Laboratory X-ray Diffraction Complex for In Situ Investigations of Structural Phase Evolution of Materials under Gaseous Atmosphere
Abstract
1. Introduction
2. Materials and Methods
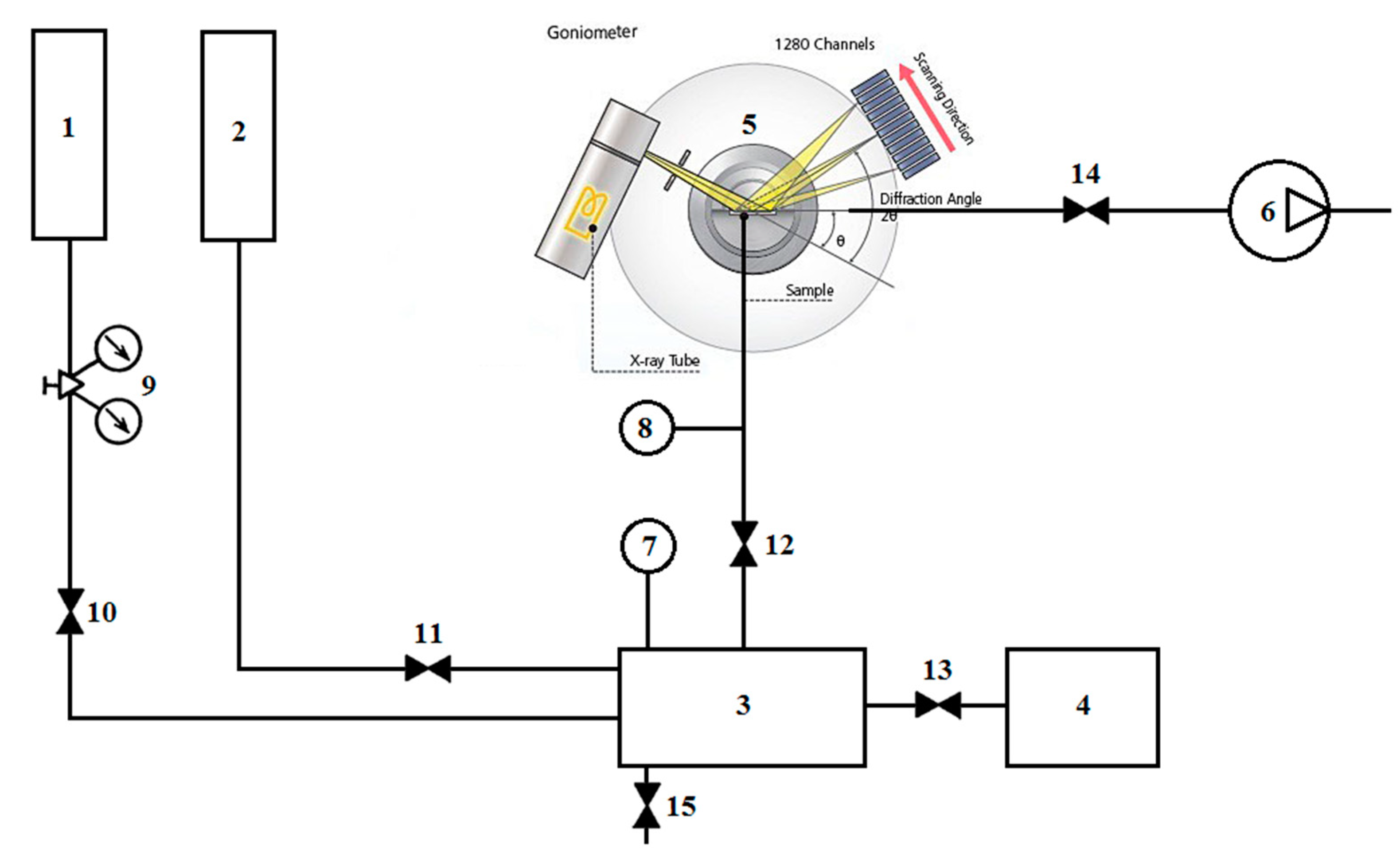
2.1. Diffraction Complex
2.2. Characterization
3. Results and Discussion
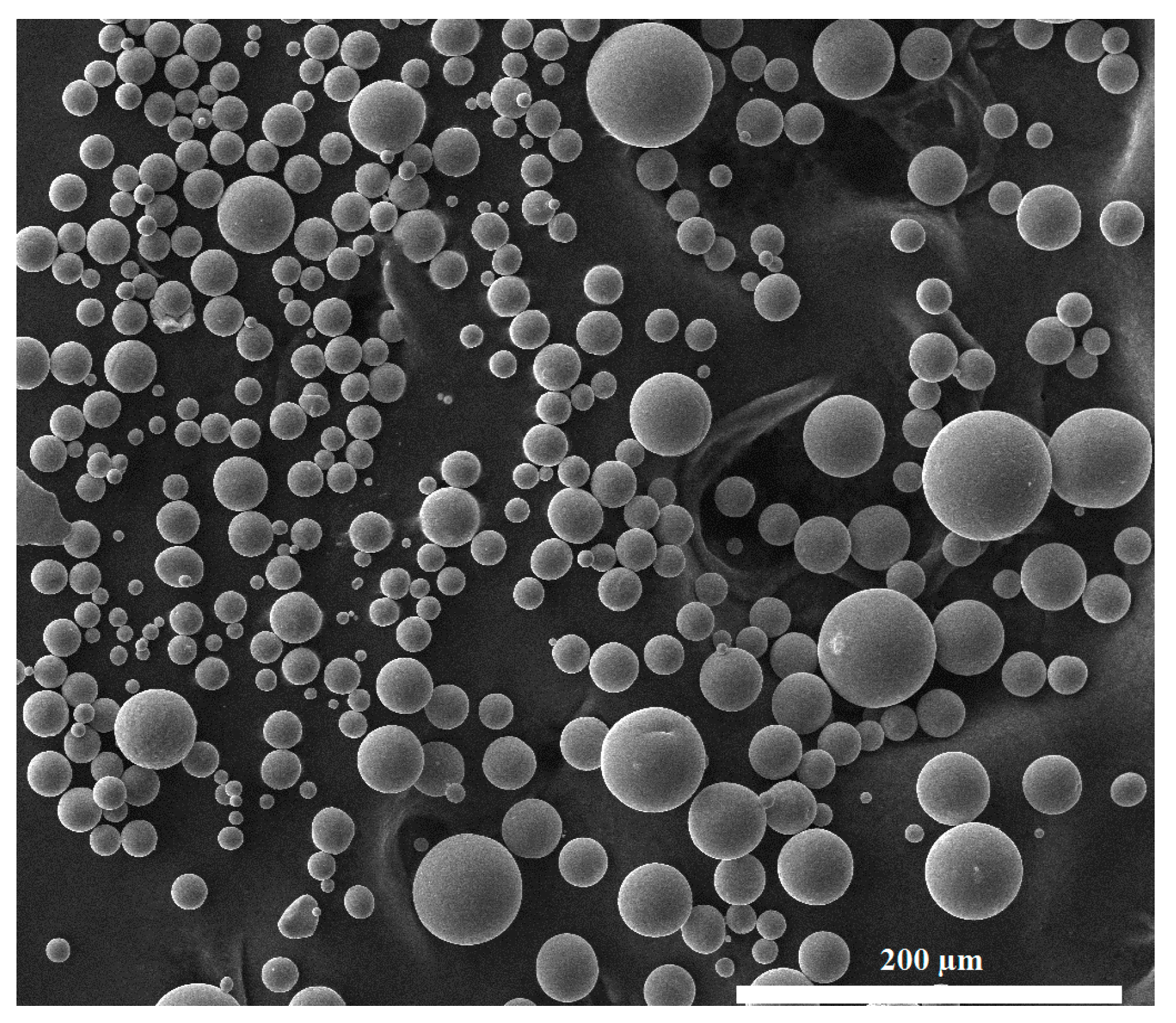
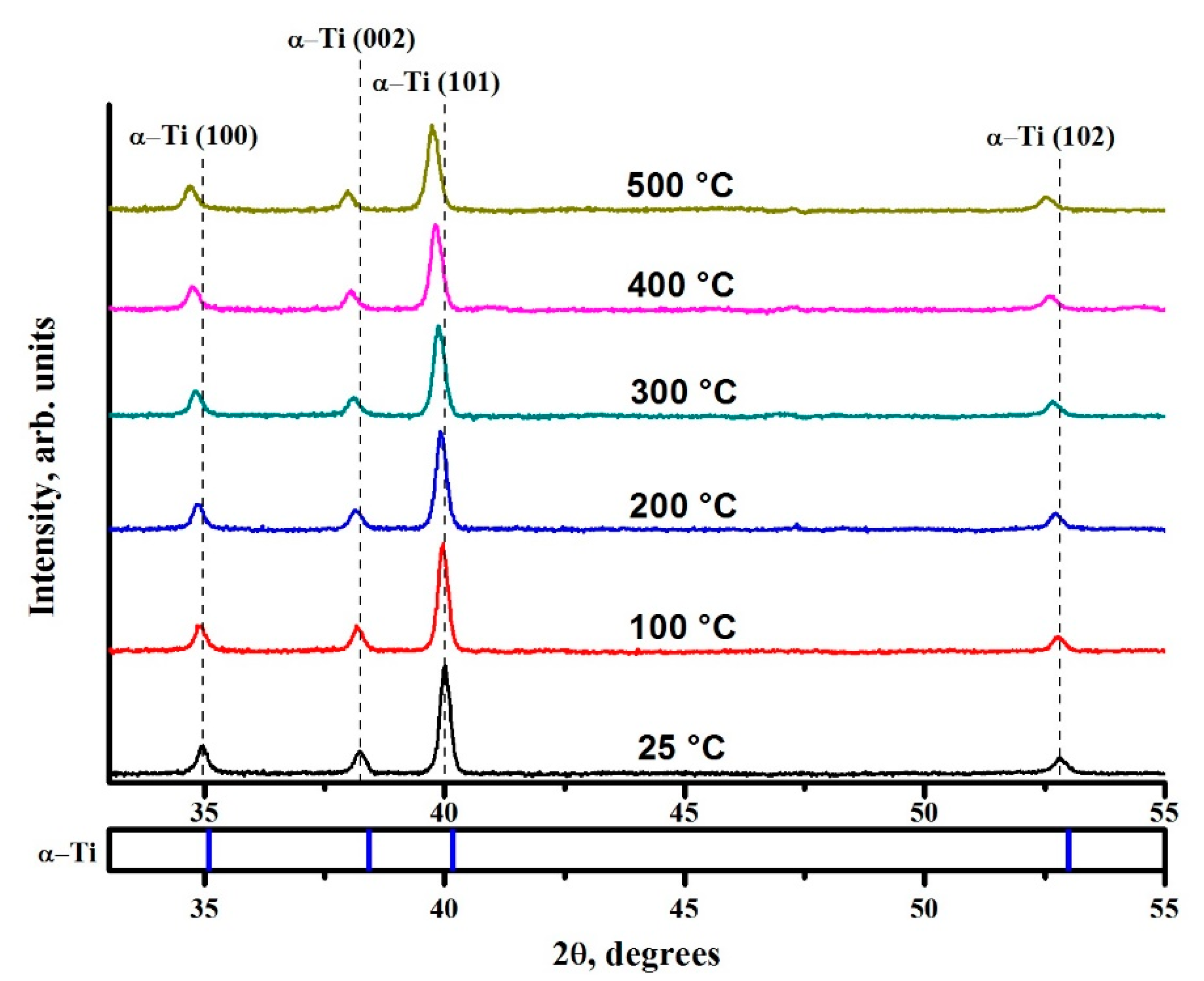
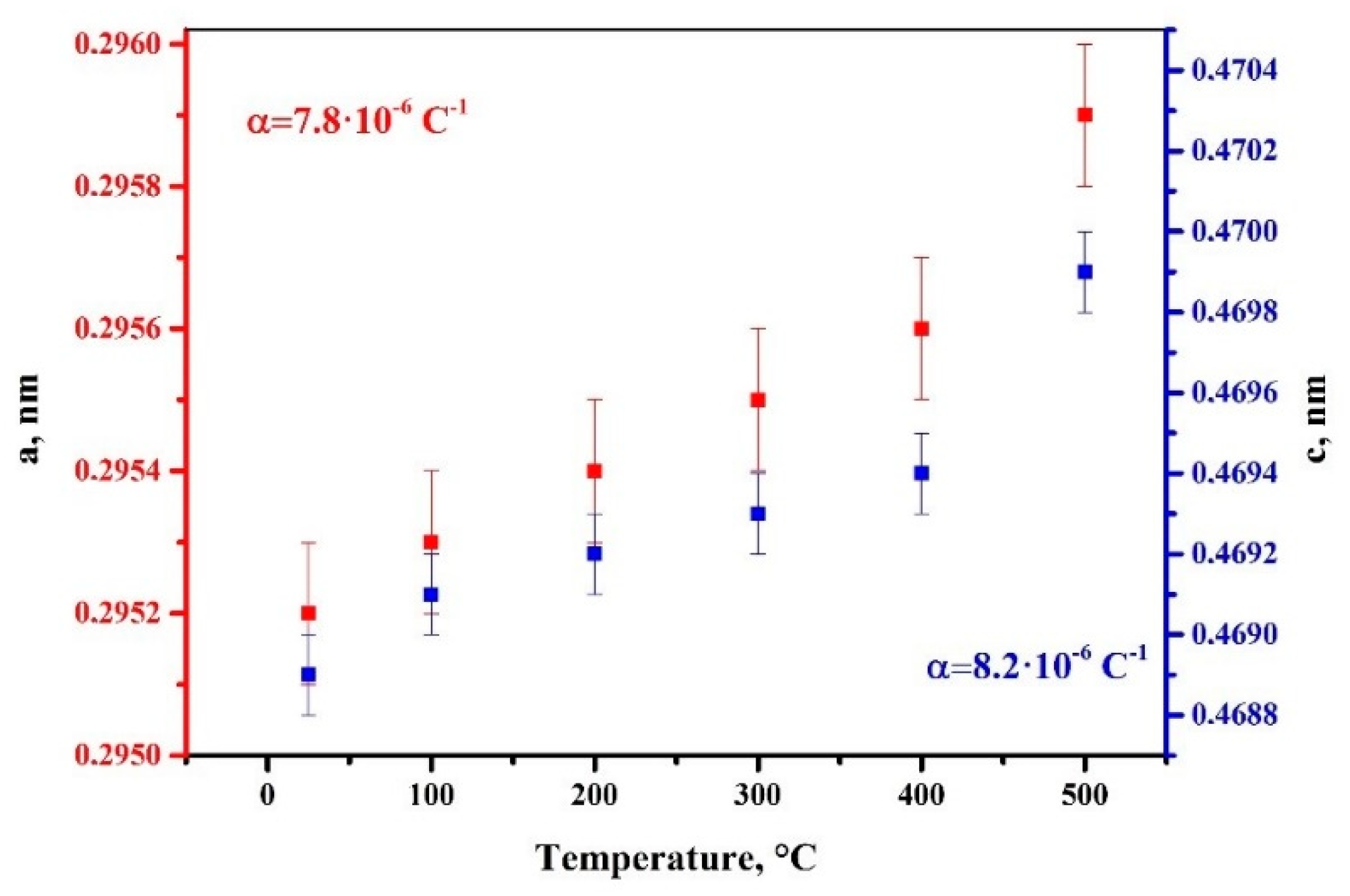
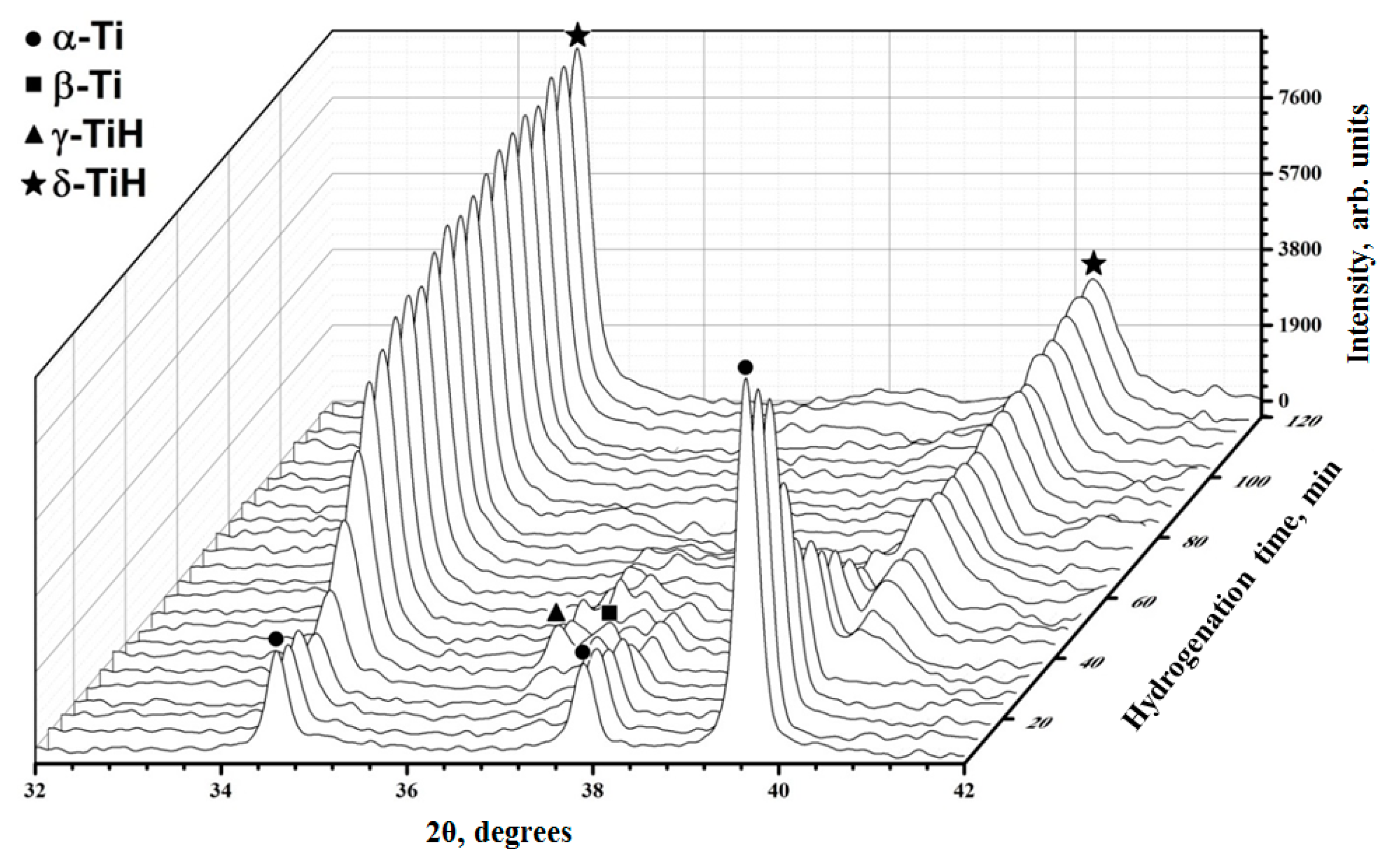
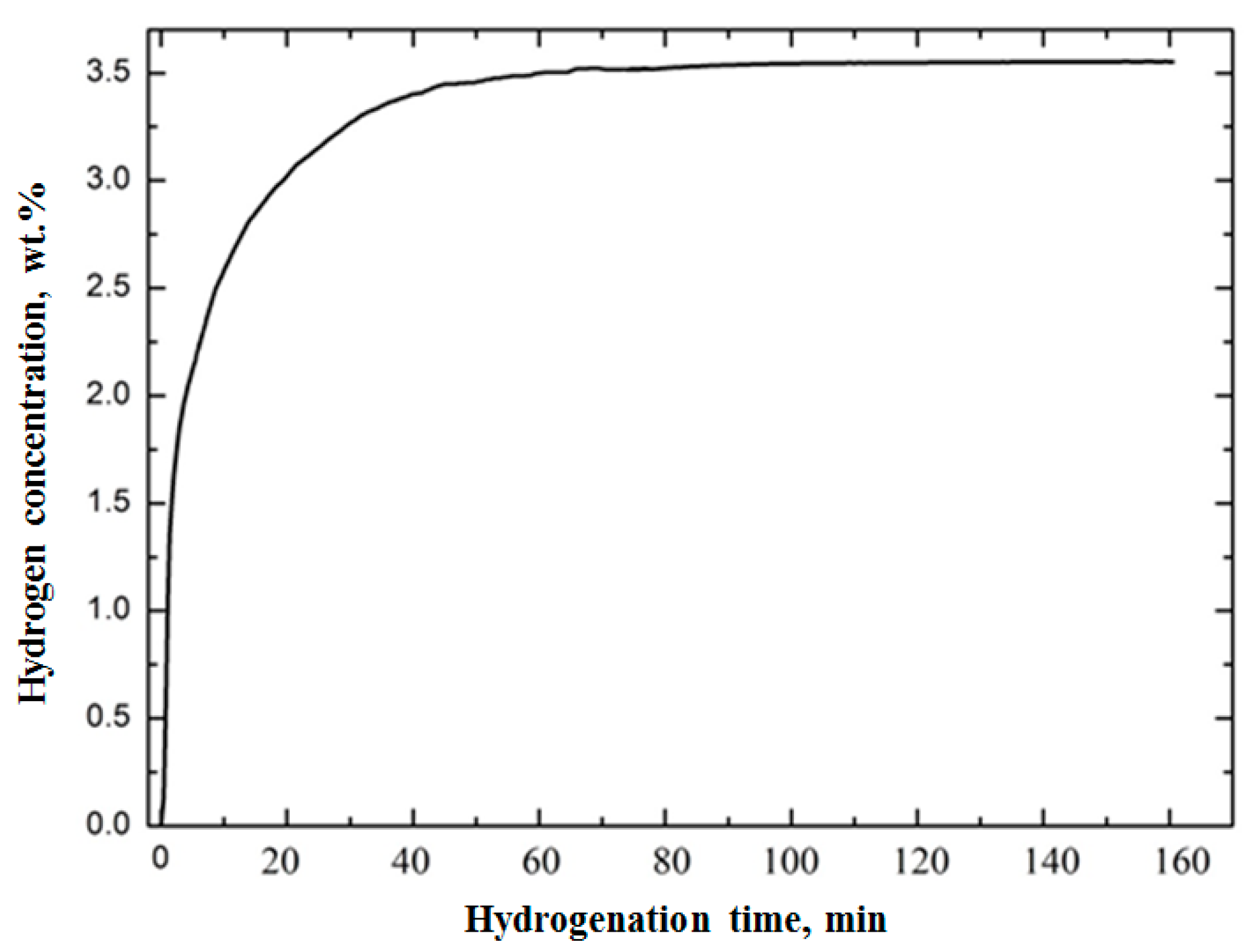
3.1. Verification of the System Using Commercially Pure Titanium Powder
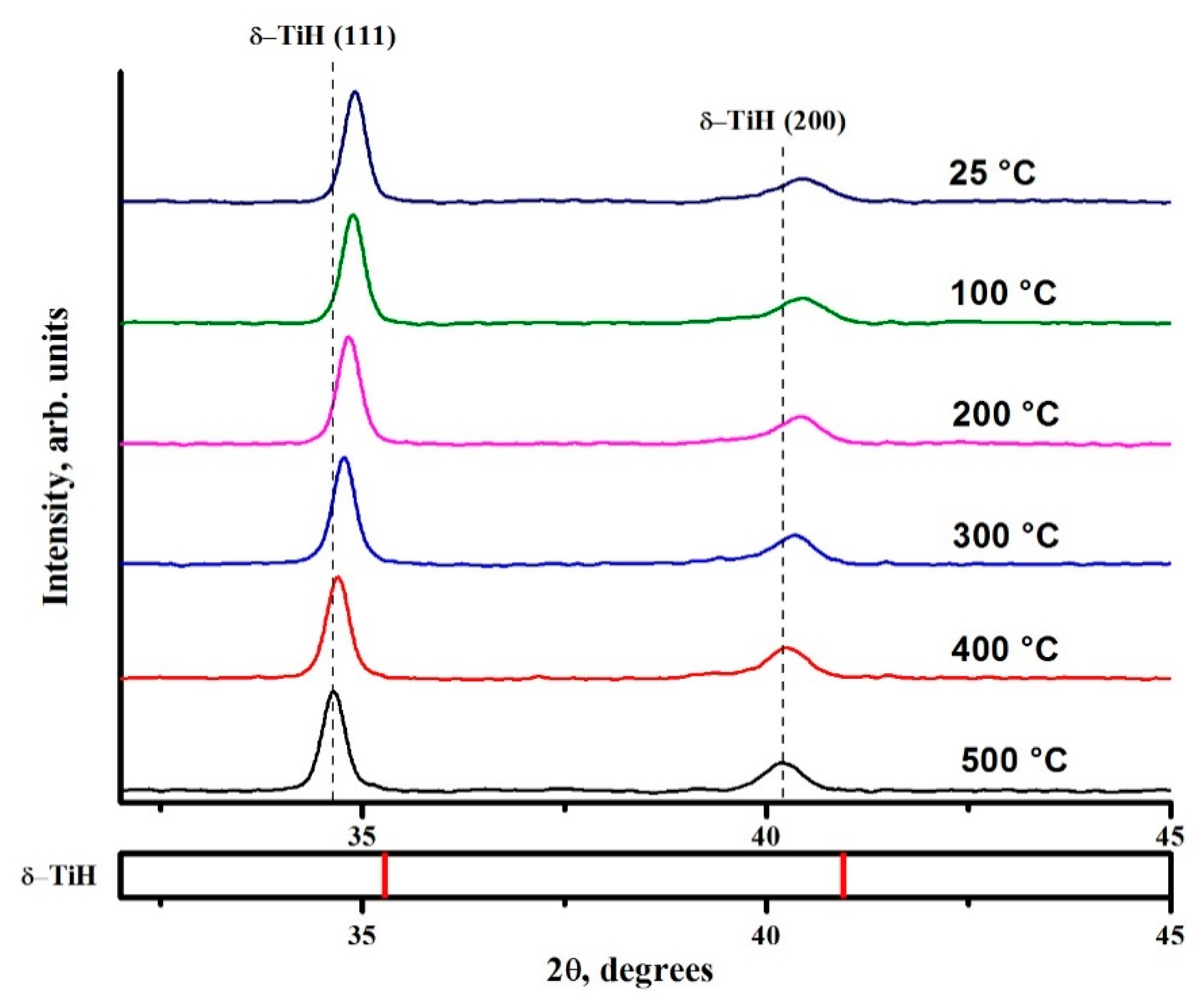
3.2. Structural and Phase State of Zr-1Nb Alloy with Nickel Coating under Hydrogenation
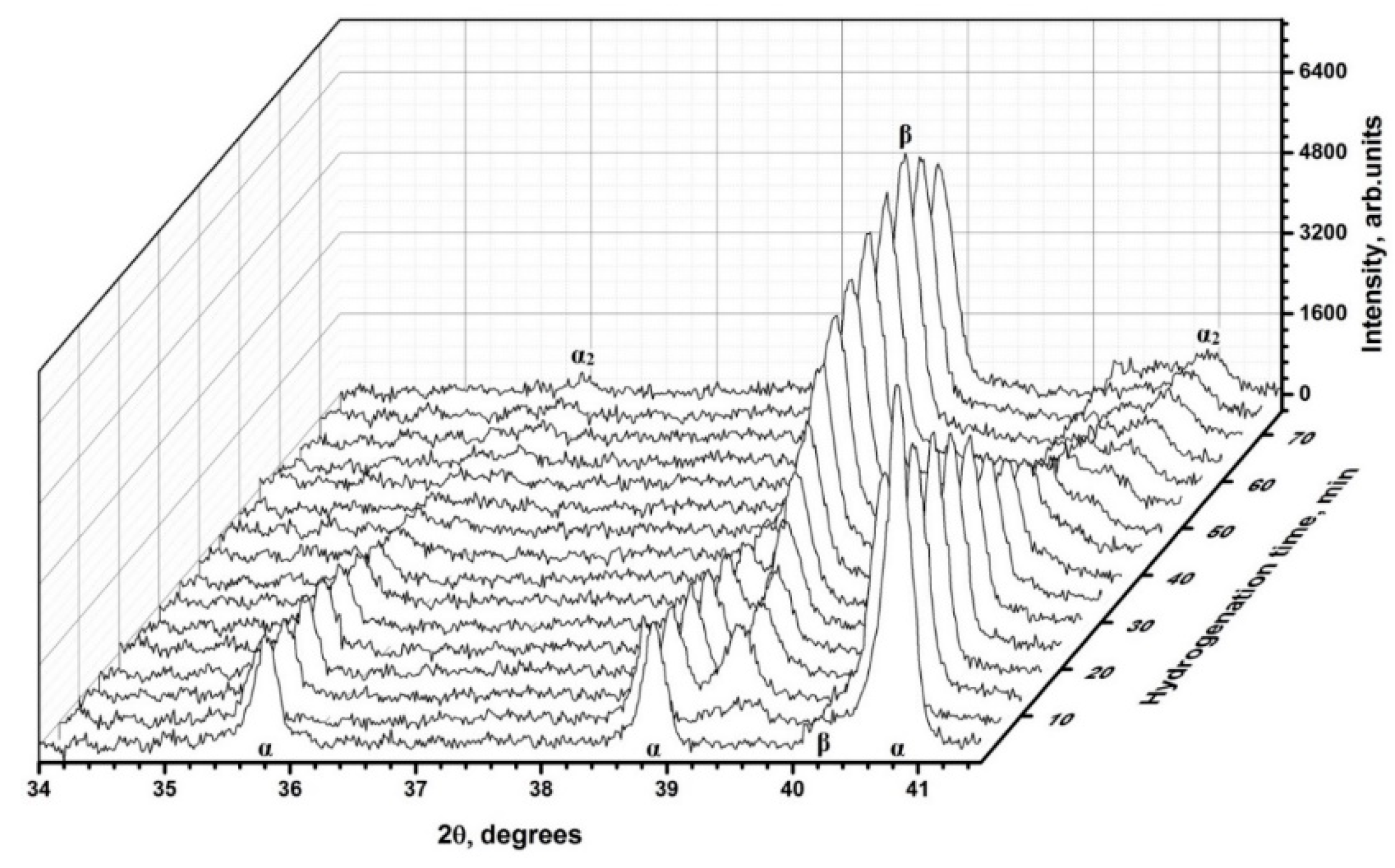
3.3. Phase Transformations and Structure Evolution of the Electron Beam Melted Titanium Ti-6Al-4V Alloy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, C.Y.; Yu, Z.H.; Liu, H.Z.; Lü, T.Q. High pressure and high temperature in situ X-ray diffraction study on the structural stability of tantalum disilicide. Solid State Commun. 2013, 157, 1–5. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Functional materials in desalination: A review. Desalination 2019, 468, 114077. [Google Scholar] [CrossRef]
- Grabovetskaya, G.P.; Stepanova, E.N.; Dubrovskaya, A.S. Effect of hydrogen on the creep of the ultrafine-grained zirconium Zr–1Nb alloy at 673 K. Int. J. Hydrogen Energy 2017, 42, 22633–22640. [Google Scholar] [CrossRef]
- Tang, D.; Hao, L.; Li, Y.; Li, Z.; Dadbakhsh, S. Dual gradient direct ink writing for formation of kaolinite ceramic functionally graded materials. J. Alloys Compd. 2019, 814, 152275. [Google Scholar] [CrossRef]
