Crystallization Process and Microstructural Evolution of Melt Spun Al-RE-Ni-(Cu) Ribbons
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
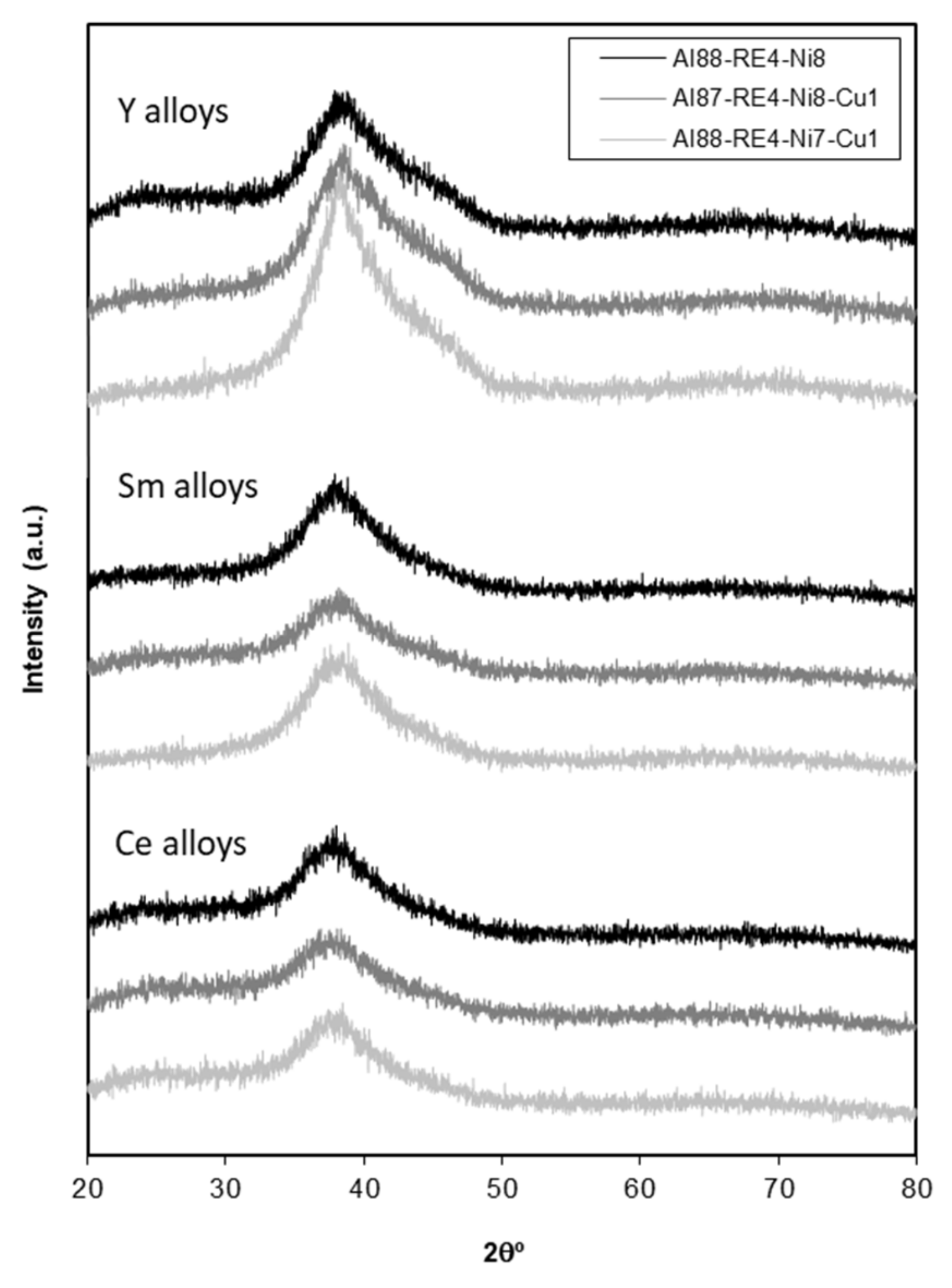
3.1. Melt-Spun Ribbons
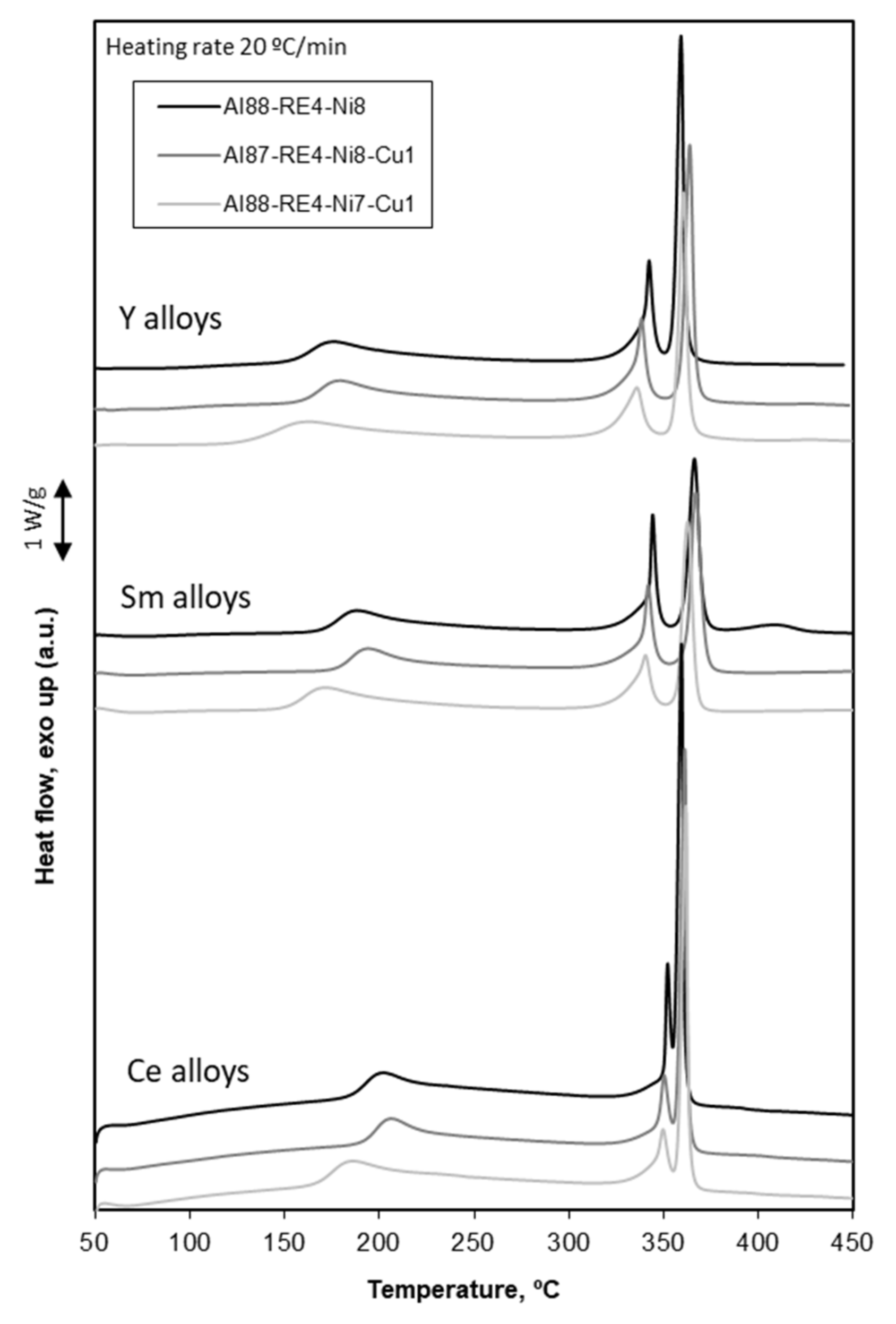
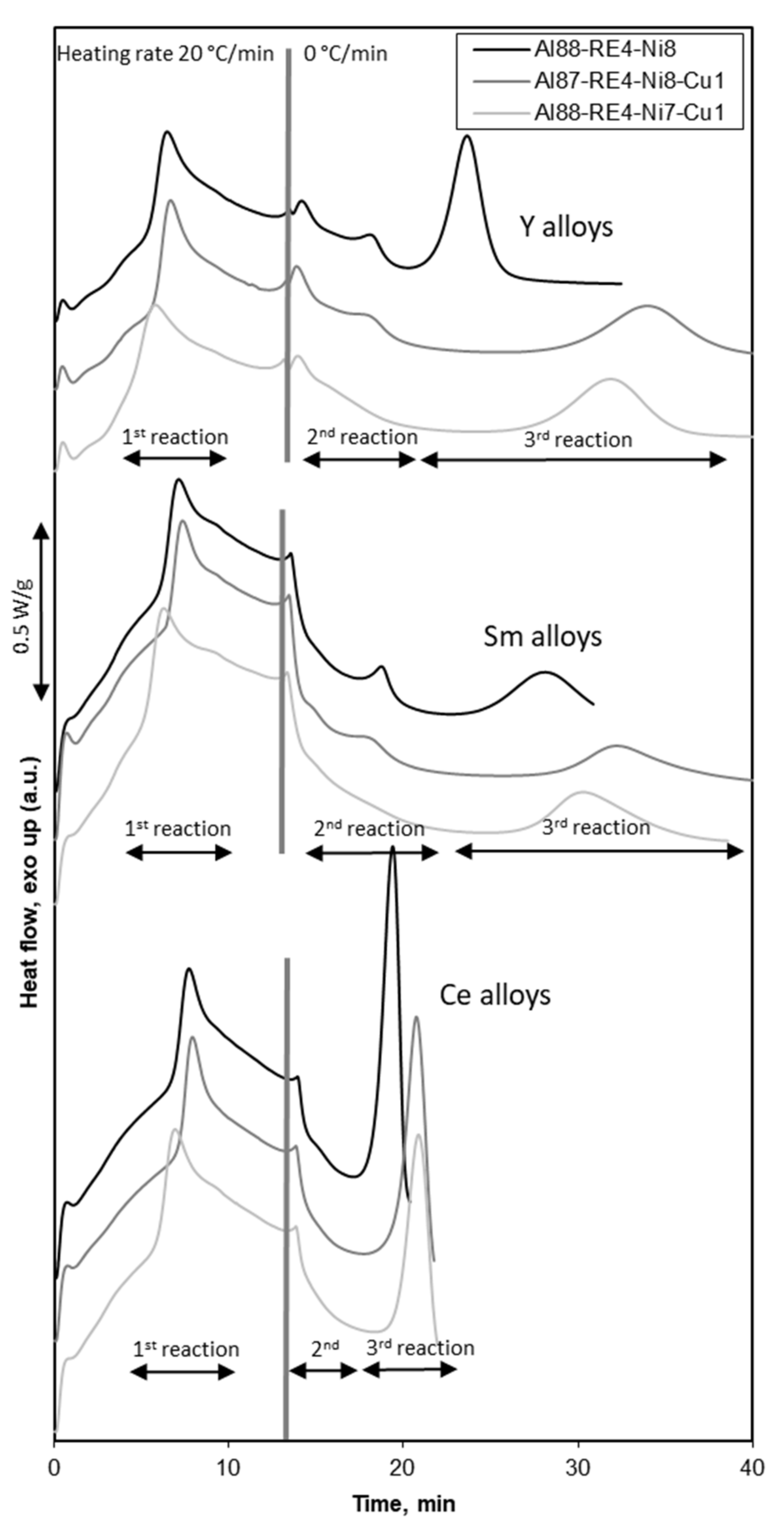
3.2. Crystallization Process
3.3. Primary Crystallization
3.4. High Temperature Crystallization Stages
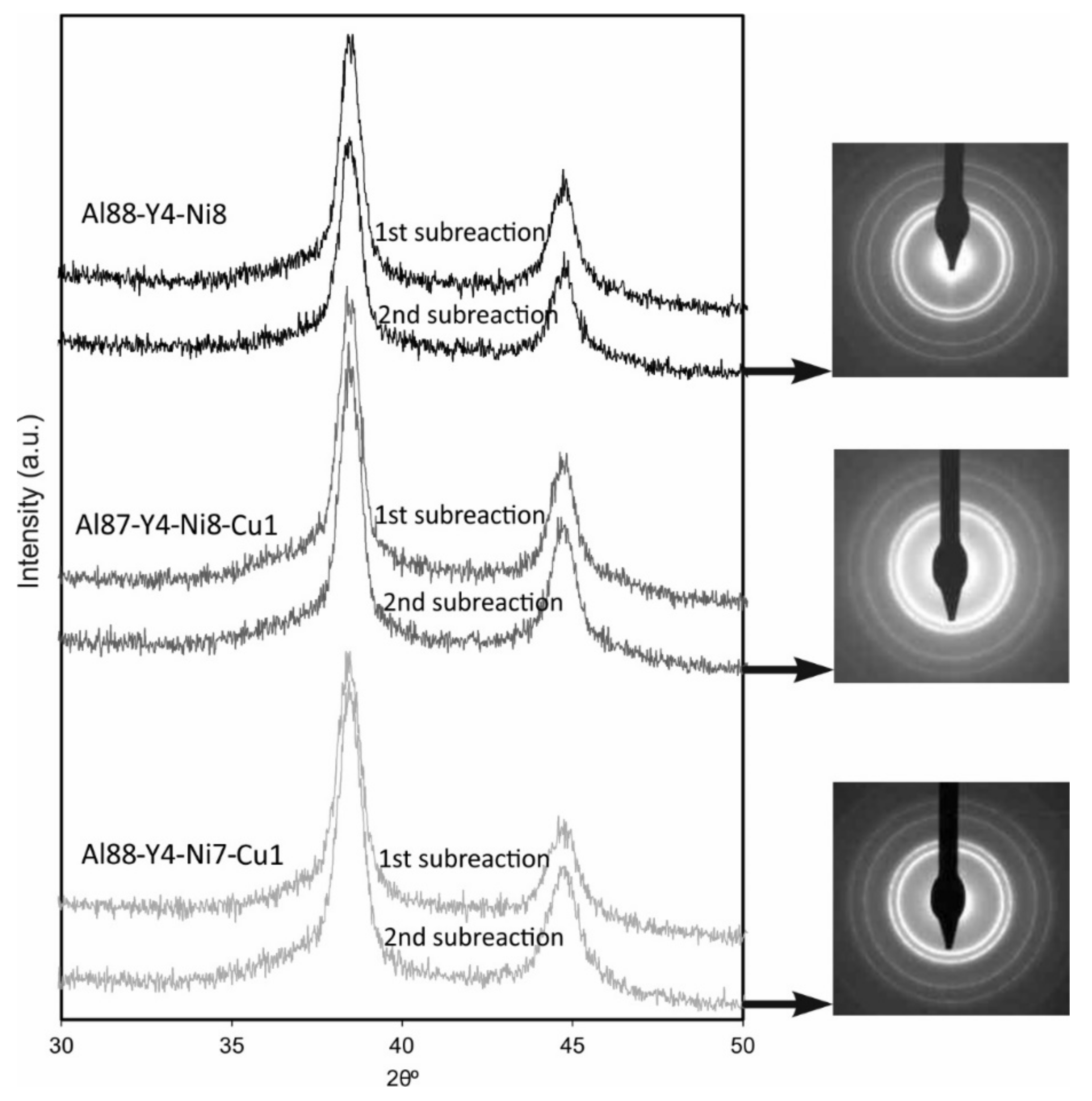
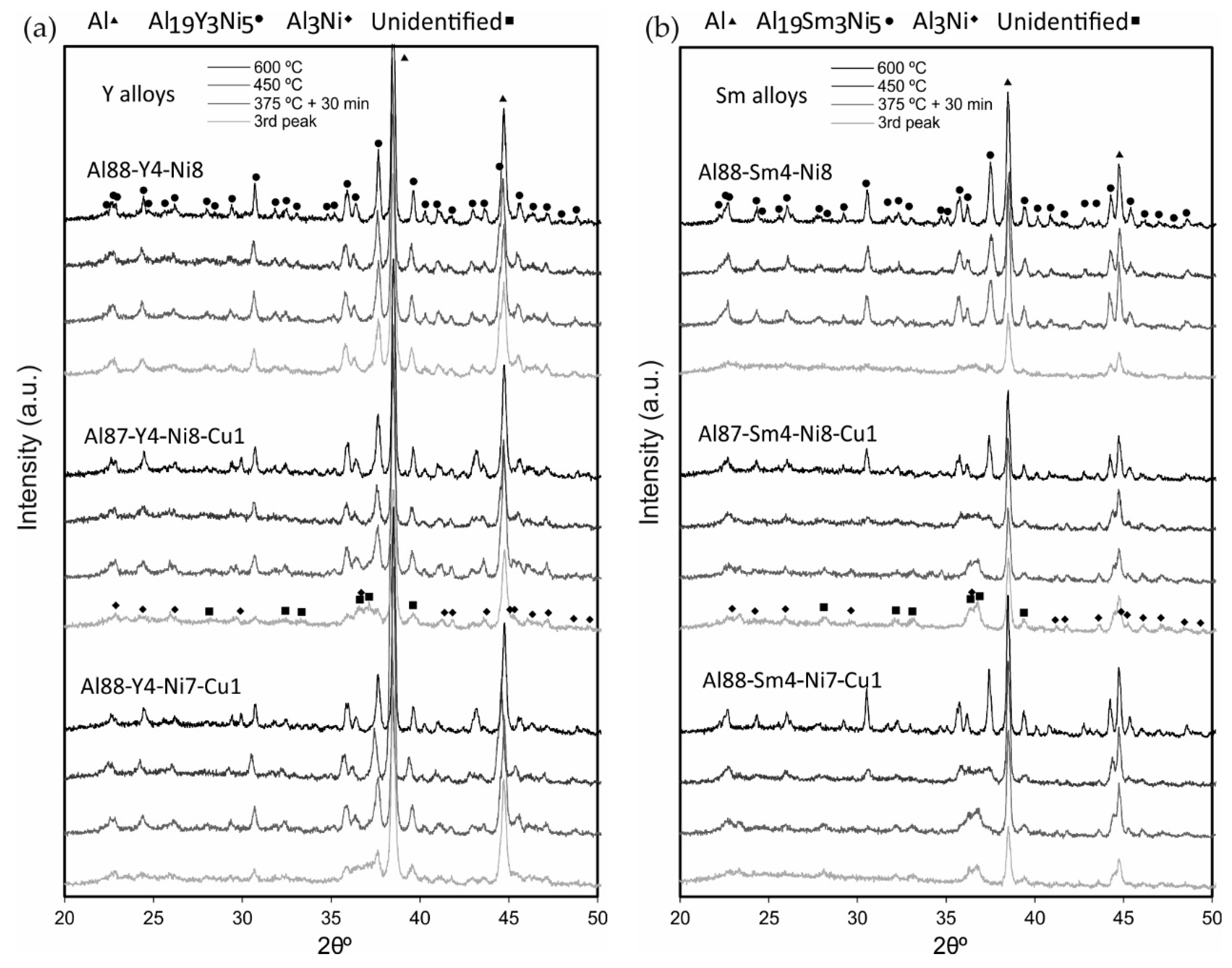
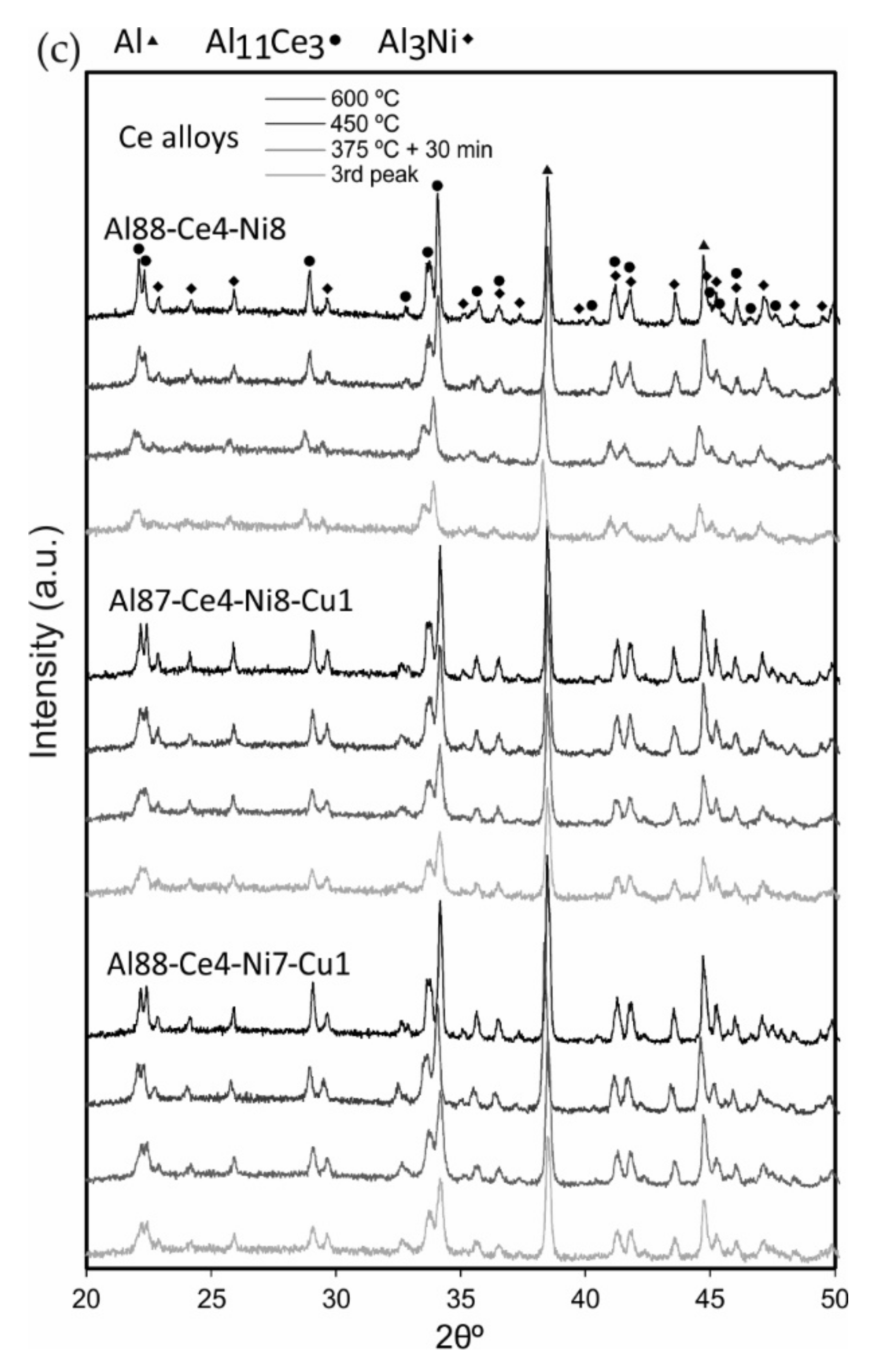
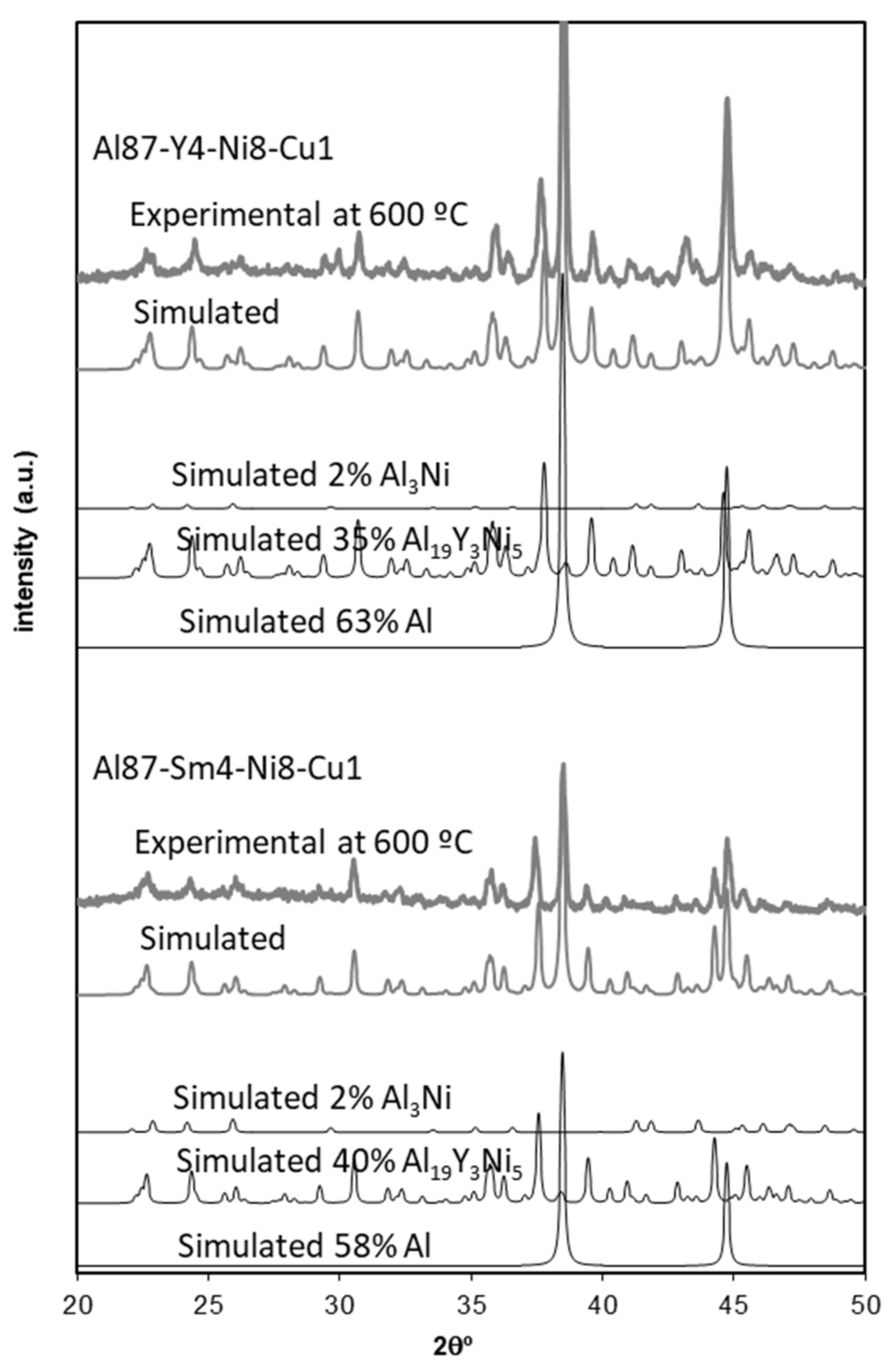
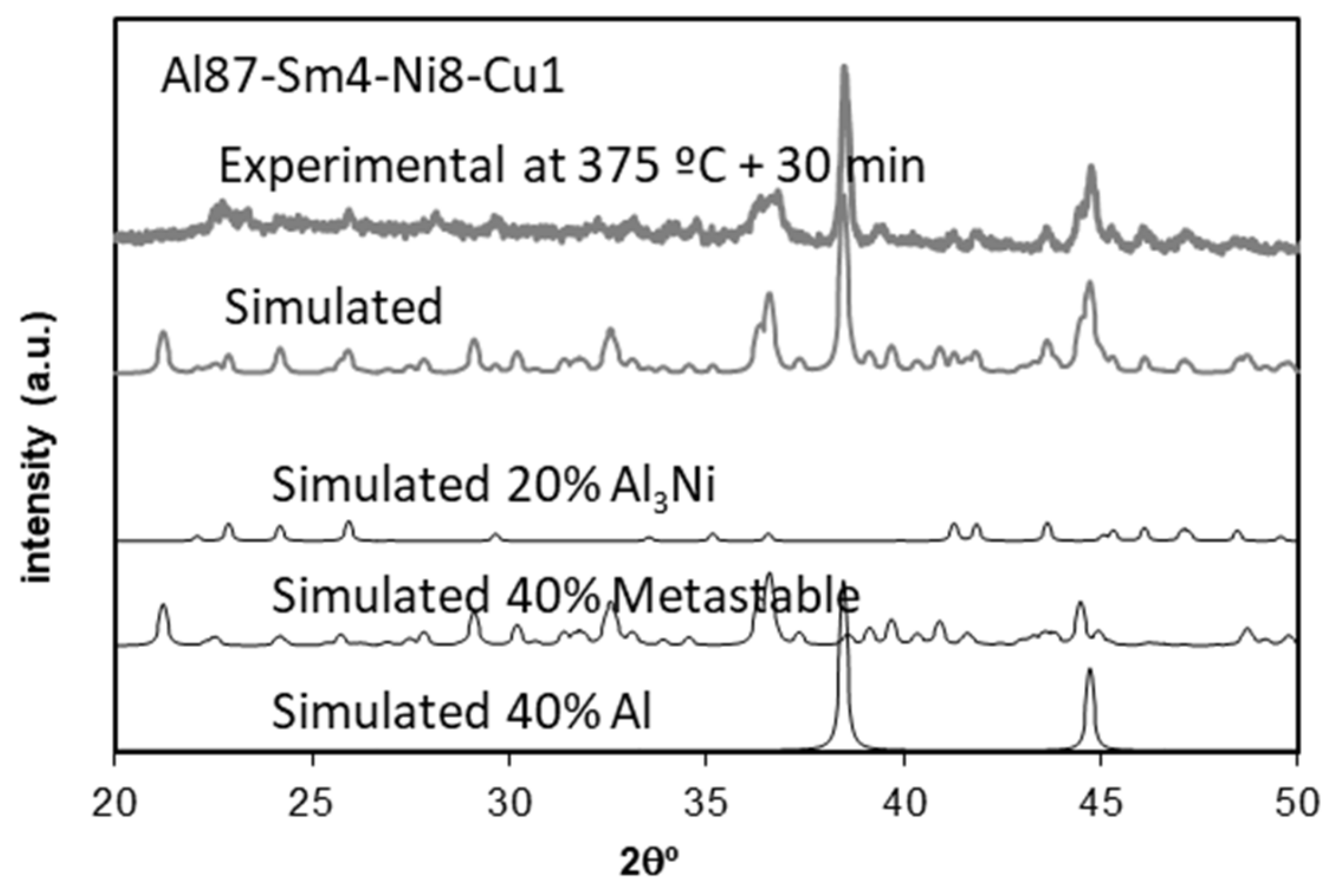
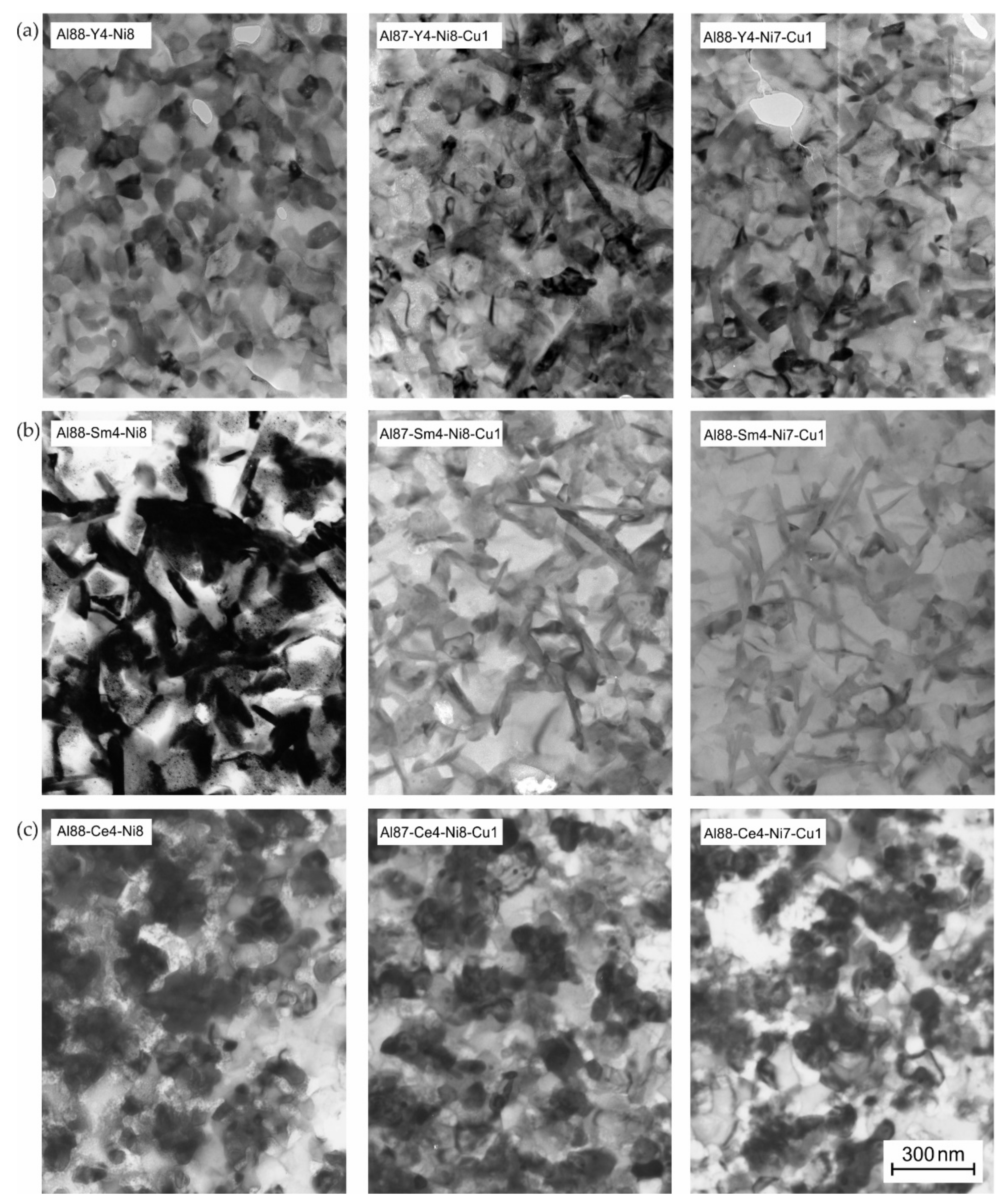
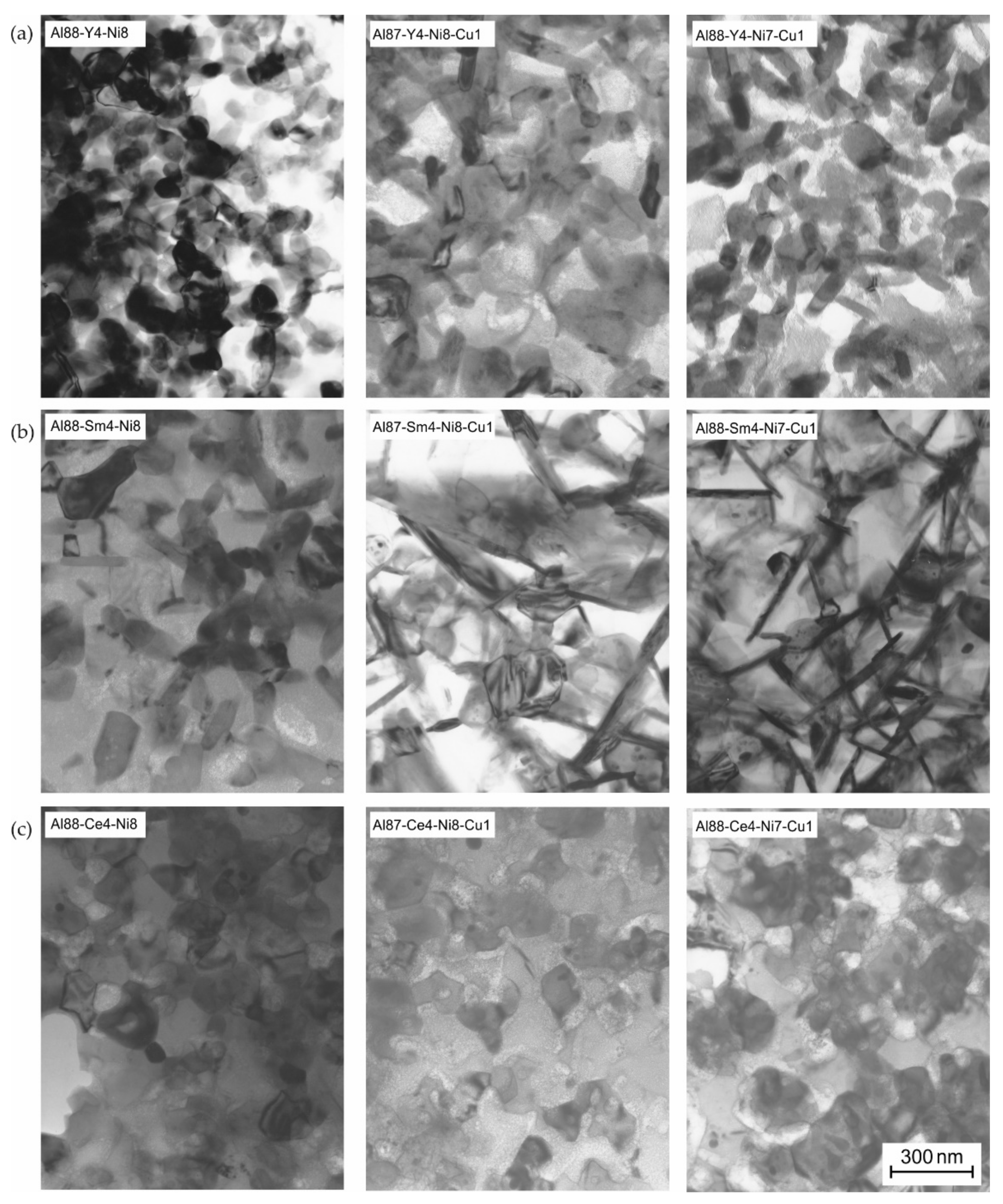
3.5. Microstructural Identification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zanotto, E.D.; Mauro, J.C. The glassy state of matter: Its definition and ultimate fate. J. Non-Cryst. Solids 2017, 471, 490–495. [Google Scholar] [CrossRef]
- Kruzic, J.J. Bulk Metallic Glasses as Structural Materials: A Review. Adv. Eng. Mater. 2016, 18, 1308–1331. [Google Scholar] [CrossRef]
- Nair, N.B.; Priyadarshini, B.G. Process, structure, property and applications of metallic glasses. AIMS Mater. Sci. 2016, 3, 1022–1053. [Google Scholar] [CrossRef]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Pershina, E.; Matveev, D.; Abrosimova, G.; Aroni, A. Formation of nanocrystals in an amorphous Al90Y10 alloy. Mater. Charact. 2017, 133, 87–93. [Google Scholar] [CrossRef]
- Inoue, A.; Ohtera, K.; Masumoto, T. New Amorphous Al-Y, Al-La and Al-Ce Alloys Prepared by Melt Spinning. Jpn. J. Appl. Phys. 1988, 27, L736. [Google Scholar] [CrossRef]
- Yin, J.; Cai, H.; Cheng, X.; Zhang, X. Al-based bulk metallic glass with large plasticity and ultrahigh strength. J. Alloy. Compd. 2015, 648, 276–279. [Google Scholar] [CrossRef]
- Eckert, J.; Das, J.; Pauly, S.; Duhamel, C. Mechanical properties of bulk metallic glasses and composites. J. Mater. Res. 2007, 22, 285–301. [Google Scholar] [CrossRef]
- Schroers, J. Processing of bulk metallic glass. Adv. Mater. 2010, 22, 1566–1597. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Komatsu, Y.; Kobayashi, K.F.; Shingu, P.H. Formation and crystallization of Al-Fe-Si amorphous alloys. J. Mater. Sci. 1983, 18, 1195–1201. [Google Scholar] [CrossRef]
