Influence of Al Additions on the Microstructure and Mechanical Properties of a C and Si-Free High-Mn Steel
Abstract
1. Introduction
2. Materials and Methods
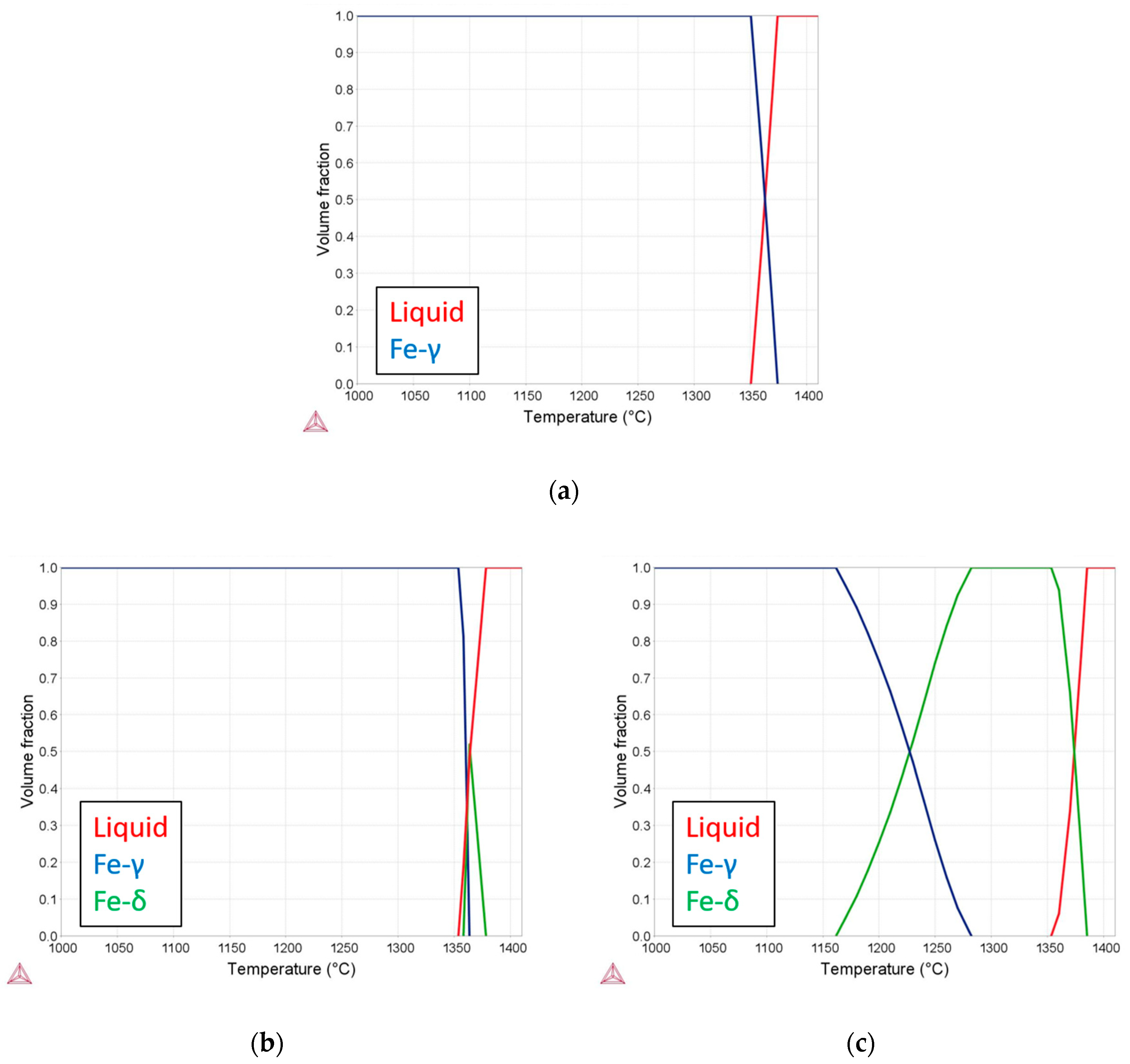
3. Results and Discussion
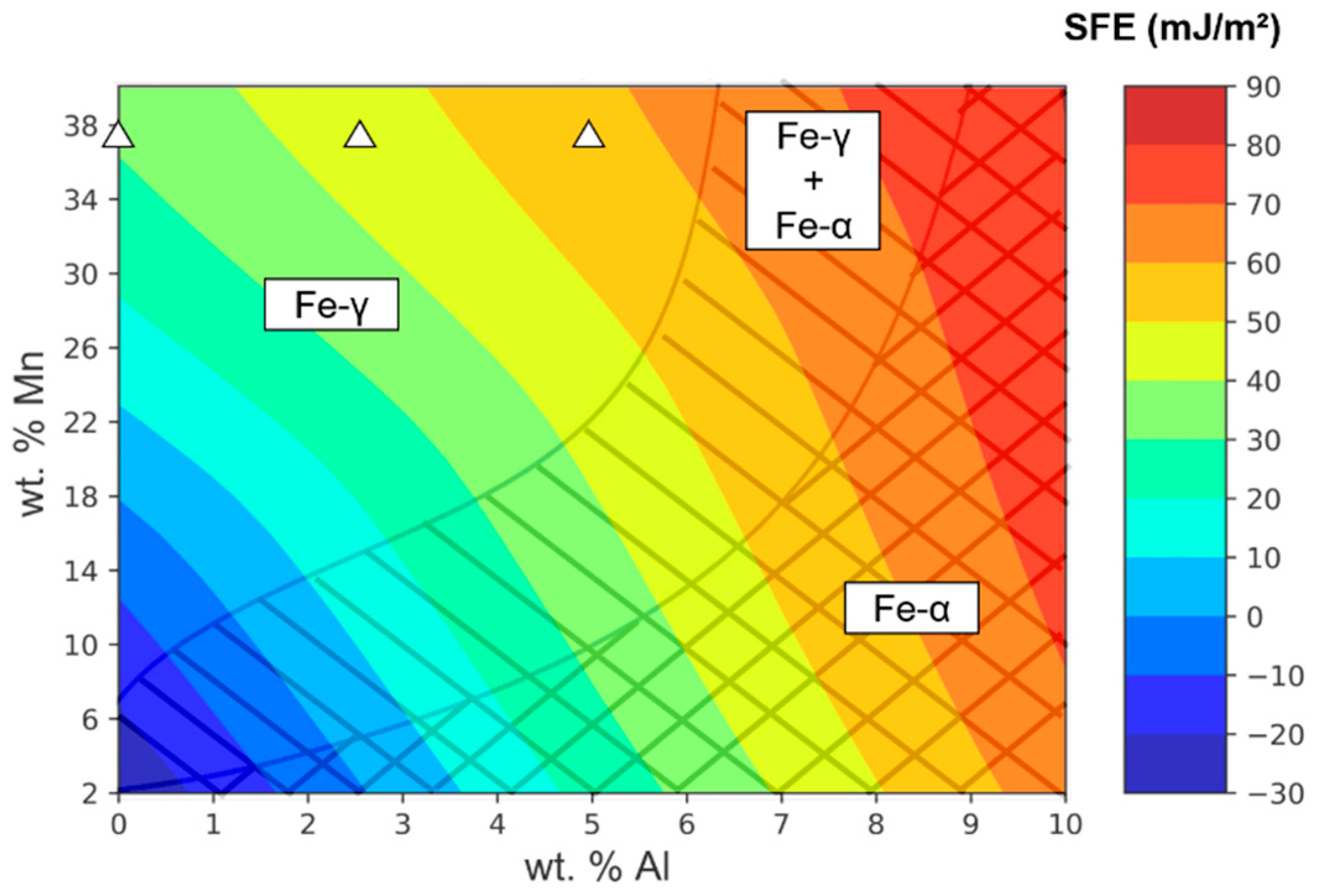
3.1. Stacking Fault Energy (SFE)
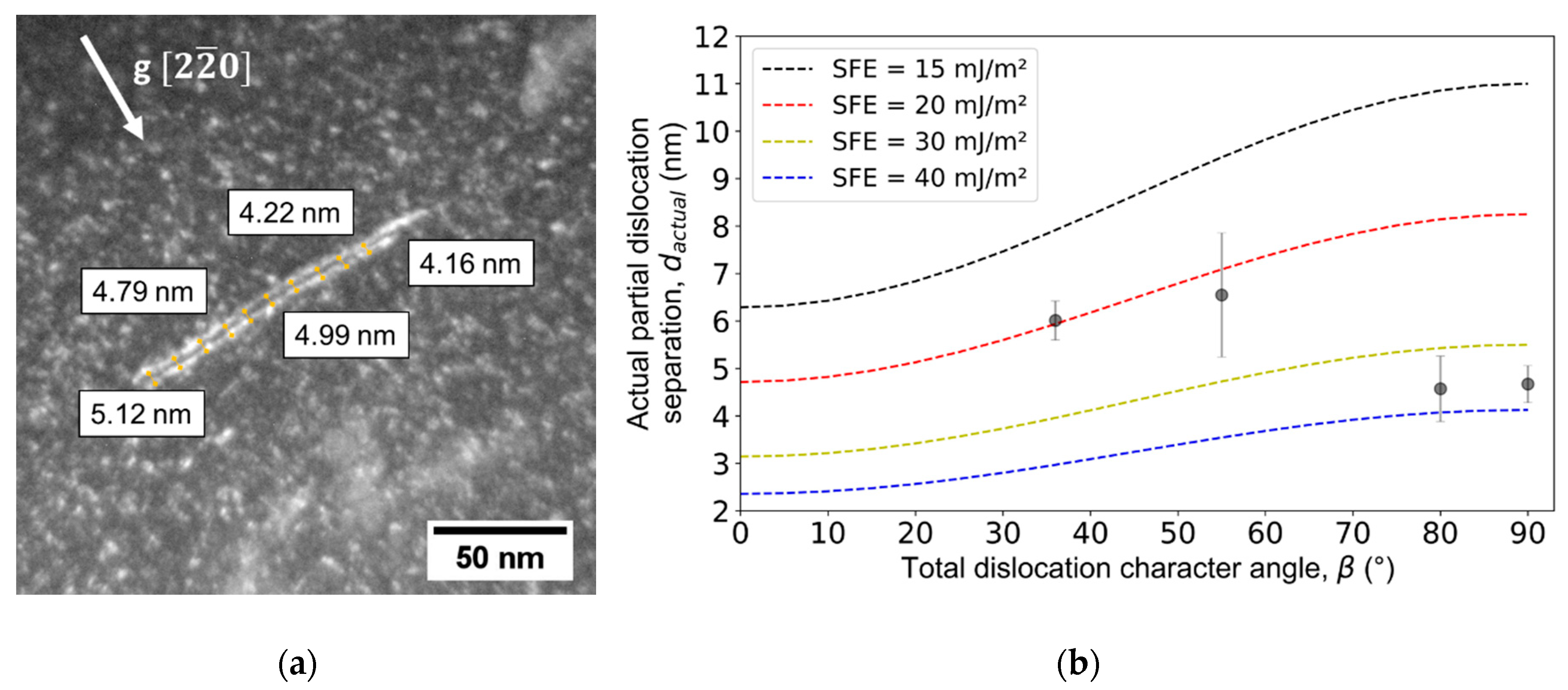
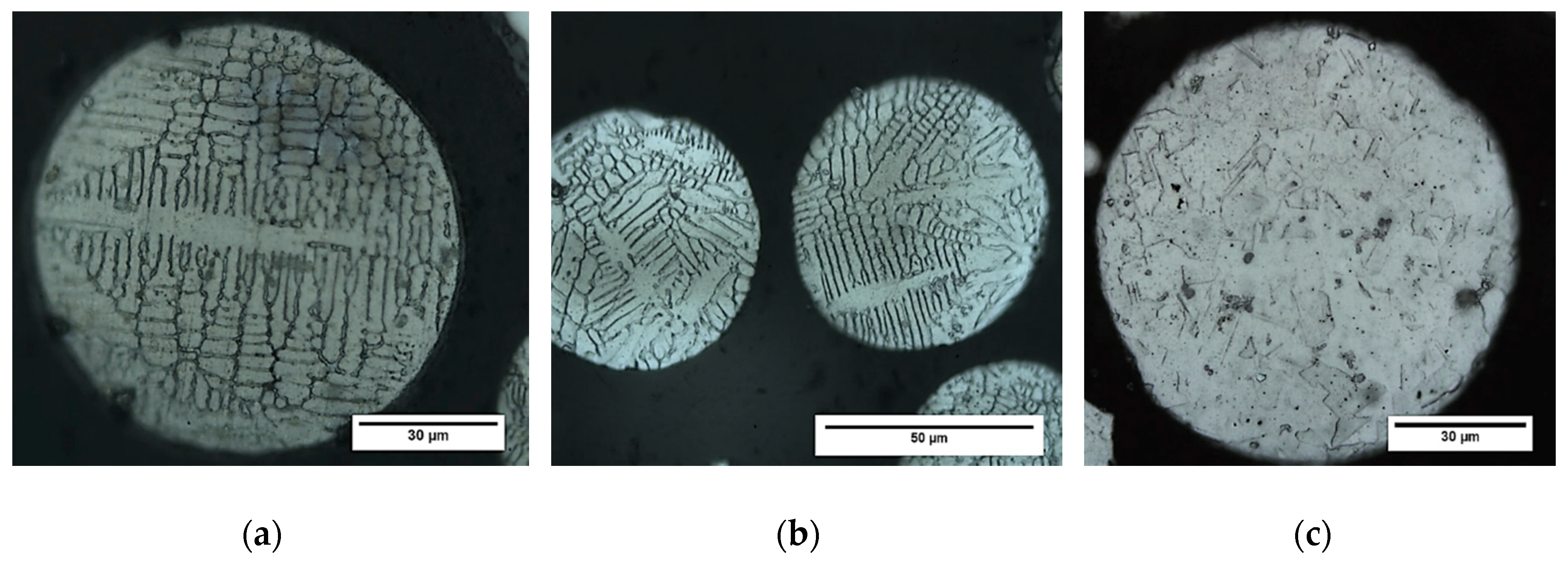
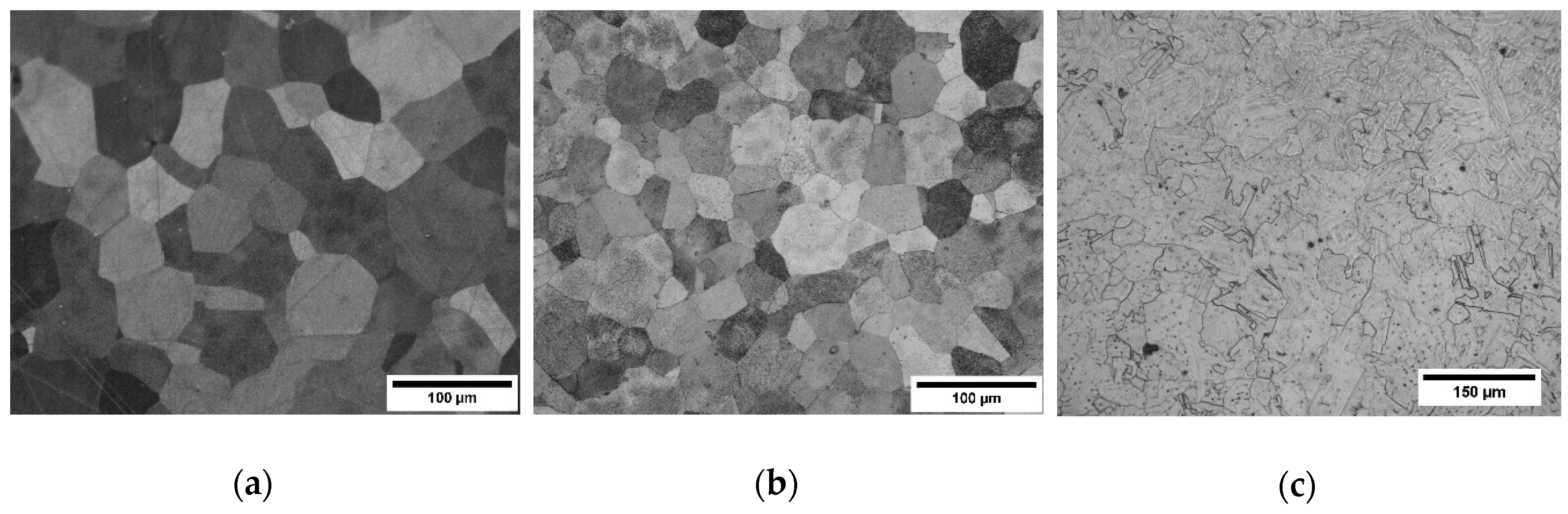
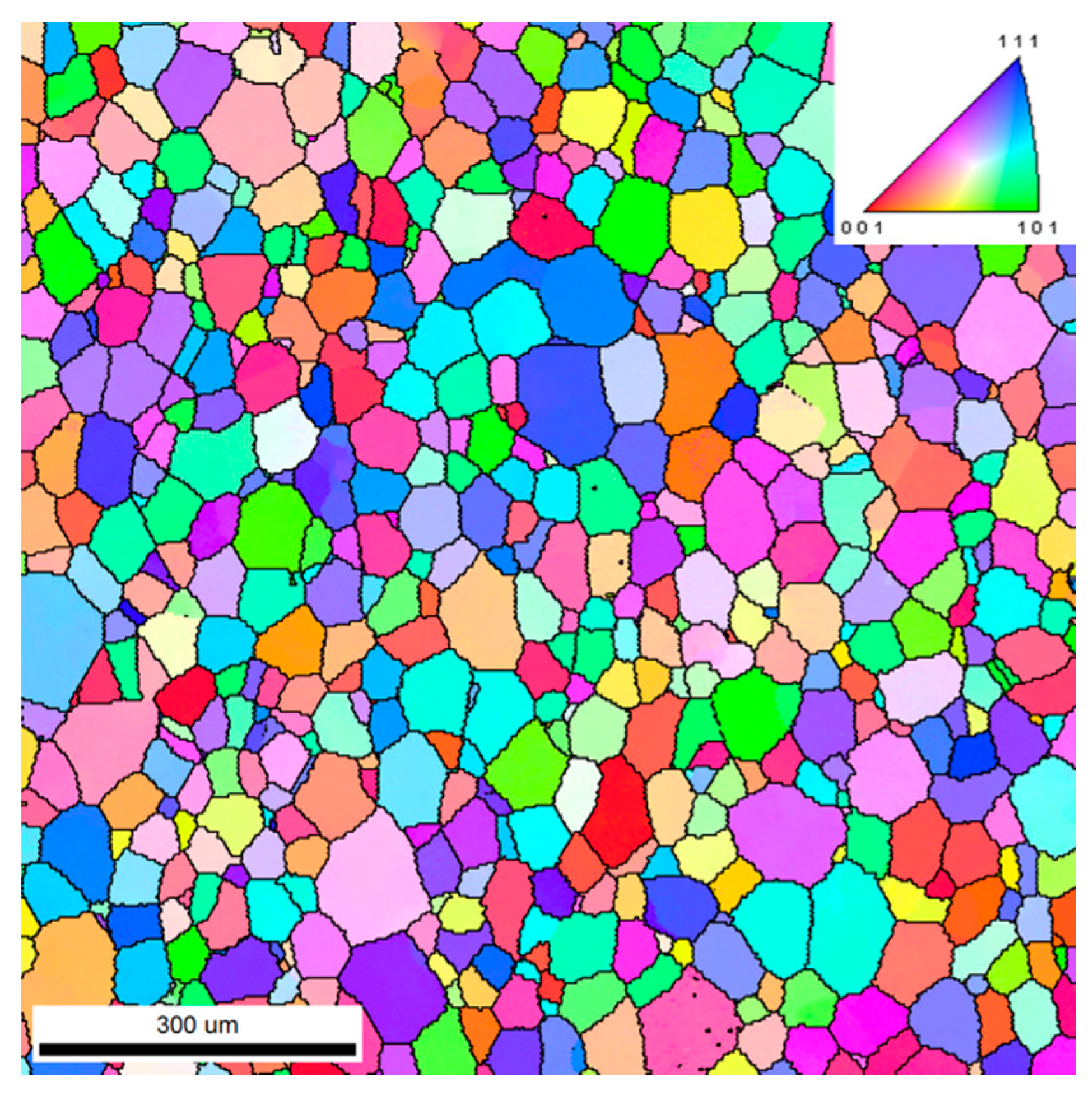
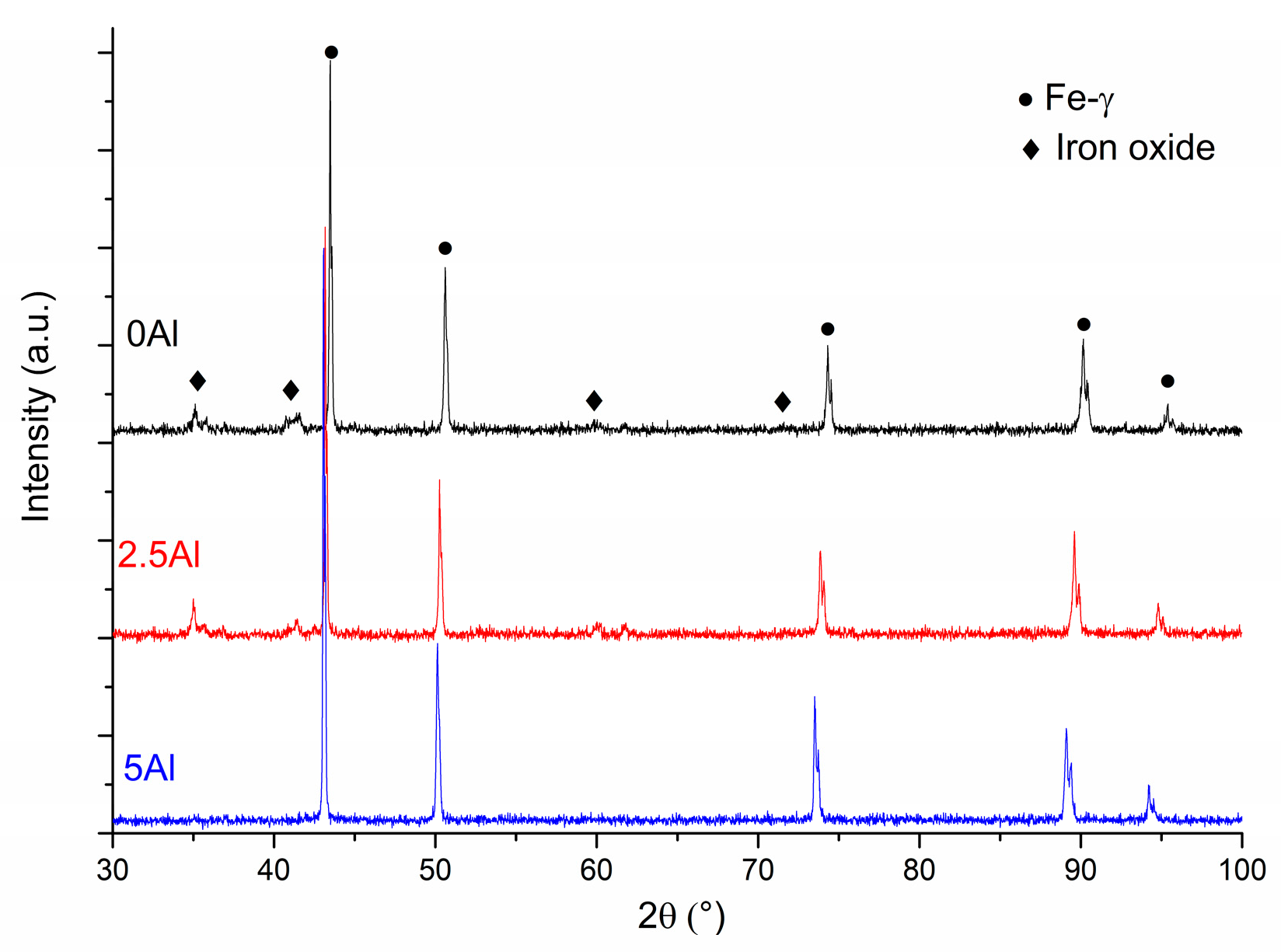
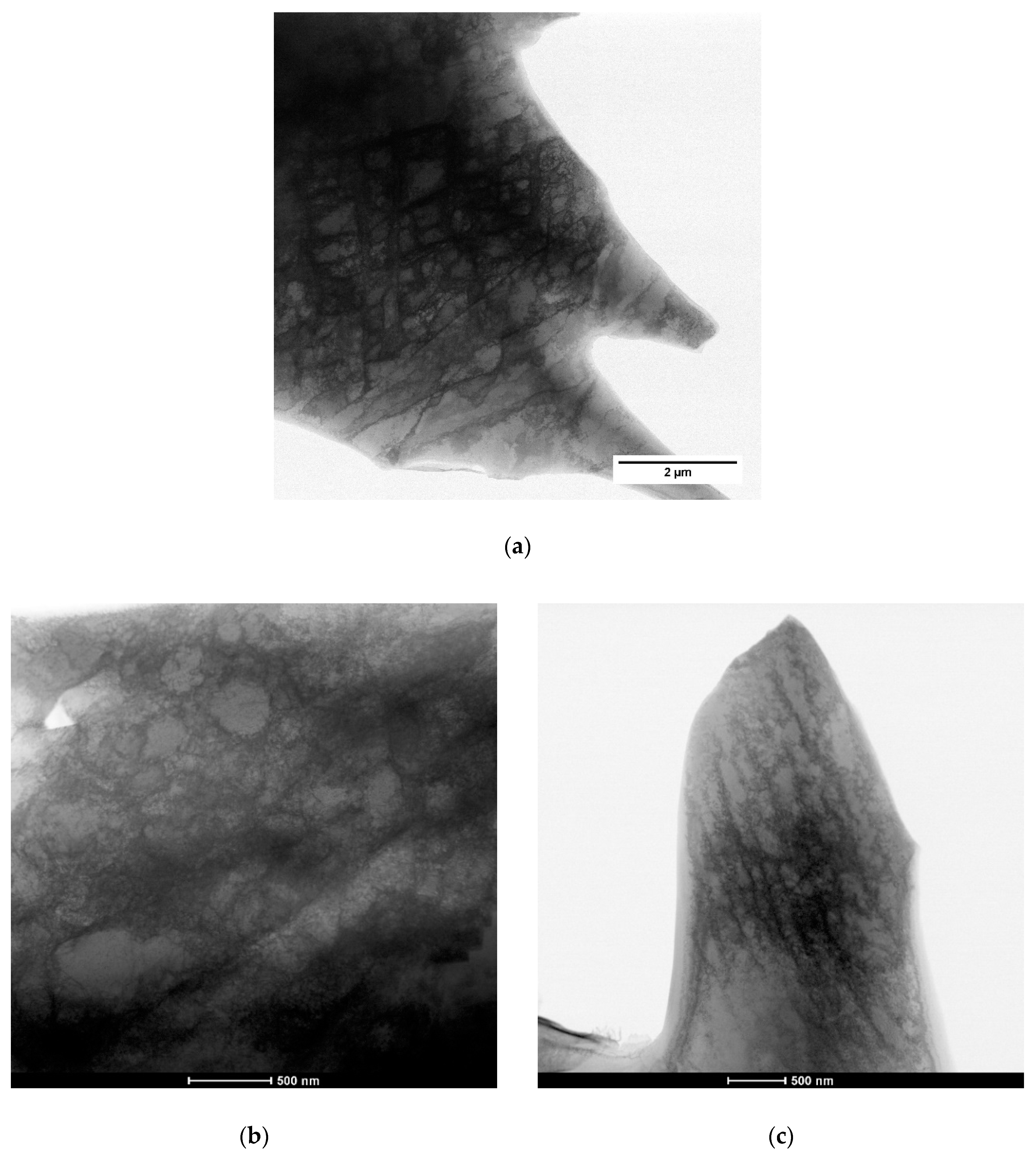
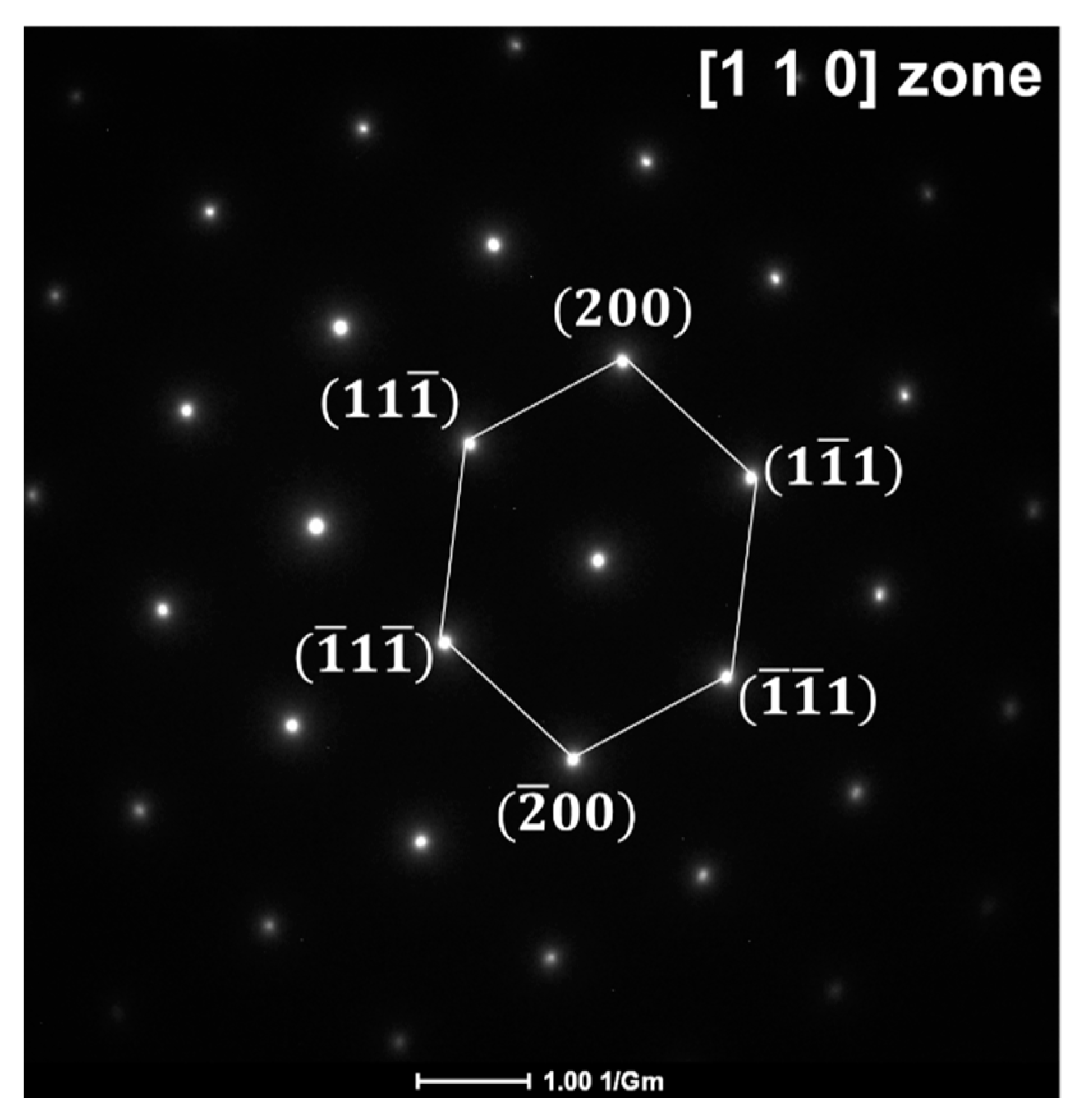
3.2. Microstructural Evolution
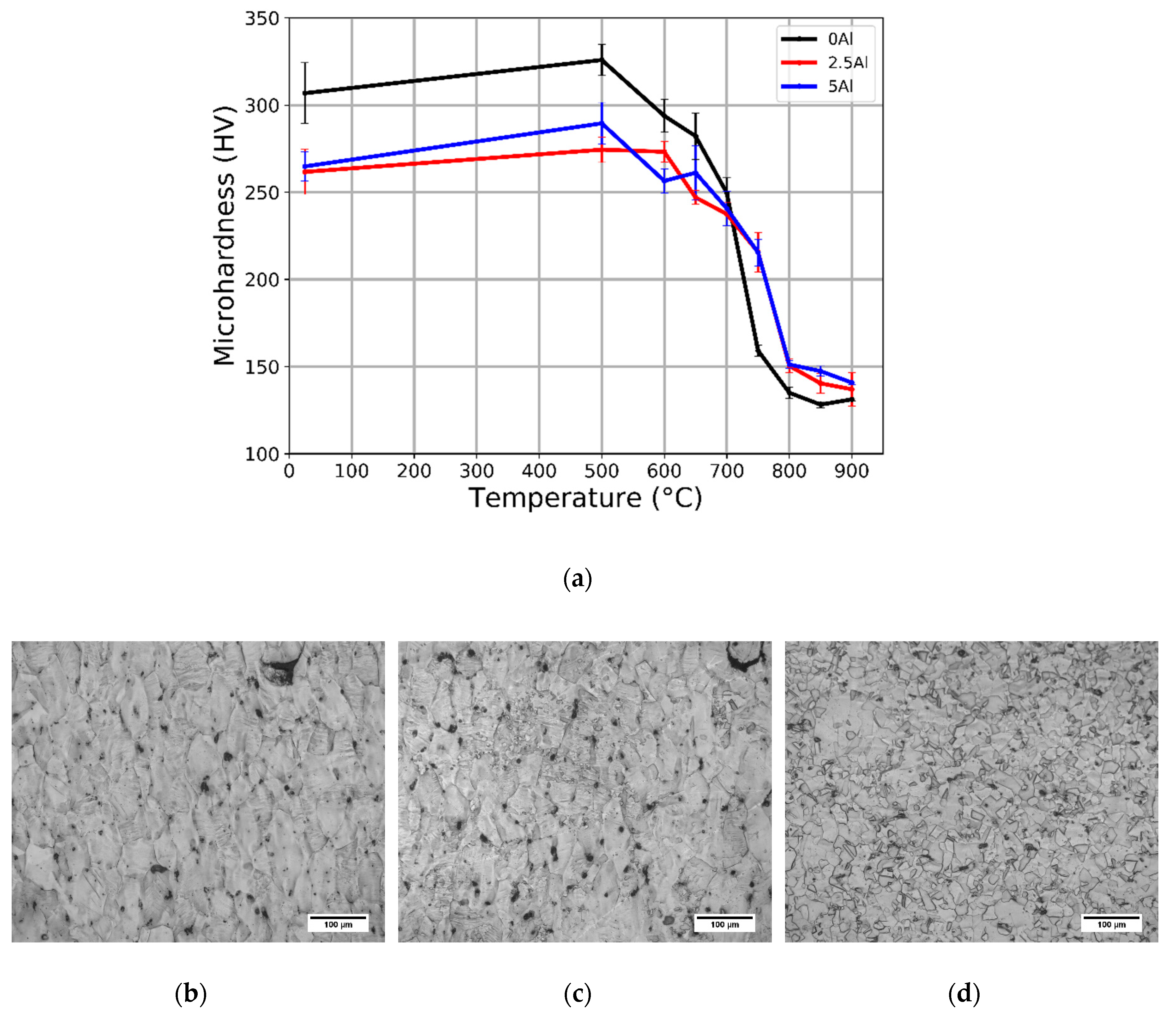
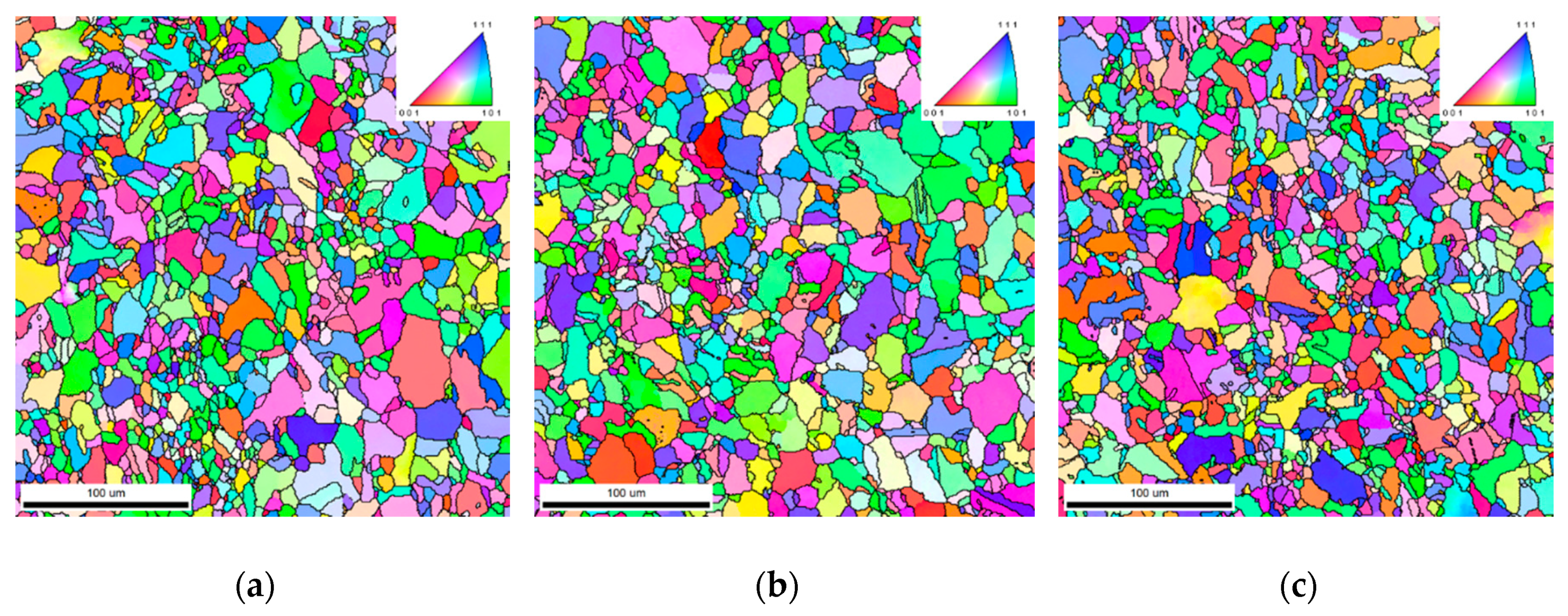
3.3. Cold Rolling and Annealing
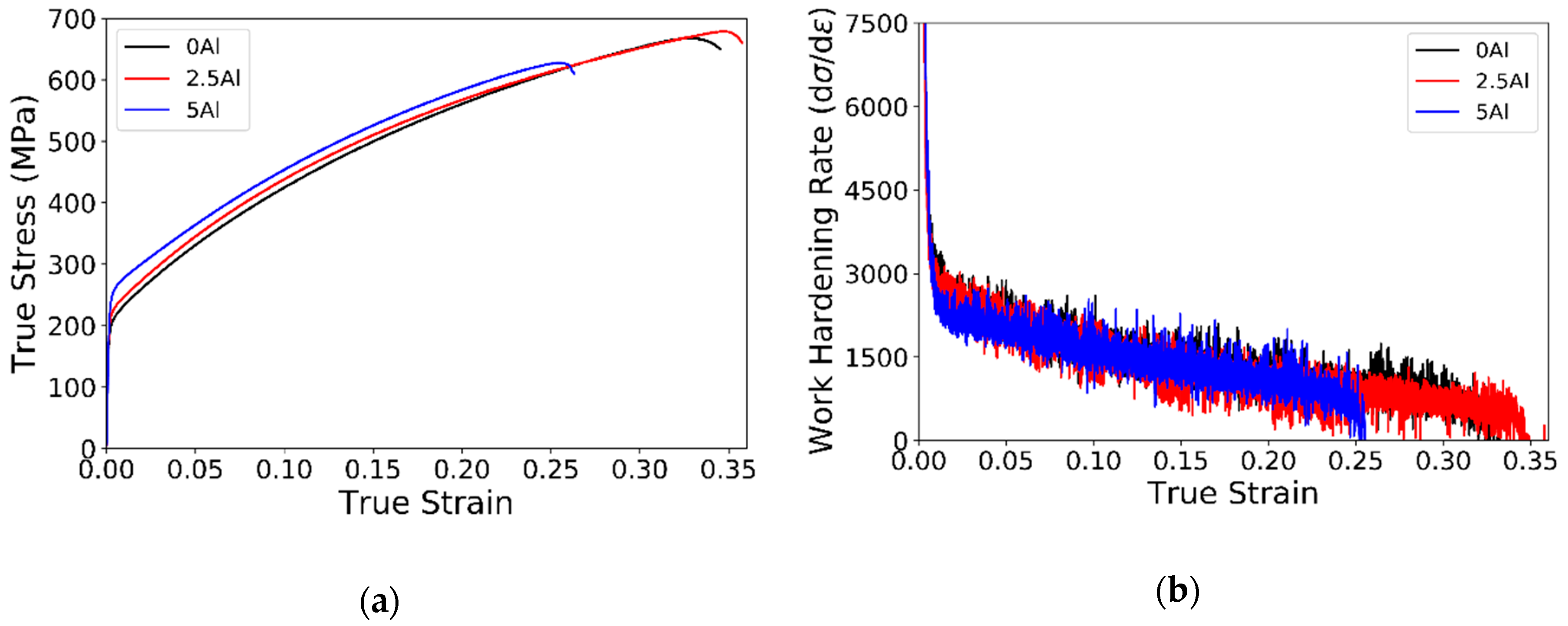
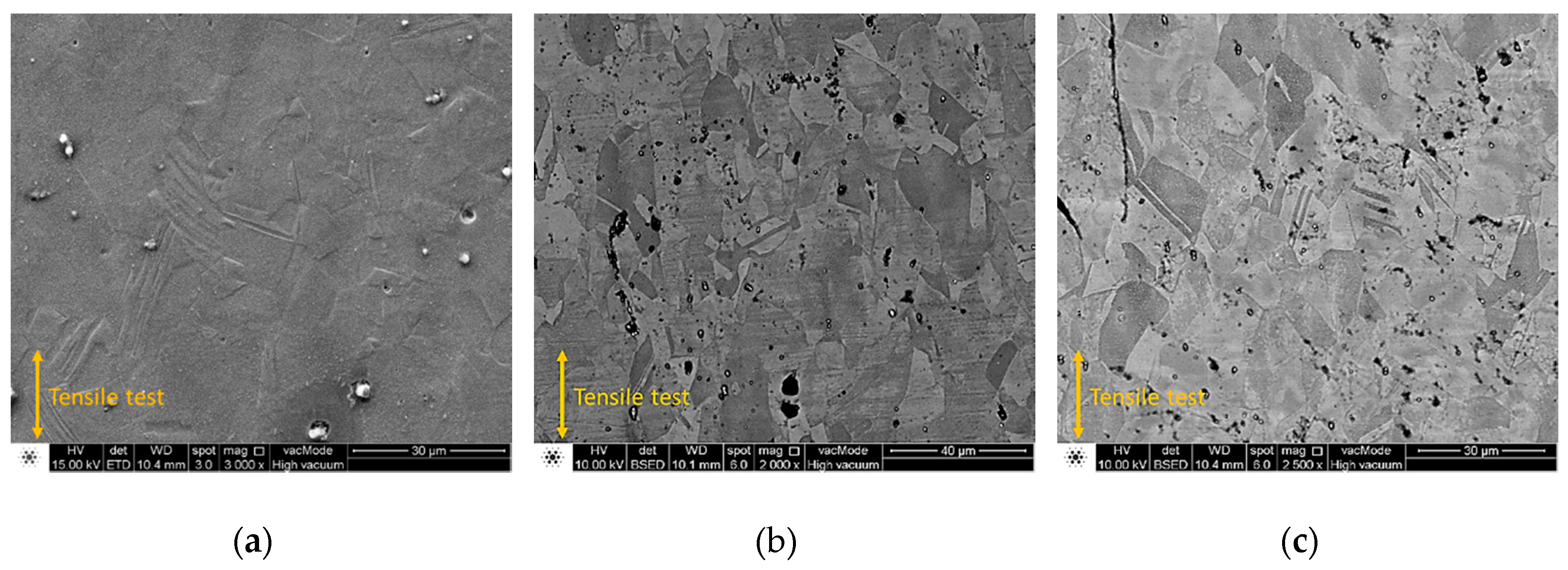
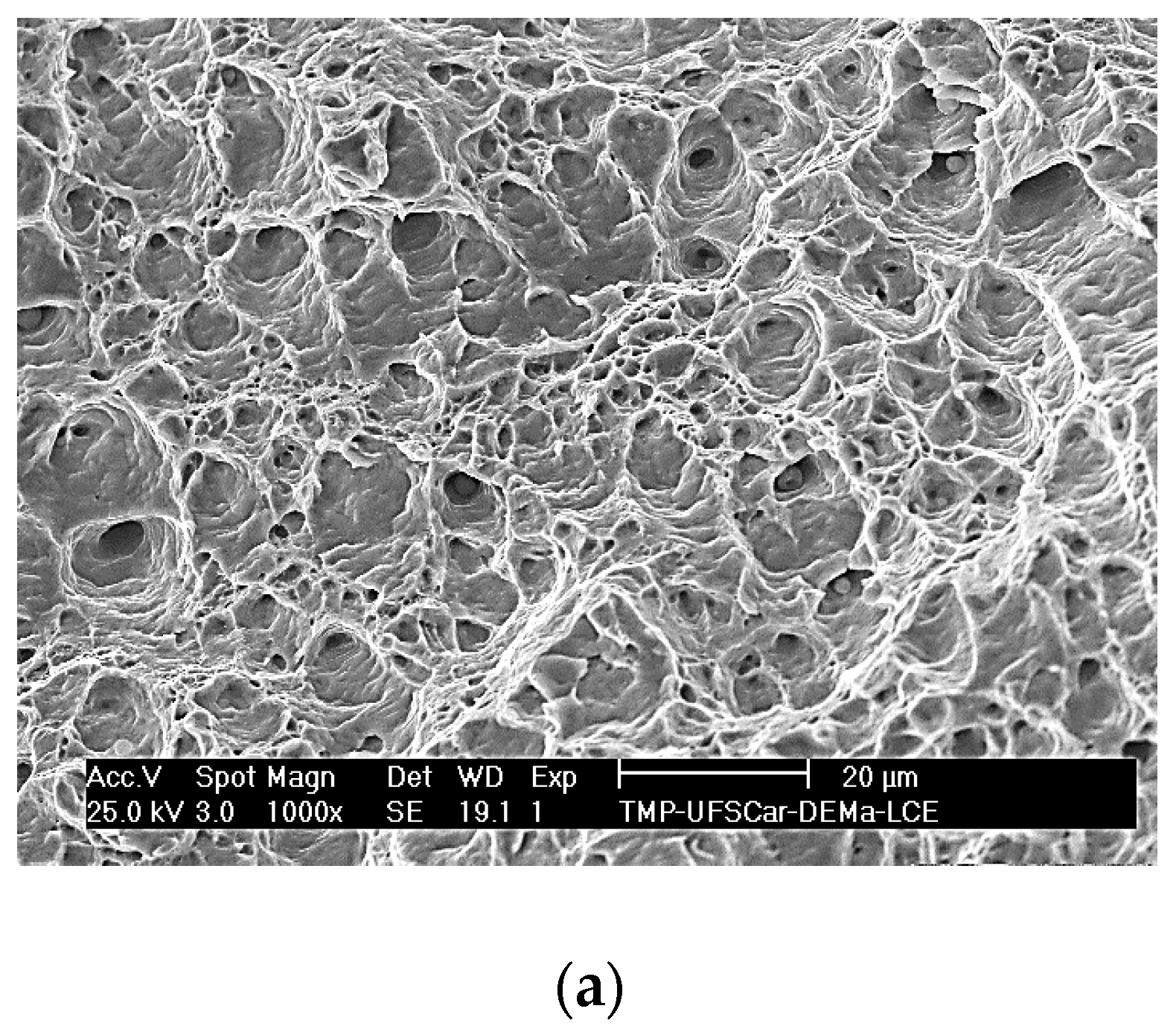
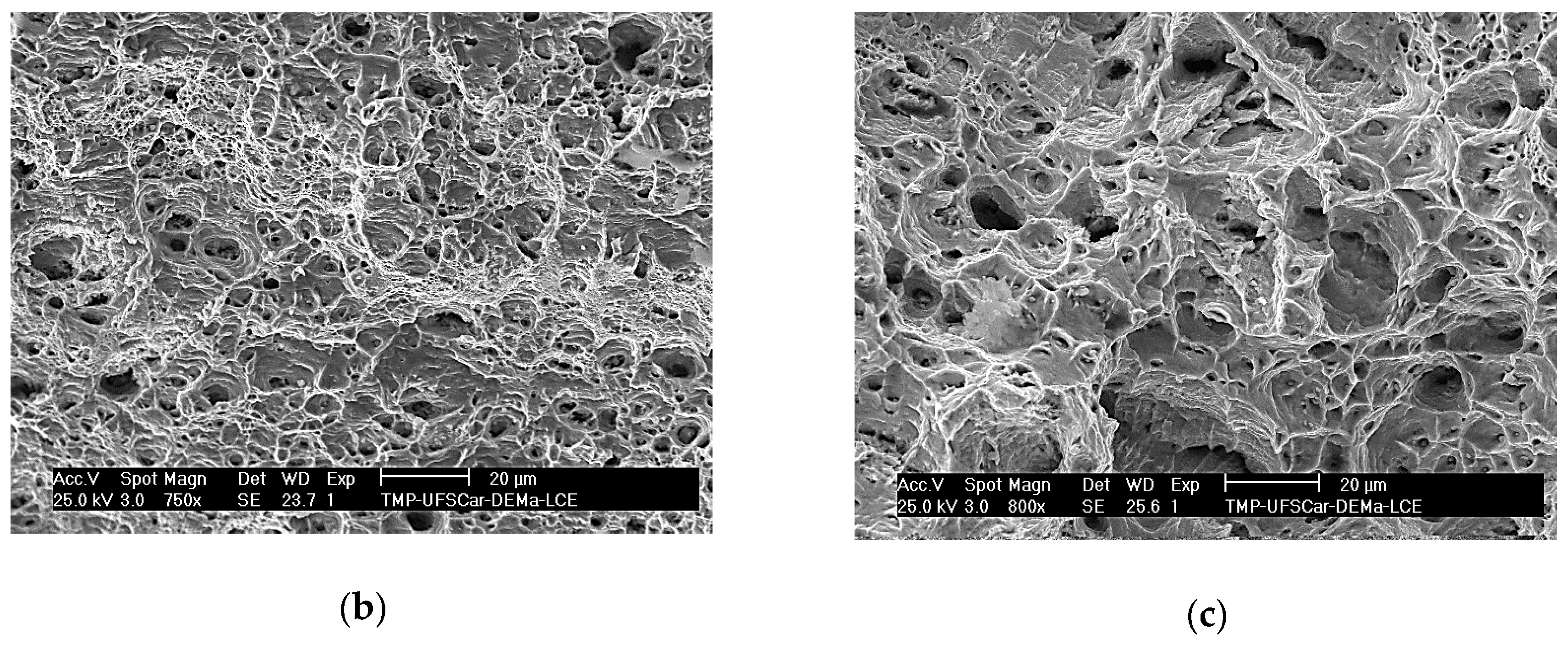
3.4. Tensile Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Value | Ref. |
|---|---|---|
| ρ | 4/(a2 N √3) | [39] |
| ΔGγ->ε | ΔGγ->εch + ΔGγ->εmag + ΔGex | [11] |
| ΔGγ->εch | XFeΔGγ->εFe + XMnΔGγ->εMn + XAlΔGγ->εAl + XCΔGγ->εC + XFeXMnΔΩγ->εFeMn + XFeXAlΔΩγ->εFeAl + XFeXCΔΩγ->εFeC + XMnXCΔΩγ->εMnC | [38] |
| ΔGγ->εFe | −2243.38 + 4.309 T | [40] |
| ΔGγ->εMn | −1000 + 1.123T | [40] |
| ΔGγ->εAl | 5481 − 1.8T | [40] |
| ΔGγ->εC | −22,166 | [38] |
| ΔΩγ->εFeMn | 2180 + 532(XFe − XMn) | [41] |
| ΔΩγ->εFeAl | 3326.28 | [38] |
| ΔΩγ->εFeC | 42,538.728 | [38] |
| ΔΩγ->εMnC | 26,910 | [39] |