- Bordulev, I.; Kudiiarov, V.; Svyatkin, L.; Syrtanov, M.; Stepanova, E.; Čížek, J.; Vlček, M.; Li, K.; Laptev, R.; Lider, A. Positron annihilation spectroscopy study of defects in hydrogen loaded Zr-1Nb alloy. J. Alloys Compd. 2019, 798, 685–694. [Google Scholar] [CrossRef]
- Shmakov, A.N.; Cherepanova, S.V.; Zyuzin, D.A.; Fedorova, Y.; Bobrikov, I.; Roger, A.-C.; Adamski, A.; Sadykov, V. The crystal structure of compositionally homogeneous mixed ceria-zirconia oxides by high resolution X-ray and neutron diffraction methods. Open Chem. 2017, 15, 438–445. [Google Scholar] [CrossRef][Green Version]
- Bösenberg, U.; Pistidda, C.; Tolkiehn, M.; Busch, N.; Saldan, I.; Suarez-Alcantara, K.; Arendarska, A.; Klassen, T.; Dornheim, M. Characterization of metal hydrides by in-situ XRD. Int. J. Hydrogen Energy 2014, 39, 9899–9903. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, Y. The application of synchrotron X-ray techniques to the study of rechargeable batteries. J. Energy Chem. 2018, 27, 1566–1583. [Google Scholar] [CrossRef]
- Boldyrev, V.V.; Gaponov, Y.A.; Lyakhov, N.Z.; Politov, A.A.; Tolochko, B.P.; Shakhtshneider, T.P.; Sheromov, M.A. Experience in use of synchrotron radiation in solid state chemistry studies. Nucl. Instrum. Methods Phys. Res. Sect. A 1987, 261, 192–199. [Google Scholar] [CrossRef]
- Bilgin, G.M.; Esen, Z.; Akın, Ş.K.; Dericioglu, A.F. Optimization of the mechanical properties of Ti-6Al-4V alloy fabricated by selective laser melting using thermohydrogen processes. Mater. Sci. Eng. A 2017, 700, 574–582. [Google Scholar] [CrossRef]
