Bonding and Stability of Ternary Structures in the CeT2Al20 (T=Ta, W, Re) and YRe2Al20 Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thiede, V.M.; Jeitschko, W.; Niemann, S.; Ebel, T. EuTa2Al20, Ca6W4Al43 and other compounds with CeCr2Al20 and Ho6Mo4Al43 type structures and some magnetic properties of these compounds. J. Alloys Compd. 1998, 267, 23–31. [Google Scholar] [CrossRef]
- Thiede, V.M.T.; Ebel, T.; Jeitschko, W. Ternary aluminides LnT2Al10 (Ln=Y, La–Nd, Sm, Gd–Lu andT=Fe, Ru, Os) with YbFe2Al10 type structure and magneticproperties of the iron-containing series. J. Mater. Chem. 1998, 8, 125–130. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; van Vucht, J.H.N.; van den Hoogenhof, W.W. Note on the crystal structure of the rare earth-3d transition metal compounds of the type RT4A18. J. Less Common Met. 1976, 50, 145–150. [Google Scholar] [CrossRef]
- Stȩpień-Damm, J.; Baran, A.; Suski, W.; Stepien-Damm, J. Crystal structure of the uranium ternary compound UFe4Al8. J. Less Common Met. 1984, 102, L5–L8. [Google Scholar] [CrossRef]
- Tamura, I.; Mizushima, T.; Isikawa, Y.; Sakurai, J. Mössbauer effect and magnetization studies of CeFe2Al8 and LaFe2Al8. J. Magn. Magn. Mater. 2000, 220, 31–38. [Google Scholar] [CrossRef]
- Suski, W. Comparison of structure and magnetic properties of the 4f and 5f electron ThMn12-type ternary aluminides. J. Alloys Compd. 1995, 223, 237–241. [Google Scholar] [CrossRef]
- Baran, A.; Suski, W. Magnetic properties of the UFenAl12-n (n = 4, 5 or 6) and ThFe4Al8 intermetallic compounds. Phys. B 1985, 130B, 219–221. [Google Scholar] [CrossRef]
- Baran, A.; Suski, W.; Mydlarz, T. MAGNETIC PROPERTIES OF THE (U, Th)(Cr, Mn)4Al8 COMPOUNDS. Anomalous Rare Earths Actin. 1987, 63–64, 196–198. [Google Scholar]
- Steglich, F.; Stockert, O.; Wirth, S.; Geibel, C.; Yuan, H.; Kirchner, S.; Si, Q. Routes to heavy-fermion superconductivity. J. Phys. Conf. Ser. 2013, 449, 012028. [Google Scholar] [CrossRef]
- Schafer, W.; Will, G. Neutron diffraction studies of the structural and magnetic properties of AnFe4Al8 (An=Th, U, Np) intermetallic compounds. J. Less Common Met. 1989, 149, 237–241. [Google Scholar] [CrossRef]
- Ott, H.R. Heavy-electron metals. Annu. Rev. Mater. Sci. 1987, 17, 13–33. [Google Scholar] [CrossRef]
