Atomic-Scale Mechanism Investigation of Mass Transfer in Laser Fabrication Process of Ti-Al Alloy via Molecular Dynamics Simulation
Abstract
1. Introduction
2. Simulation Method
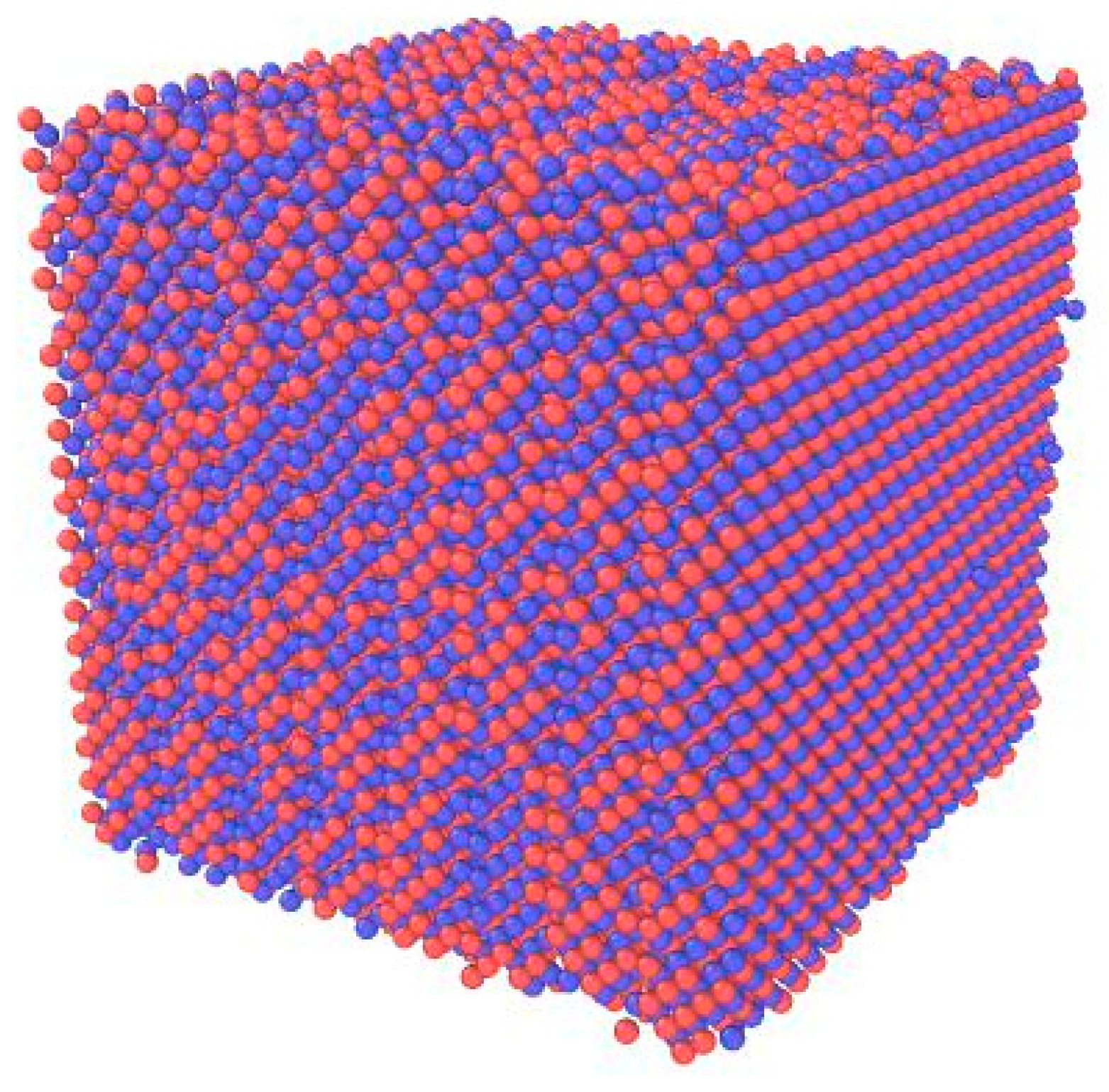
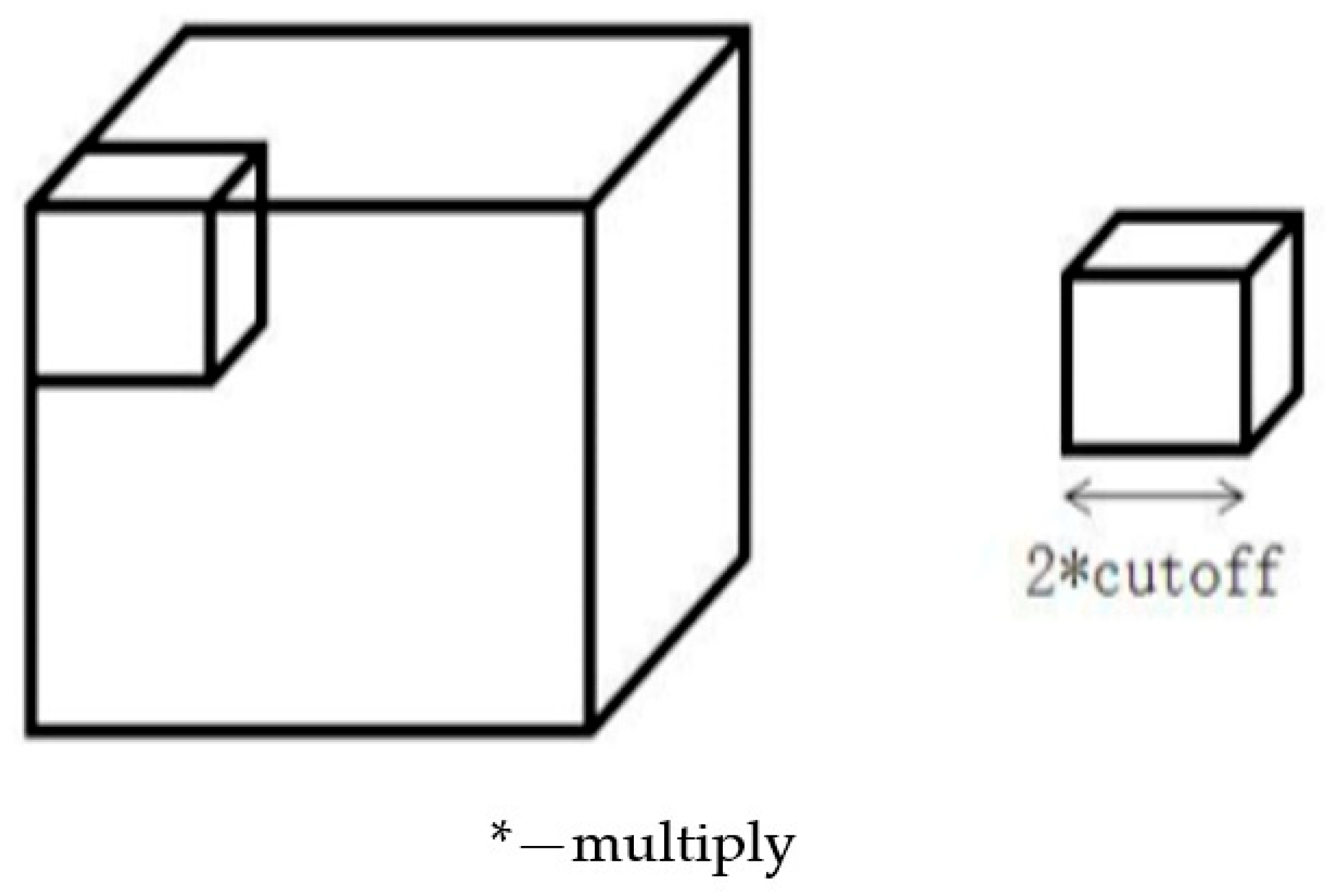
2.1. Ti-Al Cubic Simulation System
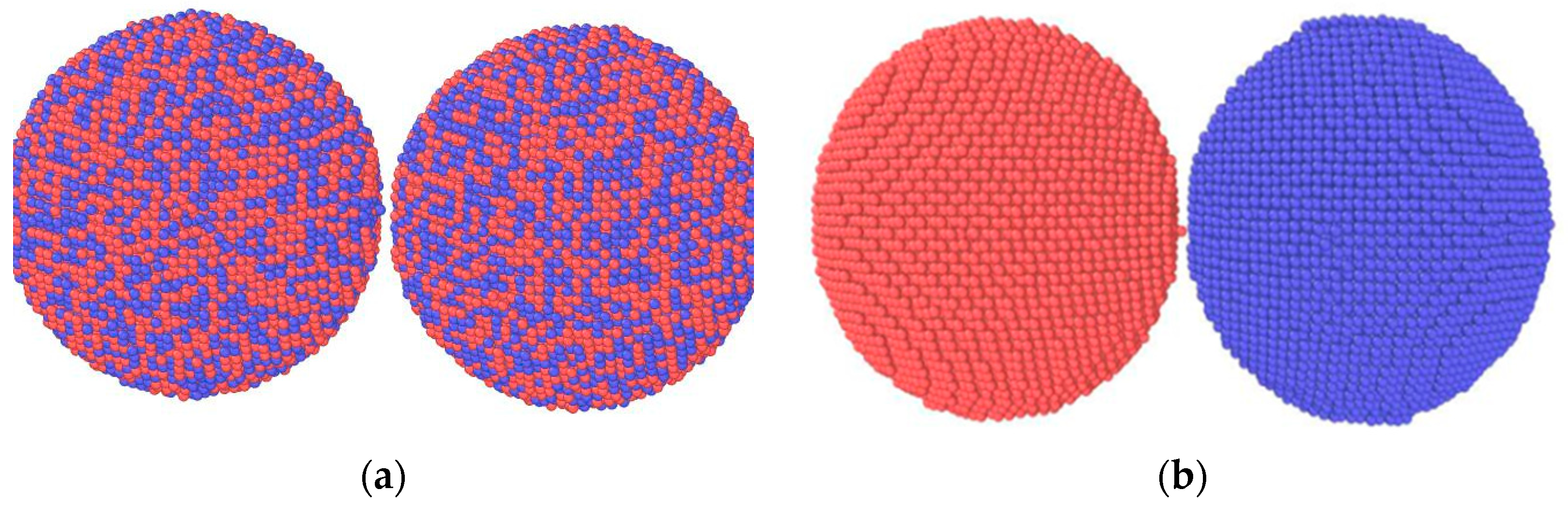
2.2. Ti-Al Spherical Particle System
3. Results and Discussion
3.1. Effect of the Heating Temperature on the Mass Transfer Behavior
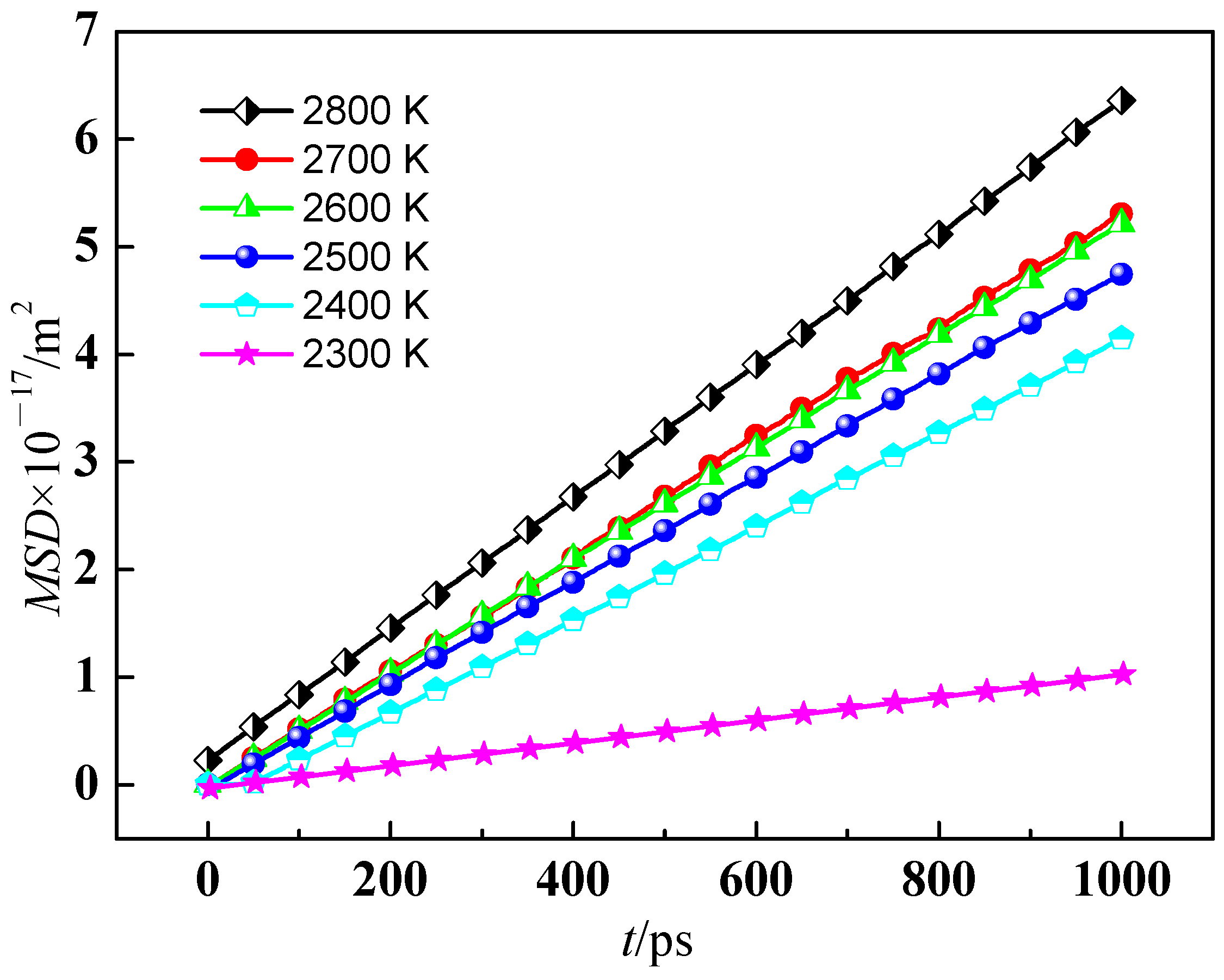
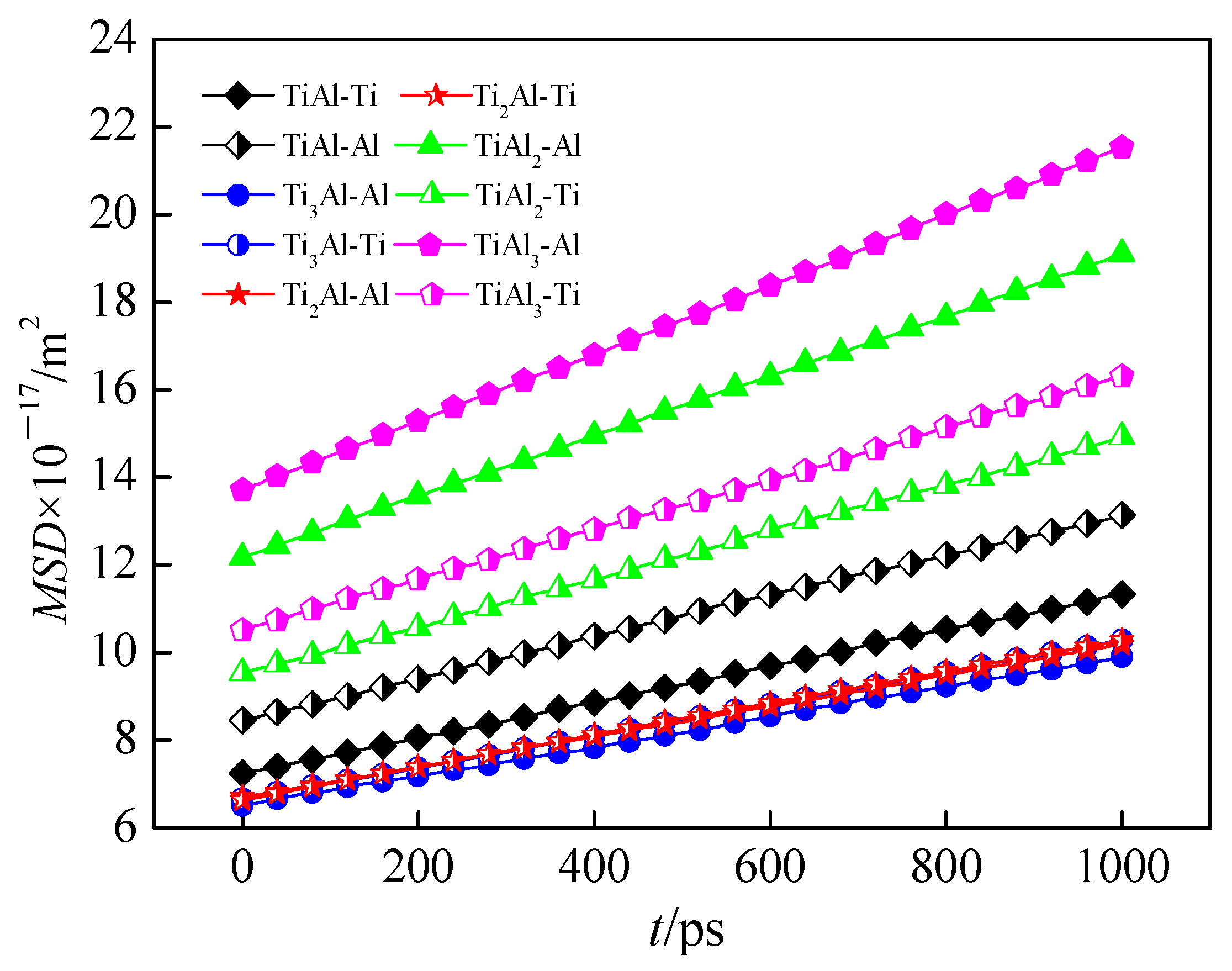
3.1.1. Diffusion Coefficient
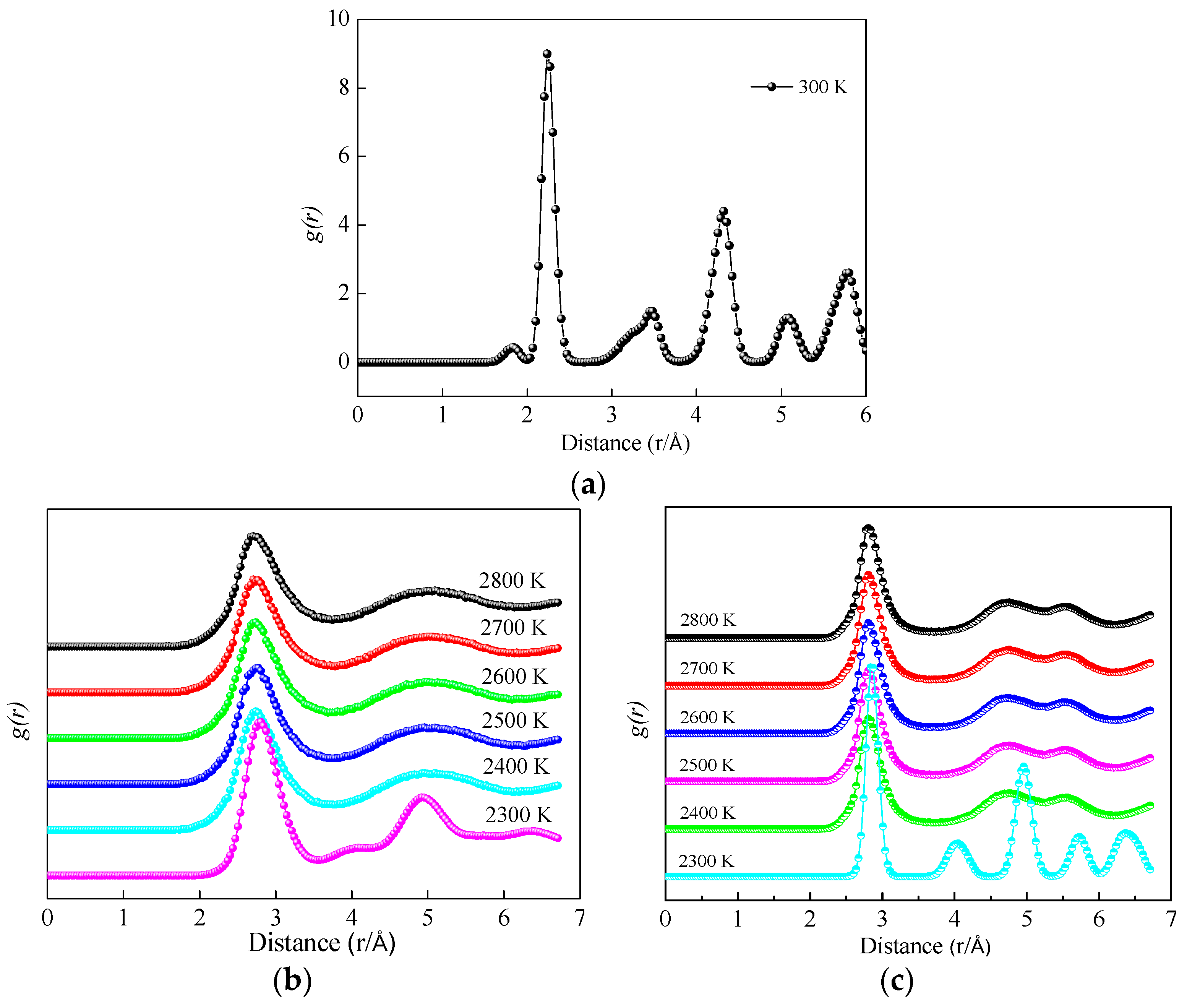
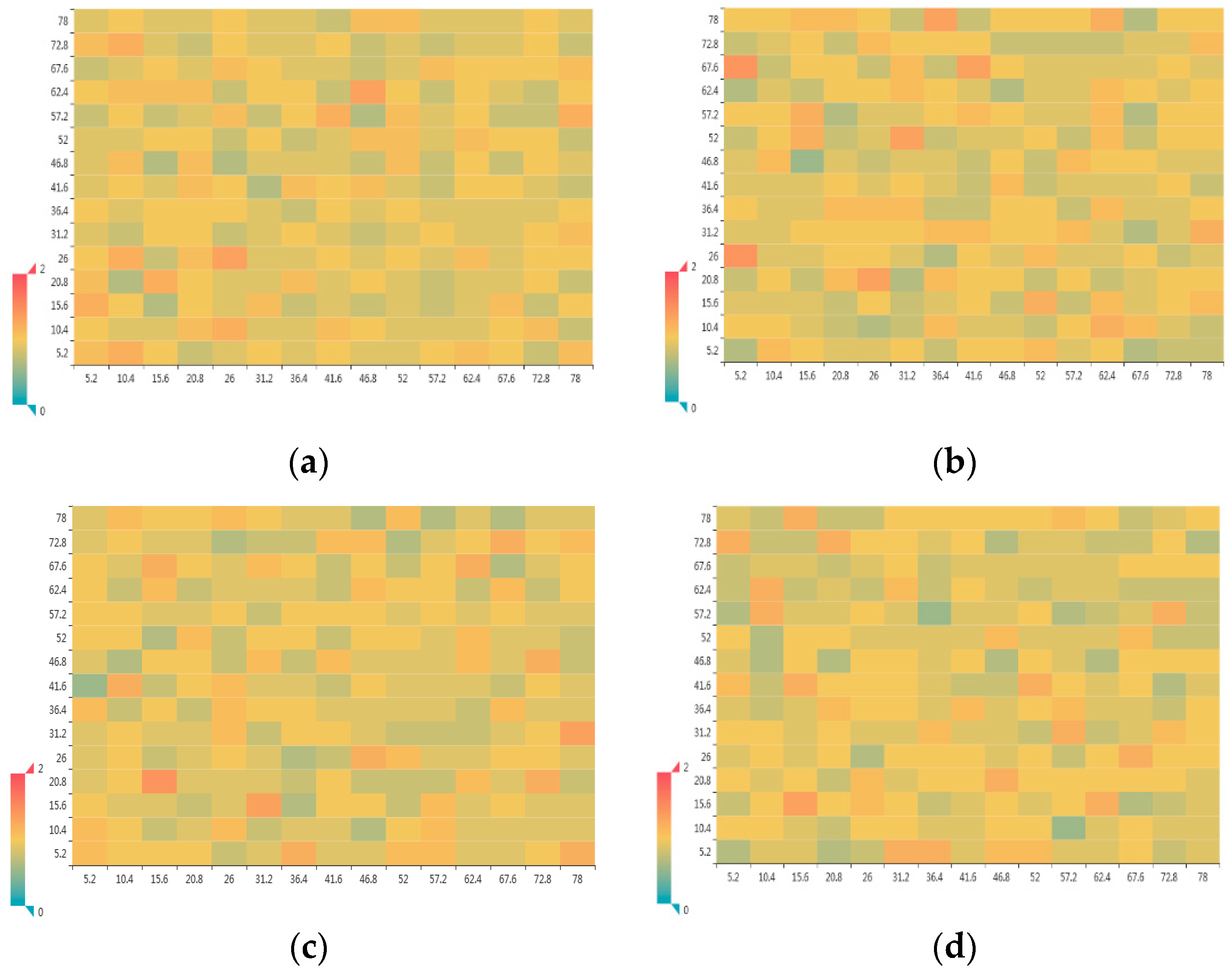
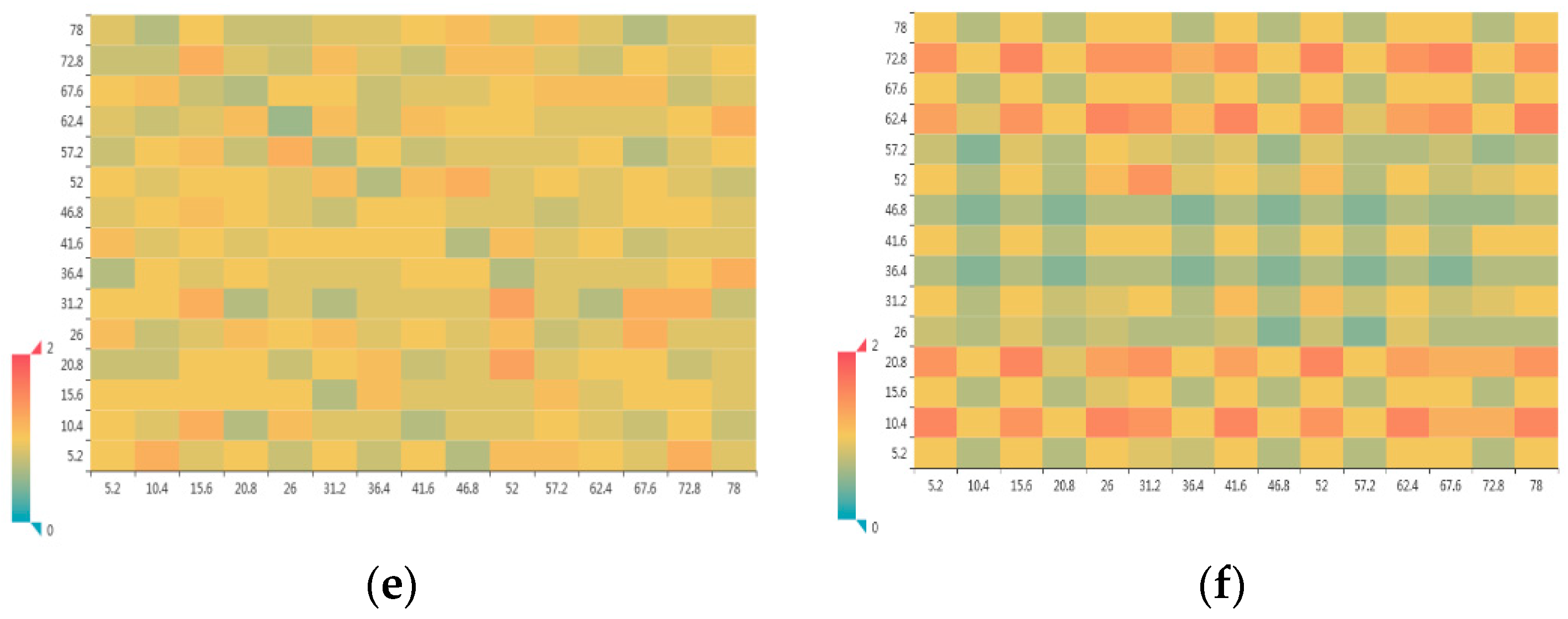
3.1.2. Radial Distribution Function
3.1.3. Atomic Number Density
3.2. Effect of Atomic Ratio on Mass Transfer Behavior
3.3. Effect of Heating Time on Mass Transfer Behavior
3.4. Atomic Scale Mass Transfer Behavior of Ti-Al Spherical Particle System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, S.S. High throughput growth and characterization of thin film materials. J. Cryst. Growth 2013, 379, 123–134. [Google Scholar] [CrossRef]