- Shen, C.C.; Perng, T.P. Pressure–composition isotherms and reversible hydrogen-induced phase transformations in Ti–6Al–4V. Acta Mater. 2007, 55, 1053–1058. [Google Scholar] [CrossRef]
- Cheng, H.; Lu, C.; Liu, J.; Yan, Y.; Han, X.; Jin, H.; Wang, Y.; Liu, Y.; Wu, C. Synchrotron radiation X-ray powder diffraction techniques applied in hydrogen storage materials-A review. Prog. Nat. Sci. Mater. Int. 2017, 27, 66–73. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.S.T.; Eng, P.J.; Osseo–Asare, K. Applications of in situ synchrotron XRD in hydrometallurgy: Literature review and investigation of chalcopyrite dissolution. Hydrometallurgy 2013, 131, 54–66. [Google Scholar] [CrossRef]
- Krysina, O.V.; Koval, N.N.; Smakov, A.N.; Vinokurov, Z.S. In situ X-ray investigation of coatings based on titanium nitride upon high-temperature oxidation in air. J. Surf. Inv. 2016, 10, 1067–1071. [Google Scholar] [CrossRef]
- Craievich, A.F. Synchrotron radiation in Brazil. Past, present and future. Radiat. Phys. Chem. 2019, 167, 108253. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Campi, G.; Cancedda, R.; Cedola, A. Synchrotron radiation techniques boost the research in bone tissue engineering. Acta Biomater. 2019, 89, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.T.W.; Ye, L.; Tsang, S.C.E. The Contribution of Synchrotron X-Ray Powder Diffraction to Modern Zeolite Applications: A Mini-review and Prospects. Chem 2018, 4, 1778–1808. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Marcelli, A.; Shang, P.P.; Liu, W.S. The complexity of thermoelectric materials: Why we need powerful and brilliant synchrotron radiation sources? Mater. Today Phys. 2018, 6, 68–82. [Google Scholar] [CrossRef]
