Characterization of a Zn-Ca5(PO4)3(OH) Composite with a High Content of the Hydroxyapatite Particles Prepared by the Spark Plasma Sintering Process
Abstract
1. Introduction
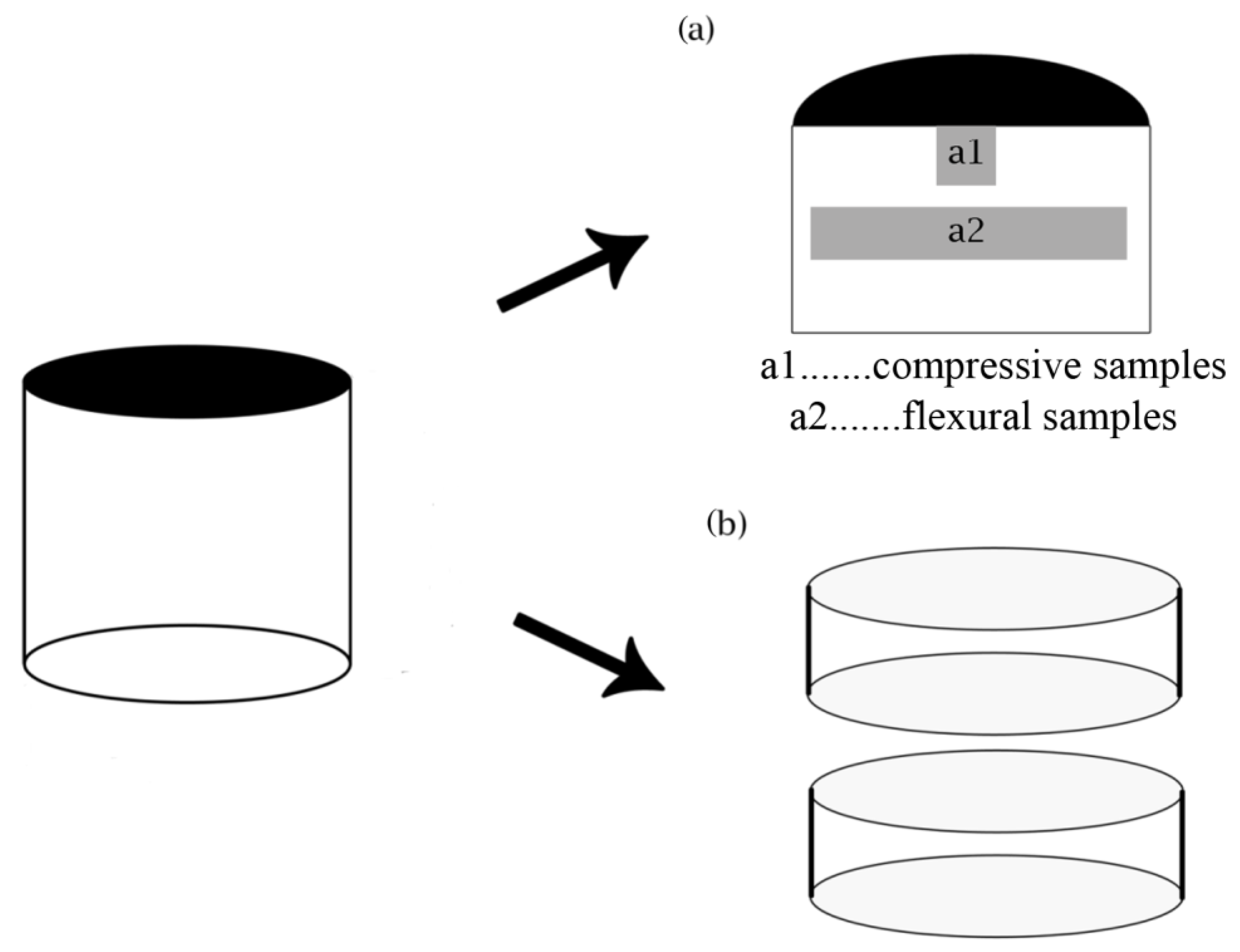
2. Materials and Methods
3. Results and Discussion
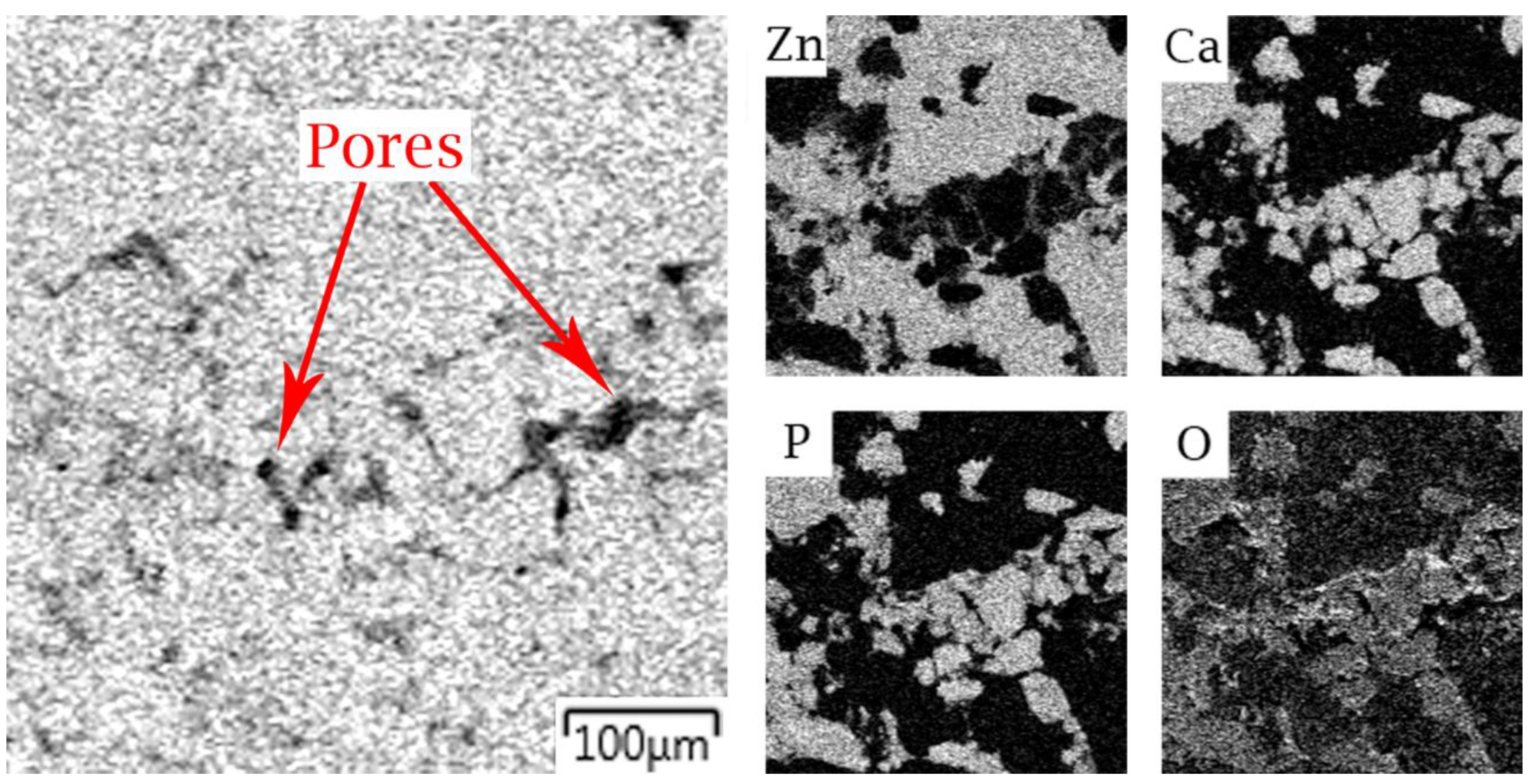
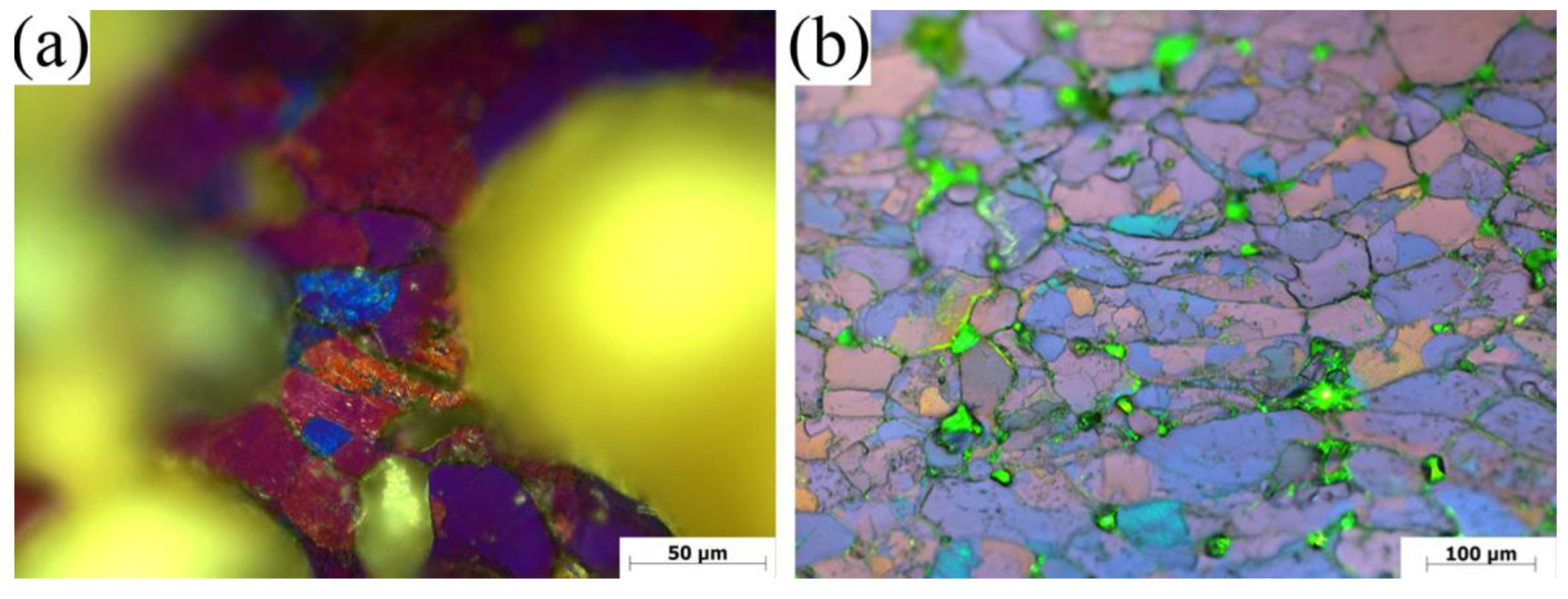
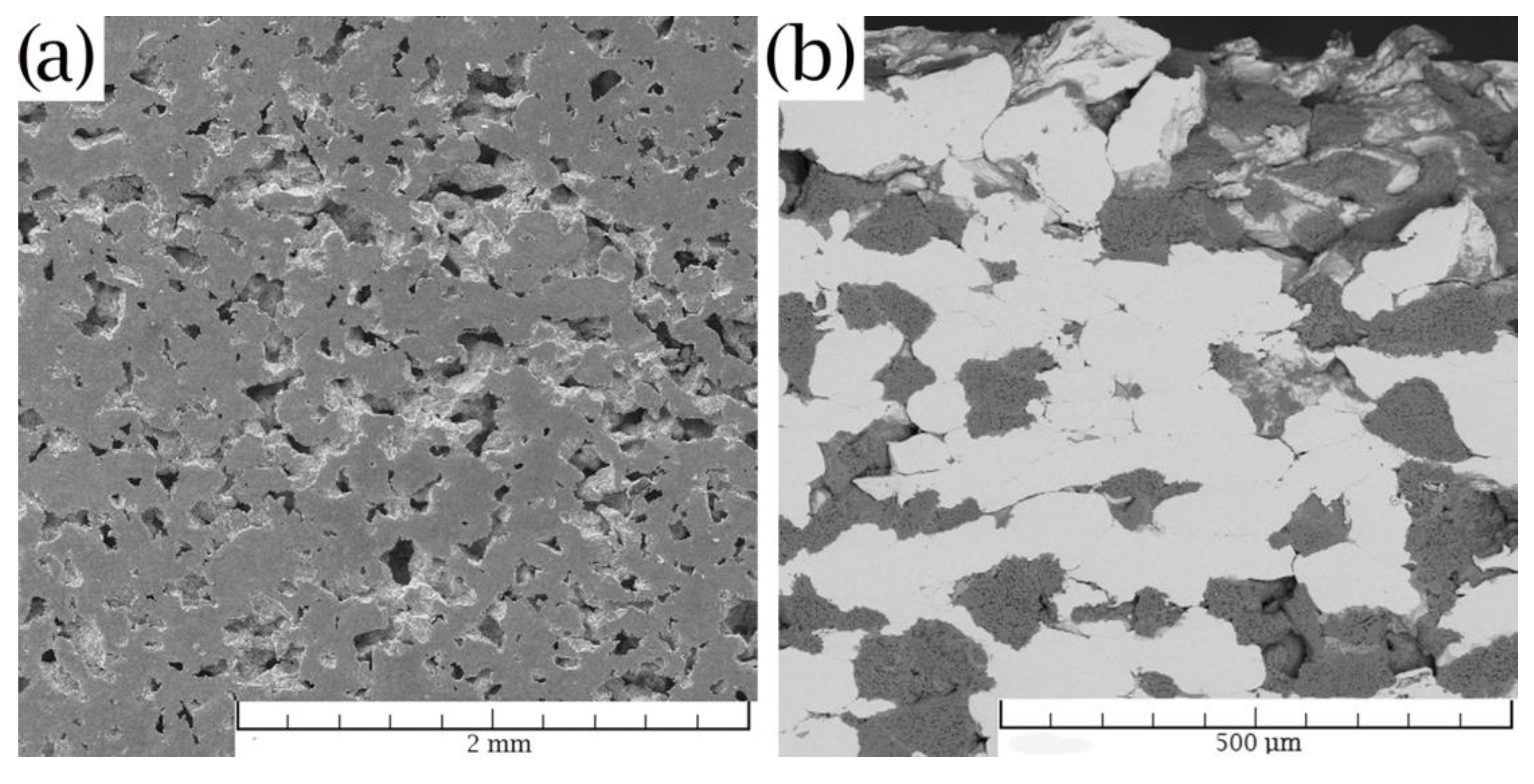
3.1. Microstructure
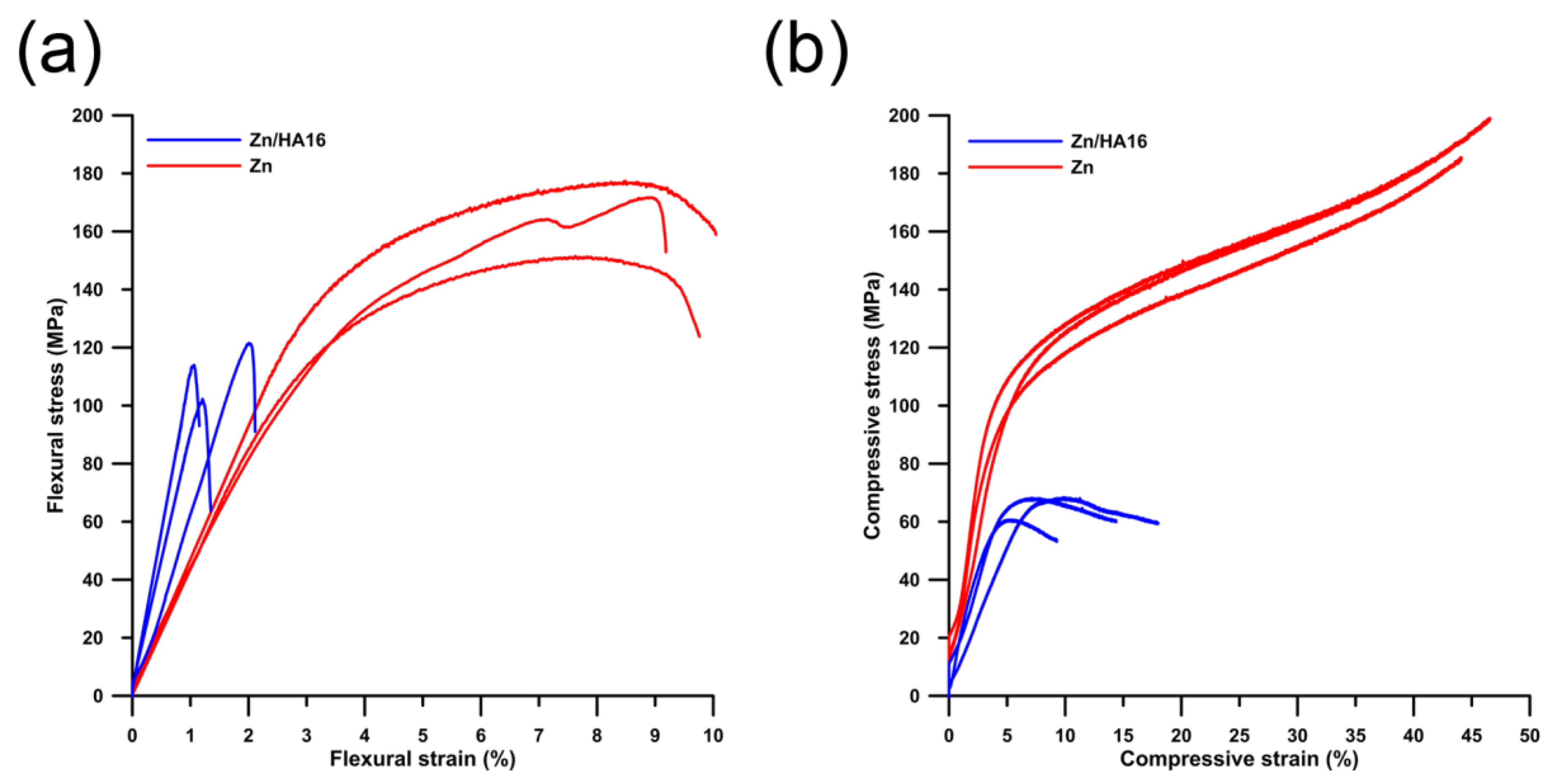
3.2. Mechanical Properties
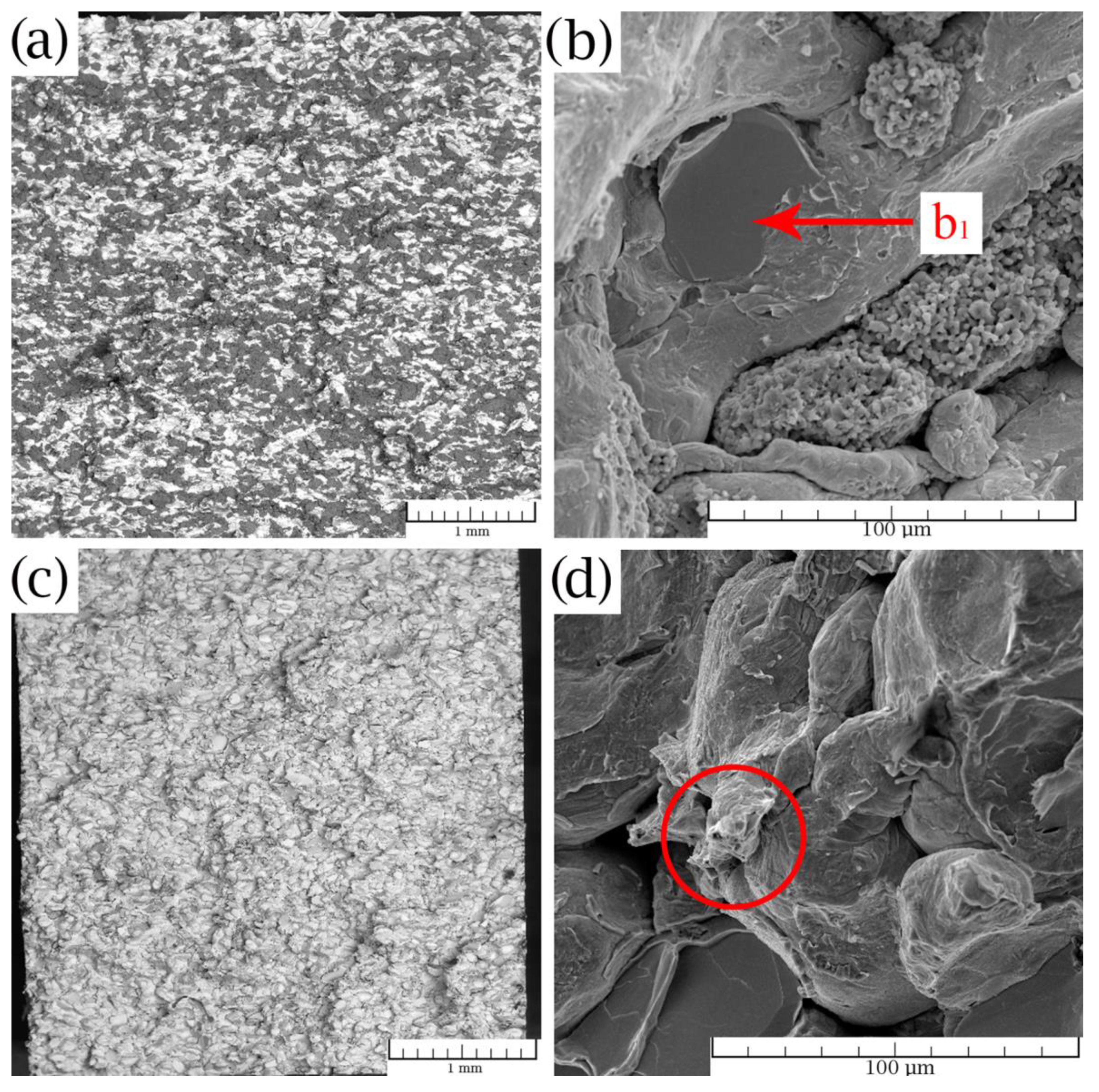
3.3. Corrosion Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R: Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable Metals for Cardiovascular Stent Application: Interests and New Opportunities. Int. J. Mol. Sci. 2011, 12, 4250. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S.A.; Ezatpour, H.R.; Torabi Parizi, M. Comparison of microstructure and mechanical properties of A356 aluminum alloy/Al2O3 composites fabricated by stir and compo-casting processes. Mater. Des. 2012, 34, 106–111. [Google Scholar] [CrossRef]
- Conner, R.D.; Dandliker, R.B.; Johnson, W.L. Mechanical properties of tungsten and steel fiber reinforced Zr41.25Ti13.75Cu12.5Ni10Be22.5 metallic glass matrix composites. Acta Mater. 1998, 46, 6089–6102. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Plunkett, N.A.; O’Brien, F.J. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur. Cells Mater. 2010, 20, 218–230. [Google Scholar] [CrossRef]
- Čapek, J.; Pinc, J.; Msallamová, Š.; Jablonská, E.; Veřtát, P.; Kubásek, J.; Vojtěch, D. Thermal plasma spraying as a new approach for preparation of zinc biodegradable scaffolds: A complex material characterization. J. Therm. Spray Technol. 2019, 28, 826–841. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1998, 41, 249–273. [Google Scholar] [CrossRef]