| ΔGγ->εmag | RT{ln[1 + βε/(μB)] f(T/ TεN) – ln[1 + βγ/(μB)] f(T/TγN)} | [41] |
| τ | T/TεNT/TγN | [41] |
| f | 1−{79τ−1/[140p] + 474/497[1/p − 1][τ3/6 + τ9/135 + τ15/600]}/D for τ ≤ 1−{τ−5/10 + τ−15/315 + τ−25/1500]}/D for τ > 1 | [41] |
| p | 0.28 | [42] |
| D | 2.342456517 | [42] |
| βε/μB | 0.62XMn − 4XC | [43] |
| βγ/μB | 0.7XFe + 0.62XMn + 0.64XFeXMn − 4 XC | [43] |
| TεN | 580 XMn | [43] |
| TγN | 10XMn3 – 898.4XMn2 + 1176XMn – 1992XC – 661XAl + 152.4 | [44] |
| 2ρΔGex | 5 | [22] |
| σγ−ε | 10 | [38] |
References
- Rana, R.; Lahaye, C.; Ray, R.K. Overview of lightweight ferrous materials: strategies and promises. JOM 2014, 66, 1734–1746. [Google Scholar] [CrossRef]
- Madivala, M.; Schwedt, A.; Prahl, U.; Bleck, W. Strain hardening, damage and fracture behavior of Al-added high Mn TWIP steels. Metals 2019, 9, 367. [Google Scholar] [CrossRef]
- He, B.B.; Hu, B.; Yen, H.W.; Cheng, G.J.; Wang, Z.K.; Luo, H.W.; Huang, M.X. High dislocation density-induced large ductility in deformed and partitioned steels. Science 2017, 357, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Bouaziz, O.; Allain, S.; Scott, C.P.; Cugy, P.; Barbier, D. High manganese austenitic twinning induced plasticity steels: A review of the microstructure properties relationships. Curr. Opin. Solid ST M 2011, 15, 141–168. [Google Scholar] [CrossRef]
- Grässel, O.; Krüger, L.; Frommeyer, G.; Meyer, L.W. High strength Fe-Mn-(Al, Si) TRIP/TWIP steels development—properties—application. Int. J. Plasticity 2000, 16, 1391–1409. [Google Scholar] [CrossRef]
