Wearing Behavior of the α-Al9FeMnSi Intermetallic Compound Formed by Reactive Sintering onto AISI 304L Stainless Steel
Abstract
1. Introduction
ΔG0 = −186238 + 59.63T (J mol−1) (878–973 K).
2. Experimental Procedure
3. Results and Discussion
3.1. Microstructure
3.2. Influence of Pressure and Temperature on the Coating Formation
3.3. Microhardness Measurements
3.4. Wearing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, Y.; Lundin, C.D.; Hariharan, V. Mechanical behavior of austenitic stainless steel weld metals with microfissures. J. Mater. Process. Technol. 2006, 171, 150–155. [Google Scholar] [CrossRef]
- Özyürek, D. An effect of weld current and weld atmosphere on the resistance spot weldability of 304L austenitic stainless steel. Mater. Des. 2008, 29, 597–603. [Google Scholar] [CrossRef]
- Jin, X.; Wang, B.; Xue, W.; Du, J.; Wu, X.; Wu, J. Characterization of wear-resistant coatings on 304 stainless steel fabricated by cathodic plasma electrolytic oxidation. Surf. Coat. Technol. 2013, 236, 22–28. [Google Scholar] [CrossRef]
- Leyland, A.; Lewis, D.; Stevensom, P.; Matthews, A. Low temperature plasma diffusion treatment of stainless steels for improved wear resistance. Surf. Coat. Technol. 1993, 62, 608–617. [Google Scholar] [CrossRef]
- Sayyedan, F.S.; Enayati, M. Evaluating oxidation behavior of amorphous aluminum phosphate coating. Appl. Surf. Sci. 2018, 455, 821–830. [Google Scholar] [CrossRef]
- Dorfman, M.R. Thermal Spray Coatings. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 469–488. [Google Scholar]