- Dini, K.; Dunlap, R.A. The relationship of the quasicrystalline icosahedral phase to the amorphous structure in rapidly quenched Al-Mn-Si and Al-Fe-Si alloys. J. Phys. F Met. Phys. 1986, 16, 1917–1925. [Google Scholar] [CrossRef]
- Tsai, A.P.; Inoue, A.; Masumoto, T. Ductile Al-Ni-Zr Amorphous Alloys with High Mechanical Strength. J. Mater. Sci. Lett. 1988, 7, 805–807. [Google Scholar] [CrossRef]
- He, Y.; Poon, S.J.; Shiflet, G.J. Synthesis and properties of metallic glasses that contain aluminium. Science 1988, 241, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ohtera, K.; Tsai, A.P.; Masumoto, T. New Amorphous Alloys with Good Ductility in Al-Y-M and Al-La-M (M=Fe, Co, Ni or Cu) Systems. Jpn. J. Appl. Phys. 1988, 27, L280. [Google Scholar] [CrossRef]
- Inoue, A.; Ohtera, K.; Kith, K.; Masumoto, T. New Amorphous Alloys with Good Ductility in Al-Ce-M (M=Nb, Fe, Co, Ni or Cu) Systems. Jpn. J. Appl. Phys. 1988, 27, L1796. [Google Scholar] [CrossRef]
- Inoue, A.; Ohtera, K.; Tsai, A.P.; Masumoto, T. Aluminum-Based Amorphous Alloys with Tensile Strength above 980 MPa (100 kg/mm2). Jpn. J. Appl. Phys. 1988, 27, L479. [Google Scholar] [CrossRef]
- Kim, Y.H.; Inoue, A.; Masumoto, T. Ultrahigh Mechanical Strengths of Al88Y2Ni10−xMx (M=Mn, Fe or Co) Amorphous Alloys Containing Nanoscale fcc-Al Particles. Mater. Trans. JIM 1991, 32, 599–608. [Google Scholar] [CrossRef]
- Inoue, A. Amorphous, nanoquasicrystalline and nanocrystalline alloys in Al-based systems. Prog. Mater. Sci. 1998, 43, 365–520. [Google Scholar] [CrossRef]
- Kim, Y.H.; Inoue, A.; Masumoto, T. Ultrahigh tensile strengths of Al88Y2Ni9Mi (M=Mn or Fe) amorphous alloys containing finely dispersed fcc-Al particles. Mater. Trans. JIM 1990, 31, 747–749. [Google Scholar] [CrossRef]
- Kim, Y.H.; Inoue, A.; Masumoto, T. Increase in Mechanical Strength of Al–Y–Ni Amorphous Alloys by Dispersion of Nanoscale fcc-Al Particles. Mater. Trans. JIM 1991, 32, 331–338. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.-Y.; Wiest, A.; Duan, G.; Lind, M.-L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Abrosimova, G.E.; Aronin, A.S.; Zver’kova, I.I.; Kir’yanov, Y.V. Phase transformations upon crystallization of amorphous Al-Ni-RE alloys. Phys. Met. Metallogr. 2002, 94, 102–107. [Google Scholar]
