Thermal and Mass Spectroscopic Analysis of BF and BOF Sludges: Study of Their Behavior under Air and Inert Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Analysis Methods
3. Results
3.1. Sludge Characterization
3.2. Thermal Analysis
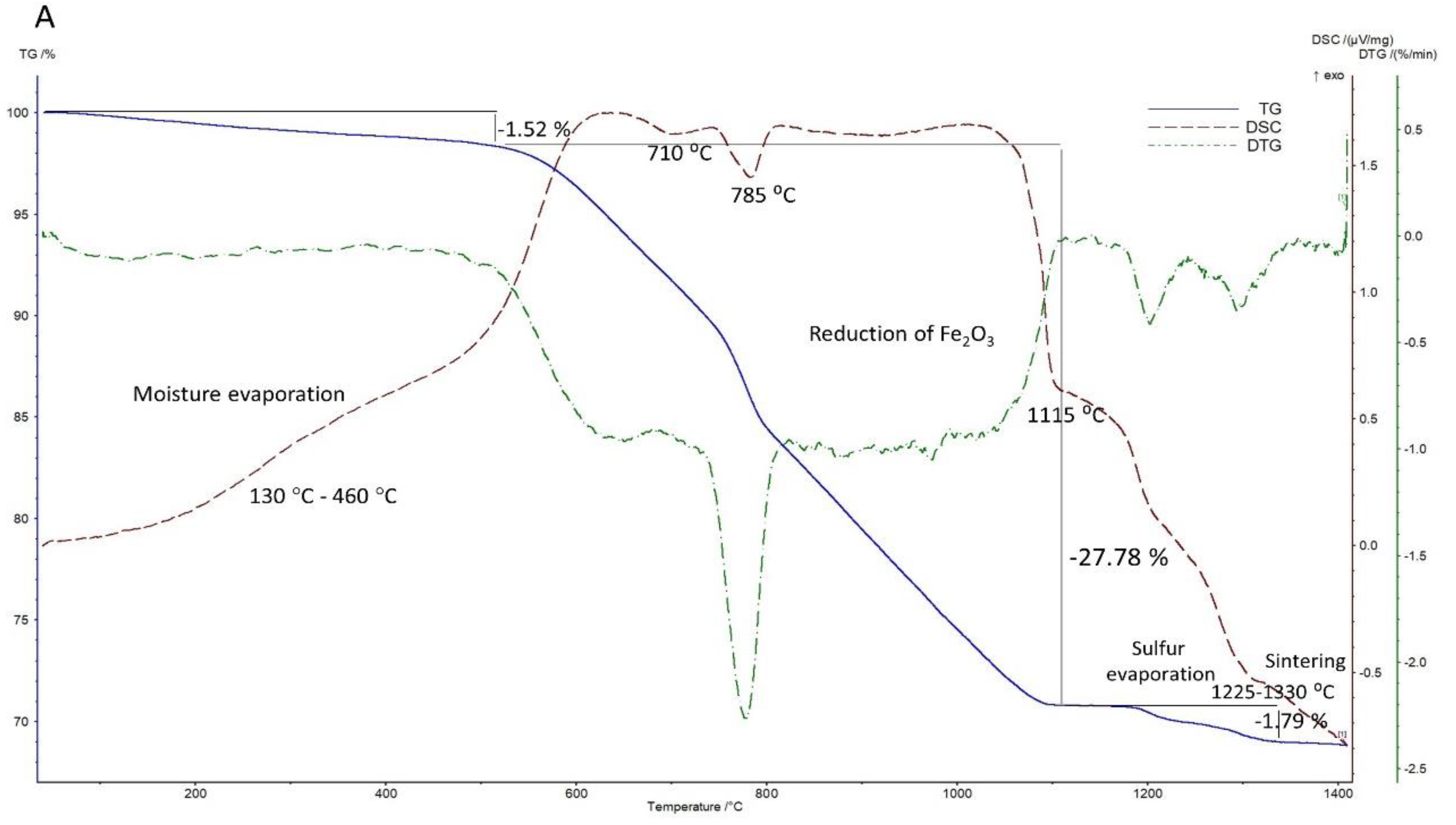
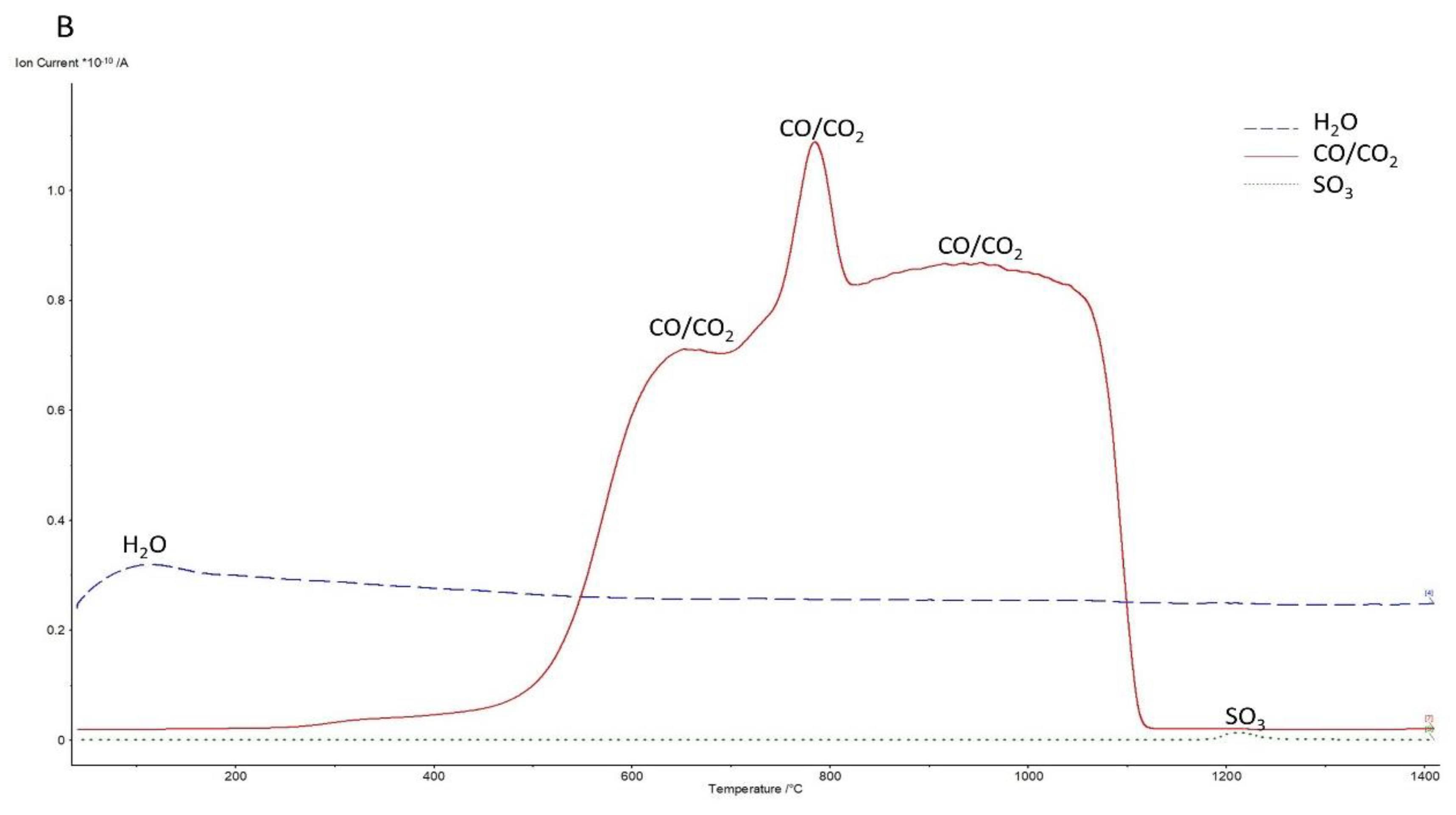
3.2.1. Under Air Atmosphere
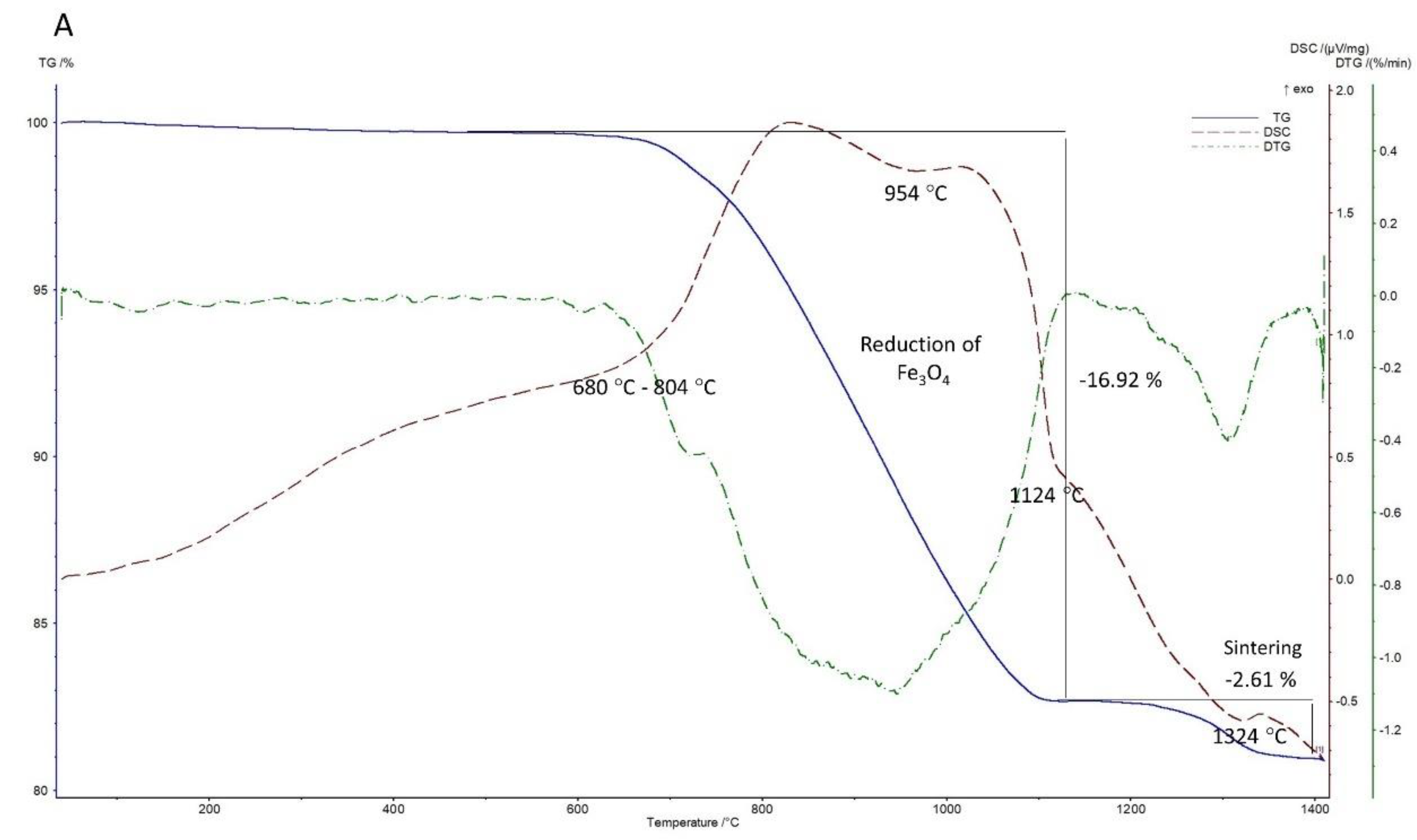
3.2.2. Under Inert/Reducing Atmosphere (Argon Atmosphere)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machado, J.G.; Brehm, F.A.; Moraes, C.A.; Santos, C.A.; Vilela, A.C.; Cunha, J.B. Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J. Hazard. Mater. 2006, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Mansfeldt, T.; Dohrmann, R. Chemical and mineralogical characterization of blast furnace sludge from an abandoned landfill. Environ. Sci. Technol. 