- Čapek, J.; Jablonská, E.; Lipov, J.; Kubatík, T.F.; Vojtěch, D. Preparation and characterization of porous zinc prepared by spark plasma sintering as a material for biodegradable scaffolds. Mater. Chem. Phys. 2018, 203, 249–258. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Sairam, K.; Sonber, J.K.; Murthy, T.S.R.C.; Subramanian, C.; Fotedar, R.K.; Nanekar, P.; Hubli, R.C. Influence of spark plasma sintering parameters on densification and mechanical properties of boron carbide. Int. J. Refract. Metals Hard Mater. 2014, 42, 185–192. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C.E. Effect of process control agent on the porous structure and mechanical properties of a biomedical Ti–Sn–Nb alloy produced by powder metallurgy. Acta Biomater. 2010, 6, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Joschek, S.; Nies, B.; Krotz, R.; Göpferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Matsunaga, K.; Murata, H.; Mizoguchi, T.; Nakahira, A. Mechanism of incorporation of zinc into hydroxyapatite. Acta Biomater. 2010, 6, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Koempel, J.A.; Patt, B.S.; O’Grady, K.; Wozney, J.; Toriumi, D.M. The effect of recombinant human bone morphogenetic protein-2 on the integration of porous hydroxyapatite implants with bone. J. Biomed. Mater. Res. 1998, 41, 359–363. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite–Carbon Nanotube Composites for Biomedical Applications: A Review. Int. J. Appl. Ceram. Tec. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Dehestani, M.; Adolfsson, E.; Stanciu, L.A. Mechanical properties and corrosion behavior of powder metallurgy iron-hydroxyapatite composites for biodegradable implant applications. Mater. Des. 2016, 109, 556–569. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; et al. Biodegradable magnesium–hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef]
- Ratna Sunil, B.; Ganapathy, C.; Sampath Kumar, T.S.; Chakkingal, U. Processing and mechanical behavior of lamellar structured degradable magnesium–hydroxyapatite implants. J. Mech. Behav. Biomed. Mater. 2014, 40, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Ulum, M.F.; Arafat, A.; Noviana, D.; Yusop, A.H.; Nasution, A.K.; Abdul Kadir, M.R.; Hermawan, H. In vitro and in vivo degradation evaluation of novel iron-bioceramic composites for bone implant applications. Mater. Sci. Eng. C. 2014, 36, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants-A Review Paper. Metals 2017, 7, 18. [Google Scholar]
- Gong, H.B.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed. Mater. Res. Part B 2015, 103, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, I.; Vojtech, D. Zinc Alloys for Biodegradable Medical Implants. In Materials Science Forum; Longauerova, M., Zubko, P., Eds.; Metallography Xv Trans Tech. Publications Ltd: Stafa-Zurich, Switzerland, 2014; pp. 457–460. [Google Scholar]
- Katarivas Levy, G.; Leon, A.; Kafri, A.; Ventura, Y.; Drelich, J.W.; Goldman, J.; Vago, R.; Aghion, E. Evaluation of biodegradable Zn-1%Mg and Zn-1%Mg-0.5%Ca alloys for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 174. [Google Scholar] [CrossRef]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. Biofactors 2012, 38, 186–193. [Google Scholar] [CrossRef]