- Li, Y.; Zhao, S.; Liu, Y.; Gong, P.; Schroers, J. How Many Bulk Metallic Glasses Are There? ACS Comb. Sci. 2017, 19, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Gao, J.E.; Wu, Y.; Wang, H.; Liu, X.J.; Lu, Z.P. Designing novel bulk metallic glass composites with a high aluminum content. Sci. Rep. 2013, 3, 3353. [Google Scholar] [CrossRef]
- Maurya, R.S.; Sahu, A.; Laha, T. Quantitative phase analysis in Al86Ni8Y6 bulk glassy alloy synthesized by consolidating mechanically alloyed amorphous powder via spark plasma sintering. Mater. Des. 2016, 93, 96–103. [Google Scholar] [CrossRef]
- Maurya, R.S.; Sahu, A.; Laha, T. Effect of consolidation pressure on phase evolution during sintering of mechanically alloyed Al86Ni8Y6 amorphous powders via spark plasma sintering. Mater. Sci. Eng. A 2016, 649, 48–56. [Google Scholar] [CrossRef]
- Lay, M.D.H.; Hill, A.J.; Saksida, P.G.; Gibson, M.A.; Bastow, T.J. 27Al NMR measurement of fcc Al configurations in as-quenched Al85Ni11Y4 metallic glass and crystallization kinetics of Al nanocrystals. Acta Mater. 2012, 60, 79–88. [Google Scholar] [CrossRef]
- Kim, Y.K.; Soh, J.R.; Kim, D.K.; Lee, H.M. Glass formation in metallic Al-Ni-Y. J. Non-Cryst. Solids 1998, 242, 122–130. [Google Scholar] [CrossRef]
- Styles, M.J.; Sun, W.W.; East, D.R.; Kimpton, J.A.; Gibson, M.A.; Hutchinson, C.R. On the competition in phase formation during the crystallisation of Al-Ni-Y metallic glasses. Acta Mater. 2016, 117, 170–187. [Google Scholar] [CrossRef]
- Shen, Y.; Perepezko, J.H. Al-based amorphous alloys: Glass-forming ability, crystallization behavior and effects of minor alloying additions. J. Alloy. Compd. 2017, 707, 3–11. [Google Scholar] [CrossRef]
- Sun, S.P.; Yi, D.Q.; Liu, H.Q.; Zang, B.; Jiang, Y. Calculation of glass forming ranges in Al–Ni–RE (Ce, La, Y) ternary alloys and their sub-binaries based on Miedema’s model. J. Alloy. Compd. 2010, 506, 377–387. [Google Scholar] [CrossRef]
- De Oliveira, M.F.; Aliaga, L.C.R.; Bolfarini, C.; Botta, W.J.; Kiminami, C.S. Thermodynamic and topological instability approaches for forecasting glass-forming ability in the ternary Al-Ni-Y system. J. Alloy. Compd. 2008, 464, 118–121. [Google Scholar] [CrossRef]