2004, 38, 5977–5984. [Google Scholar] [CrossRef] [PubMed]
- Szekely, J. A Research Program for the Minimization and Effective Utilization of Steel Plant Wastes. Iron Steelmak. 1995, 22, 25–29. [Google Scholar]
- Trung, Z.H.; Kukurugya, F.; Takacova, Z.; Orac, D.; Laubertova, M.; Miskufova, A.; Havlik, T. Acidic Leaching Both of Zinc and Iron from Basic Oxygen Furnace Sludge. J. Hazard. Mater. 2011, 192, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, P.; Vandecasteele, C.; Swennen, R.; Mortier, R. Zinc and Lead Removal from Blast Furnace Sludge with a Hydrometallurgical Process. Environ. Sci. Technol. 2000, 34, 3802–3808. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Mansfeldt, T.; Mandaliev, P.N.; Barmettler, K.; Marcus, M.A.; Voegelin, A. Speciation of Zn in Blast Furnace Sludge from Former Sedimentation Ponds Using Synchrotron X-ray Diffraction, Fluorescence, and Absorption Spectroscopy. Environ. Sci. Technol. 2012, 46, 12381–12390. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jennes, R.; Mattila, O.; Paananen, T.; Lilja, J.; Larsson, M. Investigation of Applying OxyCup® Process for an Integrated Steel Plant from a Nordic Country. In Proceedings of the METEC & 2nd European Steel Application Days (ESTAD), Dusseldorf, Germany, 15–19 June 2015; pp. 1–5. [Google Scholar]
- Omran, M.; Fabritius, T.; Paananen, T. Effect of blast furnace sludge (BFS) characteristics on suitable recycling process determining. JMMCE 2017, 5, 185–197. [Google Scholar] [CrossRef][Green Version]
