Formation Mechanism of MgO Containing Inclusions in the Molten Steel Refined in MgO Refractory Crucibles
Abstract
1. Introduction
2. Experimental Procedure
3. Variation of the Composition of Steel and Slag
4. Characteristics of Inclusions
5. Formation Mechanism of MgO-Containing Inclusions
6. Conclusions
- (1)
- In ultra-low Al ultra-low C steel ([Al] < 0.0010%, C < 0.0025%), there was no MgO-containing inclusions other than FeO formed.
- (2)
- In ultra-low Al high C steel ([Al] < 0.0010%, C > 0.5%), almost all inclusions were pure MgO. When the [Al] content in steel increased to larger than 0.0015%, inclusions were changed to spinel, some of which were rich in MgO.
- (3)
- In high Al low C steel ([Al] > 0.01%, C < 0.006%)—in the cases of slag-free and addition of MgO-free slag—inclusions were mainly MgO and MgO-rich spinel, while with the addition of 10% MgO contained slag, inclusions were almost pure MgO.
- (4)
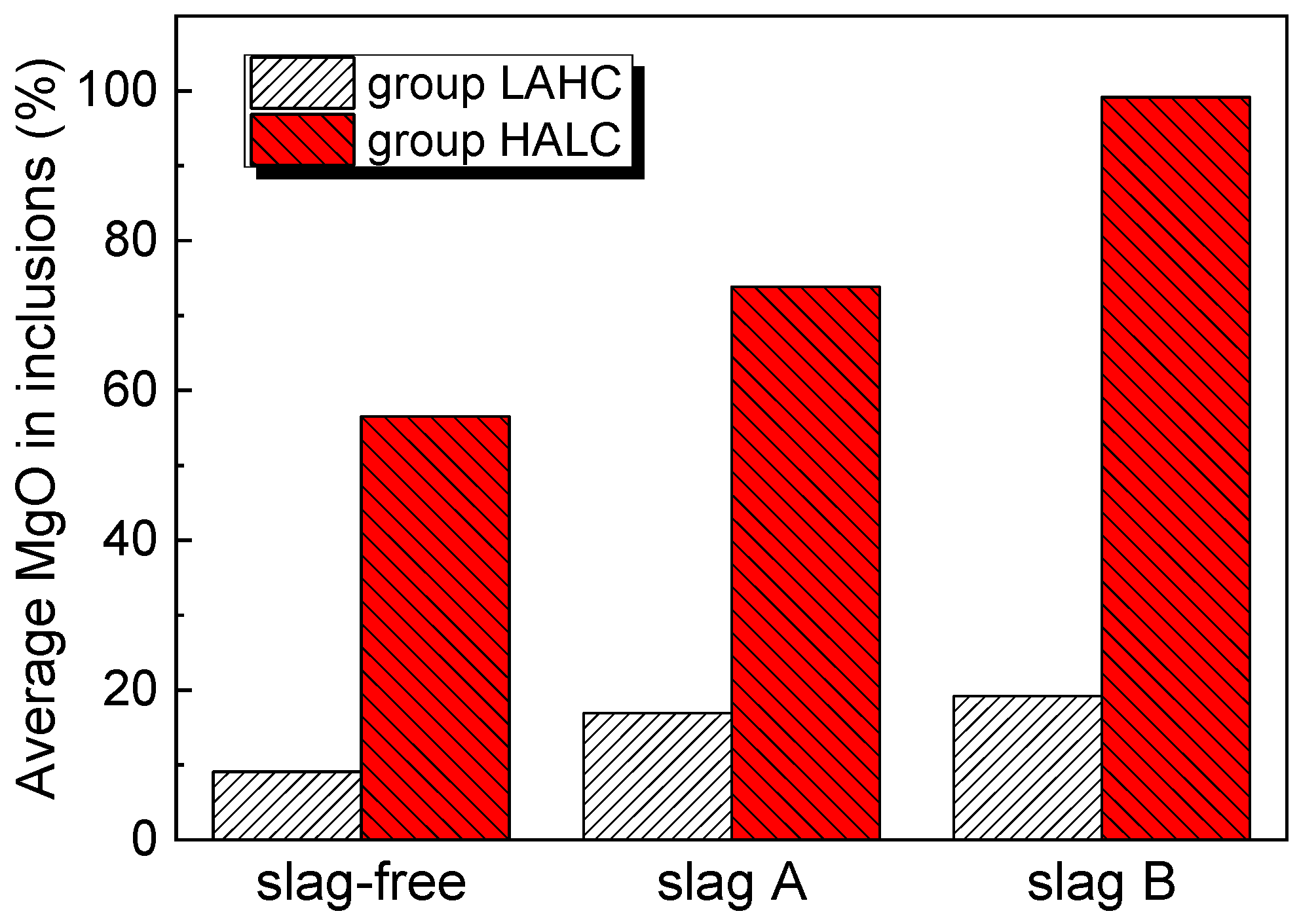
- For both the low Al high C steel and the high Al low C steel, the order of MgO content from large to small was: slag B > slag A > slag-free. Based on the experimental results, a simple formation mechanism of MgO-containing inclusions in the steel with different compositions in MgO crucibles was proposed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Pluschkell, W. Nucleation and growth kinetics of inclusions during liquid steel deoxidation. Ironmak. Steelmak. 2003, 30, 106–110. [Google Scholar] [CrossRef]
- Zhang, L.; Aoki, J.; Thomas, B.G. Inclusion removal by bubble flotation in a continuous casting mold. Metall. Mater. Trans. B 2006, 37, 361–379. [Google Scholar] [CrossRef]
- Zhang, L.; Taniguchi, S. Fundamentals of inclusion removal from liquid steel by bubble flotation. Int. Mater. Rev. 2000, 45, 59–82. [Google Scholar] [CrossRef]