- Silva, F.; Bedoya, J.; Dosta, S.; Cinca, N.; Cano, I.; Guilemany, J.; Benedetti, A. Corrosion characteristics of cold gas spray coatings of reinforced aluminum deposited onto carbon steel. Corros. Sci. 2017, 114, 57–71. [Google Scholar] [CrossRef]
- Flores-Valdés, A.; Castillejos-Escobar, A.; Acosta-González, F.; Escobedo-Bocardo, J.; Toscano-Giles, A. The Development of Surface Coatings for Co-Cr-Mo Alloys Based on Quaternary AlSiFeMn Intermetallic Compounds. In Cobalt-Base Alloys for Biomedical Applications; ASTM International: West Conshohocken, PA, USA, 2009; p. 179. [Google Scholar]
- Flores-V., A.; Sukiennik, M.; Castillejos-E., A.H.; Acosta-G., F.A.; Escobedo-B., J.C. A kinetic study on the nucleation and growth of the Al8FeMnSi2 intermetallic compound for aluminum scrap purification. Intermetallics 1998, 6, 217–227. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Effect of chromium on the formation of intermetallic phases in hot-dipped aluminide Cr–Mo steels. Appl. Surf. Sci. 2013, 277, 139–145. [Google Scholar] [CrossRef]
- Azimaee, H.-R.; Sarfaraz, M.; Mirjalili, M.; Aminian, K. Effect of silicon and manganese on the kinetics and morphology of the intermetallic layer growth during hot-dip aluminizing. Surf. Coat. Technol. 2019, 357, 483–496. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.; Chu, C.; Qiao, G.; Bao, C. Porous nano-Al2O3/Fe–Cr–Ni composites fabricated by pressureless reactive sintering. Mater. Chem. Phys. 2011, 128, 24–27. [Google Scholar] [CrossRef]