- Venediktova, O.S.; Bulavchenko, O.A.; Tsyrulnikov, P.G.; Afonasenko, T.N.; Vinokurov, Z.S.; Tsybulya, S.V. High-Temperature X-Ray Diffraction Investigation of the Decomposition Process in Manganese-Gallium Spinel Mn1.5Ga1.5O4. J. Struct. Chem. 2018, 59, 370–376. [Google Scholar] [CrossRef]
- Murashkina, T.L.; Syrtanov, M.S.; Laptev, R.S.; Stepanova, E.N.; Lider, A.M. Structure and defects evolution at temperature and activation treatments of the TiCr2 intermetallic compound of Laves phase C36-type. Int. J. Hydrogen Energy 2019, 44, 10732–10743. [Google Scholar] [CrossRef]
- Stepanova, E.; Pushilina, N.; Syrtanov, M.; Laptev, R.; Kashkarov, E. Hydrogen effect on Ti-6.5Al-3.5Mo-1.5Zr-0.3Si parts produced by electron beam melting. Int. J. Hydrogen Energy 2019, 44, 29380–29388. [Google Scholar] [CrossRef]
- Kriegner, D.; Matěj, Z.; Kužel, R.; Holý, V. Powder diffraction in Bragg–Brentano geometry with straight linear detectors. J. Appl. Crystallogr. 2015, 48, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, T. Collecting data in the home laboratory: Evolution of X-ray sources, detectors and working practices. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Bruker LYNXEYE XE 1D Detector. Available online: http://www.bruker.com (accessed on 24 March 2020).
- Ohbuchi, A. Applications of the two-dimensional detector HyPix-3000 in X-ray diffractometry. Rigaku J. 2015, 31, 4. [Google Scholar]
- Shiramata, Y. Micro-area X-ray diffraction measurement by SmartLab μHR diffractometer system with ultra-high brilliance microfocus X-ray optics and two-dimensional detector HyPix-3000. Rigaku J. 2016, 32, 1. [Google Scholar]