- Chakraborty, N. The effects of turbulence on molten pool transport during melting and solidification processes in continuous conduction mode laser welding of copper–nickel dissimilar couple. Appl. Therm. Eng. 2009, 29, 3618–3630. [Google Scholar] [CrossRef]
- Gan, Z.T.; Yu, G.; He, X.L. Numerical simulation of thermal behavior and multicomponent mass transfer in direct laser deposition of Co-base alloy on steel. Int. J. Heat Mass Transf. 2017, 104, 28–42. [Google Scholar] [CrossRef]
- Protopapas, P. Theory of transport in liquid metals. I. Calculation of self-diffusion coefficients. J. Chem. Phys. 1973, 59, 15–40. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, H.; Chen, M. A molecular dynamics examination of the relationship between self-diffusion and viscosity in liquid metals. J. Chem. Phys. 2012, 136, 80–91. [Google Scholar] [CrossRef]
- Yuan, P.; Gu, D. Molten pool behaviour and its physical mechanism during selective laser melting of TiC/AlSi10Mg nanocomposites: Simulation and experiments. J. Phys. D Appl. Phys. 2015, 48, 303–312. [Google Scholar] [CrossRef]
- Gan, Z.T.; Yu, G.; He, X.L. Surface-active element transport and its effect on liquid metal flow in laser-assisted additive manufacturing. Lett. Heat Mass Transf. 2017, 86, 206–220. [Google Scholar] [CrossRef]
- Gan, Z.T.; Liu, H.; Li, S. Modeling of thermal behavior and mass transport in multi-layer laser additive manufacturing of Ni-based alloy on cast iron. Int. J. Heat Mass Transf. 2017, 111, 709–730. [Google Scholar] [CrossRef]
- He, X.L.; Song, L.; Yu, G. Solute transport and composition profile during direct metal deposition with coaxial powder injection. Appl. Surf. Sci. 2011, 101, 260–278. [Google Scholar] [CrossRef]
- Dong, Z.B.; Wang, S.J.; Ma, R. Solute Redistribution with Shear Flow in Molten Pool of Ni-Cr Alloy. J. Mater. Sci. Technol. 2011, 27, 183–188. [Google Scholar] [CrossRef]
- Khosravian, N.; Samani, M.K.; Loh, G.C. Molecular dynamic simulation of diamond/silicon interfacial thermal conductance. J. Appl. Phys. 2013, 113, 24–42. [Google Scholar] [CrossRef]