- Triveño Rios, C.; Suriñach, S.; Baró, M.D.; Bolfarini, C.; Botta, W.J.; Kiminami, C.S. Glass forming ability of the Al–Ce–Ni system. J. Non-Cryst. Solids 2008, 354, 4874–4877. [Google Scholar]
- Zhang, Z.; Xiong, X.Z.; Zhou, W.; Lin, X.; Inoue, A.; Li, J.F. Glass forming ability and crystallization behavior of Al–Ni–RE metallic glasses. Intermetallics 2013, 42, 23–31. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.H.; Liu, J.B.; Liu, B.X. Atomistic Design of Favored Compositions for Synthesizing the Al-Ni-Y Metallic Glasses. Sci. Rep. 2015, 5, 16218. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.J.; Yao, J.H.; Zhang, J.; Yang, H.W.; Wang, J.Q.; Ma, E. Al-rich bulk metallic glasses with plasticity and ultrahighspecific strength. Scr. Mater. 2009, 61, 423–426. [Google Scholar] [CrossRef]
- Yewondwossen, M.; Dunlap, R.A.; Lloyd, D.J. Thermal and electronic properties of amorphous Al87Y8Ni5-xTMx (TM=Mn, Fe, CO, Cu). J. Phys. Condens. Matter 1992, 4, 461–472. [Google Scholar] [CrossRef]
- Hong, S.J.; Warren, P.J.; Chun, B.S. Nanocrystallization behaviour of Al–Y–Ni with Cu additions. Mater. Sci. Eng. A 2001, 304–306, 362–366. [Google Scholar] [CrossRef]
- Squire, P.J.; Chang, I.T.H. Development of rapidly solidified Al–Y–Ni-based alloys. Mater. Sci. Eng. A 2007, 449–451, 1009–1012. [Google Scholar] [CrossRef]
- Chen, S.F.; Chen, C.Y.; Lin, C.H. Insight on the glass-forming ability of Al-Y-Ni-Ce bulk metallic glass. J. Alloy. Compd. 2015, 637, 418–425. [Google Scholar] [CrossRef]
- Gögebakan, M. The Effect of Si Addition on Crystallization Behavior of Amorphous Al-Y-Ni Alloy. J. Mater. Eng. Perform. 2004, 13, 504–508. [Google Scholar] [CrossRef]
- Chen, S.F.; Chen, J.K.; Lin, S.L.; Lin, Y.L. Effects of B upon glass forming ability of Al87Y8Ni5 amorphous alloy. J. Alloy. Compd. 2013, 565, 29–36. [Google Scholar] [CrossRef]
- Jun, J.H.; Kim, J.M.; Kim, K.T.; Jung, W.J. Glass formability and thermal stability of Al–Ni–Y–Be amorphous alloys. J. Alloy. Compd. 2007, 434–435, 190–193. [Google Scholar] [CrossRef]
- Latuch, J.; Kokoszkiewicz, A.; Matyja, H. The Effect of Cu Addition on the Formation of fcc-Al Phase in Rapidly Quenched Al-Y-Ni Alloys. Mater. Sci. Eng. A 1997, 226–228, 809–812. [Google Scholar] [CrossRef]