- Bakker, M.; Van Dijk, A.; Wicherts, J.M. The Rules of the Game Called Psychological Science. Perspect. Psychol. Sci. 2012, 7, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.P.; Almeida, M.; Walker, C.; Ray, J.; Spirlet, J. Phase relations and single crystal growth of U-Fe-M (M = Al, Si) compounds with ThMn12-type structure. Mater. Lett. 1994, 19, 13–16. [Google Scholar] [CrossRef]
- Cordier, G.; Czech, E.; Ochmann, H.; Schäfer, H. Neue übergangsmetallaluminide des calciums. J. Less Common Met. 1984, 99, 173–185. [Google Scholar] [CrossRef]
- Niemann, S.; Jeitschko, W. The crystal structure of YbFe2Al10, a combined substitution and stacking variant of the ThMn 12 and CeMn 4 Al 8 type structures. Z. Krist. 1995, 210, 338–341. [Google Scholar] [CrossRef]
- Kripyakevich, P.I.; Zarechnyuk, O.S. RCr2Al20 compounds in systems of rare earth metals and calcium, and their crystal structures. Dopov. Akad. Nauk. Ukr. RSR Ser. A Fiz. Tekh. 1968, 30, 364. [Google Scholar]
- Yaniv, G.; Fuks, D.; Meshi, L. Explanation of structural differences and similarities between the AT2Al10 phases (where A = actinide, lanthanide or rare earth element and T = transition metal). Z. Krist. 2019, 234, 595–603. [Google Scholar] [CrossRef]
- Winiarski, M.; Wiendlocha, B.; Sternik, M.; Wiśniewski, P.; O’Brien, J.R.; Kaczorowski, D.; Klimczuk, T. Rattling-enhanced superconductivity in MV2Al20 (M=Sc, Lu, Y) intermetallic cage compounds. Phys. Rev. B 2016, 93, 134507. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Tsujimoto, M.; Tomita, T.; Sakai, A.; Nakatsuji, S. Heavy Fermion Superconductivity in Non-magnetic Cage Compound PrV2Al20. J. Phys. Conf. Ser. 2016, 683, 12013. [Google Scholar] [CrossRef]
- Frank, F.C.; Kasper, J.S. Complex alloy structures regarded as sphere packings. II. Analysis and classification of representative structures. Acta Crystallogr. 1959, 12, 483–499. [Google Scholar] [CrossRef]
- Luo, H.; Krizan, J.W.; Müechler, L.; Haldolaarachchige, N.; Klimczuk, T.; Xie, W.; Fuccillo, M.; Felser, C.; Cava, R.J. A large family of filled skutterudites stabilized by electron count. Nat. Commun. 2015, 6, 6489. [Google Scholar] [CrossRef] [PubMed]
- Stein, F.; Palm, M.; Sauthoff, G. Structure and stability of Laves phases. Part I. Critical assessment of factors controlling Laves phase stability. Intermetallics 2004, 12, 713–720. [Google Scholar] [CrossRef]
- Winiarski, M.; Klimczuk, T. Crystal structure and low-energy Einstein mode in ErV 2 Al 20 intermetallic cage compound. J. Solid State Chem. 2017, 245, 10–16. [Google Scholar] [CrossRef][Green Version]