- De Barbieri, F.; Cerda, F.C.; Pérez-Ipiña, J.; Artigas, A.; Monsalve, A. Temperature dependence of the microstructure and mechanical properties of a twinning-induced plasticity steel. Metals 2018, 8, 262. [Google Scholar] [CrossRef]
- Yoo, J.D.; Park, K.T. Microband-induced plasticity in a high Mn-Al-C light steel. Mat. Sci. Eng. A 2008, 496, 417–424. [Google Scholar] [CrossRef]
- Lee, S.J.; Han, J.; Lee, C.Y.; Park, I.J.; Lee, Y.K. Elastic strain energy induced by epsilon martensitic transformation and its contribution to the stacking-fault energy of austenite in Fe-15Mn-xC alloys. J. Alloys Compd. 2014, 617, 588–596. [Google Scholar] [CrossRef]
- Saeed-Akbari, A.; Imlau, J.; Prahl, U.; Bleck, W. Derivation and variation in composition-dependent stacking fault energy maps based on subregular solution model in high-manganese steels. Metall. Mater. Trans. A 2009, 40, 3076–3090. [Google Scholar] [CrossRef]
- Zambrano, O.A. Stacking fault energy maps of Fe–Mn–Al–C–Si steels: Effect of temperature, grain size, and variations in compositions. J. Eng. Mater. Tech. 2016, 138, 041010. [Google Scholar] [CrossRef]
- Olson, G.B.; Cohen, M. A general mechanism of martensitic nucleation. Metall. Trans. A 1976, 7, 1897–1904. [Google Scholar]
- De Cooman, B.C.; Estrin, Y.; Kim, S.K. Twinning-induced plasticity (TWIP) steels. Acta Mater. 2017, 142, 283–362. [Google Scholar] [CrossRef]
- Jin, J.E.; Lee, Y.K. Effects of Al on microstructure and tensile properties of C-bearing high Mn TWIP steel. Acta Mater. 2012, 60, 1680–1688. [Google Scholar] [CrossRef]