- OneSight Wide-Range High-Speed Detector, Shimadzu. Available online: http://www.shimadzu.com (accessed on 24 March 2020).
- ASTM A. F2924-14, Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium with Powder Bed Fusion; ASTM Standards: West Conshohocken, PA, USA, 2014; p. 10.
- Voskuilen, T.; Zheng, Y.; Pourpoint, T. Development of a Sievert apparatus for characterization of high pressure hydrogen sorption materials. Int. J. Hydrogen Energy 2010, 35, 10387–10395. [Google Scholar] [CrossRef]
- Souvatzis, P.; Eriksson, O.; Katsnelson, M.I. Anomalous thermal expansion in α-titanium. Phys. Rev. Lett. 2007, 99, 015901. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wei, Y.; Mei, L.; Xiao, S.; Chen, Y. XPS study of the deoxidization behavior of hydrogen in TiH2 powders. Powder Technol. 2016, 302, 423–425. [Google Scholar] [CrossRef]
- Matysina, Z.A.; Shchur, D.V. Phase Transformations α→β→γ→δ→ ε in Titanium Hydride TiHx with Increase in Hydrogen Concentration. Russ. Phys. J. 2001, 44, 1237–1243. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Kashkarov, E.B.; Syrtanov, M.S.; Lider, A.M. Hydrogen sorption by Ni-coated titanium alloy VT1-0. Int. J. Hydrogen Energy 2017, 42, 10604–10610. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Syrtanov, M.S.; Bordulev, Y.S.; Babikhina, M.N.; Lider, A.M.; Gubin, V.E.; Murashkina, T.L. The hydrogen sorption and desorption behavior in spherical powder of pure titanium used for additive manufacturing. Int. J. Hydrogen Energy 2017, 42, 15283–15289. [Google Scholar] [CrossRef]