- Zou, B.; Shen, P.; Cao, X.; Jiang, Q. The mechanism of thermal explosion (TE) synthesis of TiC–TiB2 particulate locally reinforced steel matrix composites from an Al–Ti–B4C system via a TE-casting route. Mater. Chem. Phys. 2012, 132, 51–62. [Google Scholar] [CrossRef]
- Novak, P.; Šerák, J.; Vojtěch, D.; Kubásek, J.; Michalcová, A. Where reactive sintering beats melt technology. Met. Powder Rep. 2008, 63, 20–23. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, Y.; He, Y.; Zou, J.; Xu, N.; Huang, B.; Liu, C.; Liaw, P. Oxidation behavior of porous NiAl prepared through reactive synthesis. Mater. Chem. Phys. 2010, 122, 417–423. [Google Scholar] [CrossRef]
- Novák, P.; Michalcova, A.; Marek, I.; Mudrová, M.; Saksl, K.; Bednarčík, J.; Zikmund, P.; Vojtěch, V. On the formation of intermetallics in Fe–Al system—An in situ XRD study. Intermetallics 2013, 32, 127–136. [Google Scholar] [CrossRef]
- Frutos, E.; González–Carrasco, J.; Capdevila, C.; Jiménez, J.; Houbaert, Y. Development of hard intermetallic coatings on austenitic stainless steel by hot dipping in an Al–Si alloy. Surf. Coat. Technol. 2009, 203, 2916–2920. [Google Scholar] [CrossRef]
- Sabetghadam, H.; Hanzaki, A.Z.; Araee, A.; Hadian, A. Microstructural Evaluation of 410 SS/Cu Diffusion-Bonded Joint. J. Mater. Sci. Technol. 2010, 26, 163–169. [Google Scholar] [CrossRef]
- Ahamed, H.; Senthilkumar, V. Consolidation behavior of mechanically alloyed aluminum based nanocomposites reinforced with nanoscale Y2O3/Al2O3 particles. Mater. Charact. 2011, 62, 1235–1249. [Google Scholar] [CrossRef]