- Park, K.T.; Jin, K.G.; Han, S.H.; Hwang, S.W.; Choi, K.; Lee, C.S. Stacking fault energy and plastic deformation of fully austenitic high manganese steels: Effect of Al addition. Mat. Sci. Eng. A 2010, 527, 3651–3661. [Google Scholar] [CrossRef]
- Kozłowska, A.; Grzegorczyk, B.; Morawiec, M.; Grajcar, A. Explanation of the PLC effect in advanced high-strength medium-Mn steels: A review. Materials 2019, 12, 4175. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Suh, D.; Kim, N.J. Fe–Al–Mn–C lightweight structural alloys: a review on the microstructures and mechanical properties. Sci. Technol. Adv. Mat. 2013, 14, 014205. [Google Scholar] [CrossRef]
- Grant, P.S. Solidification in Spray Forming. Metall. Mater. Trans. A 2007, 38, 1520–1529. [Google Scholar] [CrossRef]
- Pierce, D.T.; Jiménez, J.A.; Bentley, J.; Raabe, D.; Oskay, C.; Wittig, J.E. The influence of manganese content on the stacking fault and austenite/ε-martensite interfacial energies in Fe-Mn-(Al-Si) steels investigated by experiment and theory. Acta Mater. 2014, 68, 238–253. [Google Scholar] [CrossRef]
- Lu, J.; Hultman, L.; Holmström, E.; Antonsson, K.H.; Grehk, M.; Li, W.; Vitos, L.; Golpayegani, A. Stacking fault energies in austenitic stainless steels. Acta Mater. 2016, 111, 39–46. [Google Scholar] [CrossRef]