- Ma, M.; Wang, L.; Tang, B.; Lyu, P.; Xiang, W.; Wang, Y.; Tan, X. Kinetics of hydrogen desorption from titanium hydride under isothermal conditions. Int. J. Hydrogen Energy 2018, 43, 1577–1586. [Google Scholar] [CrossRef]
- Novikova, S.I. Thermal expansion. Semicond. Semimet. 1966, 2, 33–48. [Google Scholar] [CrossRef]
- Christensen, M.; Wolf, W.; Freeman, C.; Wimmer, E.; Adamson, R.B.; Hallstadius, L.; Cantonwined, P.E.; Madere, E.V. Diffusion of point defects, nucleation of dislocation loops, and effect of hydrogen in hcp-Zr: Ab initio and classical simulations. J. Nucl. Mater. 2015, 460, 82–96. [Google Scholar] [CrossRef]
- Syrtanov, M.S.; Kudiiarov, V.N.; Kashkarov, E.B.; Shmakov, A.N.; Vinokurov, Z.S.; Babikhina, M.N.; Zolotarev, K.V. Application of Synchrotron Radiation for in Situ XRD Investigation of Zirconium Hydrides Formation at Gas-phase Hydrogenation. Phys. Procedia 2016, 84, 342–348. [Google Scholar] [CrossRef]
- Pushilina, N.S.; Lider, A.M.; Kudiiarov, V.N.; Chernov, I.P.; Ivanova, S.V. Hydrogen effect on zirconium alloy surface treated by pulsed electron beam. J. Nucl. Mater. 2015, 456, 311–315. [Google Scholar] [CrossRef]
- Zhu, T.; Li, M. Effect of 0.770 wt% H addition on the microstructure of Ti–6Al–4V alloy and mechanism of δ hydride formation. J. Alloys Compd. 2009, 481, 480–485. [Google Scholar] [CrossRef]
- Yan, M.; Yu, P. An Overview of Densification, Microstructure and Mechanical Property of Additively Manufactured Ti-6Al-4V–Comparison among Selective Laser Melting, Electron Beam Melting, Laser Metal Deposition and Selective Laser Sintering, and with Conventional Powder; Sinter. Tech. Mater.; IntechOpen: London, UK, 2015; pp. 77–106. [Google Scholar] [CrossRef]
