Tribological Behavior of As-Cast and Aged AlCoCrFeNi2.1 CCA
Abstract
1. Introduction
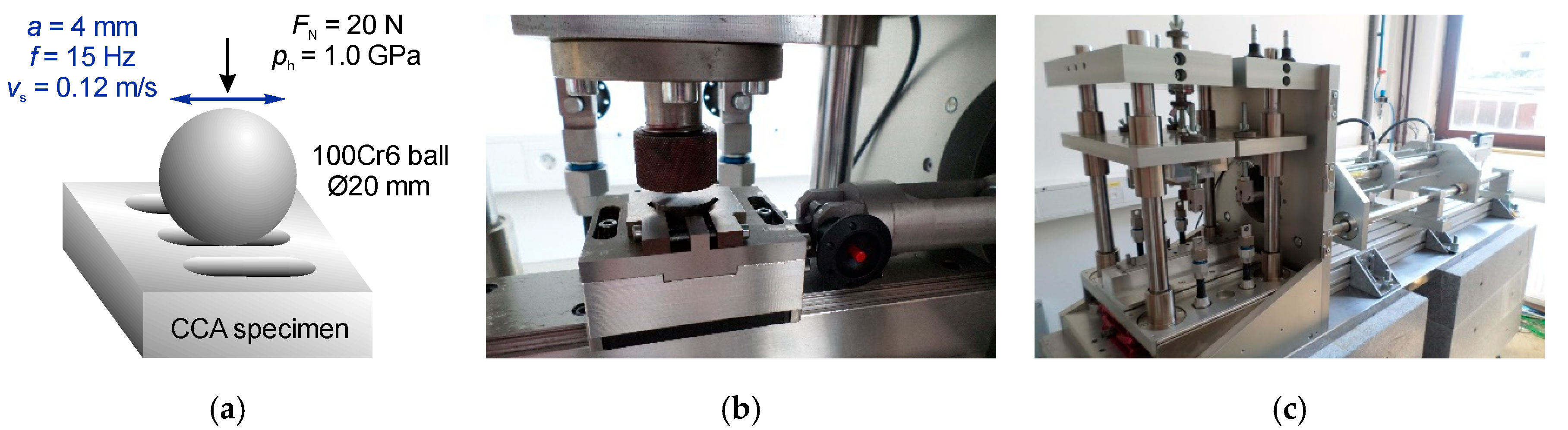
2. Materials and Methods
3. Results and Discussion
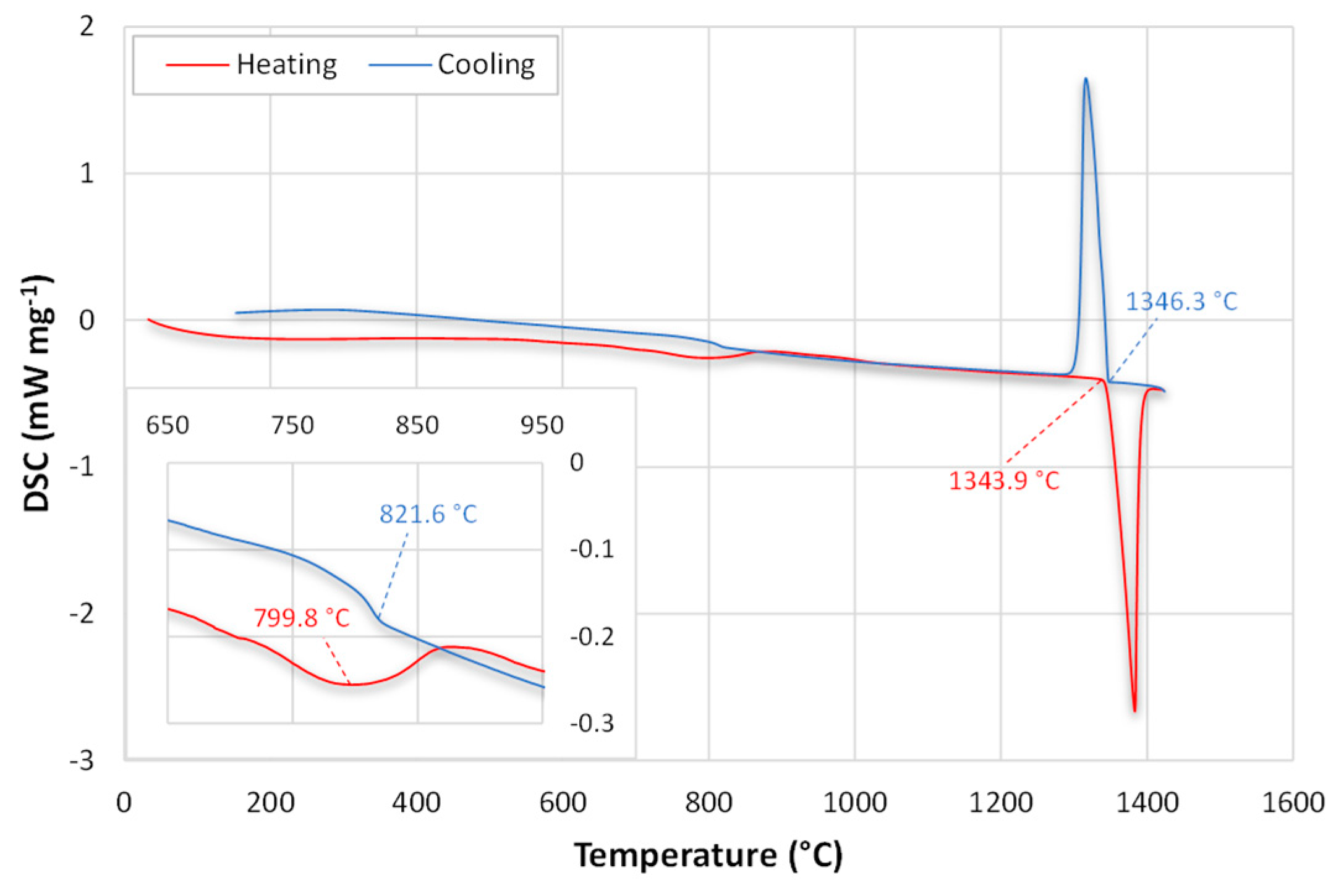
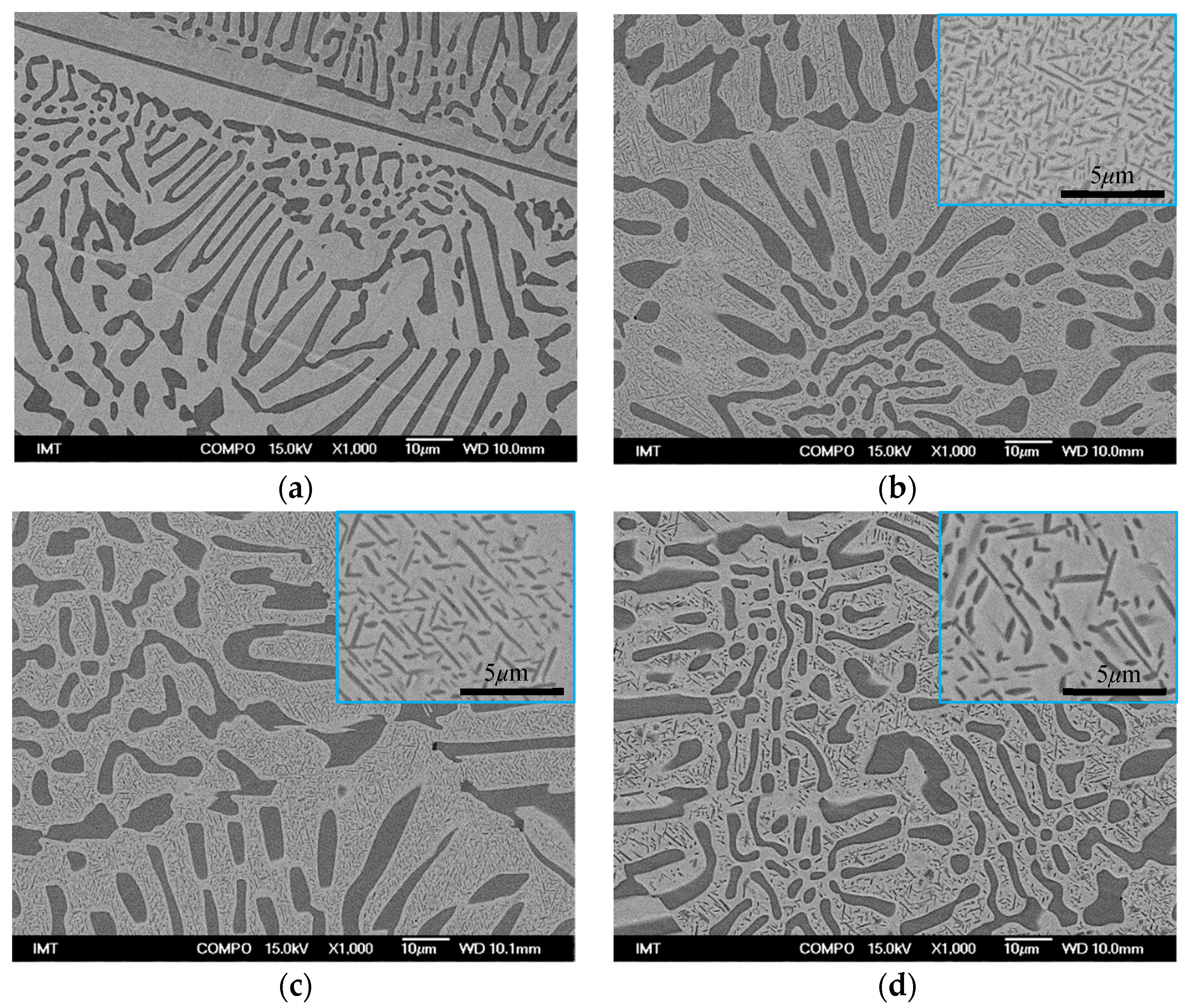
3.1. Microstructure and Hardness
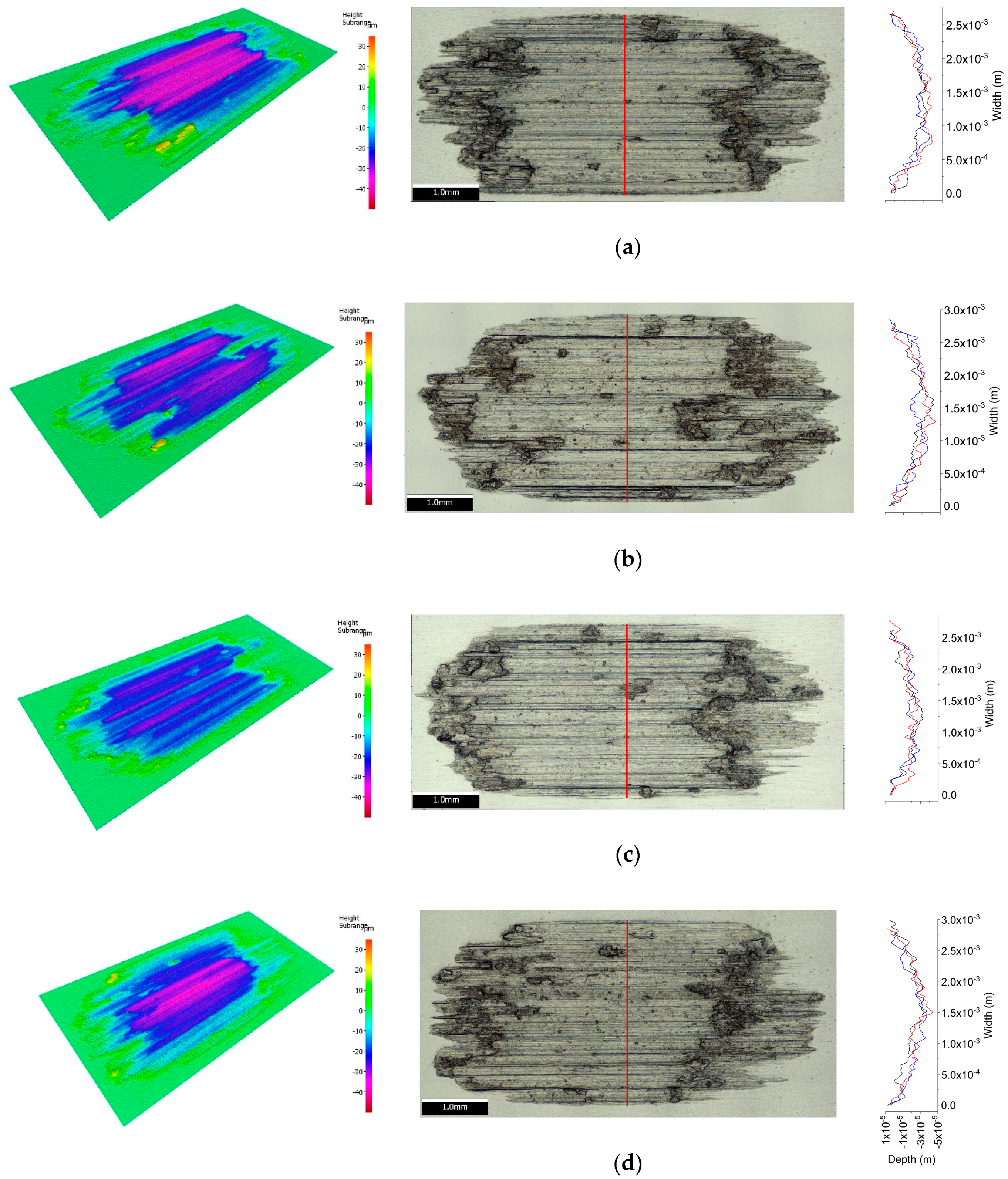
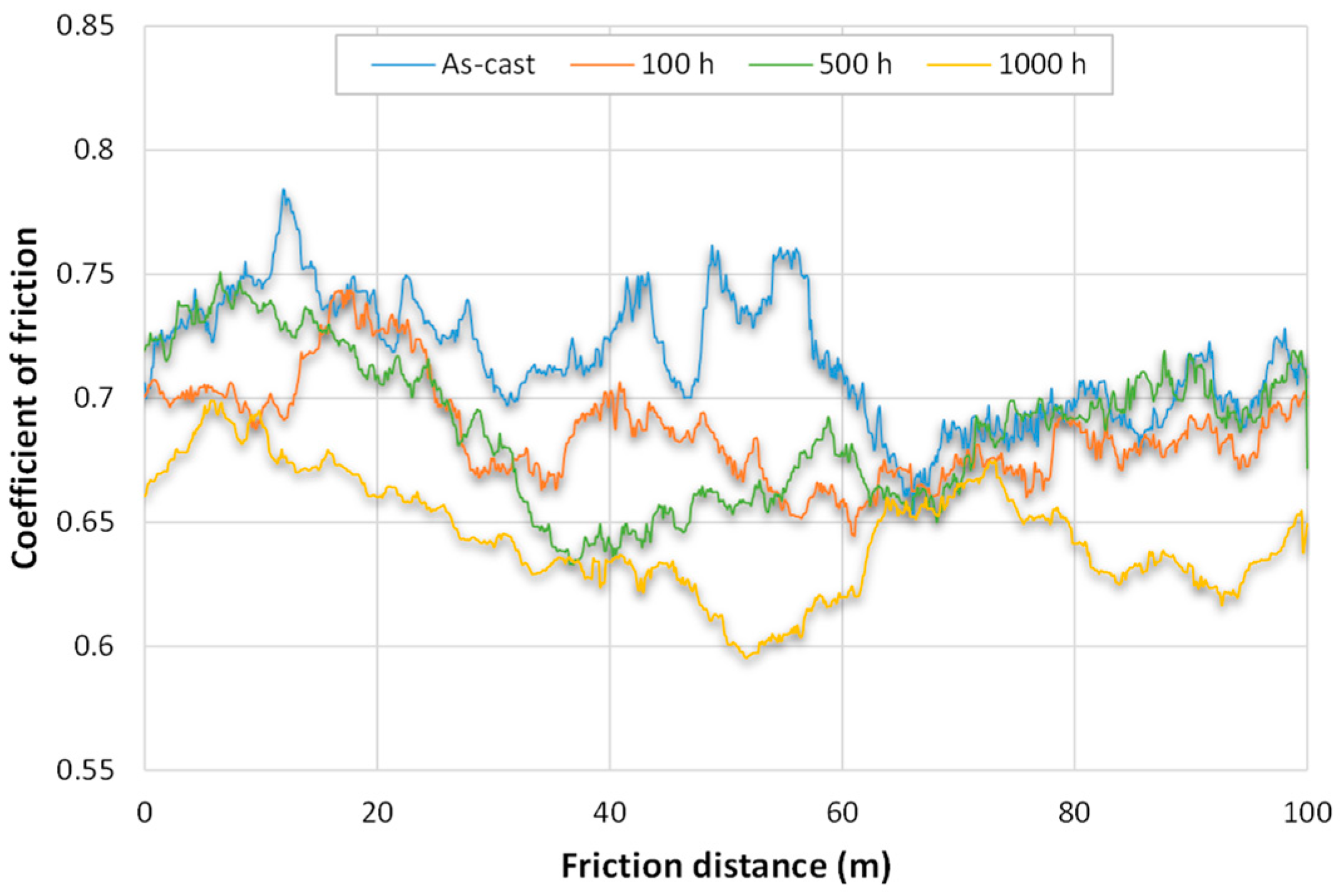
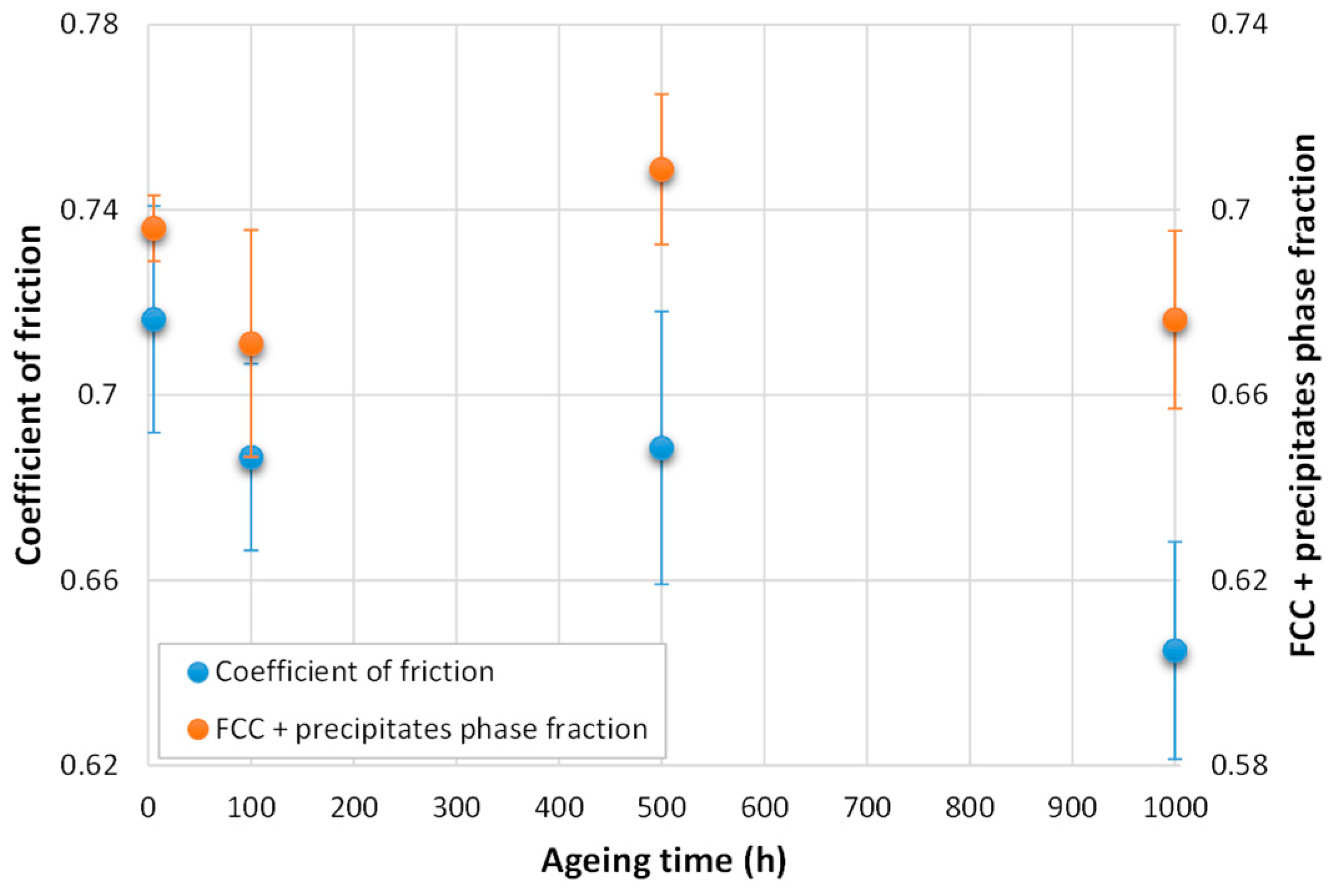
3.2. Wear Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. npj Mater Degrad 2017, 1, 15. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Grewal, H.; Arora, H.; Mukherjee, S. Corrosion, Erosion and Wear Behavior of Complex Concentrated Alloys: A Review. Metals 2018, 8, 603. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, M.-R.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Chang, S.-Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall Mat Trans A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Du, L.M.; Lan, L.W.; Zhu, S.; Yang, H.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Effects of temperature on the tribological behavior of Al0.25CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 917–925. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Pippig, R.; Lampke, T. High-Temperature Wear Behaviour of Spark Plasma Sintered AlCoCrFeNiTi0.5 High-Entropy Alloy. Entropy 2019, 21, 582. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A promising new class of high-temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, X.; Jiang, L.; Chen, Z.; Wang, T.; Jie, J.; Kang, H.; Zhang, Y.; Guo, S.; Ruan, H.; et al. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Zhang, B.; Liang, N.; Wu, G.; Sha, G.; Liu, J.; Zhao, Y. Microstructural origins of high strength and high ductility in an AlCoCrFeNi 2.1 eutectic high-entropy alloy. Acta Mater. 2017, 141, 59–66. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Wani, I.S.; Sheikh, S.; Clark, I.T.; Okawa, T.; Guo, S.; Bhattacharjee, P.P.; Tsuji, N. Simultaneous Strength-Ductility Enhancement of a Nano-Lamellar AlCoCrFeNi2.1 Eutectic High Entropy Alloy by Cryo-Rolling and Annealing. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.; Zheng, R.; Chong, Y.; Sheikh, S.; Guo, S.; Clark, I.T.; Okawa, T.; Wani, I.S.; Bhattacharjee, P.P.; Shibata, A.; et al. Effect of low temperature on tensile properties of AlCoCrFeNi2.1 eutectic high entropy alloy. Mater. Chem. Phys. 2018, 210, 207–212. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Lud, Y.P.; Chatterjee, S.; Bhattacharjeea, P.P.; Guo, S.; Tsujib, N. Ultrafine-grained AlCoCrFeNi2.1 eutectic high-entropy alloy. Mater. Res. Lett. 2016, 4, 174–179. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Tailoring nanostructures and mechanical properties of AlCoCrFeNi2.1 eutectic high