Structural Characterization of Fine γ′-Fe4N Nitrides Formed by Active Screen Plasma Nitriding
Abstract
1. Introduction
2. Materials and Methods
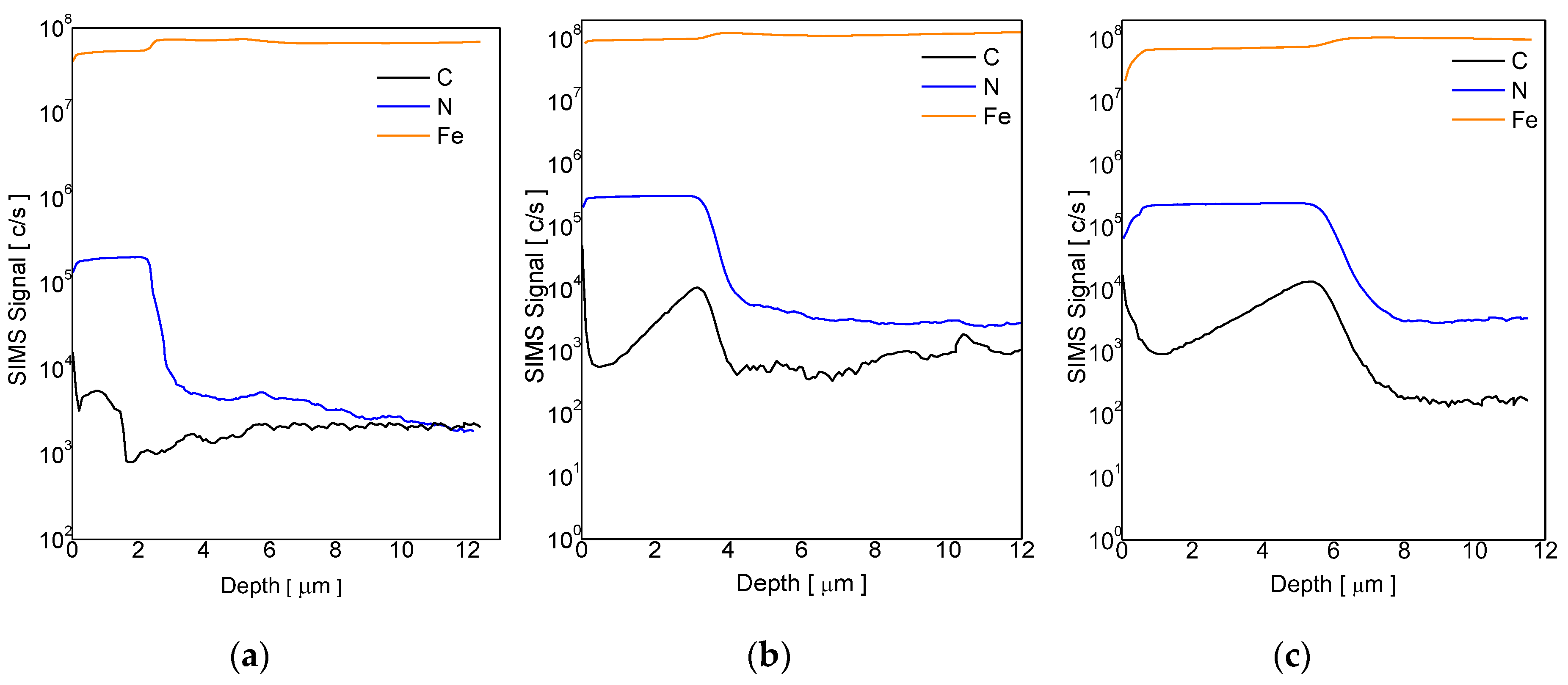
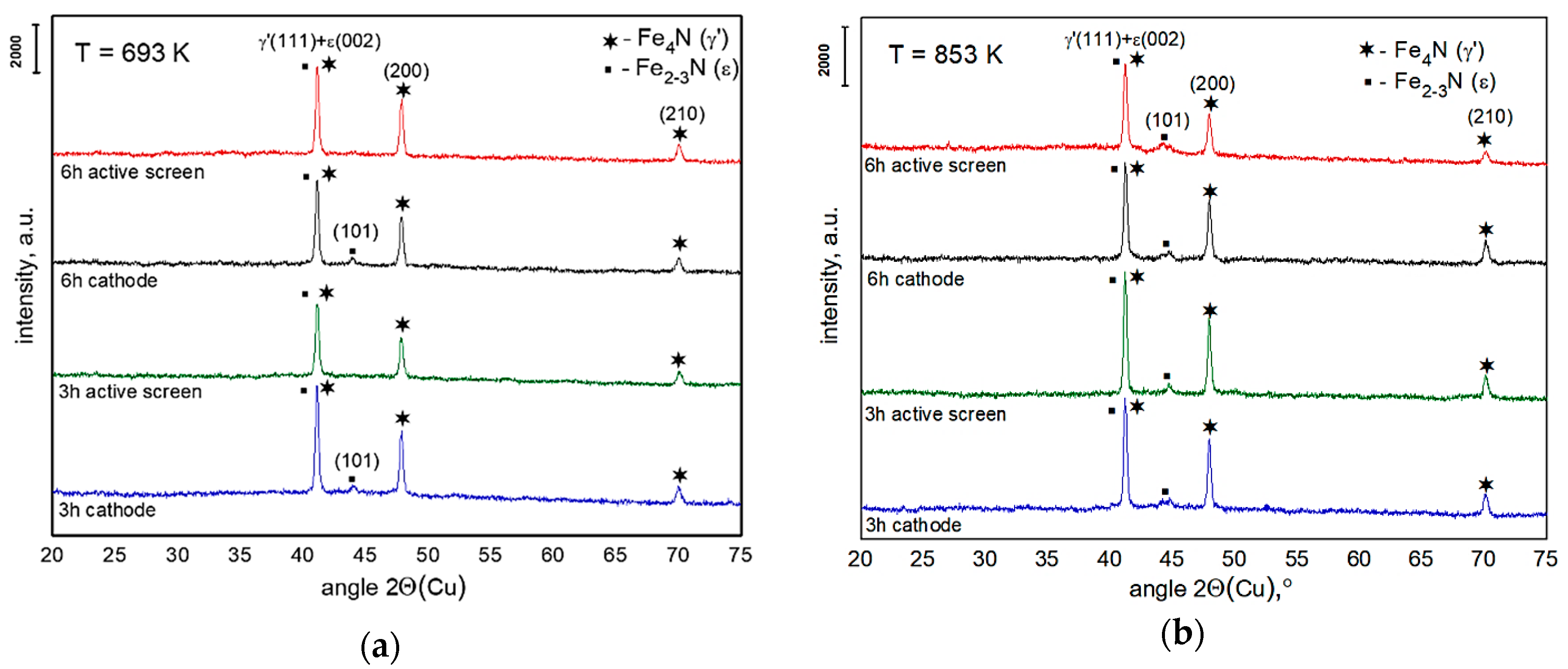
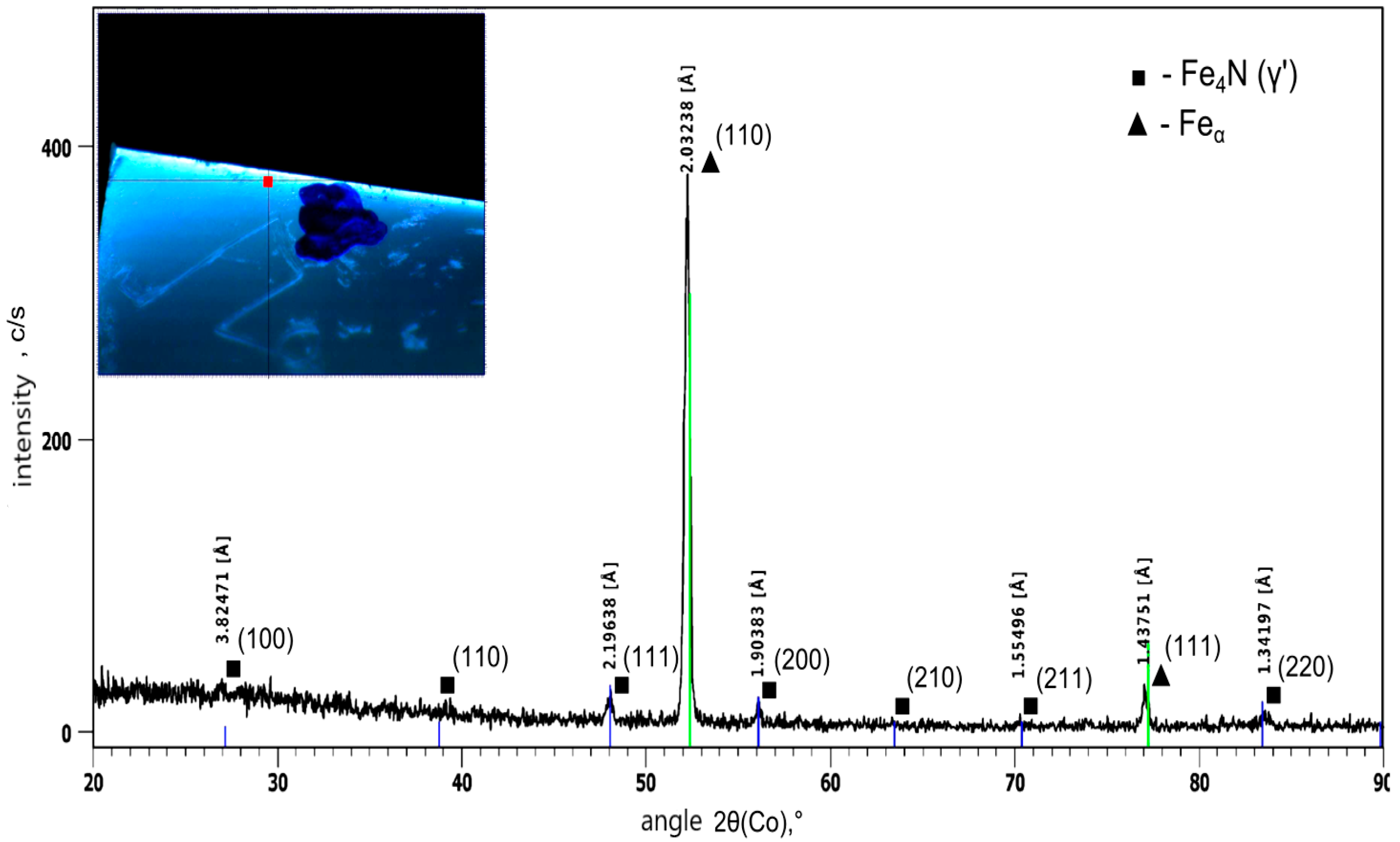
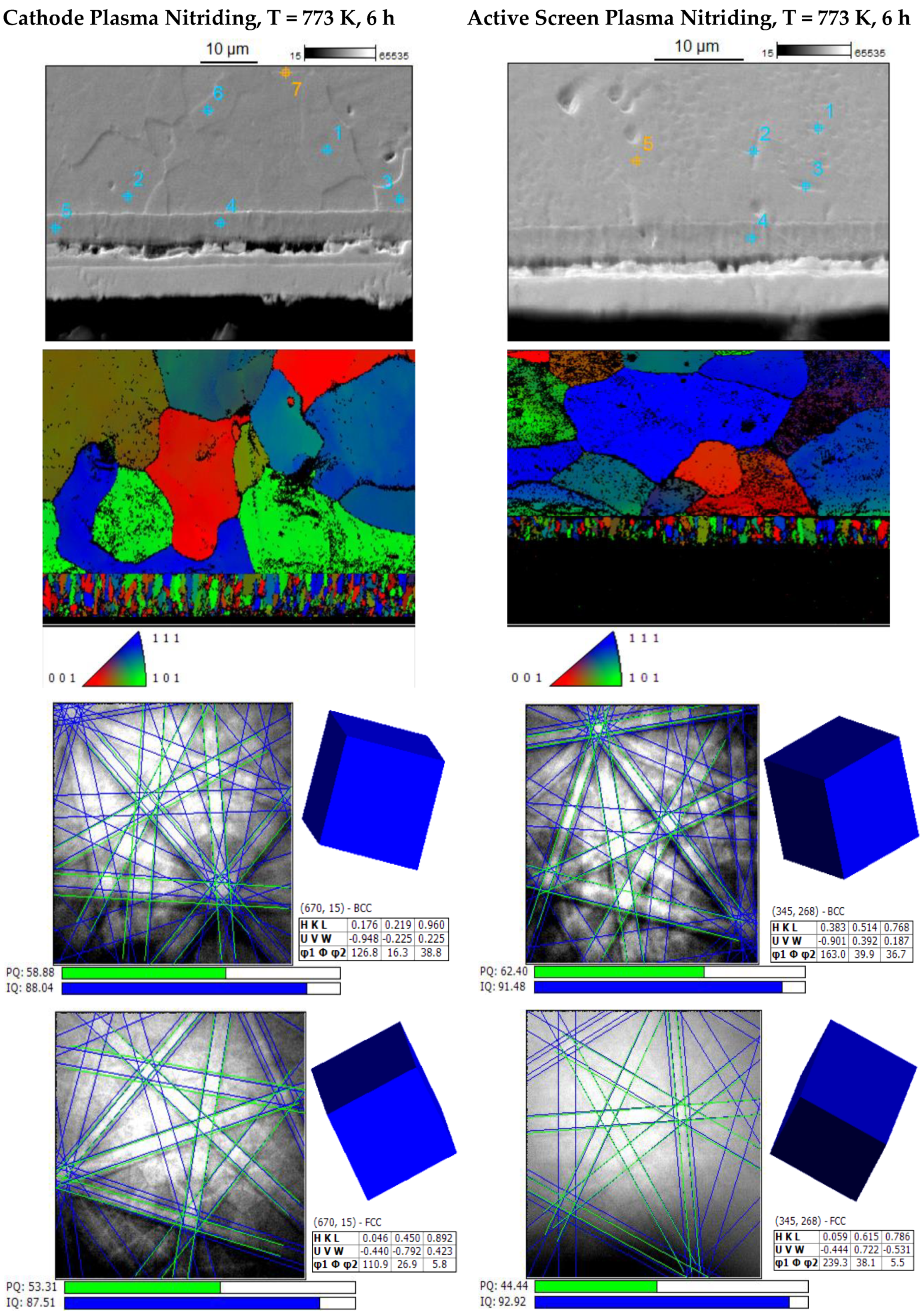
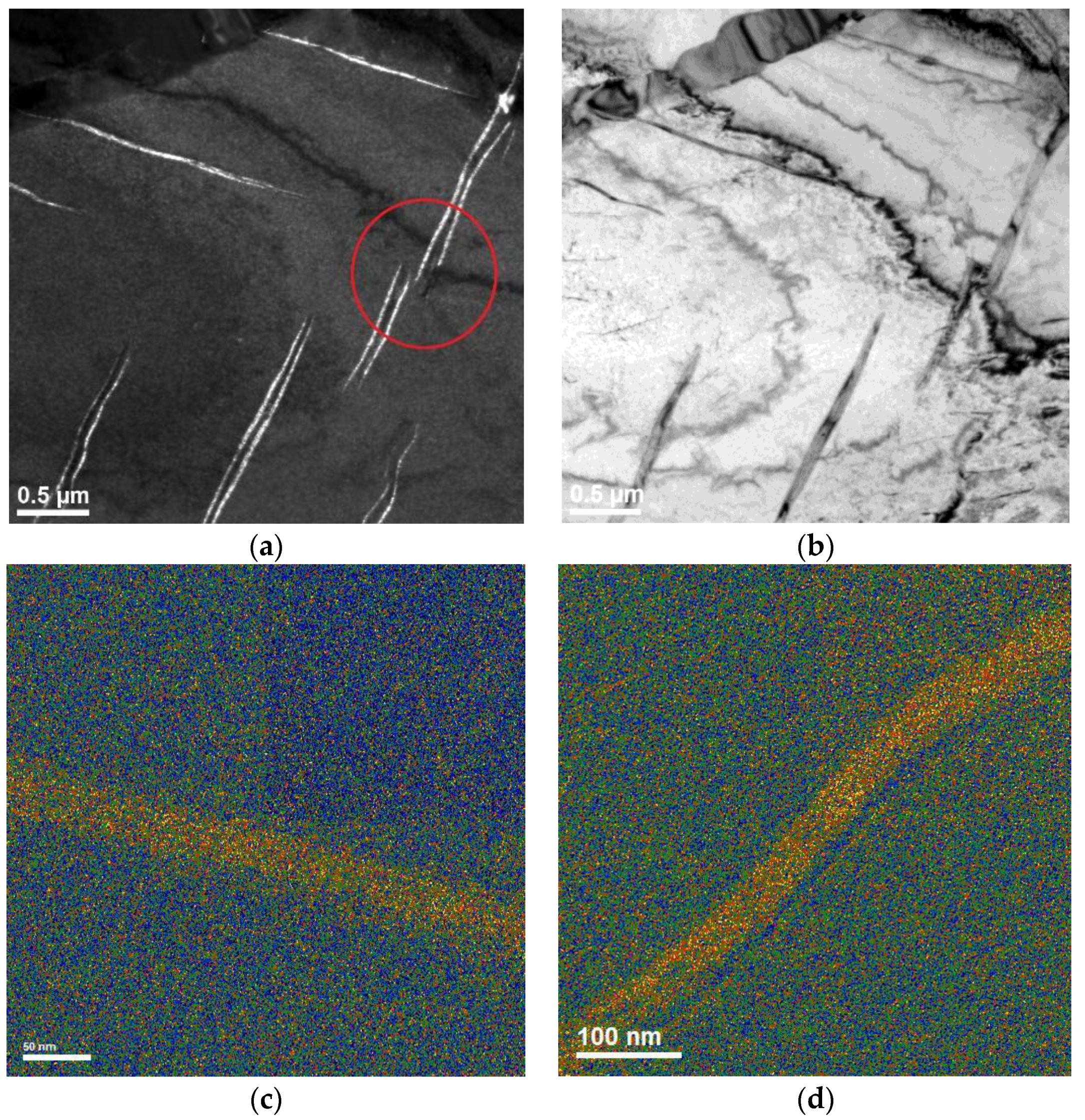
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mittemeijer, E.J.; Slycke, J.T. Thermodynamic activities of nitrogen and carbon imposed by gaseous nitriding and carburising atmospheres. Surf. Eng. 1996, 3, 67–71. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.X.; Dong, H.; Bell, T. Study on the active screen plasma nitriding and its nitriding mechanism. Surf. Coat. Technol. 2006, 201, 2320–2325. [Google Scholar] [CrossRef]
- Taherkhani, K.; Soltanieh, M. Evaluation of the formation of compound coatings on aluminium by the new method of active screen plasma nitriding. Mater. Res. Express 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Fraczek, T.; Olejnik, M.; Jasinski, J.J.; Skuza, Z. Short-term low-temperature glow discharge nitriding of 316L austenitic steel. Metalurgija 2011, 50, 151–154. [Google Scholar]