- Kim, W.T.; Gogebakan, M.; Cantor, B. Heat treatment of amorphous Al85Y5Ni10 and Al85Y10Ni5 alloys. Mater. Sci. Eng. A 1997, 226–228, 178–182. [Google Scholar] [CrossRef]
- Yang, H.W.; Wang, J.Q. Evidence of structure relaxation prior to nanocrystallization in an Al-based metallic glass. Scr. Mater. 2006, 55, 359–362. [Google Scholar] [CrossRef]
- Greer, A.L. Confusion by Design. Nature 1993, 366, 303–304. [Google Scholar] [CrossRef]
- Huang, Z.H.; Li, J.F.; Rao, Q.L.; Zhou, Y.H. Primary crystallization of Al–Ni–RE amorphous alloys with different type and content of RE. Mater. Sci. Eng. A 2008, 489, 380–388. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, W.; Xiong, X.Z.; Kong, L.T.; Li, J.F. Glass forming ability and primary crystallization behavior of Al–Ni–Ce alloys. Intermetallics 2012, 24, 1–6. [Google Scholar] [CrossRef]
- Rachek, O.P. X-ray diffraction study of amorphous alloys Al–Ni–Ce–Sc with using Ehrenfest’s formula. J. Non-Cryst. Solids 2006, 352, 3781–3786. [Google Scholar] [CrossRef]
- Gich, M.; Gloriant, T.; Suriñach, S.; Greer, A.L.; Baró, M.D. Glass forming ability and crystallisation processes within the Al-Ni-Sm system. J. Non-Cryst. Solids 2001, 289, 214–220. [Google Scholar] [CrossRef]
- Gogebakan, M.; Warren, P.J.; Cantor, B. Crystallization behaviour of amorphous Al85Y11Ni4 alloy. Mater. Sci. Eng. A 1997, 226–228, 168–172. [Google Scholar] [CrossRef]
- Sun, F.; Gloriant, T. Primary crystallization process of amorphous Al88Ni6Sm6 alloy investigated by differential scanning calorimetry and by electrical resistivity. J. Alloy. Compd. 2009, 477, 133–138. [Google Scholar] [CrossRef]
- Battezzati, L.; Rizzi, P.; Rontó, V. The difference in devitrification paths in Al87Ni7Sm6 and Al87Ni7La6 amorphous alloys. Mater. Sci. Eng. A 2004, 375–377, 927–931. [Google Scholar] [CrossRef]
- Latuch, J.; Matyja, H.; Fadeeva, V.I. Crystallization of amorphous A185Y10Ni5 and A185Y5Ni10 alloys. Mater. Sci. Eng. A 1994, 179–180, 506–510. [Google Scholar] [CrossRef]
- Saini, S.; Zaluska, A.; Altounian, Z. Effect of glass short-range order on crystallization onset in Al-Y-Ni glasses. J. Non-Cryst. Solids 1999, 250–252, 714–718. [Google Scholar] [CrossRef]