- Zhang, L.; Thomas, B.G. State of the art in evaluation and control of steel cleanliness. ISIJ Int. 2003, 43, 271–291. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, C.; Yang, W.; Ren, Y.; Ling, H. Deformability of oxide inclusions in tire cord steels. Metall. Mater. Trans. B 2018, 49, 803–811. [Google Scholar] [CrossRef]
- Li, Z.; Mukai, K.; Tao, Z. Reactions between MgO-C refractory, molten slag and metal. ISIJ Int. 2000, 40 (Suppl.), S101–S105. [Google Scholar] [CrossRef]
- Harada, A.; Miyano, G.; Maruoka, N.; Shibata, H.; Kitamura, S. Dissolution behavior of Mg from MgO into molten steel deoxidized by Al. ISIJ Int. 2014, 54, 2230–2238. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, L.; Zhang, Y.; Ren, Y. Kinetic modeling for the dissolution of MgO lining refractory in Al-killed steels. Metall. Mater. Trans. B 2017, 48, 2195–2206. [Google Scholar] [CrossRef]
- Aminfi, M.H.; Kazemzadehz, A.; Arfaei, B.; Saha-Chaudhury, N.; Sahajwalla, V. Investigations of calcium aluminate slag penetration to MgO monolithic refractories in steelmaking process. Int. J. ISSI 2006, 3, 34–42. [Google Scholar]
- Goto, K.; Argent, B.B.; Lee, W.E. Corrosion of MgO—MgAl2O4 spinel refractory bricks by calcium aluminosilicate slag. J. Am. Ceram. Soc. 1997, 80, 461–471. [Google Scholar] [CrossRef]
- Luz, A.P.; Leite, F.C.; Brito, M.A.M.; Pandolfelli, V.C. Slag conditioning effects on MgO–C refractory corrosion performance. Ceram. Int. 2013, 39, 7507–7515. [Google Scholar] [CrossRef]
- Van Ende, M.; Guo, M.; Jones, P.T.; Blanpain, B.; Wollants, P. Degradation of MgO–C refractories by MnO-rich stainless steel slags. Ceram. Int. 2009, 35, 2203–2212. [Google Scholar] [CrossRef]
