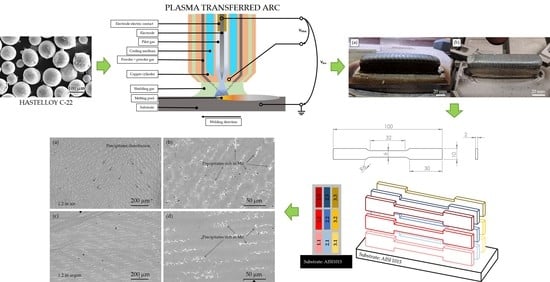
Processing by Additive Manufacturing Based on Plasma Transferred Arc of Hastelloy in Air and Argon Atmosphere
Abstract
1. Introduction
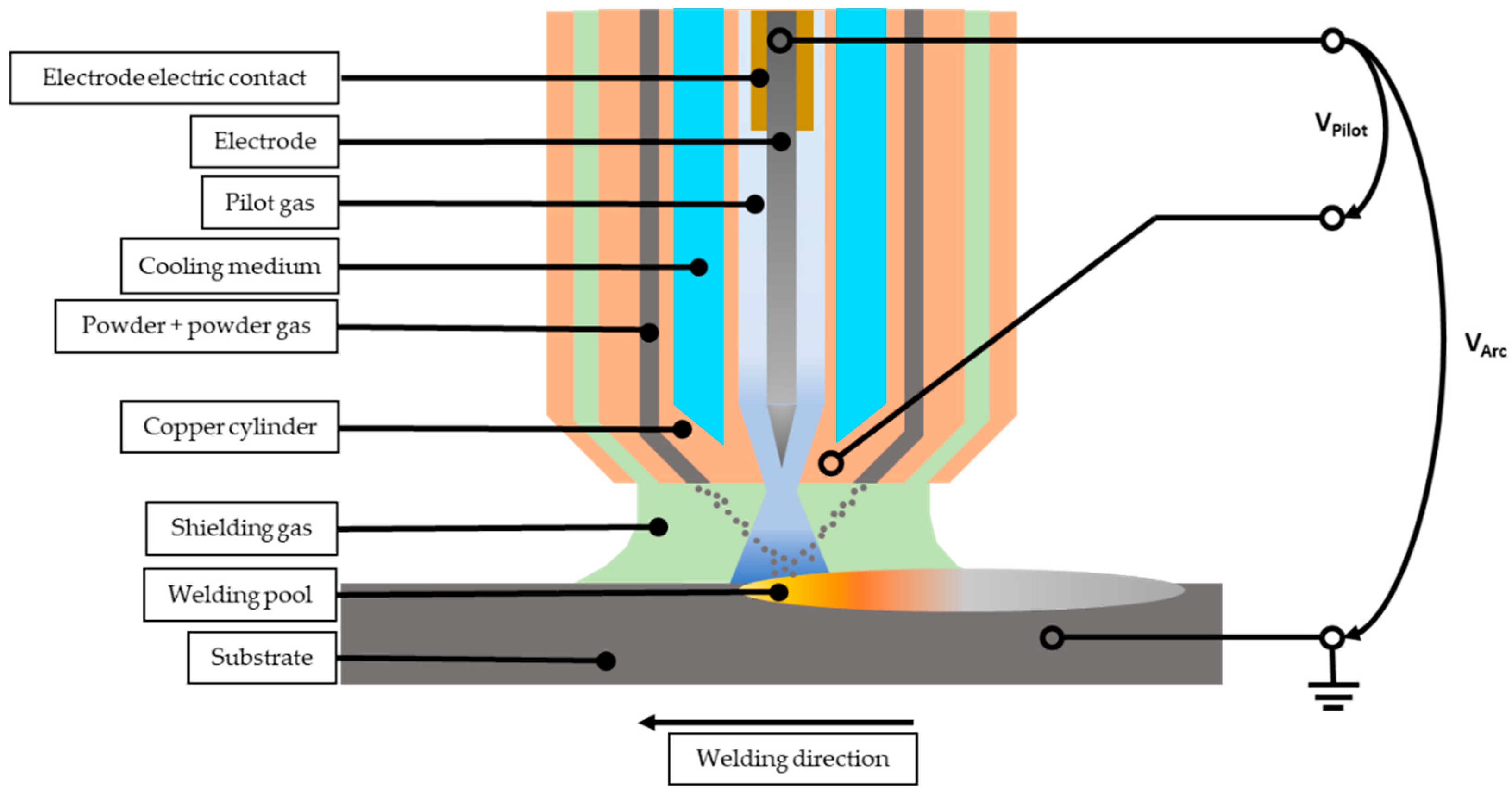
2. Materials and Methods
3. Results
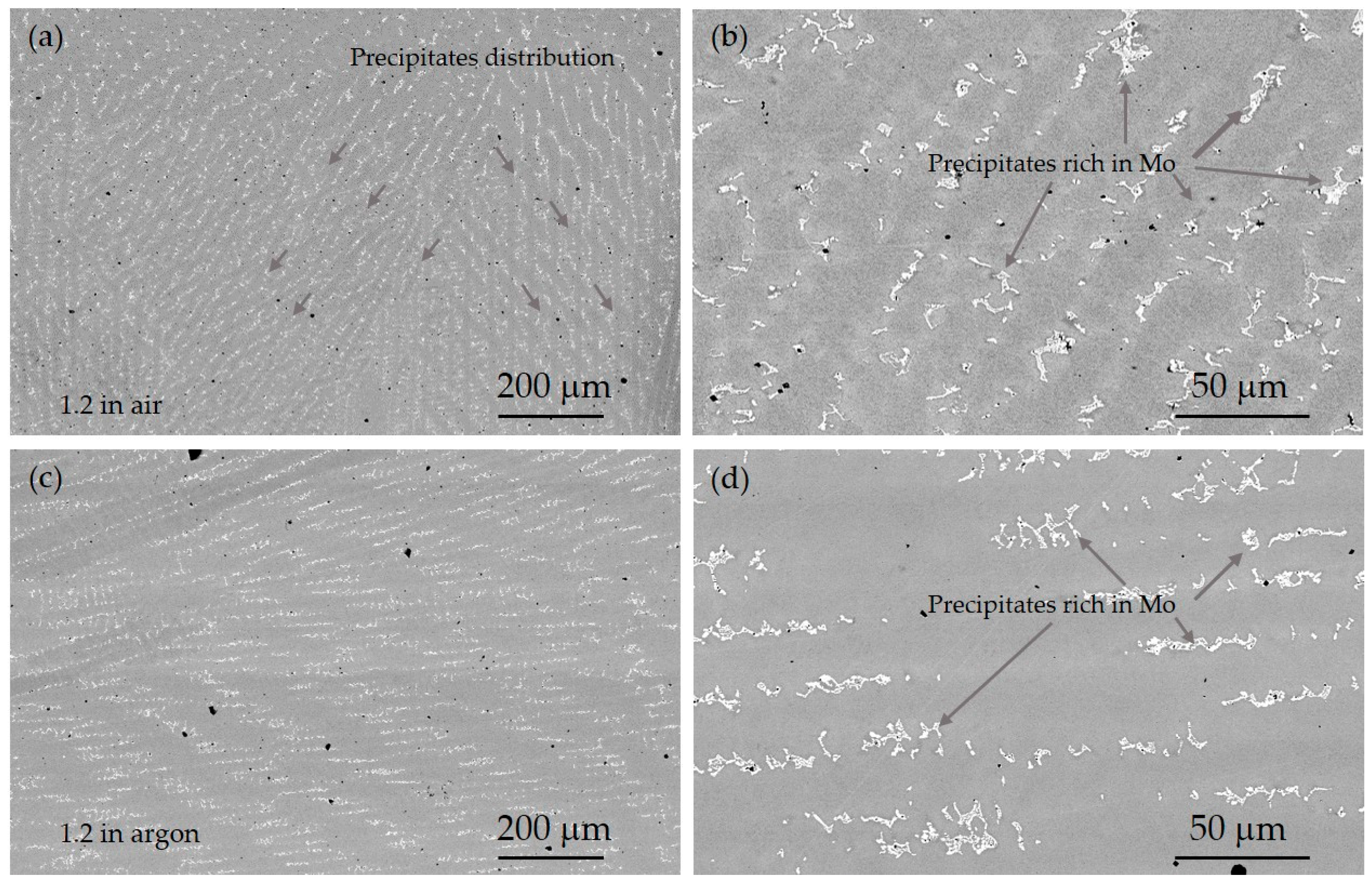
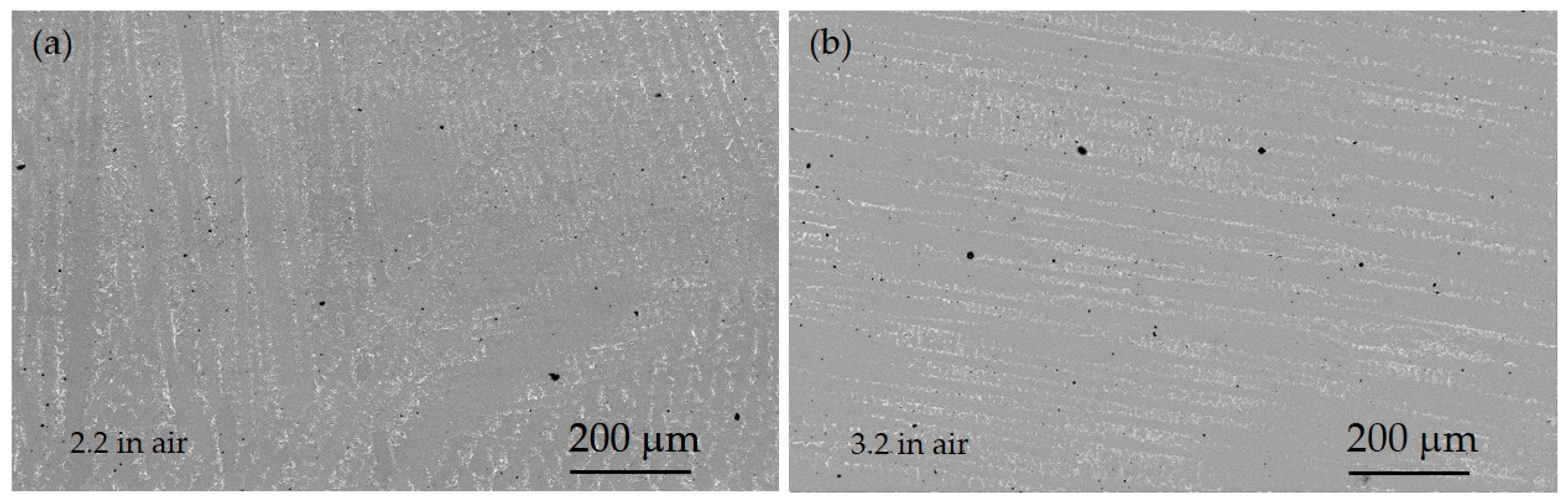
3.1. Microstructural Study and XRD Analysis
3.2. Mechanical Properties and Wear Behavior
4. Discussion
5. Conclusions
- Two walls from Hastelloy with large dimensions could be produced by plasma transferred arc in air or argon conditions.
- The atmosphere during the additive process presented lower impact on the final behavior than expected. The results of the thorough characterization of the specimens stated that their mechanical properties, as built, achieved values close to the standard ones, independently of the atmospheric conditions during the manufacturing.
- There was no effect of the thermal treatments in the final behavior of the specimens. The heat treatments were insufficient to modify and to improve the mechanical properties.