- Mansfeldt, T.; Dohrmann, R. Identification of a Crystalline Cyanide- Containing Compound in Blast-Furnace Sludge Deposits. J. Environ. Qual. 2001, 30, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Foldi, C.; Dohrmann, R.; Mansfeldt, T. Mercury in Dumped Blast Furnace Sludge. Chemosphere 2014, 99, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Salama, W.; El Aref, M.; Gaupp, R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 136, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, D.; Di Cecca, C.; Mapelli, C.; Barella, S.; Bondi, E. Experimental analysis on the use of BF-sludge for the reduction of BOF-powders to direct reduced iron (DRI) production. Process Saf. Environ. Prot. 2016, 102, 410–420. [Google Scholar] [CrossRef]
- Ökvist, L.S.; Cang, D.; Zong, Y.; Bai, H. The effect of BOF slag and BF flue dust on coal combustion efficiency. ISIJ Int. 2004, 44, 1501–1510. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Improved removal of zinc from blast furnace sludge by particle size separation and microwave heating. Miner. Engin. 2018, 127, 265–276. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Reduction of hematite (Fe2O3) to wüstite (FeO) by carbon monoxide (CO) for chemical looping combustion. Chem. Eng. J. 2014, 242, 204–210. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Leonardou, S.A. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD) Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.; Fabritius, T. Treatment of blast furnace sludge (BFS) using a microwave heating technique. Ironmak. Steelmak. 