- Higashinaka, R.; Nakama, A.; Ando, M.; Watanabe, M.; Aoki, Y.; Sato, H. Magnetic and transport properties of YbT2Al20(T= Ti, V and Cr). J. Physics: Conf. Ser. 2011, 273, 012033. [Google Scholar] [CrossRef]
- Namiki, T.; Nosaka, K.; Tsuchida, K.; Lei, Q.; Kanamori, R.; Nishimura, K. Magnetic and thermal properties of Nd T 2 AI 20 (T: Ti, V, Cr) single crystals. J. Phys. Conf. Ser. 2016, 683, 12017. [Google Scholar] [CrossRef]
- Bram, A.I.; Venkert, A.; Meshi, L. Characterization of new aluminides found in the ThT 2 Al 20 alloys (where T = Ti, V, Mn). J. Alloys Compd. 2015, 641, 1–6. [Google Scholar] [CrossRef]
- Uziel, A.; Bram, A.; Venkert, A.; Kiv, A.E.; Fuks, D.; Meshi, L. Abrupt symmetry decrease in the ThT2Al20 alloys (T = 3d transition metal). J. Alloys Compd. 2015, 648, 353–359. [Google Scholar] [CrossRef]
- Yaniv, G.; Fuks, D.; Meshi, L. Structure and peculiarities of bonding in the Al-rich A-Mn-Al alloys (where A=Y, Gd, Th and U). Intermetallics 2018, 100, 44–51. [Google Scholar] [CrossRef]
- Fehrmann, B.; Jeitschko, W. Lanthanoid Rhenium Aluminides with a High Content of Aluminum: LnRe2Al10(Ln = Ho−Lu) with a New Structure Type and NdRe2Al10with CaCr2Al10-Type Structure. Inorg. Chem. 1999, 38, 3344–3351. [Google Scholar] [CrossRef]
- Fehrmann, B.; Jeitschko, W. The Intermetallic Compounds GdRe2Al10 and TbRe2Al10, Crystallizing with a Stacking Variant of the YbFe2Al10 Type Structure. Z. Nat. B 1999, 54, 1277–1282. [Google Scholar] [CrossRef]
- Moze, O.; Tung, L.; Franse, J.; Buschow, K. Crystal structure and magnetic properties of CeV2Al20 and CeCr2Al20. J. Alloys Compd. 1998, 268, 39–41. [Google Scholar] [CrossRef]
- Fulfer, B.W.; Haldolaarachchige, N.; Young, D.P.; Chan, J.Y. Crystal growth and magnetic properties of Ln-Mn-Al (Ln=Gd, Yb) compounds of the CaCr2Al10 and ThMn12 structure types. J. Solid State Chem. 2012, 194, 143–150. [Google Scholar] [CrossRef]
- Fehrmann, B.; Jeitschko, W. ChemInform Abstract: The Intermetallic Compounds GdRe2Al10 and TbRe2Al10, Crystallizing with a Stacking Variant of the YbFe2Al10 Type Structure. Cheminform 2010, 31. [Google Scholar] [CrossRef]
- International Center for Diffraction Data. PDF4+ Commercial Database of the International Cneter for Diffractin Data; ICDD: Delaware County, PA, USA, 2019. [Google Scholar]