- In general, there were observed Mo-rich precipitates in all the specimens caused by processing; variations of the distribution of theses precipitates could be related to the mechanical properties measured.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Lane, B.; Whiting, J.; Chou, K. On thermal properties of metallic powder in laser powder bed fusion additive manufacturing. J. Manuf. Process. 2019, 47, 382–392. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basil, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharmaceut. 2017, 529, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ríos, S.; Colegrove, P.A.; Williams, S.W. Metal transfer modes in plasma Wire + Arc additive manufacture. J. Mater. Process. Technol. 2019, 264, 45–54. [Google Scholar] [CrossRef]
- Barros, R.; Silva, F.J.G.; Gouveia, R.M.; Saboori, A.; Marchese, G.; Biamino, S.; Salmi, A.; Atzeni, E. Laser powder bed fusion of Inconel 718: Residual stress analysis before and after heat treatment. Metals 2019, 9, 1290. [Google Scholar] [CrossRef]
- Waqas, A.; Qin, X.; Xiong, J.; Zheng, C.; Wang, H. Analysis of ductile fracture obtained by charpy impact test of a steel structure created by robot-assisted GMAW-based additive manufacturing. Metals 2019, 9, 1208. [Google Scholar] [CrossRef]
- Herderick, E. Additive manufacturing of metals: A review. Mater. Sci. Technol. Conf. Exhib. 2011, 2, 1413–1425. [Google Scholar]
- Gibson, I. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: Berlin, Germany, 2015. [Google Scholar]
- Vály, L.; Grech, D.; Neubauer, E.; Kitzmantel, M.; Baca, L.; Stelzer, N. Preparation of titanium metal matrix composites using additive manufacturing. Key Eng. Mater. 2017, 742, 129–136. [Google Scholar] [CrossRef]