- Ko, B.C.; Yoo, Y.C. High temperature deformation behavior of Al85Ni10Y5 alloy produced by hot extrusion using an amorphous ribbon. J. Mater. Sci. Lett. 1999, 18, 1765–1768. [Google Scholar] [CrossRef]
- Jindal, R.; Raja, V.S.; Gibson, M.A.; Styles, M.J.; Bastow, T.J.; Hutchinson, C.R. Effect of annealing below the crystallization temperature on the corrosion behavior of Al–Ni–Y metallic glasses. Corros. Sci. 2014, 84, 54–65. [Google Scholar] [CrossRef]
- Kuball, A.; Stolpe, M.; Busch, R. Crystallization behavior of the Al86Ni8Y6 metallic glass forming alloy upon rapid cooling. J. Alloy. Compd. 2018, 737, 398–404. [Google Scholar] [CrossRef]
- Cao, B.; Li, S.; Yi, D. Study on the crystallization of Al88.5Y6.5Ni5 (at.%) amorphous alloy. J. Less-Common Met. 1991, 171, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Warren, P.J.; Cerezo, A. Effect of Cu addition on nanocrystallization of Al-Ni-Sm amorphous alloy. Mater. Sci. Eng. A 2002, 327, 109–115. [Google Scholar] [CrossRef]
- Battezzati, L.; Kusy, M.; Rizzi, P.; Ronto, V. Devitrification of Al-Ni-Rare Earth amorphous alloys. J. Mater. Sci. 2004, 39, 3927–3934. [Google Scholar] [CrossRef]
- Gloriant, T.; Greer, A.L. Al-Based nanocrystalline composites by rapid solidification of Al-Ni-Sm alloys. Nanostrudured Mater. 1998, 10, 389–396. [Google Scholar] [CrossRef]
- Rontó, V.; Battezzati, L.; Yavari, A.R.; Tonegaru, M.; Lupu, N.; Heunen, G. Crystallization behaviour of Al87Ni7La6 and Al87Ni7Sm6 amorphous alloys. Scr. Mater. 2004, 50, 839–843. [Google Scholar] [CrossRef]
- Anghelus, A.; Avettand-Fènoël, M.N.; Cordier, C.; Taillard, R. Thermal crystallization of an Al88Ni6Sm6 metallic glass. J. Alloy. Compd. 2015, 651, 454–464. [Google Scholar] [CrossRef]
- Uporov, S.A.; Ryl’tsev, R.E.; Uporova, N.S.; Bykov, V.A.; Murzakaev, A.M.; Pryanichnikov, S.V. Structural and Magnetic Peculiarities of Al86Ni8Sm6 Alloy in Amorphous, Crystalline, and Liquid States. Phys. Met. Metallogr. 2015, 116, 128–135. [Google Scholar] [CrossRef]
- Révész, Á.; Varga, L.K.; Nagy, P.M.; Lendvai, J.; Bakonyi, I. Structure and thermal stability of melt-quenched Al92-xNi8(Ce,Sm)x alloys with x = 1, 2 and 4. Mater. Sci. Eng. A 2003, 351, 160–165. [Google Scholar] [CrossRef]
- Anghelus, A.; Avettand-Fènoël, M.N.; Cordier, C.; Taillard, R. Microstructural evolution of aluminium/Al–Ni–Sm glass forming alloy laminates obtained by Controlled Accumulative Roll Bonding. J. Alloy. Compd. 2015, 631, 209–218. [Google Scholar] [CrossRef]