- Guo, M.; Parada, S.; Jones, P.T.; Boydens, E.; Dyck, J.V.; Blanpain, B.; Wollants, P. Interaction of Al2O3-rich slag with MgO–C refractories during VOD refining—MgO and spinel layer formation at the slag/refractory interface. J. Eur. Ceram. Soc. 2009, 29, 1053–1060. [Google Scholar] [CrossRef]
- Beskow, K.; Tripathi, N.N.; Nzotta, M.; Sandberg, A.; Sichen, D. Impact of slag–refractory lining reactions on the formation of inclusions in steel. Ironmak. Steelmak. 2004, 31, 514–518. [Google Scholar] [CrossRef]
- Lehmann, J.; Boher, M.; Kaerle, M.C. An experimental study of the interactions between liquid steel and a MgO-based tundish refractory. CIM Bull. 1997, 90, 69–74. [Google Scholar]
- Brabie, V. A study on the mechanism of reaction between refractory materials and aluminium deoxidised molten steel. Steel Res. 1997, 68, 54–60. [Google Scholar] [CrossRef]
- Brabie, V. Mechanism of reaction between refractory materials and aluminum deoxidized molten steel. ISIJ Int. 1996, 36 (Suppl.), S109–S112. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Duan, H.; Ren, Y.; Wang, J.; Liu, X. Formation of non-metallic inclusions in the molten steel in MgO crucibles. In Proceedings of the EPD Congress 2014—TMS 2014 143rd Annual Meeting and Exhibition, San Diego, CA, USA, 16–20 February 2014; pp. 269–276. [Google Scholar]
- Liu, C.; Gao, X.; Ueda, S.; Kitamura, S. Change in composition of inclusions through the reaction between Al-killed steel and the slag of CaO and MgO saturation. ISIJ Int. 2019, 59, 268–276. [Google Scholar] [CrossRef]
- Liu, C.; Huang, F.; Wang, X. The effect of refining slag and refractory on inclusion transformation in extra low oxygen steels. Metall. Mater. Trans. B 2016, 47, 999–1009. [Google Scholar] [CrossRef]
- Liu, C.; Huang, F.; Suo, J.; Wang, X. Effect of magnesia-carbon refractory on the kinetics of MgO·Al2O3 spinel inclusion generation in extra-low oxygen steels. Metall. Mater. Trans. B 2016, 47, 989–998. [Google Scholar] [CrossRef]