- Blaha, P.; Schwarz, K.; Madsen, G.; Kvasnicka, D.; Luitz, J. WIEN2k, An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties; Schwarz, K., Ed.; Technology Universitat Wien: Wien, Austria, 2001; ISBN 3- 9501031-1-2. version 10.1. [Google Scholar]
- Desclaux, J. Hartree Fock Slater self consistent field calculations. Comput. Phys. Commun. 1970, 1, 216–222. [Google Scholar] [CrossRef]
- Koelling, D.D.; Harmon, B.N. A technique for relativistic spin-polarised calculations. J. Phys. C: Solid State Phys. 1977, 10, 3107–3114. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Henderson, T.M.; Paier, J.; Scuseria, G.E. Accurate treatment of solids with the HSE screened hybrid. Phys. Status Solidi (b) 2010, 248, 767–774. [Google Scholar] [CrossRef]
- Janesko, B.G.; Henderson, T.M.; Scuseria, G.E. Screened hybrid density functionals for solid-state chemistry and physics. Phys. Chem. Chem. Phys. 2008, 11, 443–454. [Google Scholar] [CrossRef]
- Wang, J.; Ma, L.; Ray, A.K. On the magnetic and thermodynamic properties of Americium-II: A hybrid density functional theoretic study. Phys. Lett. A 2010, 374, 4704–4712. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Solovyev, I.V.; Korotin, M.A.; Czyyk, M.T.; Sawatzky, G.A. Density-functional theory and NiO photoemission spectra. Phys. Rev. B 1993, 48, 16929–16934. [Google Scholar] [CrossRef]
- Antonov, V.N.; Harmon, B.N.; Antropov, V.P.; Perlov, A.Y.; Yaresko, A.N. Electronic structure and magneto-optical Kerr effect of Fe3O4 and Mg2+- or Al3+-substituted Fe3O4. Phys. Rev. B 2001, 64, 134410. [Google Scholar] [CrossRef]
- Anisimov, V.; Elfimov, I.S.; Hamada, N.; Terakura, K. Charge-ordered insulating state ofFe3O4from first-principles electronic structure calculations. Phys. Rev. B 1996, 54, 4387–4390. [Google Scholar] [CrossRef] [PubMed]
- Guss, P.; Foster, M.E.; Wong, B.M.; Doty, F.P.; Shah, K.S.; Squillante, M.R.; Shirwadkar, U.; Hawrami, R.; Tower, J.; Yuan, D. Results for aliovalent doping of CeBr3 with Ca2+. J. Appl. Phys. 2014, 115, 34908. [Google Scholar] [CrossRef]
- Cococcioni, M.; De Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA+U method. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Cococcioni, M. A LDA + U Study of Selected iron Compounds. Ph.D. Thesis, The university of Cambridge, Cambridge, UK, October 2002. [Google Scholar]
- Zenou, V.; Meshi, L.; Fuks, D. Why UFexAl12−x phase does not crystallize with ThMn12-structure type, when x = 2? Intermetallics 2011, 19, 713–720. [Google Scholar] [CrossRef]