- Walkowicz, J. On the mechanisms of diode plasma nitriding in N2–H2 mixtures under DC-pulsed substrate biasing. Surf. Coat. Technol. 2003, 174–175, 1211–1219. [Google Scholar] [CrossRef]
- Fraczek, T.; Ogorek, M.; Skuza, Z.; Prusak, R. Mechanism of ion nitriding of 316L austenitic steel by active screen method in a hydrogen-nitrogen atmosphere. Int. J. Adv. Manuf. Technol. 2020, 109, 1357–1368. [Google Scholar] [CrossRef]
- Georges, J. Nitriding Process and Nitriding Furnace Therefor. U.S. Patent 5,989,363, 23 September 1999. [Google Scholar]
- Tibbets, G.G. Role of nitrogen atoms in ion-nitriding. J. Appl. Phys. 1974, 45, 5072–5073. [Google Scholar] [CrossRef]
- Moszynski, D.; Moszynska, I.; Arabczyk, W. Iron nitriding and reduction of iron nitrides in nanocrystalline Fe–N system. Mater. Lett. 2012, 78, 32–34. [Google Scholar] [CrossRef]
- Bernal, J.L.; Salas, O.; Figueroa, U.; Oseguera, J. Early stages of gamma prim-Fe4N1−x nucleation and growth in a post-discharge nitriding reactor. Surf. Coat. Technol. 2004, 177–178, 665–670. [Google Scholar] [CrossRef]