- Liu, C.; Yagi, M.; Gao, X.; Kim, S.; Huang, F.; Ueda, S.; Kitamura, S. Dissolution behavior of Mg from magnesia-chromite refractory into Al-killed molten steel. Metall. Mater. Trans. B 2018, 49, 2298–2307. [Google Scholar] [CrossRef]
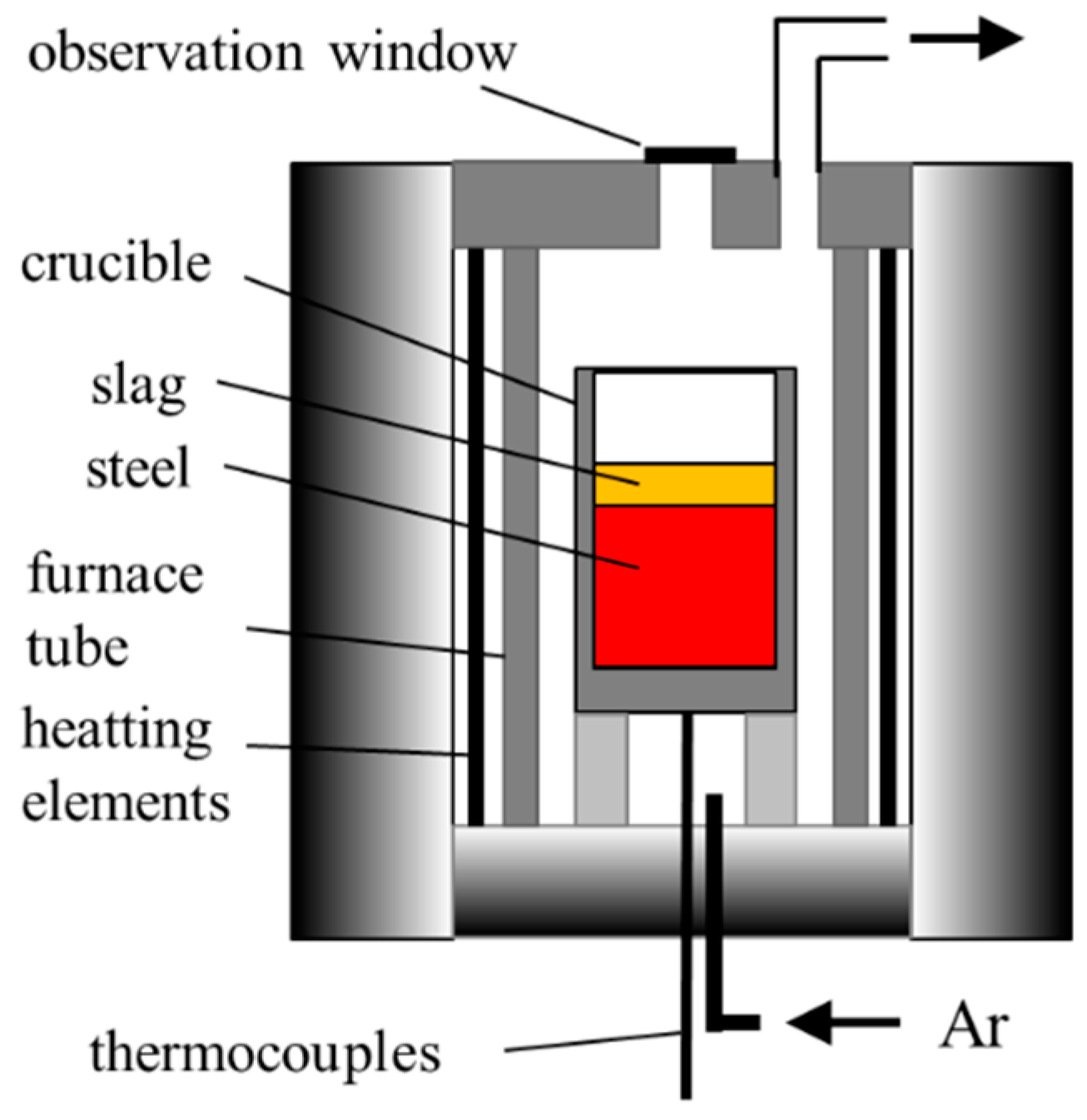


| Group | Heat no. | Slag (R = 7, 50 g) | Steel Preparation (around 100 g) |
|---|---|---|---|
| ULAULC | 1 | No slag | Electrolytic iron (EI) |
| 2 | Slag A | ||
| 3 | Slag B | ||
| LAHC | 1 | No slag | 1 kg EI + 10 g C melted in Al2O3 crucible using VIF |
| 2 | Slag A | ||
| 3 | Slag B | ||
| ULAHC | 1 | No slag | 1 kg EI melted in graphite crucible using VIF |
| 2 | Slag A | ||
| 3 | Slag B | ||
| HALC | 1 | No slag | 1 kg EI + 10 g Al melted in Al2O3 crucible using VIF |
| 2 | Slag A | ||
| 3 | Slag B |
| Element | C | Si | Mn | P | S | T.Al | T.Mg | T.O |
|---|---|---|---|---|---|---|---|---|
| Content | 0.0024 | <0.0005 | 0.0001 | 0.0004 | 0.0006 | 0.0018 | <0.0004 | 0.0105 |
| Slag | CaO | SiO2 | Al2O3 | MgO |
|---|---|---|---|---|
| A | 48.1 | 6.9 | 45.0 | 0.0 |
| B | 43.2 | 6.2 | 40.6 | 10.0 |
| Group | No. | Slag | Steel Composition | Slag Composition after Experiment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [Al] | T.Mg | C | T.O | CaO | Al2O3 | MgO | SiO2 | Fe2O3 | |||
| ULAULC | 0 | 0.0008 | <0.0004 | 0.0024 | 0.0105 | ||||||
| 1 | none | <0.0005 | <0.0005 | - | 0.0060 | ||||||
| 2 | A | <0.0005 | <0.0005 | - | 0.0041 | 48.55 | 32.20 | 9.04 | 6.69 | 3.01 | |
| 3 | B | <0.0005 | 0.0005 | - | 0.0081 | 48.19 | 31.45 | 9.43 | 6.20 | 4.35 | |
| LAHC | 0 | 0.0021 | <0.0004 | 0.84 | - | ||||||
| 1 | none | 0.0032 | 0.0068 | - | 0.0160 | ||||||
| 2 | A | 0.0016 | 0.0003 | 0.78 | 0.0123 | 50.45 | 33.40 | 7.46 | 6.64 | 1.62 | |
