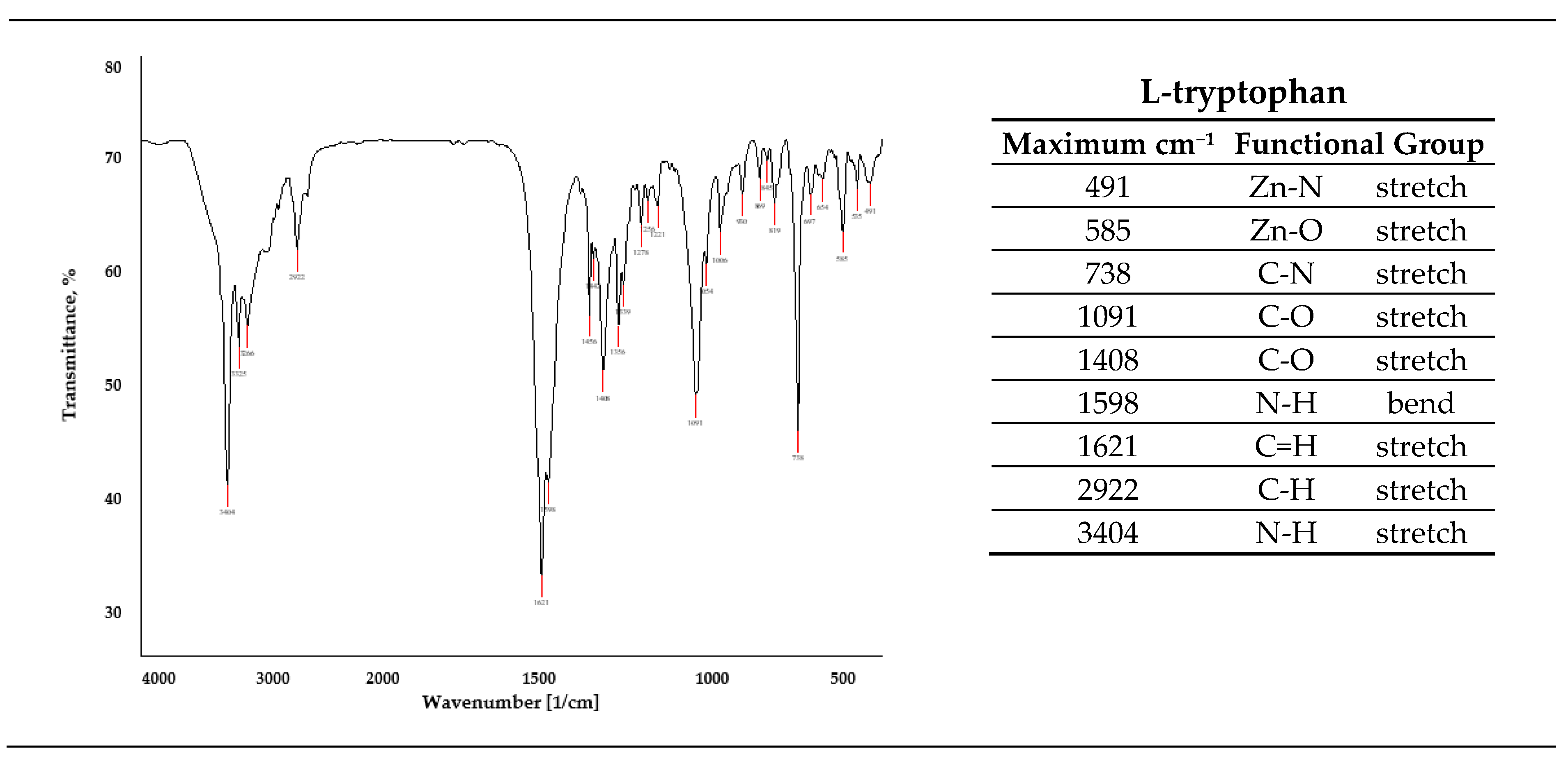
| Solution | No. Inhibitor | Inhibitor 1-VCI | Inhibitor 2 | Inhibitor 3 | L-Tryptophan |
|---|---|---|---|---|---|
| Fresh water (samples 1) |  |  |  |  |  |
| Distilled water (samples 2) |  |  |  |  |  |
| 1% NaCl (samples 3) |  |  |  |  |  |
| Sample | mbefore (g) | mafter (g) | Δm (g) | vcorr (mm/year) | η (%) |
|---|---|---|---|---|---|
| 1 | 21.7963 | 21.7909 | 0.0054 | 0.023 | - |
| 2 | 21.8112 | 21.7592 | 0.052 | 0.219 | - |
| 3 | 21.7553 | 21.5931 | 0.1622 | 0.682 | - |
| 1.1 | 21.8969 | 21.8956 | 0.0013 | 0.006 | 75.93 |
| 1.2 | 21.8322 | 21.7711 | 0.0611 | 0.257 | - |
| 1.3 | 21.8685 | 21.5812 | 0.2873 | 1.207 | - |
| 2.1 | 21.8953 | 21.8498 | 0.0455 | 0.191 | - |
| 2.2 | 21.7701 | 21.766 | 0.0041 | 0.017 | 92.12 |
| 2.3 | 21.7867 | 21.7794 | 0.0073 | 0.030 | 95.50 |
| 3.1 | 21.5936 | 21.5867 | 0.0069 | 0.029 | - |
| 3.2 | 21.6011 | 21.5833 | 0.0178 | 0.074 | 65.77 |
| 3.3 | 21.7335 | 21.7312 | 0.0023 | 0.009 | 98.58 |
| 4.1 | 21.8281 | 21.8262 | 0.0019 | 0.008 | 64.81 |
| 4.2 | 21.7714 | 21.7636 | 0.0078 | 0.032 | 85.00 |
| 4.3 | 21.8133 | 21.8119 | 0.0014 | 0.005 | 99.14 |
| Sample | Ecorr vs. SCE (mV) | βa (mV/dec) | βc (mV/dec) | jcorr (µA/cm2) | η (%) |
|---|---|---|---|---|---|
| 1 | −890 | 97.6 | 288.1 | 21.14 | - |
| 1.1 | −886 | 121.8 | 240.7 | 3.483 | 83.52 |
| 2.1 | −963 | 145.3 | 156.4 | 5.788 | 72.62 |
| 3.1 | −854 | 57.14 | 167.8 | 10.51 | 50.28 |
| 4.1 | −986 | 106.8 | 252.2 | 22.86 | - |
| Sample | Ecorr vs. SCE (mV) | βa (mV/dec) | βc (mV/dec) | jcorr (µA/cm2) | η (%) |
|---|---|---|---|---|---|