- Pierce, D.T.; Nowag, K.; Montagne, A.; Jiménez, J.A.; Wittig, J.E.; Ghisleni, R. Single crystal elastic constants of high-manganese transformation- and twinning-induced plasticity steels determined by a new method utilizing nanoindentation. Mat. Sci. Eng. A 2013, 578, 134–139. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Choi, C.-S. Driving force for γ→ε martensitic transformation and stacking fault energy of γ in Fe-Mn binary system. Metall. Mater. Trans. A 2000, 31, 355–360. [Google Scholar] [CrossRef]
- Zepon, G.; Ellendt, N.; Uhlenwinkel, V.; Bolfarini, C. Solidification sequence of spray-formed steels. Metall. Mater. Trans. A 2016, 47, 842–851. [Google Scholar] [CrossRef]
- Zepon, G.; Fernandes, J.F.M.; Otani, L.B.; Bolfarini, C. Stable eutectic formation in spray-formed cast iron. Metall. Mater. Trans. A 2020, 51, 798–808. [Google Scholar] [CrossRef]
- Asgari, S.; El-Danaf, E.; Kalidindi, S.R.; Doherty, R.D. Strain hardening regimes and microstructural evolution during large strain compression of low stacking fault energy fcc alloys that form deformation twins. Metall. Mater. Trans. A 1997, 28, 1781–1795. [Google Scholar] [CrossRef]
- Kalidindi, S.R. Modeling the strain hardening response of low SFE FCC alloys. Int. J. Plasticity 1998, 14, 1265–1277. [Google Scholar] [CrossRef]
- Lee, Y.K. Microstructural evolution during plastic deformation of twinning-induced plasticity steels. Scr. Mater. 2012, 66, 1002–1006. [Google Scholar] [CrossRef]
- Lee, S.-J.; Han, J.; Lee, S.; Kang, S.-H.; Lee, S.-M.; Lee, Y.-K. Design for Fe-high Mn alloy with an improved combination of strength and ductility. Sci. Rep.-UK 2017, 7, 3573. [Google Scholar] [CrossRef]