- Cerovic, A.; Miletic, I.; Sobajic, S.; Blagojevic, D.; Radusinovic, M.; El-Sohemy, A. Effects of zinc on the mineralization of bone nodules from human osteoblast-like cells. Biol. Trace Elem. Res. 2007, 116, 61–71. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Vojtěch, D.; Kubásek, J.; Čapek, J.; Pospíšilová, I. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Yong, L.; Wei, X.; Chengcheng, Z.; Biao, G.; Hanfeng, G.; Hao, C.; Fu, J.; Feng, L. Enhanced osseointegration and antibacterial action of zinc-loaded titania-nanotube-coated titanium substrates: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 3939–3950. [Google Scholar]
- Ann, L.C.; Mahmud, S.; Bakhori, S.K.M.; Sirelkhatim, A.; Mohamad, D.; Hasan, H.; Seeni, A.; Rahman, R.A. Antibacterial responses of zinc oxide structures against Staphylococcus aureus, Pseudomonas aeruginosa and Streptococcus pyogenes. Ceram. Int. 2014, 40, 2993–3001. [Google Scholar] [CrossRef]
- Soon, L.L.; Zuhailawati, H.; Suhaina, I.; Dhindaw, B.K. Prediction of Compressive Strength of Biodegradable Mg–Zn/HA Composite via Response Surface Methodology and Its Biodegradation. Acta Metall. Sin. 2016, 29, 464–474. [Google Scholar] [CrossRef]
- Salleh, E.M.; Zuhailawati, H.; Ramakrishnan, S.; Dhindaw, B.K. Enhanced Mechanical Properties and Corrosion Behavior of Biodegradable Mg-Zn/HA Composite. Metall. Mater. Trans. A. 2017, 48, 2519–2528. [Google Scholar] [CrossRef]
- Yang, F.; Dong, W.J.; He, F.M.; Wang, X.X.; Zhao, S.F.; Yang, G.L. Osteoblast response to porous titanium surfaces coated with zinc-substituted hydroxyapatite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 313–318. [Google Scholar] [CrossRef]