- Yu, J.; Wanderka, N.; Miehe, G.; Banhart, J. Intermetallic phases in high purity Al-10Si-0.3Fe cast alloys with and without Sr modification studied by FIB tomography and TEM. Intermetallics 2016, 72, 53–61. [Google Scholar] [CrossRef]
- Yin, F.; Zhao, M.-X.; Liu, Y.-X.; Han, W.; Li, Z. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron and molten aluminum. Trans. Nonferrous Met. Soc. China 2013, 23, 556–561. [Google Scholar] [CrossRef]
- Rasool, G.; Stack, M. Wear maps for TiC composite based coatings deposited on 303 stainless steel. Tribol. Int. 2014, 74, 93–102. [Google Scholar] [CrossRef]
- Kong, D.; Tianyuan, S. Wear behaviors of HVOF sprayed WC-12Co coatings by laser remelting under lubricated condition. Opt. Laser Technol. 2017, 89, 86–91. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Han, T.; Ranabhat, J.; Feng, X.; Shen, Y. Microstructures and oxidation behavior of NiCrAlCoY-Al composite coatings on Ti-6Al-4V alloy substrate via high-energy mechanical alloying method. J. Alloys Compd. 2017, 697, 268–281. [Google Scholar] [CrossRef]
- Toscano, J.; Flores, A.; Salinas, A.; Nava, E. Microstructure of Al9(MnFe)xSi intermetallics produced by pressure-assisted reactive sintering of elemental AlMnFeSi powder mixtures. Mater. Lett. 2003, 57, 2246–2252. [Google Scholar] [CrossRef]
- Martínez-Perales, L.G.; Flores-Valdés, A.; Salinas-Rodríguez, A.; Ochoa, R.; Toscano-Giles, J.A.; Torres-Torres, J. Formation of abrasion-resistant coatings of the AlSiFexMny intermetallic compound type on the AISI 304L alloy. Rev. Metal. 2016, 52, e061. [Google Scholar] [CrossRef]