- Wetzel, M.H.; Schwarz, M.R.; Leineweber, A. High-pressure high-temperature study of the pressure induced decomposition of the iron nitride γ′-Fe4N. J. Alloys Compd. 2019, 801, 438–448. [Google Scholar] [CrossRef]
- Jasinski, J.J.; Fraczek, T.; Kurpaska, L.; Lubas, M.; Sitarz, M. Effects of different nitriding methods on nitrided layer structure and morphology. Arch. Metall. Mater. 2018, 63, 337–345. [Google Scholar]
- Jacobs, H.; Rechenbach, D.; Zachwieja, U. Structure determination of γ′-Fe4N and ϵ-Fe3N. J. Alloys Compd. 1995, 227, 10–17. [Google Scholar] [CrossRef]
- Leineweber, A.; Gressmann, T.; Mittemeijer, E.J. Simultaneous control of the nitrogen and carbon activities during nitrocarburising of iron. Surf. Coat. Technol. 2012, 206, 2780–2791. [Google Scholar] [CrossRef]
- Keddam, M. Surface modification of the pure iron by the pulse plasma nitriding: Application of a kinetic model. Mater. Sci. Eng. A 2007, 462, 169–173. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, X.; Gu, J.; Hu, M.; Zhu, Z. Isothermal decomposition behavior of the high nitrogen concentration γ-Fe[N] prepared from pure iron. Appl. Surf. Sci. 2008, 254, 7361–7364. [Google Scholar] [CrossRef]
- Nishimoto, A.; Nagatsuka, K.; Narita, R.; Nii, H.; Akamatsu, K. Effect of the distance between screen and sample on active screen plasma nitriding properties. Surf. Coat. Technol. 2010, 205, 365–368. [Google Scholar] [CrossRef]
- Hubbard, P.; Partridge, J.G.; Doyle, E.D.; McCulloch, G.D.; Taylor, M.B.; Dowey, S.J. Investigation of nitrogen mass transfer within an industrial plasma nitriding system I: The role of surface deposits. Surf. Coat. Technol. 2010, 204, 1145–1150. [Google Scholar] [CrossRef]
- Hirsch, T.; Clarke, T.G.R.; Rocha, A.d.S. An in-situ study of plasma nitriding. Surf. Coat. Technol. 2007, 201, 6380–6386. [Google Scholar] [CrossRef]
- Belmonte, T.; Jaoul, C.; Borges, J.N. Modelling nitrogen atom flux in post-discharge nitriding processes. Surf. Coat. Technol. 2004, 188–189, 201–206. [Google Scholar] [CrossRef]