| 2 | −822 | 241.2 | 282.7 | 3.364 | - |
| 1.2 | −811 | 125.6 | 216.4 | 2.166 | 35.61 |
| 2.2 | −839 | 228.5 | 288.1 | 17.13 | - |
| 3.2 | −791 | 57.19 | 268.3 | 6.969 | - |
| 4.2 | −992 | 257.2 | 282.8 | 11.32 | - |
| Sample | Ecorr vs. SCE (mV) | βa (mV/dec) | βc (mV/dec) | jcorr (µA/cm2) | η (%) |
|---|---|---|---|---|---|
| 3 | −1010 | 36.16 | 197.1 | 17.64 | - |
| 1.3 | −989 | 11.19 | 260.6 | 31.88 | - |
| 2.3 | −1029 | 68.7 | 236.3 | 13.97 | 20.8 |
| 3.3 | −1016 | 36.08 | 187.4 | 16.11 | 8.67 |
| 4.3 | −1023 | 214.1 | 276.4 | 11.83 | 32.94 |
| Fresh Water | Distilled Water | 1% NaCl | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | vcorr (mm/year) | Rp (kΩcm2) | Sample | vcorr (mm/year) | Rp (kΩcm2) | Sample | vcorr (mm/year) | Rp (kΩcm2) |
| 1 | 0.32 | 4.762 | 2 | 0.048 | 26.63 | 3 | 0.263 | 0.466 |
| 1.1 | 0.052 | 11.39 | 1.2 | 0.033 | 61.59 | 1.3 | 0.496 | 0.216 |
| 2.1 | 0.087 | 9.31 | 2.2 | 0.253 | 9.463 | 2.3 | 0.202 | 0.972 |
| 3.1 | 0.159 | 17.94 | 3.2 | 0.104 | 29.66 | 3.3 | 0.248 | 0.416 |
| 4.1 | 0.346 | 8.661 | 4.2 | 0.168 | 19.41 | 4.3 | 0.172 | 1.013 |
| Sample | Rs | Rcoat | Rct | Rcpl | Rsum | Ccoat (µF/cm2) | CPE1 | CPE2 | W (Ωcm2s−1/2) | χ2 | η (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (kΩcm2) | Q1 (µF/cm2) | n1 | Q2 (µF/cm2) | n2 | |||||||||
| Zn in fresh water | 0.245 | 1.73 | 0.937 | - | 2.91 | - | 0.916 | 1 | 30 | 0.75 | - | 0.02 | - |
| Zn in 1% NaCl | 0.012 | 0.236 | 0.277 | - | 0.525 | - | 27.1 | 1 | 221 | 0.76 | 3.89 × 10−3 | 0.03 | - |
| Inh. 1 in fresh water | 0.721 | 74.4 | 236 | - | 311 | - | 0.311 | 1 | 2.24 | 0.91 | - | 0.03 | 99.06 |