- Frutos, E.; Álvarez, D.; Fernandez, L.; González-Carrasco, J. Effects of bath composition and processing conditions on the microstructure and mechanical properties of coatings developed on 316 LVM by hot dipping in melted AlSi alloys. J. Alloy Compd. 2014, 617, 646–653. [Google Scholar] [CrossRef]
- Dong, L.; Chen, W.; Hou, L.; Liu, Y.; Luo, Q. Metallurgical process analysis and microstructure characterization of the bonding interface of QAl9-4 aluminum bronze and 304 stainless steel composite materials. J. Mater. Process. Technol. 2016, 238, 325–332. [Google Scholar] [CrossRef]
- Wang, J.; Xing, J.; Cao, L.; Su, W.; Gao, Y. Dry sliding wear behavior of Fe3Al alloys prepared by mechanical alloying and plasma activated sintering. Wear 2010, 268, 473–480. [Google Scholar] [CrossRef]
- Balamurugan, G.; Duraiselvam, M.; Anandakrishnan, V. Comparison of high temperature wear behaviour of plasma sprayed WC–Co coated and hard chromium plated AISI 304 austenitic stainless steel. Mater. Des. 2012, 35, 640–646. [Google Scholar] [CrossRef]
- Jeshvaghani, R.A.; Harati, E.; Shamanian, M. Effects of surface alloying on microstructure and wear behavior of ductile iron surface-modified with a nickel-based alloy using shielded metal arc welding. Mater. Des. 2011, 32, 1531–1536. [Google Scholar] [CrossRef]


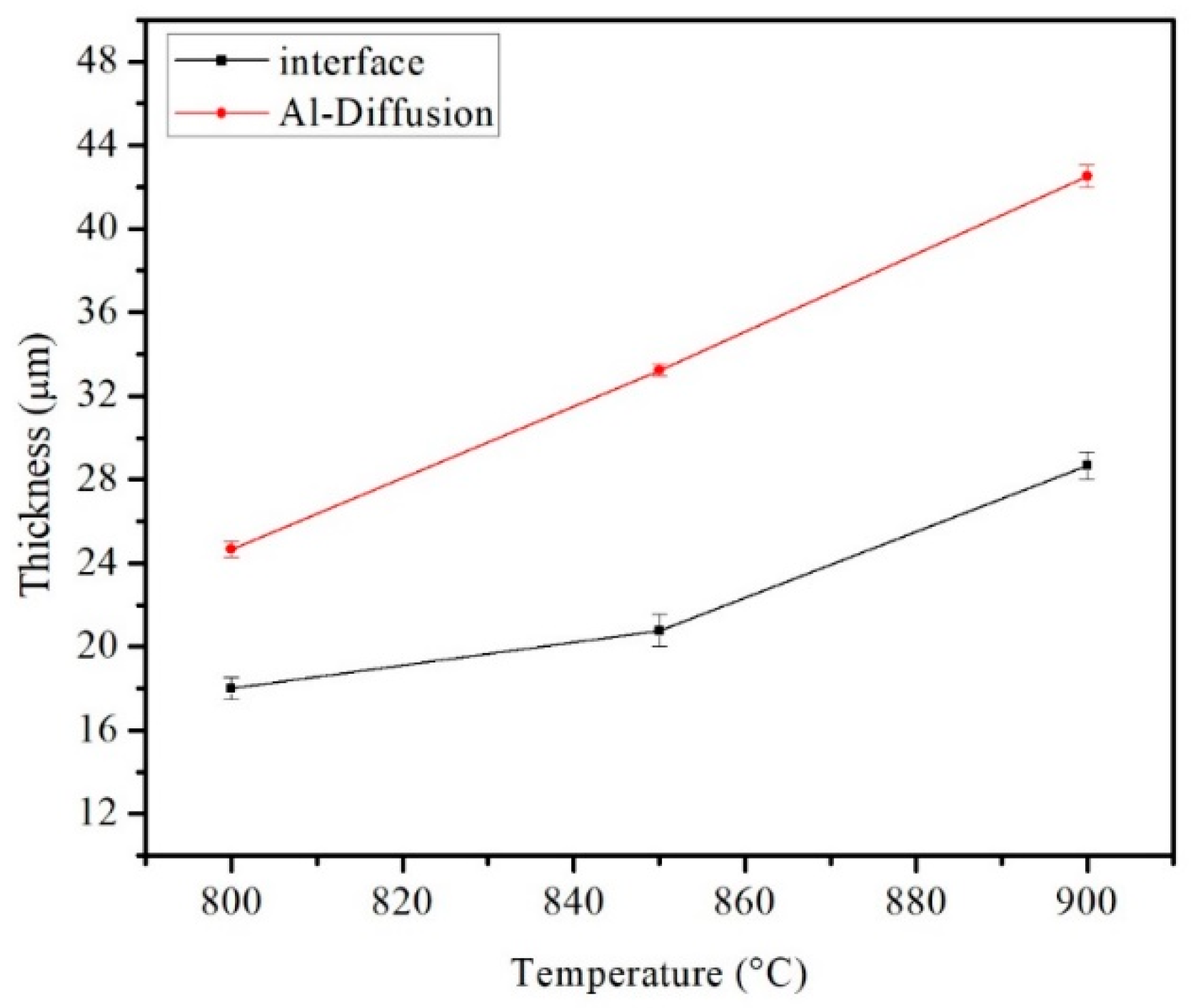
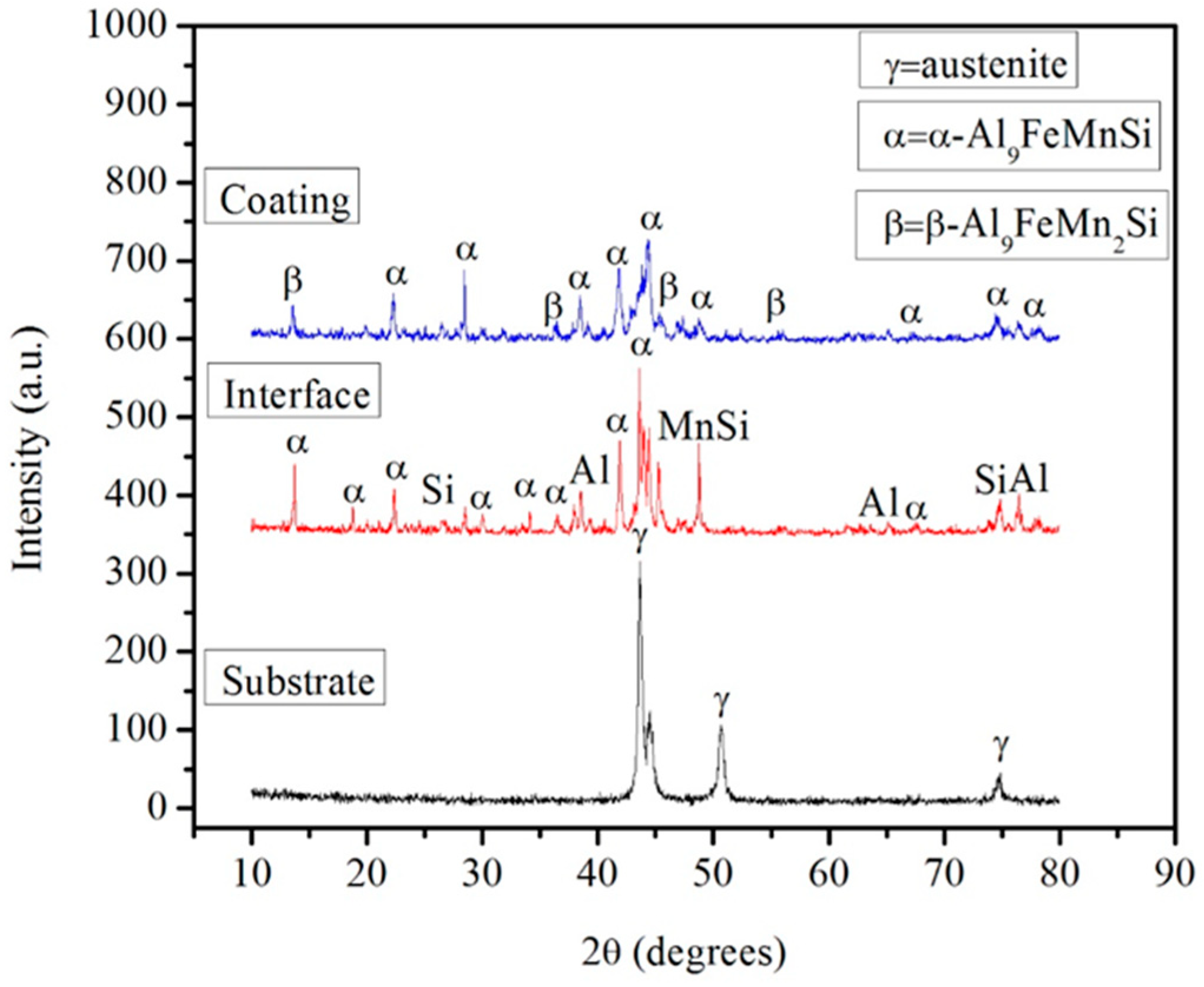








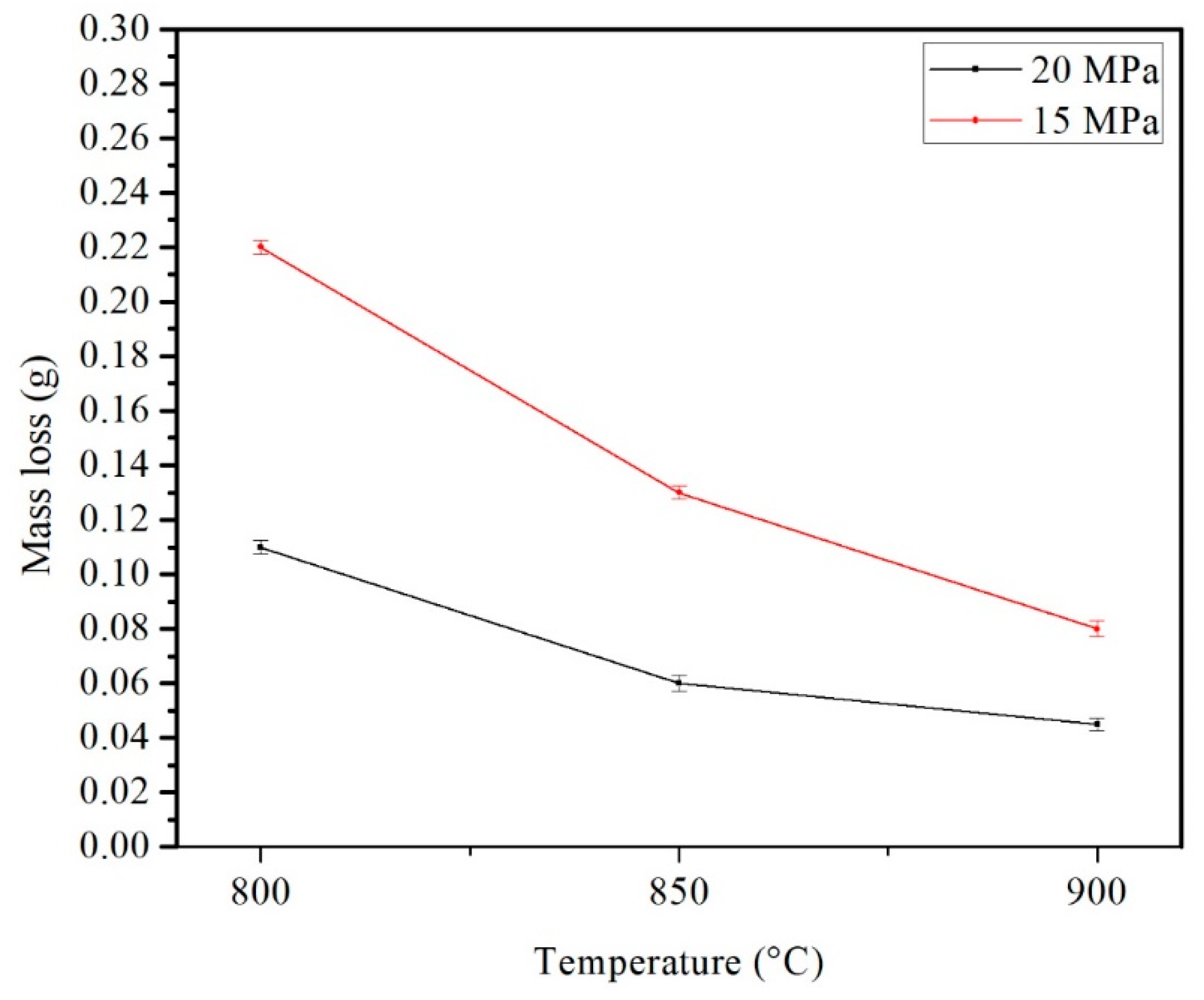