- Mercado Rojas, J.G.; Wolfe, T.; Fleck, B.A.; Qureshi, A.J. Plasma transferred arc additive manufacturing of Nickel metal matrix composites. Manuf. Lett. 2018, 18, 31–34. [Google Scholar] [CrossRef]
- Sharples, R.V. The Plasma Transferred Arc (PTA) Weld. Surfacing Process; Welding Institute: Cambridge, UK, 1985. [Google Scholar]
- Alberti, E.A.; Bueno, B.M.P.; D’Oliveira, A.S.C.M. Additive manufacturing using plasma transferred arc. Int. J. Adv. Manuf. Technol. 2016, 83, 1861–1871. [Google Scholar] [CrossRef]
- Dinovitzer, M.; Chen, X.; Laliberte, J.; Huang, X.; Frei, H. Effect of wire and arc additive manufacturing (WAAM) process parameters on bead geometry and microstructure. Addit. Manuf. 2019, 26, 138–146. [Google Scholar] [CrossRef]
- ISO/ASTM 52900:2015 (ASTM F2792). Additive manufacturing-General principles-Terminology. International Organization for Standardization. Available online: https://www.iso.org/standard/69669.html (accessed on 14 January 2020).
- Hoefer, K.; Mayr, P. Additive Manufacturing of titanium parts using 3D plasma metal deposition. Mater. Sci. Forum 2018, 941, 2137–2141. [Google Scholar] [CrossRef]
- Rodriguez, J.; Hoefer, K.; Haelsig, A.; Mayr, P. Functionally graded SS 316L to Ni-based structures produced by 3D plasma metal deposition. Metals 2019, 9, 620. [Google Scholar] [CrossRef]
- Tabernero, I.; Paskual, A.; Álvarez, P.; Suárez, A. Study on arc welding processes for high deposition rate additive manufacturing. Procedia CIRP 2018, 68, 358–362. [Google Scholar] [CrossRef]