- Czerwiec, T.; Michel, H.; Bergmann, E. Low-pressure, high-density plasma nitriding: Mechanisms, technology and results. Surf. Coat. Technol. 1998, 108–109, 182–190. [Google Scholar] [CrossRef]
- Hosseini, S.R.; Ashrafizadeh, F. Accurate measurement and evaluation of the nitrogen depth profile in plasma nitrided iron. Vacuum 2009, 83, 1174–1178. [Google Scholar] [CrossRef]
- Somers, M.A.J.; Kooi, B.J.; Maldzinski, L.; Mittemeijer, E.J.; Van Der Horst, A.A.; van der Kraan, A.M.; van der Pers, N.M. Thermodynamics and long-range order of interstitials in an h.c.p. lattice Nitrogen in ε-Fe2N1–z. Acta Mater. 1997, 45, 2013–2025. [Google Scholar] [CrossRef]
- Keshavarz Hedayati, M.; Mahboubi, F.; Nickchi, T. Comparison of conventional and active screen plasma nitriding of hard chromium electroplated steel. Vacuum 2009, 83, 1123–1128. [Google Scholar] [CrossRef]
- Neumann, G.; Tuijn, C. Self-Diffusion and Impurity Diffusion in Pure Metals: Handbook of Experimental Data; Pergamon Materials Series 14; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Jasinski, J.J.; Fraczek, T.; Kurpaska, L.; Lubas, M.; Sitarz, M. Investigation of nitrogen transport in active screen plasma nitriding processes—Uphill diffusion effect. J. Mol. Struct. 