- Zhou, X.W.; Jones, R.E.; Duda, J.C. Molecular dynamics studies of material property effects on thermal boundary conductance. J. Phys. Chem. 2013, 15, 11078–11087. [Google Scholar] [CrossRef] [PubMed]
- Duda, J.C.; English, T.S.; Piekos, E.S. Implications of cross-species interactions on the temperature dependence of Kapitza conductance. Phys. Rev. B 2011, 84, 4193–4198. [Google Scholar] [CrossRef]
- Shi, W.; Shuai, L.; Qing, W. Effect of Molten Pool Boundaries on the Mechanical Properties of Selective Laser Melting Parts. J. Mater. Process. Technol. 2014, 214, 2660–2667. [Google Scholar]
- Loh, L.E.; Chua, C.K.; Yeong, W.Y.; Song, J.; Mapar, M.; Sing, S.-L.; Liu, Z.-H.; Zhang, D.-Q. Numerical investigation and an effective modelling on the Selective Laser Melting (SLM) process with aluminium alloy 6061. Int. J. Heat Mass Transf. 2015, 80, 288–300. [Google Scholar] [CrossRef]
- Wang, H.P.; Yang, S.J.; Hu, L. Molecular dynamics prediction and experimental evidence for density of normal and metastable liquid zirconium. Chem. Phys. Lett. 2016, 653, 112–124. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhu, Y. Molecular dynamics simulation of the melting behavior of copper nanorod. Comput. Mater. Sci. 2018, 143, 248–262. [Google Scholar] [CrossRef]
- Essajai, R.; Hassanain, N. Molecular dynamics study of melting properties of gold nanorods. J. Mol. Liq. 2018, 261, 402–413. [Google Scholar] [CrossRef]
- Joshi, A.; James, S. Molecular dynamics simulation study of cold spray process. J. Manuf. Process. 2018, 33, 136–145. [Google Scholar] [CrossRef]
- Foroughi, A.; Tavakoli, R.; Aashuri, H. Molecular dynamics study of structural formation in Cu50–Zr50 bulk metallic glass. J. Non-Cryst. Solids. 2016, 432, 334–359. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.T.; Inoue, A. Structural properties and energy analysis of ZrxCu92−xAl8 ternary metallic glasses. Comput. Mater. Sci. 2017, 139, 260–272. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, H.F.; Hu, Z.Q. Molecular dynamic simulation studies of glass formation and atomic-level structures in Pd-Ni alloy. Phys. Lett. A 2004, 327, 506–520. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.J.; Lu, Z.P. Atomic structural evolution during glass formation of a Cu–Zr binary metallic glass. Comput. Mater. Sci. 2014, 85, 147–162. [Google Scholar] [CrossRef]
- Panwisawas, C.; Qiu, C.L.; Sovani, Y. On the role of thermal fluid dynamics into the evolution of porosity during selective laser melting. Scr. Mater. 2015, 105, 14–22. [Google Scholar] [CrossRef]
- Malek, K.; Coppens, M.O. Molecular simulations of solute transport in xylose isomerase crystals. J. Phys. Chem. B 2008, 112, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Agrawal, A.; Flores, K. Are Hints about Glass Forming Ability Hidden in the Liquid Structure? Acta Mater. 2019, 62, 99–115. [Google Scholar] [CrossRef]
- Sorkin, A.; Tan, J.L.; Wong, C.H. Multi-material modelling for selective laser melting. Procedia Eng. 2017, 216, 51–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.J.; Li, M. Atomic-scale structural evolution in selective laser melting of Cu50Zr50 metallic glass. Comput. Mater. Sci. 2018, 150, 62–73. [Google Scholar] [CrossRef]