- Ho, A.; Zhao, H.; Fellowes, J.W.; Martina, F.; Davis, A.E.; Prangnell, P.B. On the origin of microstructural banding in Ti-6Al4V wire-arc based high deposition rate additive manufacturing. Acta Mater. 2019, 166, 306–323. [Google Scholar] [CrossRef]
- Chen, L.; Bai, S.L.; Ge, Y.Y.; Wang, Q.Y. Erosion-corrosion behaviour and electrochemical performance of Hastelloy C22 coatings under impingement. Appl. Surf. Sci. 2018, 456, 985–998. [Google Scholar] [CrossRef]
- Chen, L.; Bai, S.L. The anti-corrosion behaviour under multi-factor impingement of Hastelloy C22 coating prepared by multilayer laser cladding. Appl. Surf. Sci. 2018, 437, 1–12. [Google Scholar] [CrossRef]
- Han, Q.; Mertens, R.; Montero-Sistiaga, M.L.; Yang, S.; Setchi, R.; Vanmeensel, K. Laser powder bed fusion of Hastelloy X: Effects of hot isostatic pressing and the hot cracking mechanism. Mater. Sci. Eng. A Struct. 2018, 732, 228–239. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Bai, S.L.; Zhao, Y.H.; Liu, Z.D. Effect of mechanical polishing on corrosion behavior of Hastelloy C22 coating prepared by high power diode laser cladding. Appl. Surf. Sci. 2014, 303, 312–318. [Google Scholar] [CrossRef]
- Molin, S.; Dunst, K.J.; Karczewski, J.; Jasinski, P. High temperature corrosion evaluation of porous Hastelloy X alloy in air and humidified hydrogen atmospheres. J. Electrochem. Soc. 2016, 163, C296–C302. [Google Scholar] [CrossRef]
- Tu, R.; Goto, T. Corrosion behavior of Hastelloy-XR alloy in O2 and SO2 atmosphere. Mater. Trans. 2005, 46, 1882–1889. [Google Scholar] [CrossRef]
- Hussain, N.; Qureshi, A.H.; Shahid, K.A.; Chugtai, N.A.; Khalid, F.A. High-temperature oxidation Behavior of Hastelloy C-4 in steam. Oxid. Met. 2004, 61, 355–364. [Google Scholar] [CrossRef]