2018, 1164, 37–44. [Google Scholar] [CrossRef]
- Campos, I.; Torres, R.; Bautista, O.; Ramirez, G.; Zuniga, L. Evaluation of the diffusion coefficient of nitrogen in Fe4N1−x nitride layers during microwave post-discharge nitriding. Appl. Surf. Sci. 2005, 249, 54–59. [Google Scholar] [CrossRef]
- Taherkhani, K.; Soltanieh, M. Composite coatings created by new method of active screen plasma nitriding on aluminium alloy 6061. J. Alloys Compd. 2018, 741, 1247–1257. [Google Scholar] [CrossRef]
- Tessier, F.; Navrotsky, A.; Niewa, R.; Leineweber, A.; Jacobs, H.; Kikkawa, S.; Takahashi, M.; Kanamaru, F.; di Salvo, F.J. Energetics of binary iron nitride. Solid State Sci. 2000, 2, 457–462. [Google Scholar] [CrossRef]


| Parameter | Sputtering | Plasma Nitriding |
|---|---|---|
| Atmosphere [%] | 90% H2–10% Ar | 25% N2–75% H2 |
| Temperature [K] | heating up to process | 693, 773, 853 |
| Treatment time [h] | ~0.8–1 | 6 |
| Pressure [Pa] | 150 | 150 |