- Rahman, K.M.; Vorontsov, V.A.; Dye, D. The effect of grain size on the twin initiation stress in a TWIP steel. Acta Mater. 2015, 89, 247–257. [Google Scholar] [CrossRef]
- Narita, N.; Takamura, J. Deformation twinning in silver-and copper-alloy crystals. Philos. Mag. 1974, 29, 1001–1028. [Google Scholar]
- Mahajan, S.; Chin, G.Y. Formation of deformation twins in f.c.c. crystals. Acta Metall. 1973, 21, 1353–1363. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Zaefferer, S.; Raabe, D. The effect of grain size and grain orientation on deformation twinning in a Fe–22wt. % Mn–0.6wt. % C TWIP steel. Mat. Sci. Eng. A 2010, 527, 3552–3560. [Google Scholar] [CrossRef]
- Kim, J.K.; Kwon, M.H.; De Cooman, B.C. On the deformation twinning mechanisms in twinning-induced plasticity steel. Acta Mater. 2017, 141, 444–455. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, J.; Ding, H.; Cai, Z.; Zhang, D.; Misra, D. Effect of Deformation Temperature on Mechanical Properties and Deformation Mechanisms of Cold-Rolled Low C High Mn TRIP/TWIP Steel. Metals 2018, 8, 476. [Google Scholar] [CrossRef]
- Kim, J.K.; De Cooman, B.C. Stacking fault energy and deformation mechanisms in Fe-xMn-0.6C-yAl TWIP steel. Mat. Sci. Eng. A 2016, 676, 216–231. [Google Scholar] [CrossRef]
- Zhang, L.; Song, R.; Zhao, C.; Yang, F. Work hardening behavior involving the substructural evolution of an austenite–ferrite Fe–Mn–Al–C steel. Mat. Sci. Eng. A 2015, 640, 225–234. [Google Scholar] [CrossRef]