| Inh. 1 in 1% NaCl | 0.029 | 0.746 | 1.19 | - | 1.97 | - | 15.6 | 0.75 | 1480 | 0.61 | - | 0.02 | 73.35 |
| Inh.2 in fresh water | 0.171 | 39.2 | 32.1 | - | 71.47 | - | 0.182 | 1 | 1.36 | 1 | - | 0.18 | 95.93 |
| Inh.2 in 1% NaCl | 0.002 | 1.96 | 0.767 | - | 2.73 | - | 11.7 | 1 | 2780 | 0.72 | - | 0.09 | 80.77 |
| Inh.3 in fresh water | 0.022 | 90.9 | 20.7 | - | 112 | - | 0.53 | 1 | 23.8 | 1 | - | 0.17 | 97.40 |
| Inh.3 in 1% NaCl | 0.011 | 0.072 | 0.517 | 0.367 | 0.967 | 0.855 | 21.58 | 0.8 | 4092 | 0.56 | - | 0.01 | 45.71 |
| L-trypt. in fresh water | 0.011 | 95,100 | 239,000 | - | 334,100 | - | 6.15 × 10−3 | 1 | 3.22 × 10−5 | 1 | 6.61 × 10−15 | 0.09 | 99.99 |
| L-trypt. in 1% NaCl | 0.11 | 8210 | 33,500 | - | 41,710 | - | 0.29 × 10−3 | 0.96 | 1.14 × 10−3 | 0.93 | 4.81 × 10−8 | 0.07 | 99.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alar, V.; Stojanović, I.; Mezdić, D. A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions. Metals 2020, 10, 448. https://doi.org/10.3390/met10040448
Alar V, Stojanović I, Mezdić D. A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions. Metals. 2020; 10(4):448. https://doi.org/10.3390/met10040448
Chicago/Turabian StyleAlar, Vesna, Ivan Stojanović, and Dražen Mezdić. 2020. "A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions" Metals 10, no. 4: 448. https://doi.org/10.3390/met10040448
APA StyleAlar, V., Stojanović, I., & Mezdić, D. (2020). A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions. Metals, 10(4), 448. https://doi.org/10.3390/met10040448