- Jakse, N.; Pasturel, A. Transport properties and Stokes-Einstein relation in Al-rich liquid alloys. J. Chem. Phys. 2016, 144, 39–52. [Google Scholar] [CrossRef]
- Zope, R.R.; Mishin, Y. Interatomic potentials for atomistic simulations of the Ti-Al system. Phys. Rev. B 2003, 68, 366–391. [Google Scholar] [CrossRef]
- Meyer, A.; Petry, W.; Koza, M. Fast diffusion in ZrTiCuNiBe melts. Appl. Phys. Lett. 2003, 83, 3894–3906. [Google Scholar] [CrossRef]
- Liu, L.Q. Study on the Mechanical Behavior of Cu-Zr Metallic Glasses via Molecular Dynamics Simulations. Master’s Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]

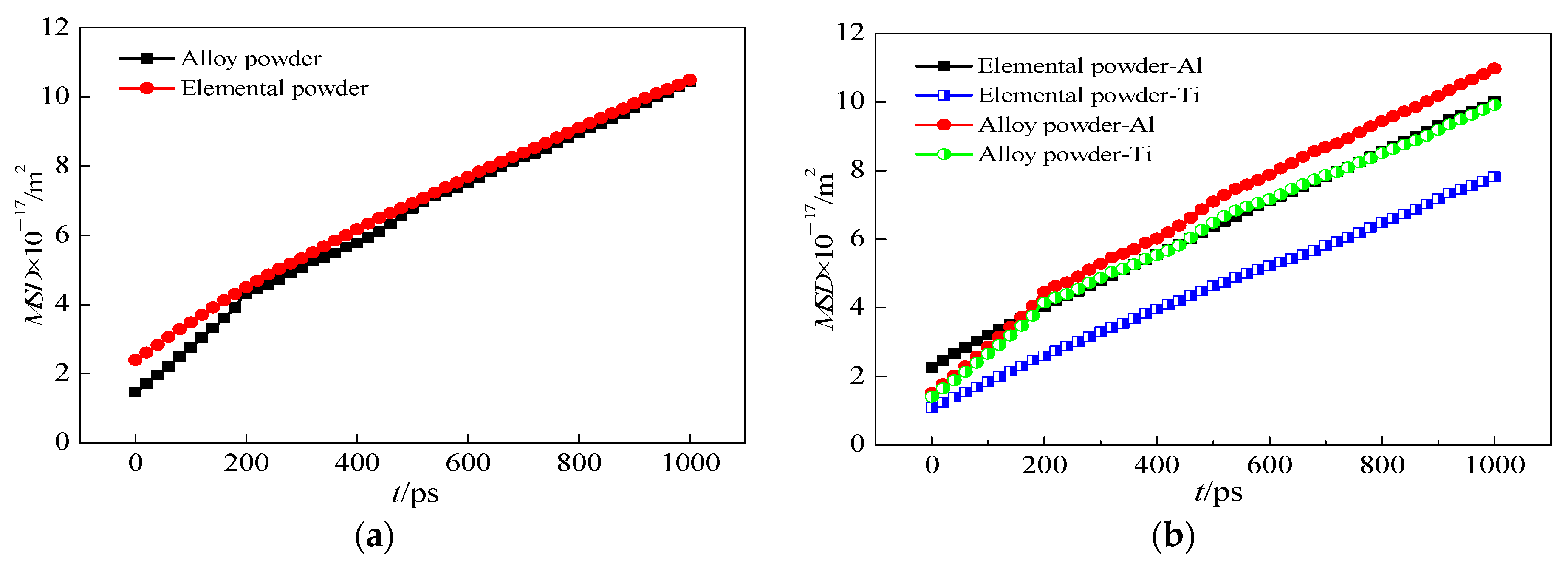
| Temperature (T/K) | Diffusion Coefficient (D × 10−6/m2·s−1) | Activation Energy (Q × 105/KJ·mol−1) |
|---|---|---|
| 2300 | 1.56 | 5.36 |
| 2400 | 7.19 | 4.91 |
| 2500 | 8.01 | 4.73 |
| 2600 | 8.72 | 4.57 |
| 2700 | 8.92 | 4.51 |
| 2800 | 10.20 | 4.41 |
| Heating Time (t/ps) | Diffusion Coefficient of Alloy Powder (DA/m2·s−1) | Diffusion Coefficient of Elemental Powder (DE/m2·s−1) |
|---|---|---|