- Luo, Z.C.; Huang, M.X. Revisit the role of deformation twins on the work-hardening behaviour of twinning-induced plasticity steels. Scripta Mater. 2018, 142, 28–31. [Google Scholar] [CrossRef]
- Yang, W.S.; Wan, C.M. The influence of aluminium content to the stacking fault energy in Fe-Mn-Al-C alloy system. J. Mater. Sci. 1990. [Google Scholar] [CrossRef]
- Allain, S.; Chateau, J.P.; Bouaziz, O.; Migot, S.; Guelton, N. Correlations between the calculated stacking fault energy and the plasticity mechanisms in Fe-Mn-C alloys. Mat. Sci. Eng. A 2004, 387–389, 158–162. [Google Scholar] [CrossRef]
- Dinsdale, A.T. Sgte data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Huang, W. An assessment of the Fe-Mn system. Calphad 1989, 13, 243–252. [Google Scholar] [CrossRef]
- Li, L.; Hsu(Xu Zuyao), T.Y. Gibbs free energy evaluation of the fcc(γ) and hcp(ε) phases in Fe-Mn-Si alloys. Calphad 1997, 21, 443–448. [Google Scholar] [CrossRef]
- Dumay, A.; Chateau, J.P.; Allain, S.; Migot, S.; Bouaziz, O. Influence of addition elements on the stacking-fault energy and mechanical properties of an austenitic Fe-Mn-C steel. Mat. Sci. Eng. A 2008, 483–484, 184–187. [Google Scholar] [CrossRef]
- Kusakin, P.; Belyakov, A.; Molodov, D.A.; Kaibyshev, R. On the effect of chemical composition on yield strength of TWIP steels. Mater. Sci. Eng. A 2017, 687, 82–84. [Google Scholar] [CrossRef]
| Alloy | Mn | Al | C | Fe |
|---|---|---|---|---|
| 0Al | 36.89 | 0 | 0.02 | Bal. |
| 2.5Al | 38.22 | 2.46 | 0.01 | Bal. |
| 5Al | 38.26 | 4.96 | 0.01 | Bal. |
| Pouring temperature | 1793 K (1520 °C) |
| Atomization gas | Nitrogen |