- Illeková, E.; Duhaj, P.; Mrafko, P.; Svec, P. Influence of Pd on crystallization of Al–Ni–Sm-based ribbons. J. Alloy. Compd. 2009, 483, 20–23. [Google Scholar] [CrossRef]
- Hono, K.; Zhang, Y.; Inoue, A.; Sakurai, T. Atom Probe Studies of Nanocrystalline Microstructural Evolution in Some Amorphous Alloys. Mater. Trans. JIM 1995, 36, 909–917. [Google Scholar] [CrossRef]
- Cuevas, F.G.; Lozano-Perez, S.; Aranda, R.M.; Ternero, F. Crystallisation of amorphous Al-Y-Ni-(Cu) alloys. J. Non-Cryst. Solids 2019, 512, 15–24. [Google Scholar] [CrossRef]
- Matsuura, M.; Sakurai, M.; Suzuki, K.; Tsai, A.P.; Inoue, A. Local structure change of Ce and Cu in the course of nanocrystalline formation from amorphous Al87Ni8Ce3Cu2. Mater. Sci. Eng. A 1997, 226–228, 511–514. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Imhoff, S.D.; Hebert, R.J. Nanostructure development during devitrification and deformation. J. Alloy. Compd. 2010, 495, 360–364. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, W.T.; Park, E.S.; Mattern, N.; Eckert, J. Phase separation in metallic glasses. Prog. Mater. Sci. 2013, 58, 1103–1172. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Influence of a supercooled liquid on crystallization behaviour of Al–Y–Ni–Co metallic glass. Mater. Lett. 2002, 54, 75–80. [Google Scholar] [CrossRef]
- Bassim, N.; Kiminami, C.S.; Kaufman, M.J.; Oliveira, M.F.; Perdigao, M.N.R.V.; Botta Filho, W.J. Crystallization behavior of amorphous Al84Y9Ni5Co2 alloy. Mater. Sci. Eng. A 2001, 304–306, 332–337. [Google Scholar] [CrossRef]
- Raggio, R.; Borzone, G.; Ferro, R. The Al-rich region in the Y-Ni-Al system: Microstructures and phase equilibria. Intermetallics 2000, 8, 247–257. [Google Scholar] [CrossRef]
- Vasiliev, A.L.; Aindow, M.; Blackburn, M.J.; Watson, T.J. Phase Stability and Microstructure in Devitrified Al-Rich Al-Y-Ni Alloys. Intermetallics 2004, 12, 349–362. [Google Scholar] [CrossRef]
- Mudry, S.; Kulyk, Y.; Mykhaylyuk, V.; Tsizh, B. Structure changes in Al80Ni15Y5 amorphous alloy. J. Non-Cryst. Solids 2008, 354, 4488–4490. [Google Scholar] [CrossRef]