- Bal, K.S.; Majumdar, J.D.; Choudhury, A.R. Effect of post-weld heat treatment on the tensile strength of laser beam welded Hastelloy C-276 sheets at different heat inputs. J. Manuf. Process. 2019, 37, 578–594. [Google Scholar] [CrossRef]
- Wang, F. Mechanical property study on rapid additive layer manufacture Hastelloy X alloy by selective laser melting technology. Int. J. Adv. Manuf. Technol. 2012, 58, 545–551. [Google Scholar] [CrossRef]
- ASTM B574. ASTM and SAE-AMS Standards and Specifications for Nickel Based Alloys; ASTM: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Montero-Sistiaga, M.L.; Pourbabak, S.; Van Humbeeck, J.; Schryvers, D.; Vanmeensel, K. Microstructure and mechanical properties of Hastelloy X produced by HP-SLM (high power selective laser melting). Mater. Des. 2019, 165, 107598. [Google Scholar] [CrossRef]
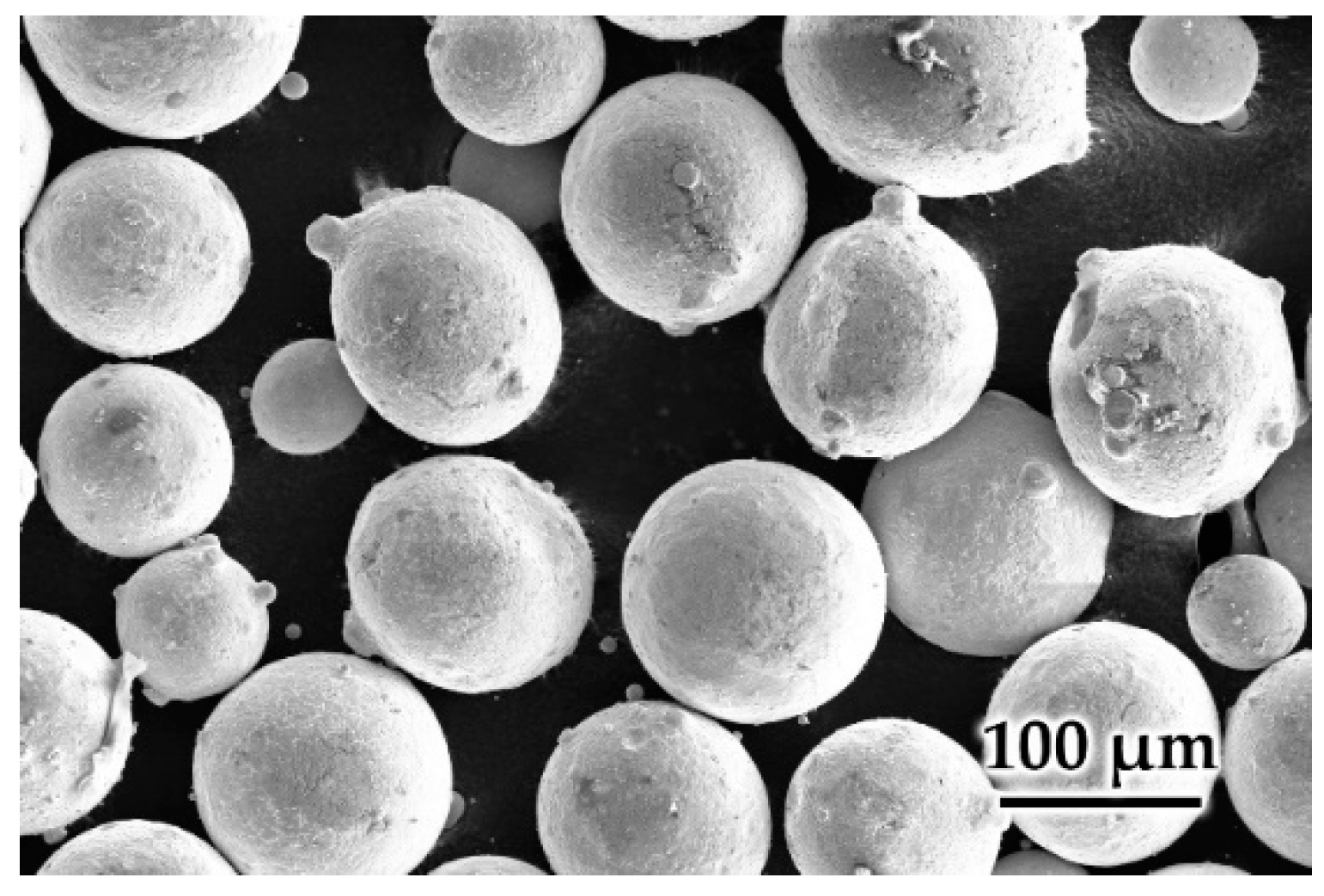

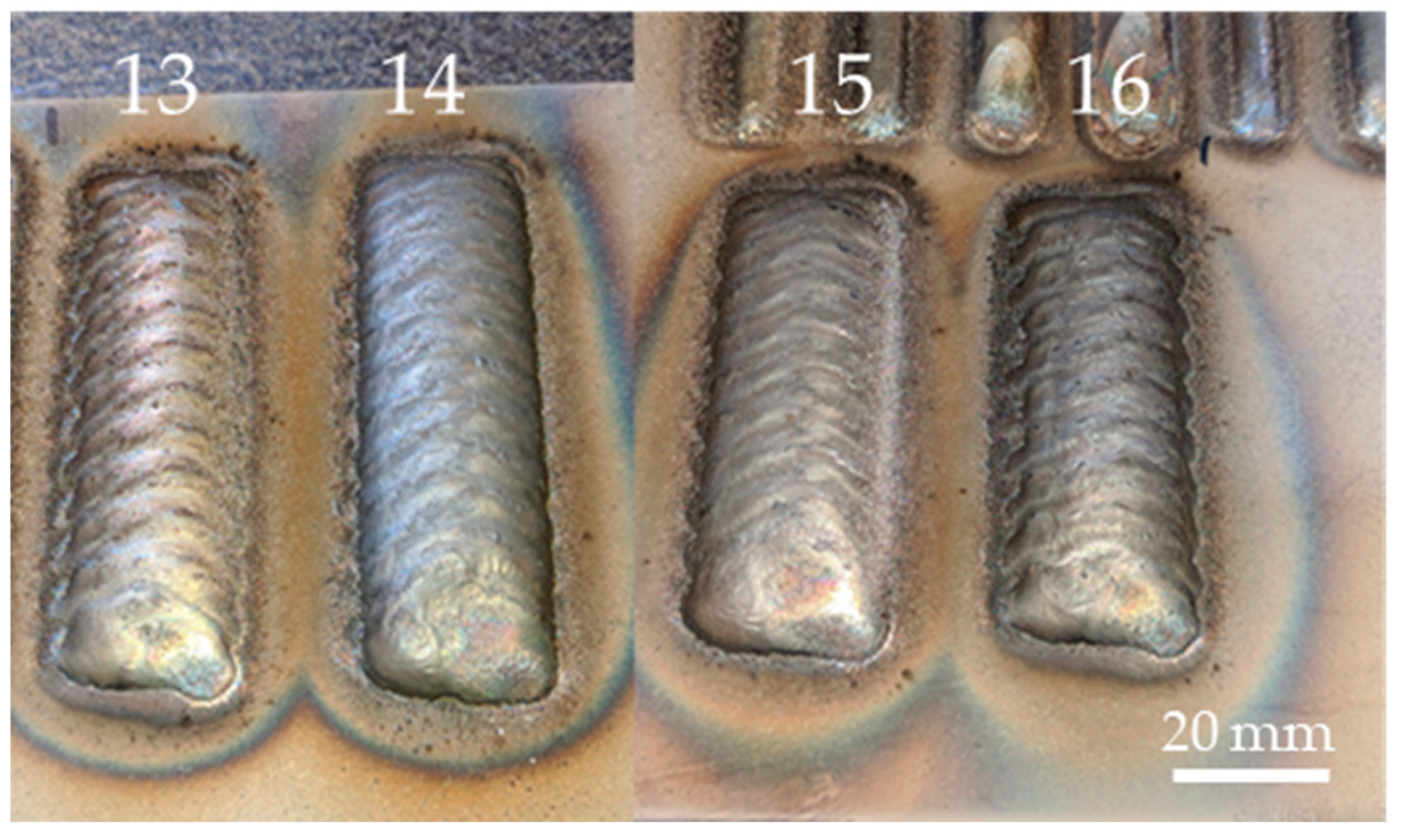

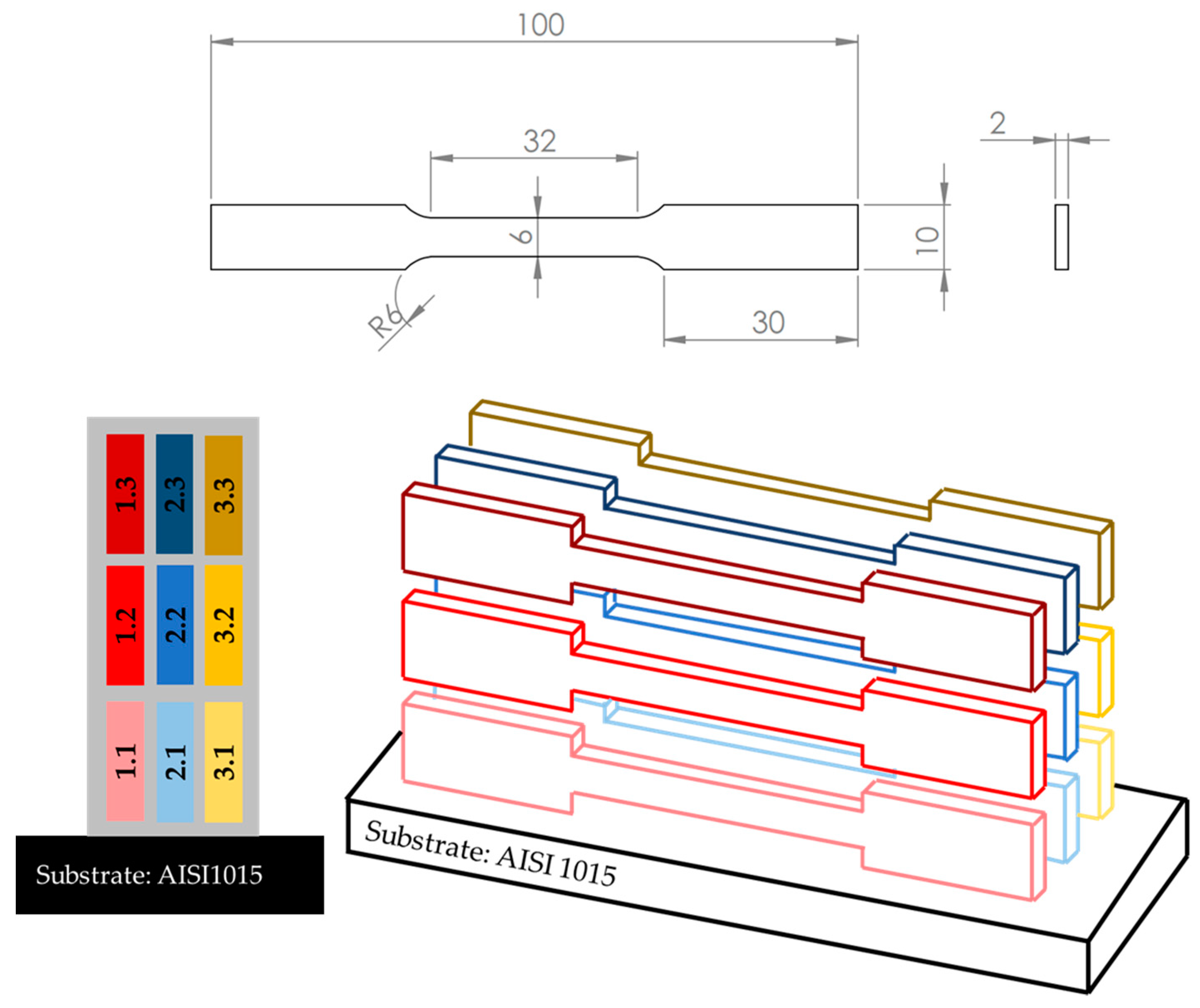

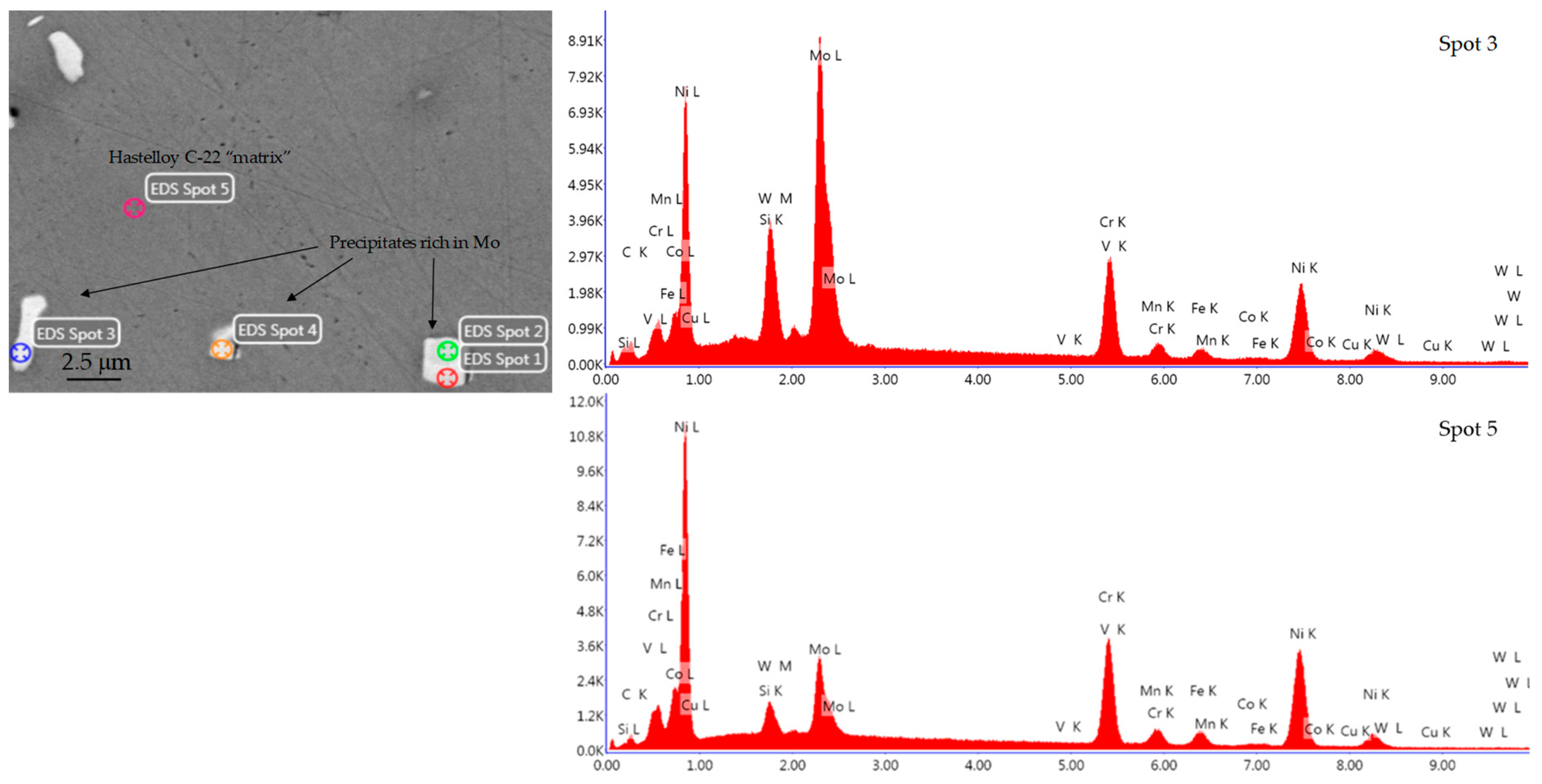
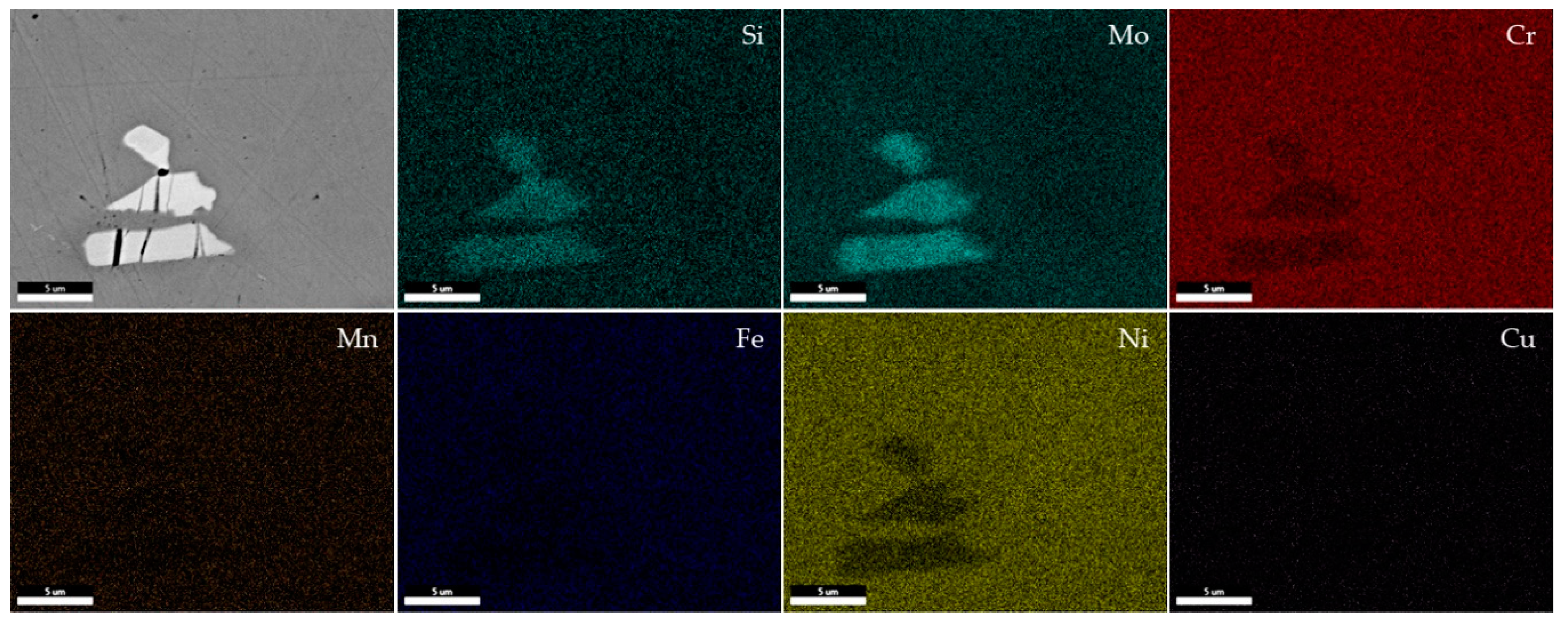



| Element | Supplied Hastelloy C-22 [%wt.] | Standard Hastelloy C-22 [%wt.] |
|---|---|---|
| Ni | Bal. | Bal. |
| C | 0.007 | max. 0.015 |
| Co | 0.40 | max. 2.50 |
| Cr | 22.11 | 20.00–22.50 |
| Fe | 5.00 | 2.00–6.00 |
| Mn | 0.65 | max. 0.50 |
| Mo | 12.37 | 12.50–14.50 |
| Si | 0.91 | max. 0.08 |
| V | <0.02 | max. 0.35 |
| W | 3.63 | 2.50–4.00 |
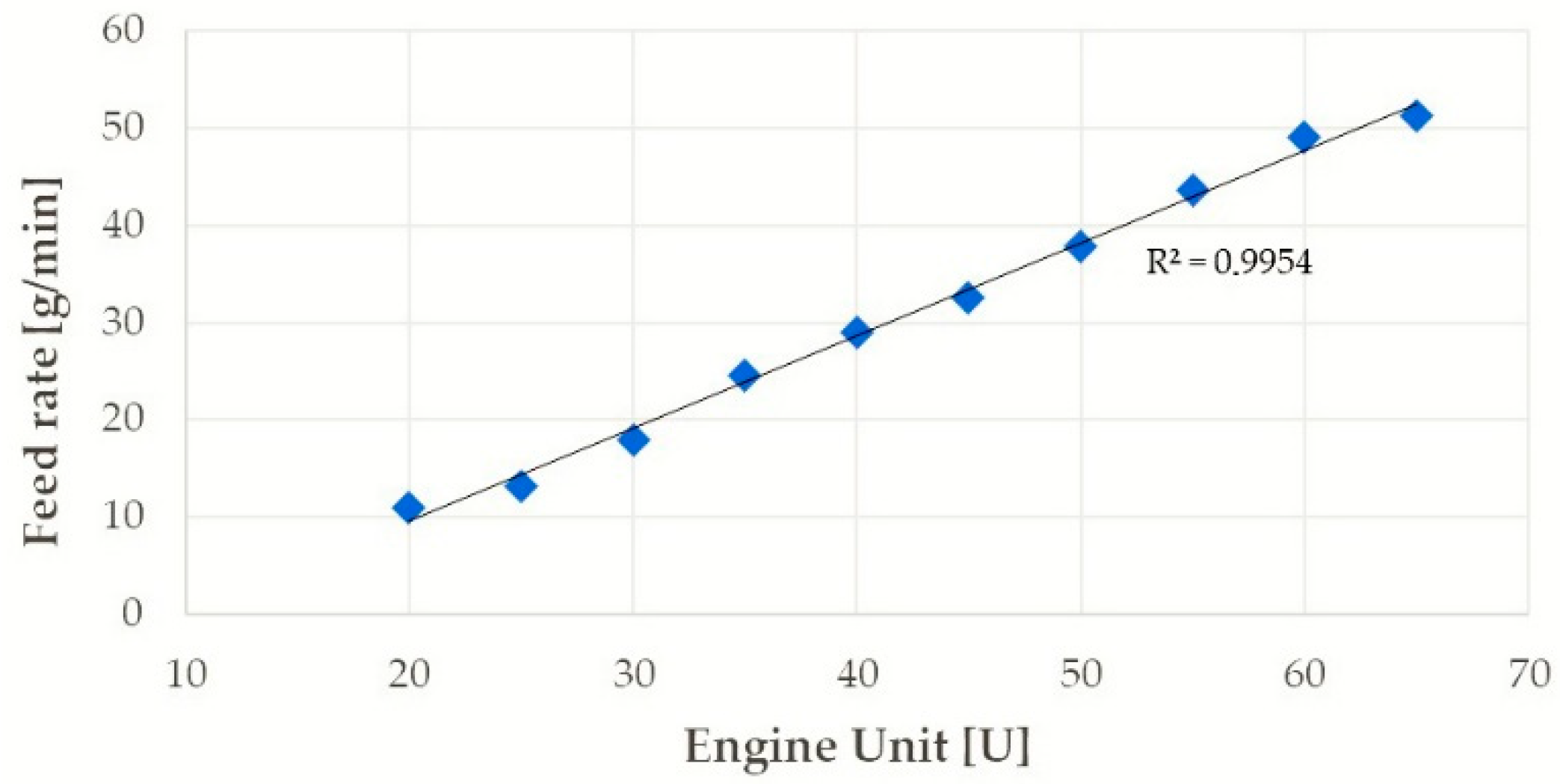
| Layer | Current [A] | Travel Speed [mm/min] | Feed Rate [g/min] |
|---|---|---|---|
| 1 | 120 | 200 | 13.5 |
| 2 | 140 | 200 | 13.5 |
| 3 | 180 | 200 | 13.5 |
| 4 | 220 | 200 | 13.5 |
| 5 | 120 | 200 | 11.0 |
| 6 | 120 | 100 | 11.0 |
| 7 | 140 | 100 | 11.0 |
| 8 | 160 | 100 | 11.0 |