| 100 | 9.11 × 10−8 | 1.06 × 10−7 |
| 1000 | 8.38 × 10−8 | 8.27 × 10−8 |
| 2000 | 7.74 × 10−8 | 7.28 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Zhou, X.; Meng, Q. Atomic-Scale Mechanism Investigation of Mass Transfer in Laser Fabrication Process of Ti-Al Alloy via Molecular Dynamics Simulation. Metals 2020, 10, 1660. https://doi.org/10.3390/met10121660
Cui Z, Zhou X, Meng Q. Atomic-Scale Mechanism Investigation of Mass Transfer in Laser Fabrication Process of Ti-Al Alloy via Molecular Dynamics Simulation. Metals. 2020; 10(12):1660. https://doi.org/10.3390/met10121660
Chicago/Turabian StyleCui, Ziqi, Xianglin Zhou, and Qingbo Meng. 2020. "Atomic-Scale Mechanism Investigation of Mass Transfer in Laser Fabrication Process of Ti-Al Alloy via Molecular Dynamics Simulation" Metals 10, no. 12: 1660. https://doi.org/10.3390/met10121660
APA StyleCui, Z., Zhou, X., & Meng, Q. (2020). Atomic-Scale Mechanism Investigation of Mass Transfer in Laser Fabrication Process of Ti-Al Alloy via Molecular Dynamics Simulation. Metals, 10(12), 1660. https://doi.org/10.3390/met10121660