entropy alloy using thermo-mechanical processing. Mater. Sci. Eng. A 2016, 675, 99–109. [Google Scholar] [CrossRef]
- Li, P.; Sun, H.; Wang, S.; Hao, X.; Dong, H. Rotary friction welding of AlCoCrFeNi2.1 eutectic high entropy alloy. J. Alloys Compd. 2020, 814, 152322. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Ayyagari, A.V.; Muskeri, S.; Salloom, R.; Mukherjee, S. Surface degradation mechanisms in a eutectic high entropy alloy at microstructural length-scales and correlation with phase-specific work function. npj Materials Degradation 2019, 3, 16. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Meth. 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Clark, I.T.; Park, M.H.; Okawa, T.; Guo, S.; Bhattacharjee, P.P.; Tsuji, N. Cold-rolling and recrystallization textures of a nano-lamellar AlCoCrFeNi2.1 eutectic high entropy alloy. Intermetallics 2017, 84, 42–51. [Google Scholar] [CrossRef]

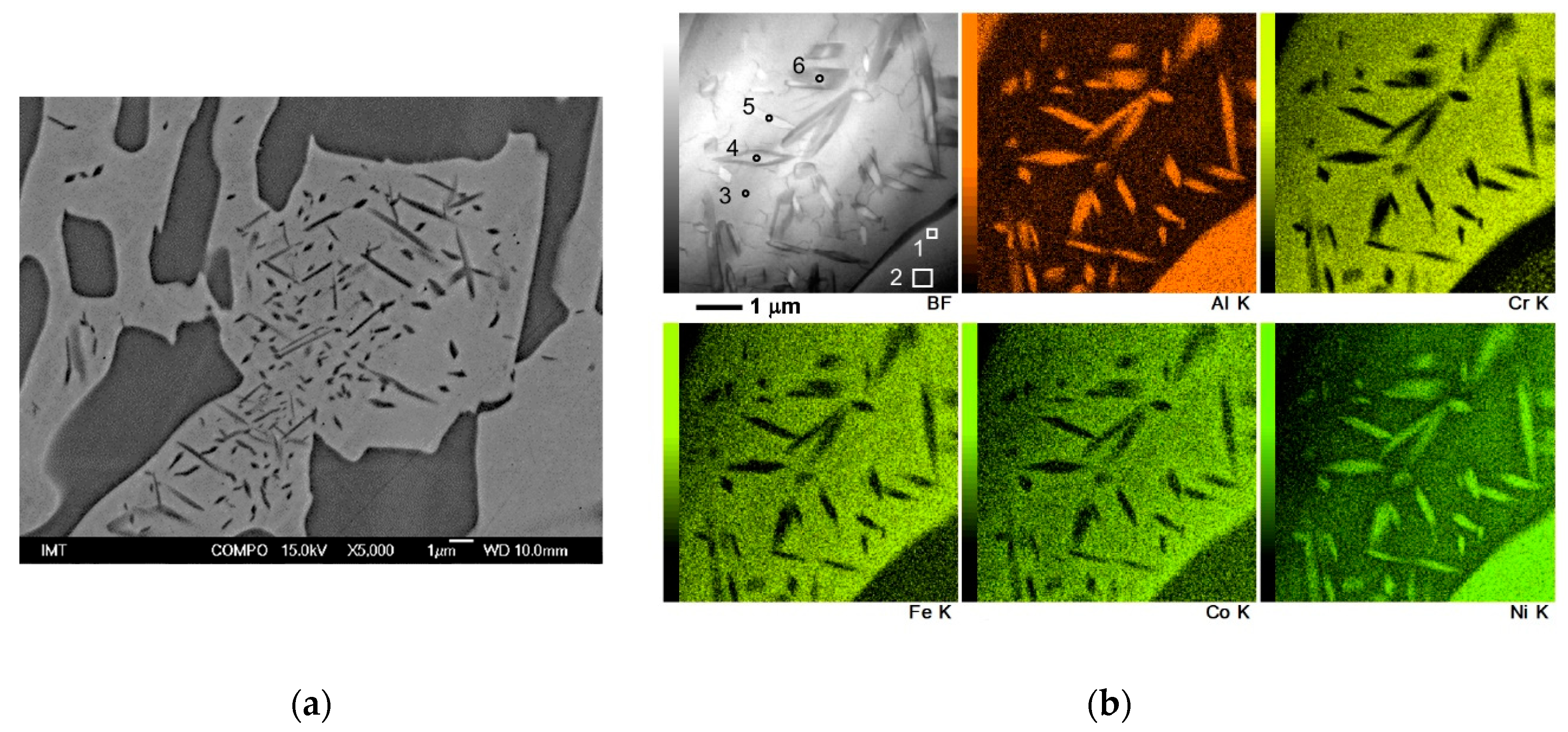


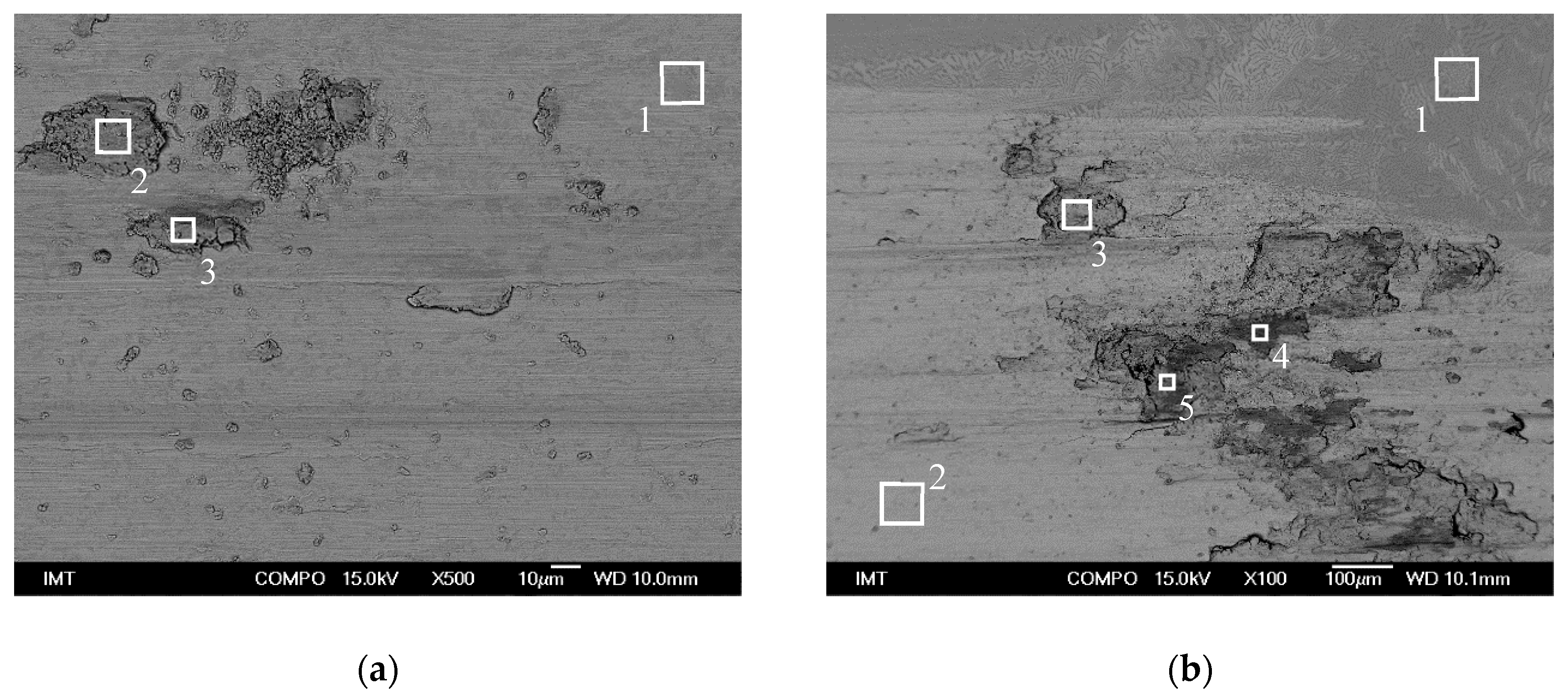

| Spectrum | Al | Co | Cr | Fe | Ni | Total |
|---|---|---|---|---|---|---|
| 1 | 13.4 | 13.8 | 4.9 | 9.4 | 58.4 | 100 |
| 2 | 13.2 | 12.7 | 8.7 | 10.8 | 54.6 | 100 |
| 3 | 3.5 | 18.3 | 22.6 | 20.4 | 35.3 | 100 |
| 4 | 19.8 | 10.1 | 3.7 | 8.7 | 57.8 | 100 |
| 5 | 18.6 | 11.1 | 5.2 | 9.7 | 55.3 | 100 |
| 6 | 18.6 | 11.1 | 4.2 | 9.5 | 56.6 | 100 |
| Spectrum | O | Al | Cr | Fe | Co | Ni | Total |