- Zenou, V.; Rafailov, G.; Dahan, I.; Kiv, A.; Meshi, L.; Fuks, D. Ordered U(Al, Si)3 phase: Structure and bonding. J. Alloys Compd. 2017, 690, 884–889. [Google Scholar] [CrossRef]
- Söderlind, P.; Sadigh, B.; Lordi, V.; Landa, A.; Turchi, P. Electron correlation and relativity of the 5f electrons in the U–Zr alloy system. J. Nucl. Mater. 2014, 444, 356–358. [Google Scholar] [CrossRef]
- Xie, W.; Marianetti, C.A.; Morgan, D. Reply to “Comment on ‘Correlation and relativistic effects in U metal and U-Zr alloy: Validation of ab initio approaches’”. Phys. Rev. B 2016, 93, 157101. [Google Scholar] [CrossRef]
- Söderlind, P.; Landa, A.; Perron, A.; Sadigh, B.; Heo, T.W. Ground-State and Thermodynamical Properties of Uranium Mononitride from Anharmonic First-Principles Theory. Appl. Sci. 2019, 9, 3914. [Google Scholar] [CrossRef]
- Fuks, D.; Mastrikov, Y.; Kotomin, E.; Maier, J. Ab initio thermodynamic study of (Ba,Sr)(Co,Fe)O3 perovskite solid solutions for fuel cell applications. J. Mater. Chem. A 2013, 1, 14320. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Thiede, V.M.; Jeitschko, W. Ternary Intermetallic Compounds LnMn2Al10 (Ln = Y, La-Nd, Sm, Gd-Dy) and LnRe2Al10 (Ln = Ce, Pr, Sm) with CaCr2Al10-Type Structure. Z. Nat. B 1998, 53, 673–678. [Google Scholar]
- Sefat, A.S.; Bud’Ko, S.L.; Canfield, P.C. Properties of RRe2Al10 (R=Y, Gd–Lu) crystals. Phys. Rev. B 2009, 79, 174429. [Google Scholar] [CrossRef]
- Niemann, S.; Jeitschko, W. Ternary aluminide AT2Al20 (A=rare earth elements and Uranium; T=Ti, Nb, Ta, Mo, and W) with CeCr2Al20-type structure. J. Solid State Chem. 1995, 114, 337–341. [Google Scholar] [CrossRef]
- Kiv, A.; Ezersky, V.; Talianker, M. The stability of structures with icosahedral local order in Al-based alloys with transition metals. Mater. Sci. Eng. A 2003, 352, 100–104. [Google Scholar] [CrossRef]
- Silberberg, M. The Molecular Nature of Matter and Change, 6th ed.; McGraw-Hill Science/Engineering/Math: New York, NY, USA, 2011. [Google Scholar]
- Makovicky, E.; Balic-Zunic, T. New Measure of Distortion for Coordination Polyhedra. Acta Crystallogr. Sect. B Struct. Sci. 1998, 54, 766–773. [Google Scholar] [CrossRef]
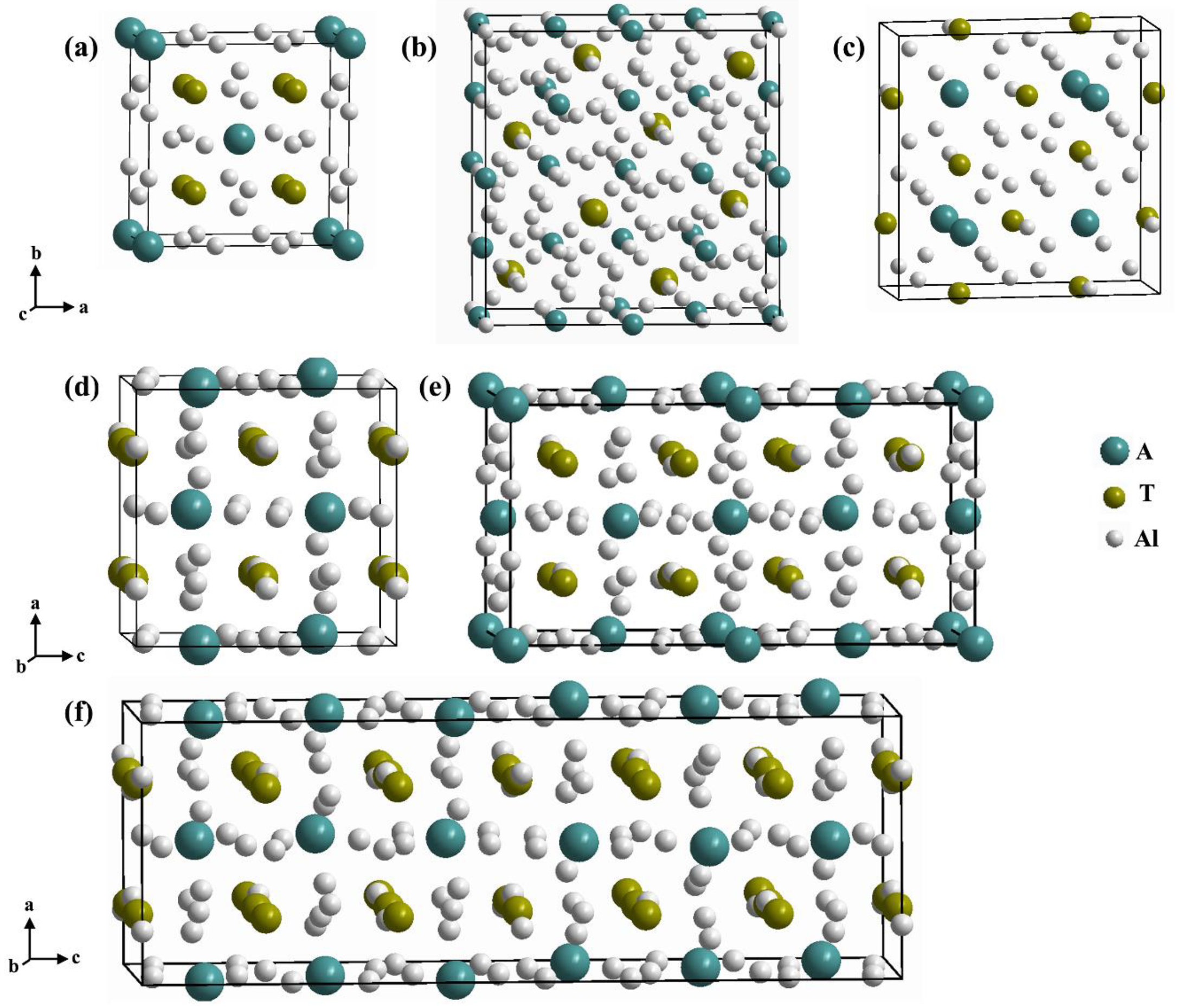


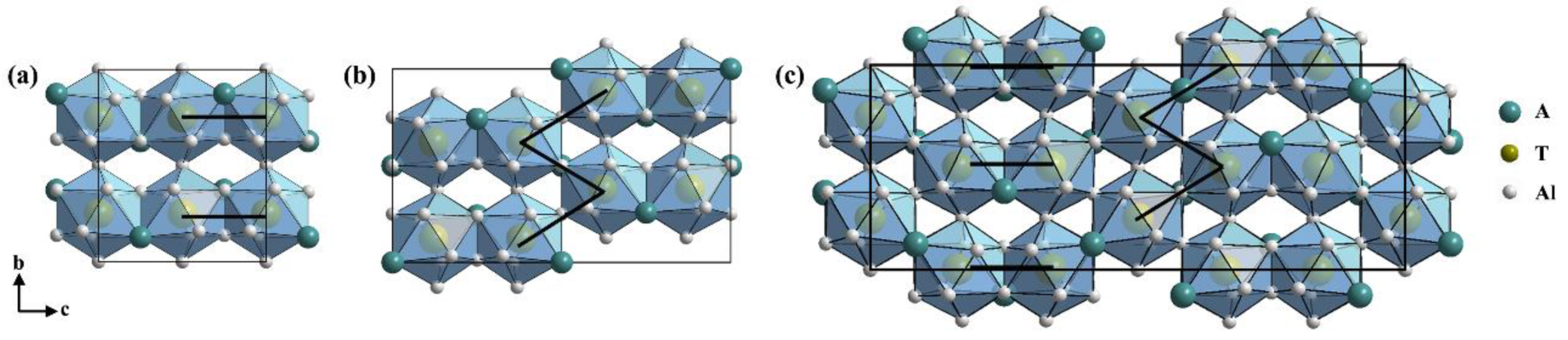
| Geometry and Stoichiometry | Structure Type | Space Group | Representative Structure | Lattice Parameters Å |
|---|---|---|---|---|