- Candidato, R.T.; Thouzellier, C.; Pawlowski, L. Evaluation of the in-vitro behavior of nanostructured hydroxyapatite and zinc doped hydroxyapatite coatings obtained using solution precursor plasma spraying. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 2101–2108. [Google Scholar] [CrossRef]
- Uysal, I.; Severcan, F.; Tezcaner, A.; Evis, Z. Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. Mater. Int. 2014, 24, 340–349. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, J.; Qin, C.; Xu, A.; Zhang, Z.; Lin, Y.; Chen, Z.; Lin, S.; Yuan, Z.; Ren, X.; et al. One-pot synthesis and characterization of Zn-doped hydroxyapatite nanocomposites. Mater. Chem. Phys. 2017, 199, 122–130. [Google Scholar] [CrossRef]
- Müller, L.; Müller, F.A. Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Thümmler, F.; Oberacker, R. Porosity and Pore-related properties. In Introduction to Powder Metallurgy; Maney Publishing for IOM3; The Institute of Materials, Minerals and Mining: London, UK, 1993; ISBN 978-0-901716-26-2. [Google Scholar]
- Zhang, L.; He, Z.Y.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Rapidly sintering of interconnected porous Ti-HA biocomposite with high strength and enhanced bioactivity. Mater. Sci. Eng. C 2016, 67, 104–114. [Google Scholar] [CrossRef]
- Silva, V.V.; Domingues, R.Z.; Lameiras, F.S. Microstructural and mechanical study of zirconia-hydroxyapatite (ZH) composite ceramics for biomedical applications. Compos. Sci. Technol. 2001, 61, 301–310. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R. Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.W.; Li, J.G.; Sun, X.D. Preparation and mechanical property of a novel 3D porous magnesium scaffold for bone tissue engineering. Mater. Sci. Eng. C 2014, 42, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Vojtěch, D. Effect of sintering conditions on the microstructural and mechanical characteristics of porous magnesium materials prepared by powder metallurgy. Mater. Sci. Eng. C. 2014, 35, 21–28. [Google Scholar] [CrossRef]
- Del Campo, R.; Savoini, B.; Muñoz, A.; Monge, M.A.; Pareja, R. Processing and mechanical characteristics of magnesium-hydroxyapatite metal matrix biocomposites. J. Mech. Behav. Biomed. Mater. 2017, 69, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Elzinga, E.J.; Reeder, R.J. Sorption Mechanisms of Zinc on Hydroxyapatite: Systematic Uptake Studies and EXAFS Spectroscopy Analysis. Environ. Sci. Technol. 2005, 39, 4042–4208. [Google Scholar] [CrossRef]
- Wei, X.; Yates, M.Z. Yttrium-Doped Hydroxyapatite Membranes with High Proton Conductivity. Chem. Mater. 2012, 24, 1738–1743. [Google Scholar] [CrossRef]
- Wellinghausen, N. Immunobiology of gestational zinc deficiency. Br. J. Nutr. 2007, 85, S81–S86. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Meng, Y.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.N. Initial formation of corrosion products on pure zinc in simulated body fluid. J. Mater. Sci. Technol. 2018, 34, 2271–2282. [Google Scholar] [CrossRef]
- Turianicová, E.; Kaňuchová, M.; Zorkovská, A.; Holub, M.; Bujňáková, Z.; Dutková, E.; Baláž, M.; Findoráková, L.; Balintová, M.; Obut, A. CO2 utilization for fast preparation of nanocrystalline hydrozincite. J. CO2 Util. 2016, 16, 328–335. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Basirun, W.J.; Alias, Y.; Ebadi, M. Facile fabrication of Zn/Zn5(OH)8Cl2·H2O flower-like nanostructure on the surface of Zn coated with poly (N-methyl pyrrole). Appl. Surf. Sci. 2011, 257, 10539–10544. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, G.; Qiu, Y.; Guo, X. The crevice corrosion behaviour of stainless steel in sodium chloride solution. Corros. Sci. 2011, 53, 4065–4672. [Google Scholar] [CrossRef]


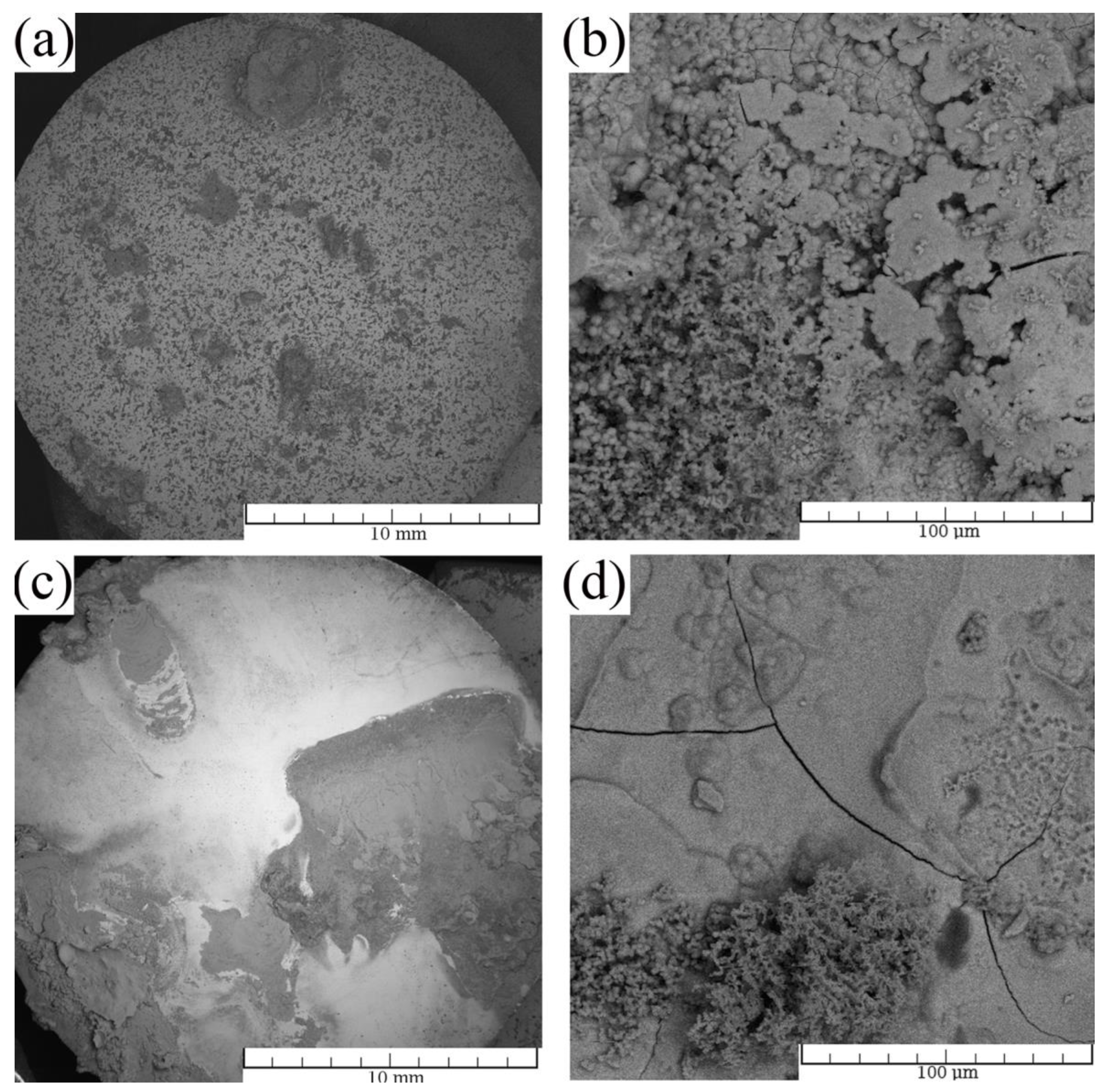
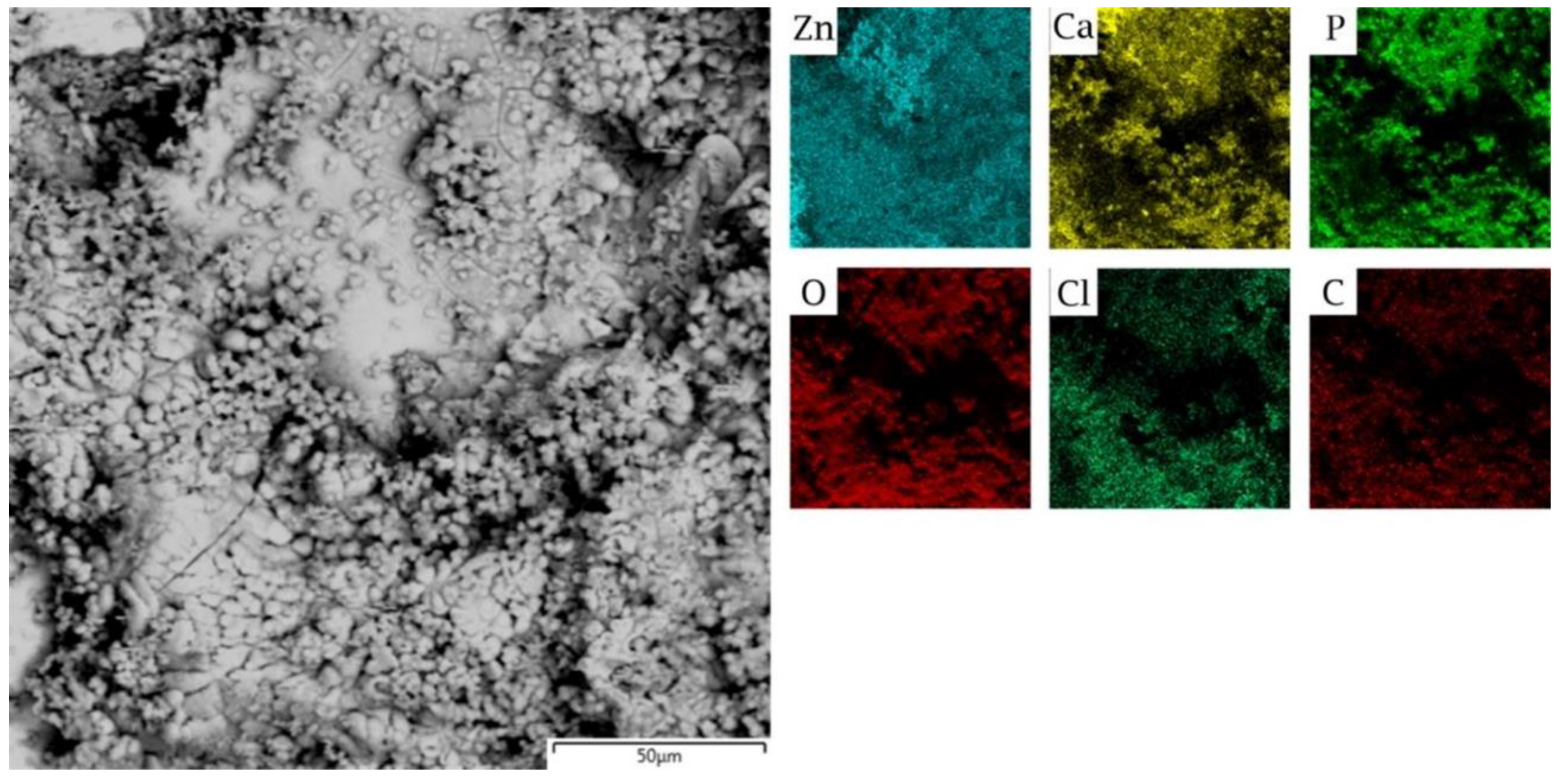
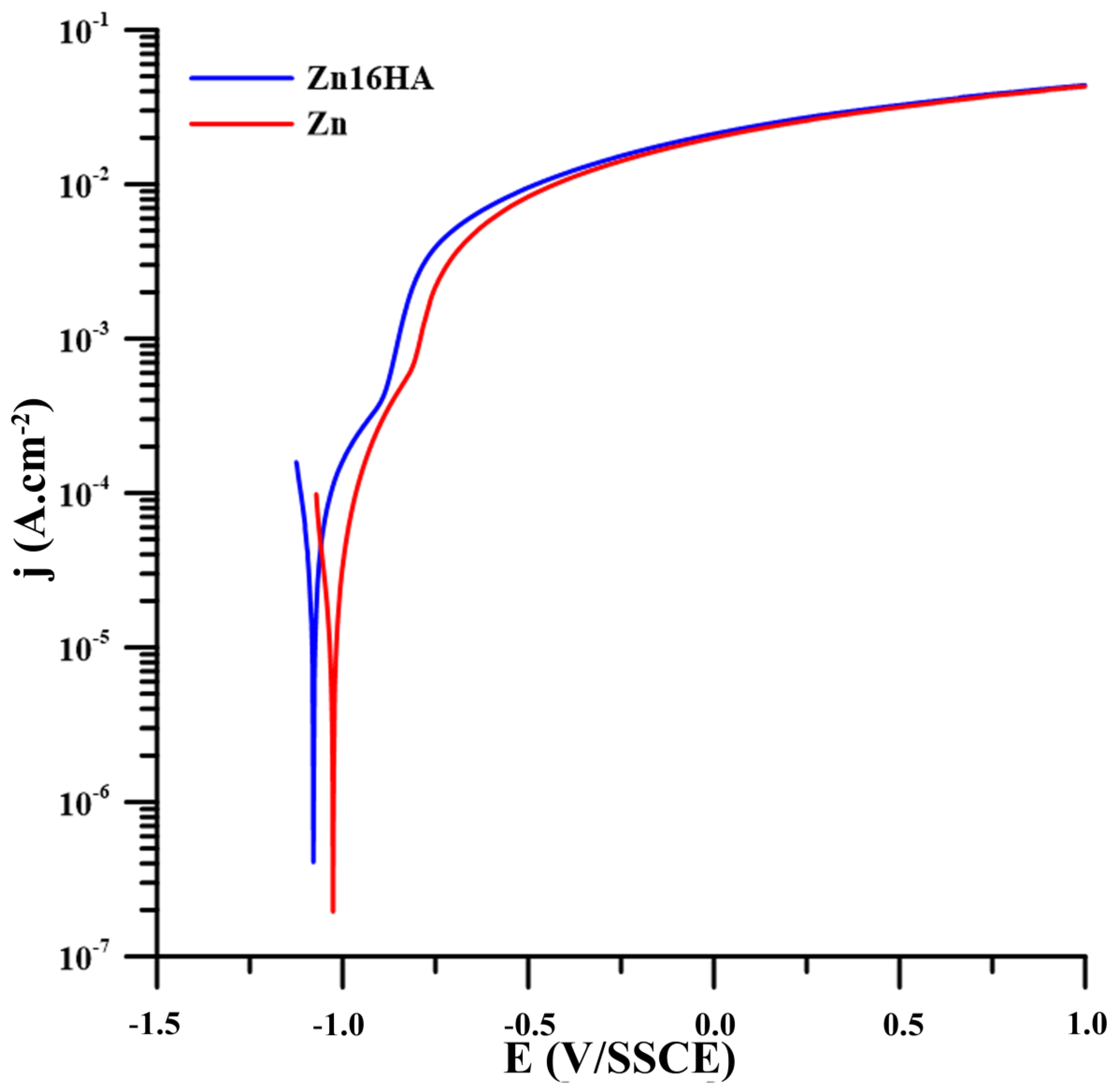
| Ions | Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | HPO42− |
|---|---|---|---|---|---|---|---|---|
| (mmol/L) | 142 | 5 | 2.5 | 1 | 109 | 27 | 1 | 1 |
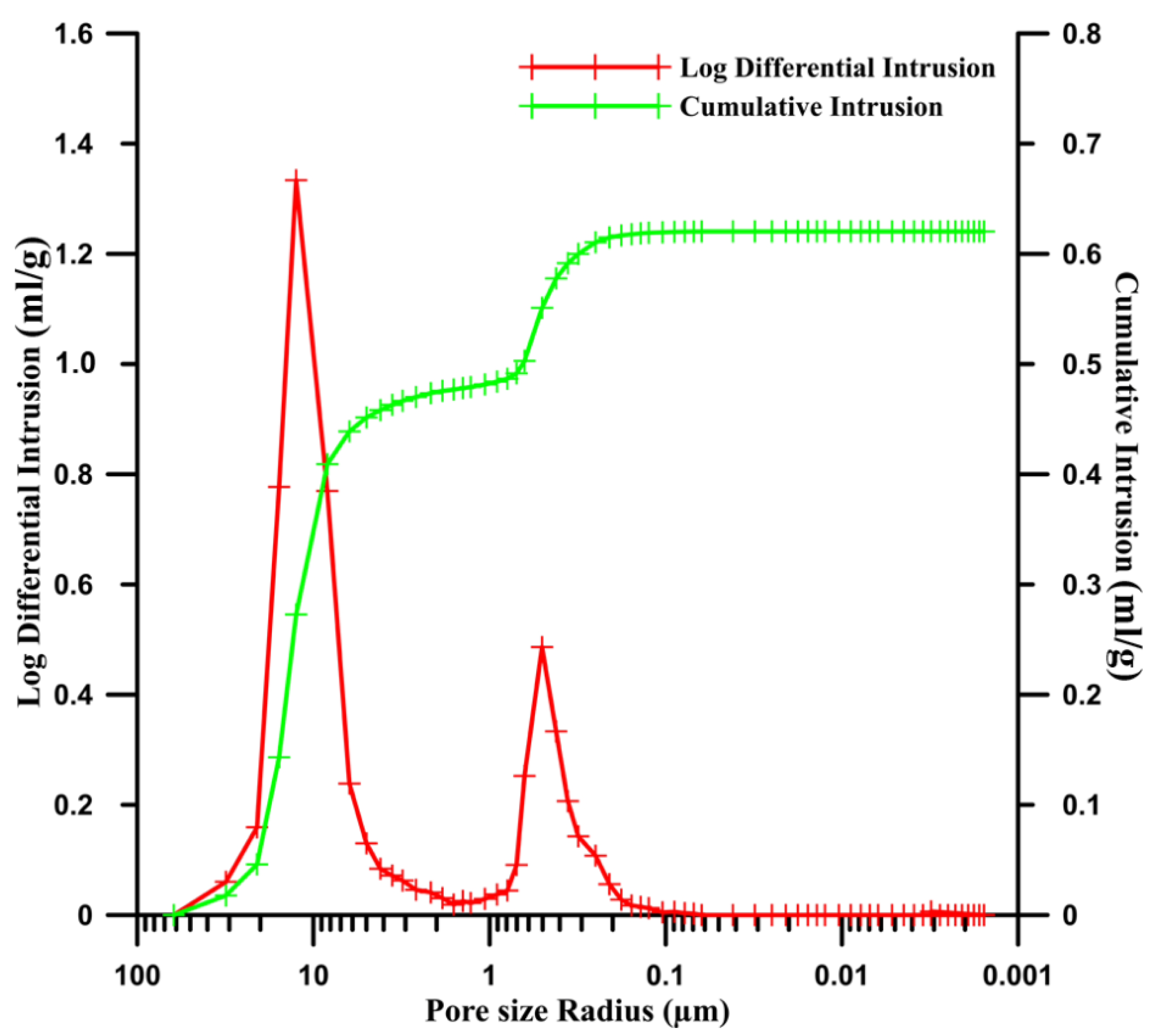
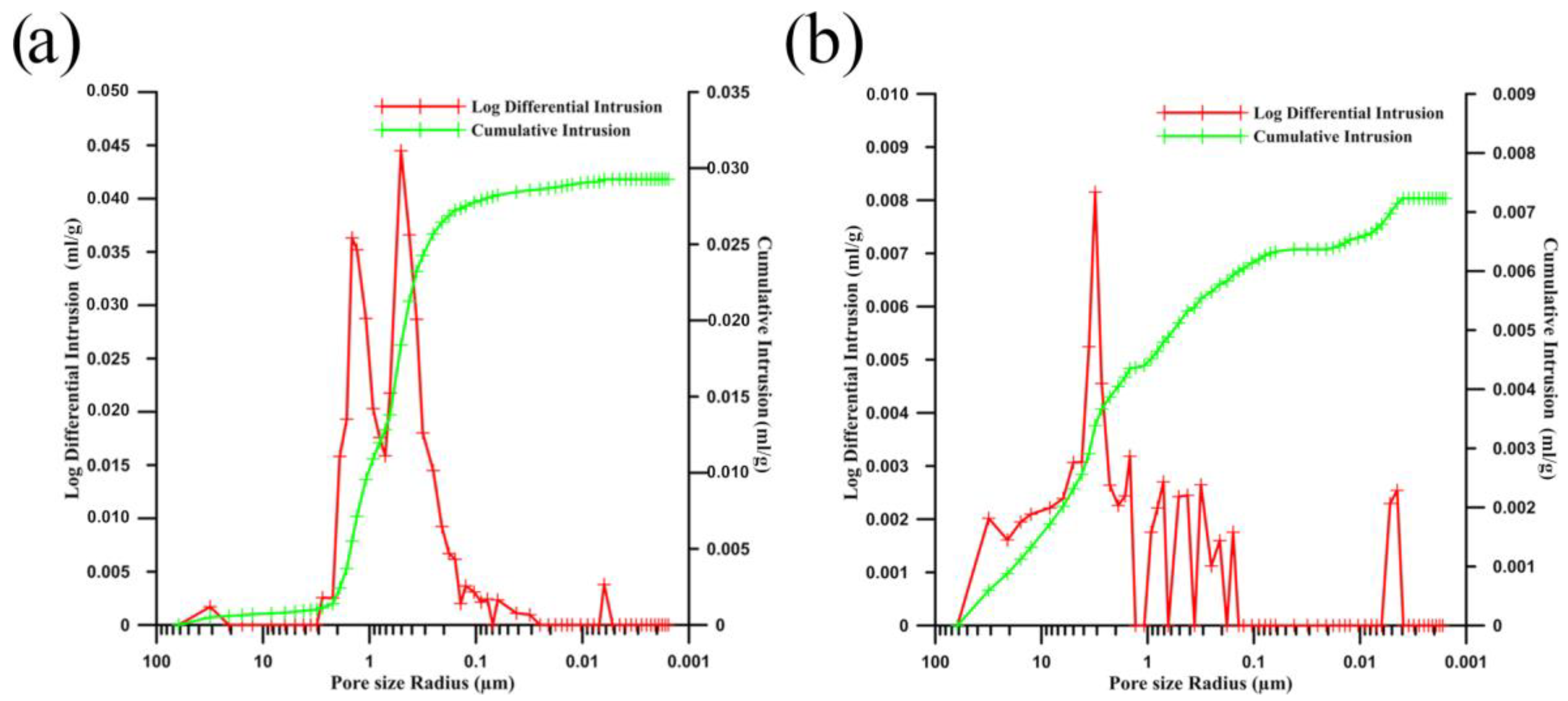
| Sample | Theoretical Porosity | Porosity Evaluated by Image Analysis | Porosity Evaluated by Mercury Porosimetry | Average Pore Size (µm) | ||
|---|---|---|---|---|---|---|
| Pure zinc | 2.7% | 1.7% | 5% | 21 | ||
| Zinc matrix | 12.6% | 18% | 10.2% | 10.6% | 15.2% | <1 (Zn–Zn) ~19 (Zn–HA) |
| HA | 5.4% | - | 4.6% | <1 | ||
| Materials | UFS (MPa) | Hardness (HV) | CYS (MPa) | UCS (MPa) | Reference |
|---|---|---|---|---|---|
| Cortical bone | 160 | - | - | 88–230 | [45,46] |
| Cancellous bone | - | - | 2–12 | 0.2–80 | [47,48] |
| PM Zinc | 167 ± 11 | 33 ± 2 (HV5) | 81 ± 5 | - | This study |
| PM Magnesium | 9 | 27 (HV 3) | 33 | 45 | [48] |
| Zn/1HA * | - | 46 (HV0.1) | 70 | 157 | [23] |
| Zn/5HA * | - | 45 (HV0.1) | 42 | 109 | [23] |
| Zn/10HA * | - | 45 (HV0.1) | 47 | 72 | [23] |
| Zn/16HA | 113 ± 8 | 24 ± 5 (HV5) | 46 ± 3 | 65 ± 4 | This study |