| 9 | 140 | 300 | 29.0 |
| 10 | 180 | 300 | 29.0 |
| 11 | 220 | 300 | 29.0 |
| 12 | 250 | 400 | 43.5 |
| 13 | 140 | 300 | 11.0 |
| 14 | 180 | 400 | 29.0 |
| 15 | 180 | 400 | 24.5 |
| 16 | 140 | 300 | 13.5 |
| Specimen | Layer Number | Current [A] | Travel Speed [mm/min] | Feed Rate [g/min] |
|---|---|---|---|---|
| Two Hastelloy walls produced in air and in argon | 1 | 180 | 400 | 29.0 |
| 2 | 160 | 500 | 24.5 | |
| 3–9 | 150 | 600 | 18.0 | |
| 10–16 | 150 | 700 | 13.5 |
| Element | Spot 5 [%wt.] | Spot 3 in a Precipitate [%wt.] |
|---|---|---|
| C K | 4.46 | 6.30 |
| Si K | 0.84 | 2.53 |
| W M | 1.81 | 5.63 |
| Mo L | 8.73 | 28.70 |
| V K | 0.11 | 0.07 |
| Cr K | 22.04 | 17.80 |
| Mn K | 1.43 | 0.79 |
| Fe K | 4.70 | 2.79 |
| Co K | 0.68 | 0.45 |
| Ni K | 54.41 | 34.59 |
| Cu K | 0.80 | 0.36 |
| Specimen | Treatment | E [GPa] | Yield Strength [MPa] | UTS [MPa] | Deformation [%] |
|---|---|---|---|---|---|
| 1.1 | As built | 209.30 | 352.52 | 611.84 | 16.59 |
| 1.2 | 201.23 | 334.46 | 650.37 | 24.04 | |
| 1.3 | 181.23 | 309.97 | 516.84 | 9.32 | |
| Average set 1 | 197.49 | 332.32 | 593.02 | 16.65 | |
| 2.1 | TT1 | 158.21 | 307.42 | 715.87 | 13.18 |
| 2.2 | 135.33 | 271.24 | 717.40 | 15.74 | |
| 2.3 | 147.47 | 295.84 | 547.38 | 3.89 | |
| Average set 2 | 147.00 | 291.50 | 660.22 | 10.94 | |
| 3.1 | TT2 | 183.64 | 298.92 | 690.13 | 10.89 |
| 3.2 | 200.45 | 307.52 | 686.08 | 12.29 | |
| 3.3 | 218.20 | 303.95 | 556.75 | 4.31 | |
| Average set 3 | 200.76 | 303.46 | 644.32 | 9.17 |
| Specimen | Treatment | E [GPa] | Yield Strength [MPa] | UTS [MPa] | Deformation [%] |
|---|---|---|---|---|---|
| 1.1 | As built | 167.18 | 347.97 | 620.20 | 23.46 |