| Tetragonal, ATxAl12-x | ThMn12 | I4/mmm | UFe4Al8 | a = 8.749 c = 5.036 [4] |
| Cubic, AT2Al20 | CeCr2Al20 | Fdm | CeV2Al20 | a = 14.558 [31] |
| Tetragonal, AT2Al10 | CaCr2Al10 | P4/mnm | GdMn2Al10 | a = 12.733 c = 5.128 [32] |
| Orthorhombic, AT2Al10 | YbFe2Al10 | Cmcm | YbFe2Al10 | a = 8.966 b = 10.153 c = 9.003 [15] |
| Orthorhombic 2c, AT2Al10 | TbRe2Al10 | Cmcm | TbRe2Al10 | a = 9.322 b = 10.304 c = 18.032 [33] |
| Orthorhombic 3c, AT2Al10 | LuRe2Al10 | Cmcm | LuRe2Al10 | a = 9.291 b = 10.277 c = 26.841 [29] |
| Phase | Cubic | Tetragonal | Ortho 1c | Ortho 2c | Ortho 3c | Pure Al | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Type | Fdm | Unit Cell | P4/nmm | Unit Cell | Cmcm | Unit Cell | Cmcm | Unit Cell | Cmcm | Unit Cell | Fmm | Unit Cell |
| Total number of atoms | 184 | 46 | 52 | 52 | 52 | 26 | 104 | 52 | 156 | 78 | 4 | 1 |
| A atoms | 8 | 2 | 4 | 4 | 4 | 2 | 8 | 4 | 12 | 6 | 0 | 0 |
| T atoms | 16 | 4 | 8 | 8 | 8 | 4 | 16 | 8 | 24 | 12 | 0 | 0 |
| Al atoms | 160 | 40 | 40 | 40 | 40 | 20 | 80 | 40 | 120 | 60 | 4 | 1 |
| System | Structure | ΔE without SOC | ΔE with SOC |
|---|---|---|---|
| Ce-Ta-Al | Cubic | 0 | 0 |
| Tetragonal | 0.206 | 0.310 | |
| Orthorhombic 1c | 0.351 | 0.457 | |
| Ce-W-Al | Cubic | 0 | 0 |
| Tetragonal | 0.043 | 0.036 | |
| Orthorhombic 1c | 0.131 | 0.124 | |
| Ce-Re-Al1 | Cubic | 0 | 0 |
| Tetragonal | −0.1469 | −0.2897 | |
| Orthorhombic 1c | −0.1447 | −0.2876 | |
| Orthorhombic 2c | −0.144 | −0.286 | |
| Orthorhombic 3c | −0.142 | −0.283 | |
| Y-Re-Al | Cubic | 0 | 0 |
| Tetragonal | −0.242 | −0.242 | |
| Orthorhombic 1c | −0.212 | −0.213 | |
| Orthorhombic 2c | −0.240 | −0.240 | |
| Orthorhombic 3c | −0.246 | −0.246 |
| Phase | DFT Calculations | Experimental Results |
|---|---|---|
| CeTa2Al20 | a = 14.787 | a = 14.748 [1] |
| CeW2Al20 | a = 14.647 | a = 14.589 [57] |
| CeRe2Al10 | a = 12.955 c = 5.198 | a = 12.956 c = 5.172 [55] |
| YRe2Al10 | a = 9.337 b = 10.342 c = 27.026 | a = 9.306 b = 10.308 c = 26.936 [56] |
| Studied Systems | Main Structures | Structural Derivatives | ||||
|---|---|---|---|---|---|---|
| System type | T atom | Cubic | Tetragonal | Orthorhombic 1c | Orthorhombic 2c | Orthorhombic 3c |
| Ce-Ta-Al | Ta | 0.00701 | 0.00854 | 0.01025 | - | - |
| Ce-W-Al | W | 0.00937 | 0.01867 | 0.01182 | - | - |
| Ce-Re-Al | Re(1) | 0.00743 | 0.00123 | 0.00288 | 0.00830 | 0.00744 |
| Re(2) | - | - | - | - | 0.00796 | |