| Mg/5HA | - | 64 (HV1) | 205 | 330 | [49] |
| Methods | Weight Loss | AAS | Polarization Resistance | ||
|---|---|---|---|---|---|
| Samples | mm/year | mg/(cm2·day) | mg/(cm2·day) | mm/year | mg/(cm2·day) |
| SPS Zinc | 0.26 | 0.45 ± 0.05 | 0.40 ± 0.03 | 0.85 | 1.72 ± 0.18 |
| SPS Zn/HA16 composite | 0.41 | 0.54 ± 0.04 | 0.45 ± 0.07 | 1.52 | 2.81 ± 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinc, J.; Čapek, J.; Kubásek, J.; Průša, F.; Hybášek, V.; Veřtát, P.; Sedlářová, I.; Vojtěch, D. Characterization of a Zn-Ca5(PO4)3(OH) Composite with a High Content of the Hydroxyapatite Particles Prepared by the Spark Plasma Sintering Process. Metals 2020, 10, 372. https://doi.org/10.3390/met10030372
Pinc J, Čapek J, Kubásek J, Průša F, Hybášek V, Veřtát P, Sedlářová I, Vojtěch D. Characterization of a Zn-Ca5(PO4)3(OH) Composite with a High Content of the Hydroxyapatite Particles Prepared by the Spark Plasma Sintering Process. Metals. 2020; 10(3):372. https://doi.org/10.3390/met10030372
Chicago/Turabian StylePinc, Jan, Jaroslav Čapek, Jiří Kubásek, Filip Průša, Vojtěch Hybášek, Petr Veřtát, Ivona Sedlářová, and Dalibor Vojtěch. 2020. "Characterization of a Zn-Ca5(PO4)3(OH) Composite with a High Content of the Hydroxyapatite Particles Prepared by the Spark Plasma Sintering Process" Metals 10, no. 3: 372. https://doi.org/10.3390/met10030372
APA StylePinc, J., Čapek, J., Kubásek, J., Průša, F., Hybášek, V., Veřtát, P., Sedlářová, I., & Vojtěch, D. (2020). Characterization of a Zn-Ca5(PO4)3(OH) Composite with a High Content of the Hydroxyapatite Particles Prepared by the Spark Plasma Sintering Process. Metals, 10(3), 372. https://doi.org/10.3390/met10030372