| Atomization pressure | 0.5 MPa |
| Feedstock mass | 4.0 kg |
| Gas–metal ratio (GMR) | 1.7 |
| Flight distance | 200 mm |
| Substrate material | Mild steel |
| Diameter of the substrate | 300 mm |
| Rotation of the substrate | 20 rpm |
| Alloy | σy | UTS | εtotal |
|---|---|---|---|
| 0Al | 207 MPa | 484 MPa | 42.5% |
| 2.5Al | 236 MPa | 497 MPa | 37.5% |
| 5Al | 253 MPa | 488 MPa | 30.9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otani, L.; Vidilli, A.; Coury, F.; Kiminami, C.; Botta, W.; Zepon, G.; Bolfarini, C. Influence of Al Additions on the Microstructure and Mechanical Properties of a C and Si-Free High-Mn Steel. Metals 2020, 10, 352. https://doi.org/10.3390/met10030352
Otani L, Vidilli A, Coury F, Kiminami C, Botta W, Zepon G, Bolfarini C. Influence of Al Additions on the Microstructure and Mechanical Properties of a C and Si-Free High-Mn Steel. Metals. 2020; 10(3):352. https://doi.org/10.3390/met10030352
Chicago/Turabian StyleOtani, Lucas, André Vidilli, Francisco Coury, Claudio Kiminami, Walter Botta, Guilherme Zepon, and Claudemiro Bolfarini. 2020. "Influence of Al Additions on the Microstructure and Mechanical Properties of a C and Si-Free High-Mn Steel" Metals 10, no. 3: 352. https://doi.org/10.3390/met10030352
APA StyleOtani, L., Vidilli, A., Coury, F., Kiminami, C., Botta, W., Zepon, G., & Bolfarini, C. (2020). Influence of Al Additions on the Microstructure and Mechanical Properties of a C and Si-Free High-Mn Steel. Metals, 10(3), 352. https://doi.org/10.3390/met10030352