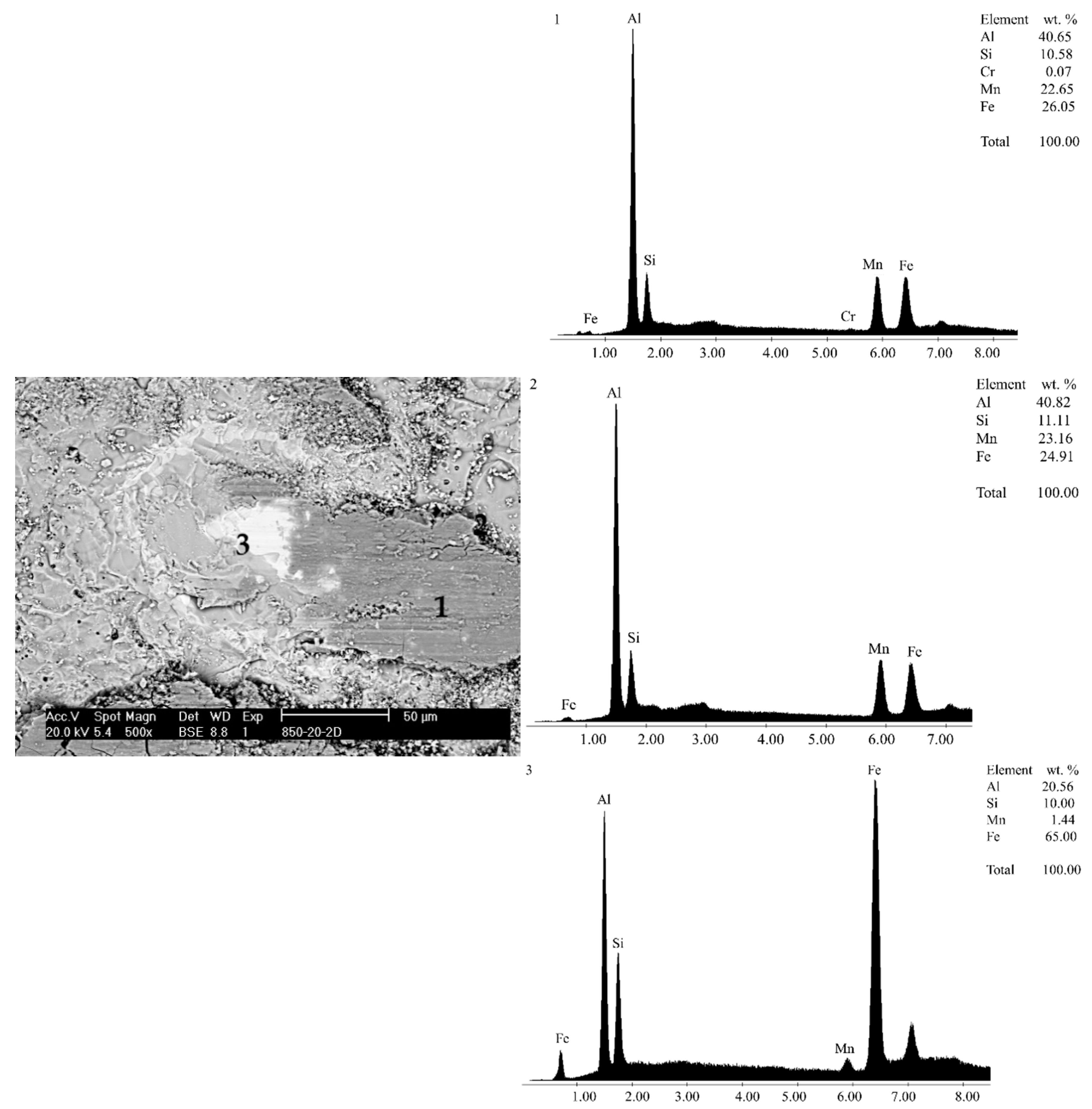

| Temperature | Substrate | STD | Al-Diffusion | STD | Interface | STD | Coating | STD |
|---|---|---|---|---|---|---|---|---|
| 800 | 95.78 | 5 | 255.84 | 5.84 | 400.68 | 8.57 | 444.48 | 5.02 |
| 850 | 118.8 | 3.52 | 258.01 | 7.19 | 430.02 | 6.35 | 497.38 | 10.37 |
| 900 | 125 | 3.85 | 275 | 5.72 | 450 | 5.32 | 540 | 4.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar Ibarra, M.; Flores Valdés, A.; Escobedo Bocardo, J.C.; Flores Saldívar, A.A. Wearing Behavior of the α-Al9FeMnSi Intermetallic Compound Formed by Reactive Sintering onto AISI 304L Stainless Steel. Metals 2020, 10, 1567. https://doi.org/10.3390/met10121567
Salazar Ibarra M, Flores Valdés A, Escobedo Bocardo JC, Flores Saldívar AA. Wearing Behavior of the α-Al9FeMnSi Intermetallic Compound Formed by Reactive Sintering onto AISI 304L Stainless Steel. Metals. 2020; 10(12):1567. https://doi.org/10.3390/met10121567
Chicago/Turabian StyleSalazar Ibarra, Martín, Alfredo Flores Valdés, José Concepción Escobedo Bocardo, and Alfredo Alan Flores Saldívar. 2020. "Wearing Behavior of the α-Al9FeMnSi Intermetallic Compound Formed by Reactive Sintering onto AISI 304L Stainless Steel" Metals 10, no. 12: 1567. https://doi.org/10.3390/met10121567
APA StyleSalazar Ibarra, M., Flores Valdés, A., Escobedo Bocardo, J. C., & Flores Saldívar, A. A. (2020). Wearing Behavior of the α-Al9FeMnSi Intermetallic Compound Formed by Reactive Sintering onto AISI 304L Stainless Steel. Metals, 10(12), 1567. https://doi.org/10.3390/met10121567