2017, 44, 619–629. [Google Scholar] [CrossRef]
- Mikhail, S.A.; Turcotte, A.-M. Thermal reduction of steel-making secondary materials I. Basic-oxygen-furnace dust. Thermochim. Acta 1998, 311, 113–119. [Google Scholar] [CrossRef]
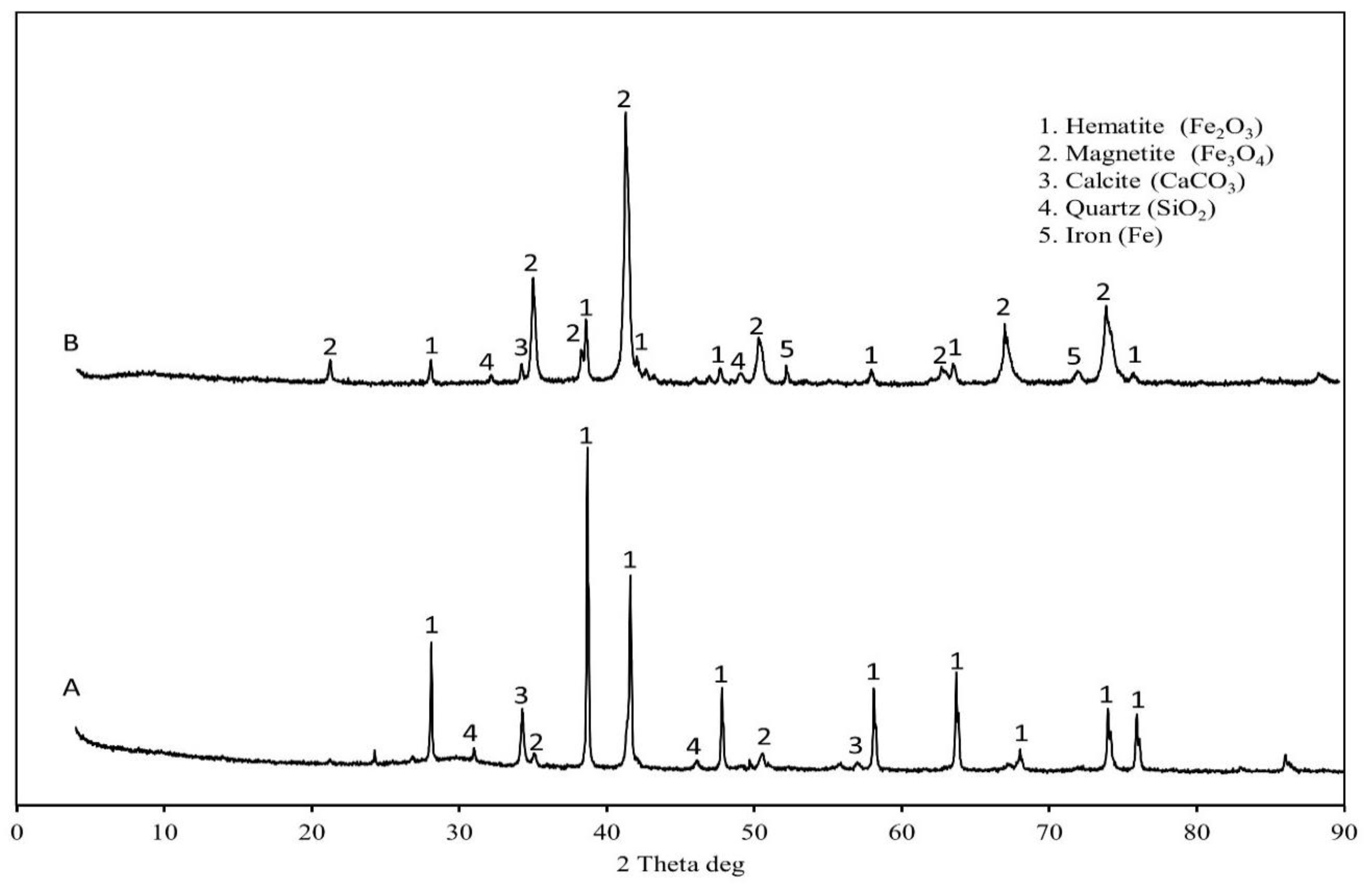
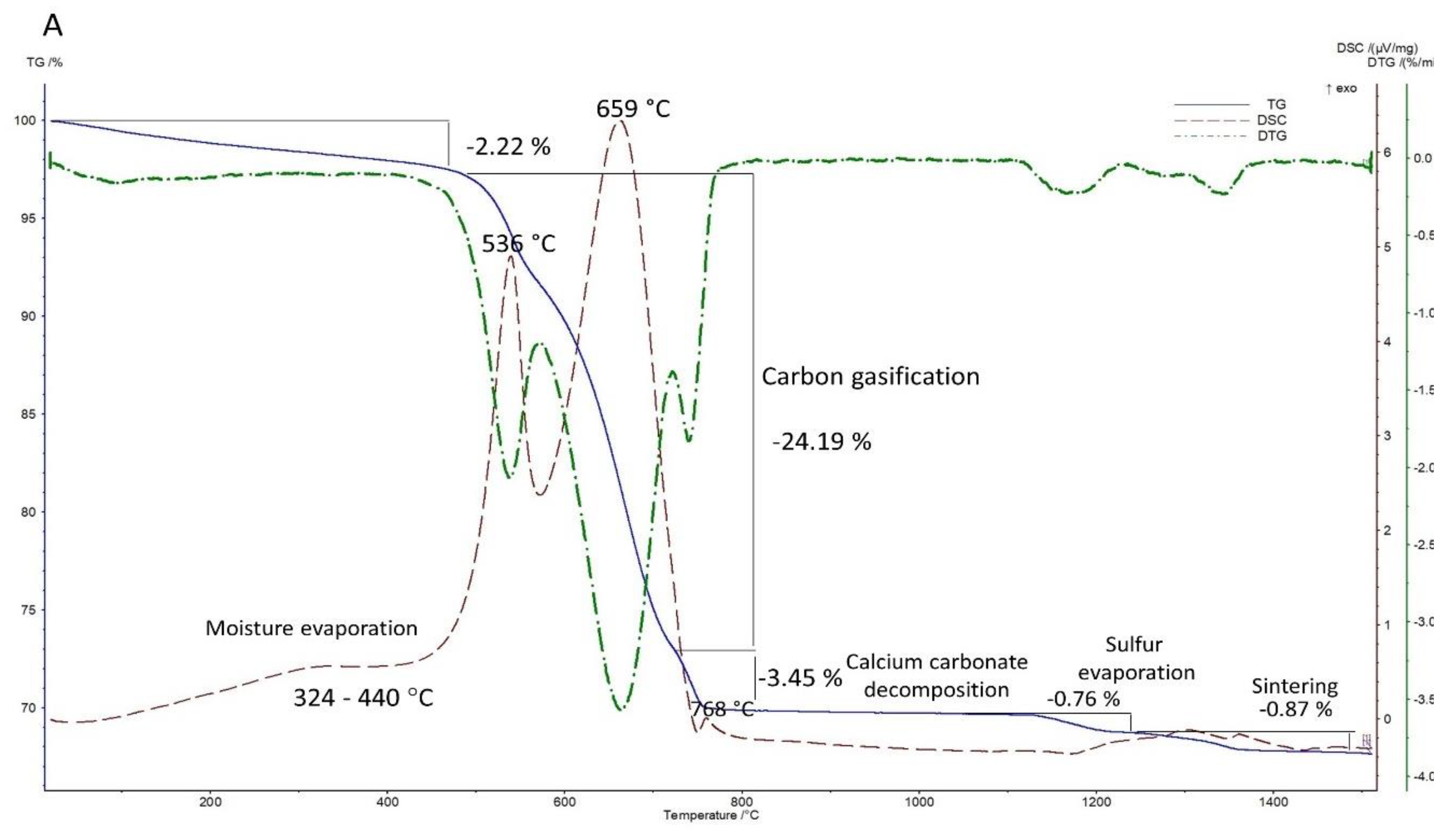
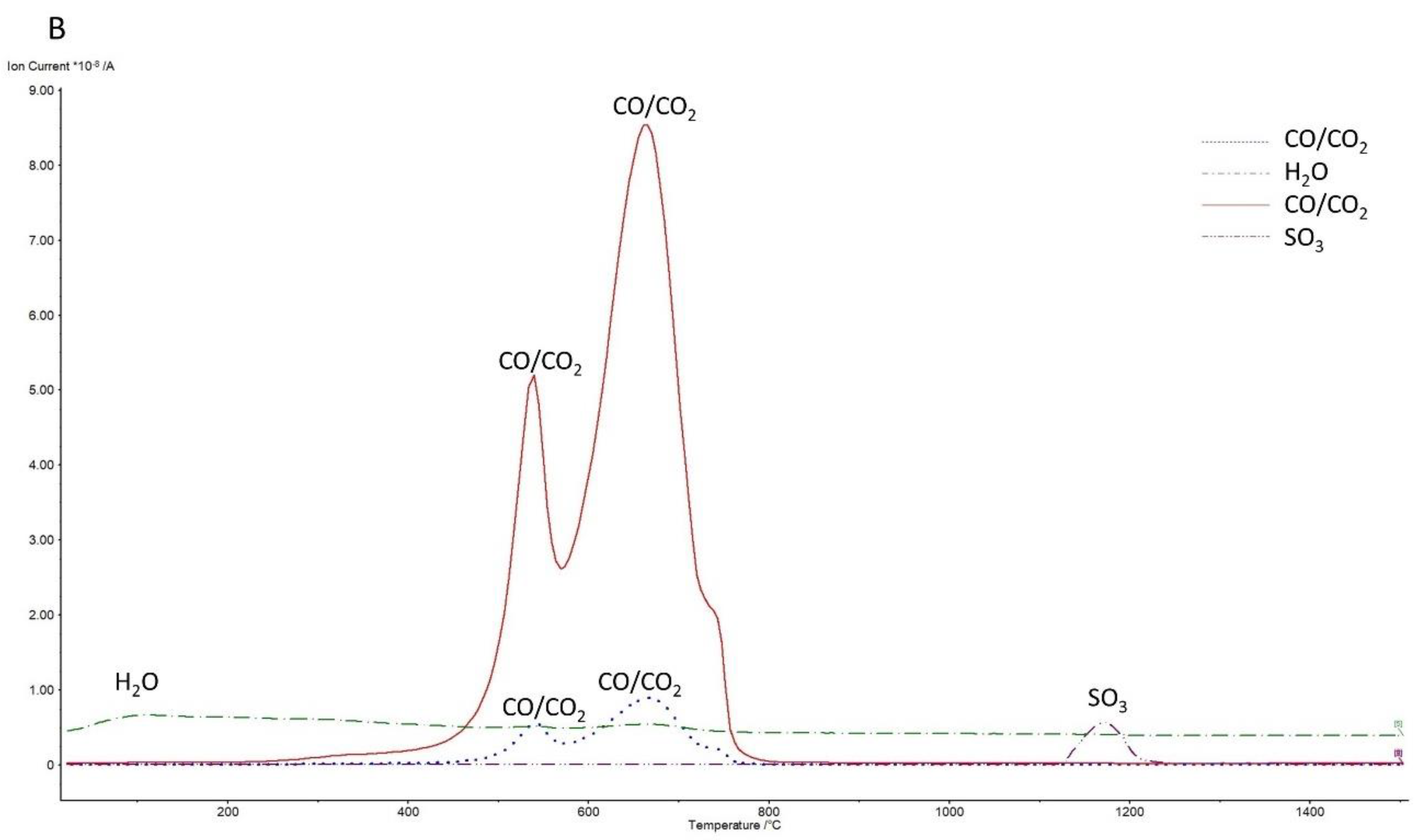
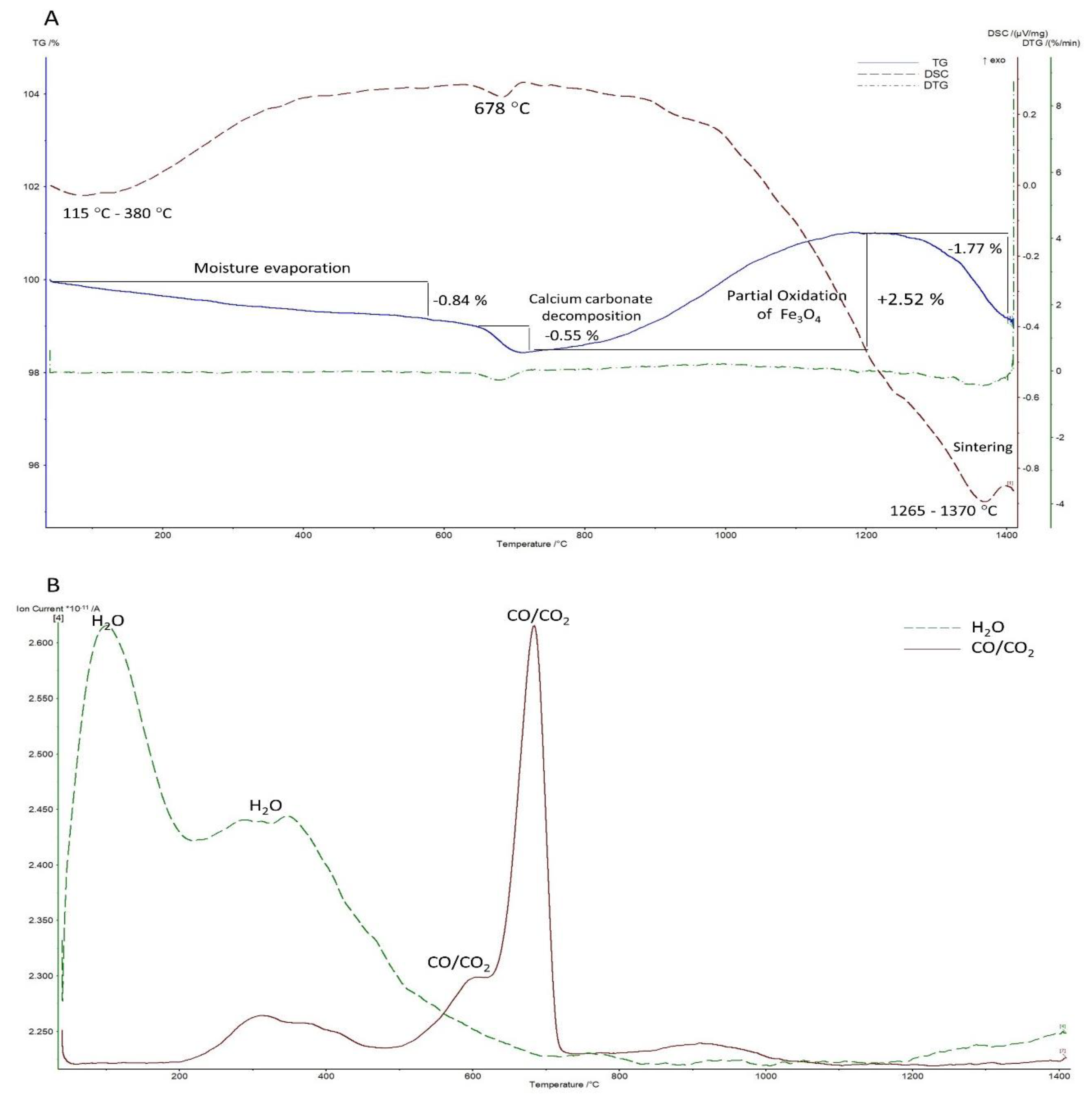

| Component | BOFS | BFS | Analysis Technique |
|---|---|---|---|
| Major Element (wt. %) | |||
| Fe2O3 | 80.79 | 58.57 | XRF |
| C | 0.36 | 22.05 | Leco |
| CaO | 4.25 | 9.53 | XRF |
| SiO2 | 2.04 | 9.05 | XRF |
| MgO | 1.10 | 2.54 | XRF |
| Al2O3 | 0.18 | 3.58 | XRF |
| SO3 | 0.06 | 1.46 | Leco |