|---|---|---|---|---|---|---|---|
| 1 | 0.0 | 24.1 | 12.3 | 13.0 | 12.7 | 37.8 | 100 |
| 2 | 29.0 | 9.6 | 11.6 | 20.2 | 9.4 | 20.1 | 100 |
| 3 | 23.9 | 8.7 | 13.4 | 24.9 | 9.3 | 19.9 | 100 |
| Spectrum | O | Al | Si | Cr | Mn | Fe | Co | Ni | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.9 | 16.2 | 0.0 | 15.1 | 0.0 | 15.9 | 16.6 | 34.3 | 100 |
| 2 | 0.0 | 15.6 | 0.0 | 15.9 | 0.0 | 16.6 | 17.1 | 34.8 | 100 |
| 3 | 15.0 | 3.3 | 0.6 | 16.3 | 0.9 | 48.9 | 3.5 | 11.5 | 100 |
| 4 | 47.6 | 3.0 | 0.4 | 9.9 | 0.5 | 26.4 | 3.8 | 8.4 | 100 |
| 5 | 44.1 | 4.2 | 0.5 | 10.7 | 0.4 | 25.7 | 4.4 | 10.0 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafexhiu, F.; Podgornik, B.; Feizpour, D. Tribological Behavior of As-Cast and Aged AlCoCrFeNi2.1 CCA. Metals 2020, 10, 208. https://doi.org/10.3390/met10020208
Kafexhiu F, Podgornik B, Feizpour D. Tribological Behavior of As-Cast and Aged AlCoCrFeNi2.1 CCA. Metals. 2020; 10(2):208. https://doi.org/10.3390/met10020208
Chicago/Turabian StyleKafexhiu, Fevzi, Bojan Podgornik, and Darja Feizpour. 2020. "Tribological Behavior of As-Cast and Aged AlCoCrFeNi2.1 CCA" Metals 10, no. 2: 208. https://doi.org/10.3390/met10020208
APA StyleKafexhiu, F., Podgornik, B., & Feizpour, D. (2020). Tribological Behavior of As-Cast and Aged AlCoCrFeNi2.1 CCA. Metals, 10(2), 208. https://doi.org/10.3390/met10020208