| Voltage [V] | ~750 | ~860 |
| Effective power [kW] | ~5.4 | ~5.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasinski, J.J.; Kurpaska, L.; Fraczek, T.; Lubas, M.; Sitarz, M. Structural Characterization of Fine γ′-Fe4N Nitrides Formed by Active Screen Plasma Nitriding. Metals 2020, 10, 1656. https://doi.org/10.3390/met10121656
Jasinski JJ, Kurpaska L, Fraczek T, Lubas M, Sitarz M. Structural Characterization of Fine γ′-Fe4N Nitrides Formed by Active Screen Plasma Nitriding. Metals. 2020; 10(12):1656. https://doi.org/10.3390/met10121656
Chicago/Turabian StyleJasinski, Jaroslaw Jan, Lukasz Kurpaska, Tadeusz Fraczek, Malgorzata Lubas, and Maciej Sitarz. 2020. "Structural Characterization of Fine γ′-Fe4N Nitrides Formed by Active Screen Plasma Nitriding" Metals 10, no. 12: 1656. https://doi.org/10.3390/met10121656
APA StyleJasinski, J. J., Kurpaska, L., Fraczek, T., Lubas, M., & Sitarz, M. (2020). Structural Characterization of Fine γ′-Fe4N Nitrides Formed by Active Screen Plasma Nitriding. Metals, 10(12), 1656. https://doi.org/10.3390/met10121656