| Y-Re-Al | Re(1) | 0.00530 | 0.00031 | 0.00259 | 0.00049 | 0.00015 |
| Re(2) | - | - | - | - | 0.00006 | |
| Parameters | Cubic (EuTa2Al20) | Tetragonal (NdRe2Al10) | Ortho 1c (LnOs2Al10) | Ortho 2c (GdRe2Al10) | Ortho 3c (YRe2Al10) | |
|---|---|---|---|---|---|---|
| T atom type | Ta | Re | Os | Re | Re(1) | Re(2) |
| Vertex 1 | Al 2.624 | Al 2.5721 | Al 2.591 | Al 2.5474 | Al 2.5389 | Al 2.5679 |
| Vertex 2 | Al 2.624 | Al 2.5721 | Al 2.591 | Al 2.6017 | Al 2.5967 | Al 2.5679 |
| Vertex 3 | Al 2.624 | Al 2.5945 | Al 2.578 | Al 2.6021 | Al 2.6071 | Al 2.6096 |
| Vertex 4 | Al 2.624 | Al 2.6044 | Al 2.578 | Al 2.6127 | Al 2.6212 | Al 2.6096 |
| Vertex 5 | Al 2.624 | Al 2.6551 | Al 2.643 | Al 2.6461 | Al 2.6441 | Al 2.6511 |
| Vertex 6 | Al 2.624 | Al 2.6551 | Al 2.643 | Al 2.6771 | Al 2.6778 | Al 2.6511 |
| Vertex 7 | Al 2.883 | Al 2.7521 | Al 2.687 | Al 2.6889 | Al 2.6878 | Al 2.7042 |
| Vertex 8 | Al 2.883 | Al 2.7521 | Al 2.687 | Al 2.7061 | Al 2.7127 | Al 2.7042 |
| Vertex 9 | Al 2.883 | Al 2.7682 | Al 2.755 | Al 2.7348 | Al 2.7282 | Al 2.736 |
| Vertex 10 | Al 2.883 | Al 2.7682 | Al 2.755 | Al 2.8013 | Al 2.793 | Al 2.736 |
| Vertex 11 | Al 2.883 | Nd 3.3791 | Gd 3.494 | Gd 3.4418 | Y 3.3925 | Y 3.5063 |
| Vertex 12 | Al 2.883 | Nd 3.6171 | Gd 3.494 | Gd 3.5141 | Y 3.5124 | Y 3.5063 |
| r parameter, Å | 2.7535 | 2.8075 | 2.7913 | 2.7978 | 2.7927 | 2.7959 |
| Volume, Å3 | 52.95 | 56.12 | 55.16 | 55.55 | 55.24 | 55.43 |
| Polyhedral distortion,% | 0 | −6.00 | −4.18 | −4.91 | −4.33 | −4.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaniv, G.; Vidal, D.; Fuks, D.; Meshi, L. Bonding and Stability of Ternary Structures in the CeT2Al20 (T=Ta, W, Re) and YRe2Al20 Alloys. Metals 2020, 10, 422. https://doi.org/10.3390/met10040422
Yaniv G, Vidal D, Fuks D, Meshi L. Bonding and Stability of Ternary Structures in the CeT2Al20 (T=Ta, W, Re) and YRe2Al20 Alloys. Metals. 2020; 10(4):422. https://doi.org/10.3390/met10040422
Chicago/Turabian StyleYaniv, Gili, Daniel Vidal, David Fuks, and Louisa Meshi. 2020. "Bonding and Stability of Ternary Structures in the CeT2Al20 (T=Ta, W, Re) and YRe2Al20 Alloys" Metals 10, no. 4: 422. https://doi.org/10.3390/met10040422
APA StyleYaniv, G., Vidal, D., Fuks, D., & Meshi, L. (2020). Bonding and Stability of Ternary Structures in the CeT2Al20 (T=Ta, W, Re) and YRe2Al20 Alloys. Metals, 10(4), 422. https://doi.org/10.3390/met10040422