| ZnO | 1.36 | 0.98 | AAS |
| Minor Element (ppm) | |||
| NaO | 3965 | 4258 | XRF |
| PbO | 137 | 876 | XRF |
| K2O | 2089 | 3740 | XRF |
| MnO | 9470 | 1375 | XRF |
| P2O5 | 1581 | 1523 | XRF |
| TiO2 | 909 | 2126 | XRF |
| Ni | 102 | 150 | XRF |
| Cu | 20 | 67 | XRF |
| V2O5 | 3684 | 1699 | XRF |
| Rb | 104 | 143 | XRF |
| Sr | 84 | 115 | XRF |
| As | 50 | 119 | XRF |
| Ga | 26 | 35 | XRF |
| Ba | 130 | 184 | XRF |
| Cs | 53 | 90 | XRF |
| Zr | 19 | 20 | XRF |
| Sb | 38 | 38 | XRF |
| Ce | 7 | 35 | XRF |
| Nd | 11 | 27 | XRF |
| Bi | 14 | 30 | XRF |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omran, M.; Fabritius, T.; Yu, Y.; Chen, G. Thermal and Mass Spectroscopic Analysis of BF and BOF Sludges: Study of Their Behavior under Air and Inert Atmosphere. Metals 2020, 10, 397. https://doi.org/10.3390/met10030397
Omran M, Fabritius T, Yu Y, Chen G. Thermal and Mass Spectroscopic Analysis of BF and BOF Sludges: Study of Their Behavior under Air and Inert Atmosphere. Metals. 2020; 10(3):397. https://doi.org/10.3390/met10030397
Chicago/Turabian StyleOmran, Mamdouh, Timo Fabritius, Yaowei Yu, and Guo Chen. 2020. "Thermal and Mass Spectroscopic Analysis of BF and BOF Sludges: Study of Their Behavior under Air and Inert Atmosphere" Metals 10, no. 3: 397. https://doi.org/10.3390/met10030397
APA StyleOmran, M., Fabritius, T., Yu, Y., & Chen, G. (2020). Thermal and Mass Spectroscopic Analysis of BF and BOF Sludges: Study of Their Behavior under Air and Inert Atmosphere. Metals, 10(3), 397. https://doi.org/10.3390/met10030397