| 1.2 | 137.53 | 336.75 | 516.90 | 11.51 | |
| 1.3 | 130.11 | 329.71 | 513.90 | 12.26 | |
| Average set 1 | 144.94 | 338.14 | 550.33 | 15.74 | |
| 2.1 | TT1 | 131.23 | 348.52 | 711.72 | 15.22 |
| 2.2 | 130.92 | 350.08 | 646.63 | 9.39 | |
| 2.3 | 155.37 | 366.25 | 625.52 | 7.96 | |
| Average set 2 | 139.18 | 354.95 | 661.29 | 10.86 | |
| 3.1 | TT2 | 161.62 | 365.35 | 628.71 | 7.04 |
| 3.2 | 152.37 | 349.25 | 604.39 | 6.86 | |
| 3.3 | 135.85 | 337.09 | 625.98 | 10.50 | |
| Average set 3 | 149.95 | 350.56 | 619.69 | 8.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Soriano, E.M.; Ariza, E.; Arevalo, C.; Montealegre-Melendez, I.; Kitzmantel, M.; Neubauer, E. Processing by Additive Manufacturing Based on Plasma Transferred Arc of Hastelloy in Air and Argon Atmosphere. Metals 2020, 10, 200. https://doi.org/10.3390/met10020200
Perez-Soriano EM, Ariza E, Arevalo C, Montealegre-Melendez I, Kitzmantel M, Neubauer E. Processing by Additive Manufacturing Based on Plasma Transferred Arc of Hastelloy in Air and Argon Atmosphere. Metals. 2020; 10(2):200. https://doi.org/10.3390/met10020200
Chicago/Turabian StylePerez-Soriano, Eva M., Enrique Ariza, Cristina Arevalo, Isabel Montealegre-Melendez, Michael Kitzmantel, and Erich Neubauer. 2020. "Processing by Additive Manufacturing Based on Plasma Transferred Arc of Hastelloy in Air and Argon Atmosphere" Metals 10, no. 2: 200. https://doi.org/10.3390/met10020200
APA StylePerez-Soriano, E. M., Ariza, E., Arevalo, C., Montealegre-Melendez, I., Kitzmantel, M., & Neubauer, E. (2020). Processing by Additive Manufacturing Based on Plasma Transferred Arc of Hastelloy in Air and Argon Atmosphere. Metals, 10(2), 200. https://doi.org/10.3390/met10020200