| 3 | B | 0.0075 | 0.0039 | - | 0.0106 | 47.97 | 33.60 | 9.91 | 6.33 | 1.73 | |
| ULAHC | 0 | 0.0006 | <0.0004 | 4.37 | 0.0181 | ||||||
| 2 | A | 0.0012 | 0.0004 | 3.77 | 0.0167 | 50.61 | 33.78 | 9.09 | 6.52 | 1.86 | |
| 3 | B | 0.0012 | 0.0007 | 3.85 | 0.0133 | 50.52 | 33.67 | 9.54 | 6.27 | ||
| HALC | 0 | 0.40 | <0.0004 | 0.0062 | - | ||||||
| 1 | none | 0.32 | 0.0010 | - | 0.00050 | ||||||
| 2 | A | 0.0096 | <0.0005 | - | 0.00044 | 50.31 | 33.26 | 8.16 | 5.61 | 1.73 | |
| 3 | B | 0.0110 | 0.0006 | - | 0.00043 | 48.52 | 34.49 | 9.81 | 5.14 | 1.58 | |
| Group | No. | Slag | Inclusion | |
|---|---|---|---|---|
| Morphology | Type | |||
| ULAULC | 1 | none |  | FeO |
| 2 | A | FeO | ||
| 3 | B | FeO | ||
| LAHC | 1 | none |  | spinel |
| 2 | A | spinel | ||
| 3 | B | spinel | ||
| ULAHC | 2 | A |  | MgO |
| 3 | B | MgO | ||
| HALC | 1 | none | 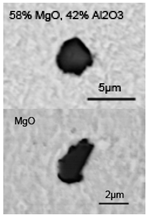 | MgO + spinel |
| 2 | A | MgO + spinel | ||
| 3 | B | MgO | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, W.; Zhang, L. Formation Mechanism of MgO Containing Inclusions in the Molten Steel Refined in MgO Refractory Crucibles. Metals 2020, 10, 444. https://doi.org/10.3390/met10040444
Li Y, Yang W, Zhang L. Formation Mechanism of MgO Containing Inclusions in the Molten Steel Refined in MgO Refractory Crucibles. Metals. 2020; 10(4):444. https://doi.org/10.3390/met10040444
Chicago/Turabian StyleLi, Yiyan, Wen Yang, and Lifeng Zhang. 2020. "Formation Mechanism of MgO Containing Inclusions in the Molten Steel Refined in MgO Refractory Crucibles" Metals 10, no. 4: 444. https://doi.org/10.3390/met10040444
APA StyleLi, Y., Yang, W., & Zhang, L. (2020). Formation Mechanism of MgO Containing Inclusions in the Molten Steel Refined in MgO Refractory Crucibles. Metals, 10(4), 444. https://doi.org/10.3390/met10040444