- Johnson, H.H.; Morlet, J.G.; Troiano, A.R. Hydrogen, crack initiation, and delayed failure in steel. Trans. Met. Soc. AIME 1958, 212, 528–536. [Google Scholar]
- Liu, H.; Cao, J.; He, P.; Feng, J.C. Effect of hydrogen on diffusion bonding of commercially pure titanium and hydrogenated Ti6Al4V alloys. Int. J. Hydrogen Energy 2009, 34, 1108–1113. [Google Scholar] [CrossRef]
- Sun, P.; Fang, Z.Z.; Koopman, M.; Paramore, J.; Chandran, K.R.; Ren, Y.; Lu, J. An experimental study of the (Ti–6Al–4V)–xH phase diagram using in situ synchrotron XRD and TGA/DSC techniques. Acta Mater. 2015, 84, 29–41. [Google Scholar] [CrossRef]






| Model | FD-1001 |
|---|---|
| Sensitive element | Line of reverse biased p-n junctions |
| Detection principle | Single photon count |
| Number of channels | 1280 |
| Active area | 64 × 8 mm2 |
| Strip width | 50 μm |
| Energy range | 5–30 keV |
| Cooling | not required |
| Weight | 280 g |
| One shot mode | yes |
| Angle resolutionDimensions | 0.0143°W 72 mm × H 100 mm × D 24 mm |
| Sensitive Element | MicroPirani (MEMS Thermal Conductivity) |
|---|---|
| Piezo Absolute (MEMS diaphragm) | |
| Measuring Range | (1 × 10−5 ÷ 1500) Torr |
| Gaseous | Air, Argon, Helium, Nitrogen, H2, H2O vapor, CO2, Xenon, Neon |
| Operating Temperature Range | 0–40 °C |
| Digital Communication | RS485/RS232 (4800 to 230,400 Baud) |
| Analog Output (Absolute Pressure) | 1 to 9.2 VDC, 1 VDC/decade, 100 Ω maximum output impedance |
| Analog Output Resolution | 16-bit |
| Relay Response | 100 ms maximum |