- Golumbfskie, W.J.; Arroyave, R.; Shin, D.; Liu, Z.-K. Finite-temperature thermodynamic and vibrational properties of Al–Ni–Y compounds via first-principles calculations. Acta Mater. 2006, 54, 2291–2304. [Google Scholar] [CrossRef]
- Shin, D.; Golumbfskie, W.J.; Ryba, E.R.; Liu, Z.-K. First-principles study of Al–Ni–Y ternary compounds for crystal structure validation. J. Alloy. Comp. 2008, 462, 262–266. [Google Scholar] [CrossRef]
- Golumbfskie, W.J.; Prins, S.N.; Eden, T.J.; Liu, Z.-K. Predictions of the Al-rich region of the Al–Co–Ni–Y system based upon first-principles and experimental data. CALPHAD 2009, 33, 124–135. [Google Scholar] [CrossRef]
- Sha, P.F.; Qi, Z.; Zhang, Z.H. Effect of Ag or Pd additions on the microstructure, crystallization and thermal stability of Al–Ni–Ce amorphous alloys. Intermetallics 2010, 18, 1699–1706. [Google Scholar] [CrossRef]
- Triveño Rios, C.; Suriñach, S.; Baró, M.D.; Bolfarini, C.; Botta, W.J.; Kiminami, C.S. Crystallization behavior of amorphous alloys in the Al-Ce-Ni system. Rev. Adv. Mater. Sci. 2008, 18, 469–475. [Google Scholar]
- Delsante, S.; Raggio, R.; Borzone, G. Phase relations of the Sm–Ni–Al ternary system at 500 °C in the 40–100 at.% Al region. Intermetallics 2008, 16, 1250–1257. [Google Scholar] [CrossRef]
- Nolze, G.; Klaus, W. PowderCell 2.0 for Windows. Powder Diffr. 1998, 13, 256–259. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, F.G.; Lozano-Perez, S.; Aranda, R.M.; Astacio, R. Crystallization Process and Microstructural Evolution of Melt Spun Al-RE-Ni-(Cu) Ribbons. Metals 2020, 10, 443. https://doi.org/10.3390/met10040443
Cuevas FG, Lozano-Perez S, Aranda RM, Astacio R. Crystallization Process and Microstructural Evolution of Melt Spun Al-RE-Ni-(Cu) Ribbons. Metals. 2020; 10(4):443. https://doi.org/10.3390/met10040443
Chicago/Turabian StyleCuevas, Francisco G., Sergio Lozano-Perez, Rosa María Aranda, and Raquel Astacio. 2020. "Crystallization Process and Microstructural Evolution of Melt Spun Al-RE-Ni-(Cu) Ribbons" Metals 10, no. 4: 443. https://doi.org/10.3390/met10040443
APA StyleCuevas, F. G., Lozano-Perez, S., Aranda, R. M., & Astacio, R. (2020). Crystallization Process and Microstructural Evolution of Melt Spun Al-RE-Ni-(Cu) Ribbons. Metals, 10(4), 443. https://doi.org/10.3390/met10040443