| Power Requirements | 9 to 30 VDC, <1.2 watts max |
| Accuracy | 5 × 10−4 to 1 × 10−3 Torr ±10% of reading |
| 10−3 to 11 Torr ±5% of reading | |
| 11 to 1000 Torr ±0.75% of reading | |
| Repeatability | 5 × 10−4 to 10−3 Torr ±8% of reading |
| 10−3 Torr to 11 Torr ±2% of reading | |
| 11 to 1000 Torr ±0.2% of reading | |
| Overpressure Limit | 2250 Torr (Absolute) |
| Weight | 170 g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syrtanov, M.; Garanin, G.; Kashkarov, E.; Pushilina, N.; Kudiiarov, V.; Murashkina, T. Laboratory X-ray Diffraction Complex for In Situ Investigations of Structural Phase Evolution of Materials under Gaseous Atmosphere. Metals 2020, 10, 447. https://doi.org/10.3390/met10040447
Syrtanov M, Garanin G, Kashkarov E, Pushilina N, Kudiiarov V, Murashkina T. Laboratory X-ray Diffraction Complex for In Situ Investigations of Structural Phase Evolution of Materials under Gaseous Atmosphere. Metals. 2020; 10(4):447. https://doi.org/10.3390/met10040447
Chicago/Turabian StyleSyrtanov, Maxim, Georgiy Garanin, Egor Kashkarov, Natalia Pushilina, Viktor Kudiiarov, and Tatyana Murashkina. 2020. "Laboratory X-ray Diffraction Complex for In Situ Investigations of Structural Phase Evolution of Materials under Gaseous Atmosphere" Metals 10, no. 4: 447. https://doi.org/10.3390/met10040447
APA StyleSyrtanov, M., Garanin, G., Kashkarov, E., Pushilina, N., Kudiiarov, V., & Murashkina, T. (2020). Laboratory X-ray Diffraction Complex for In Situ Investigations of Structural Phase Evolution of Materials under Gaseous Atmosphere. Metals, 10(4), 447. https://doi.org/10.3390/met10040447







