Improved Friction and Wear Properties of Al6061-Matrix Composites Reinforced by Cu-Ni Double-Layer-Coated Carbon Fibers
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Electroless Cu, Ni, and Cu-Ni Coating of SCFs
2.3. Composite Manufacturing
2.4. Wear Tests
2.5. Composite Characterization
3. Results and Discussion
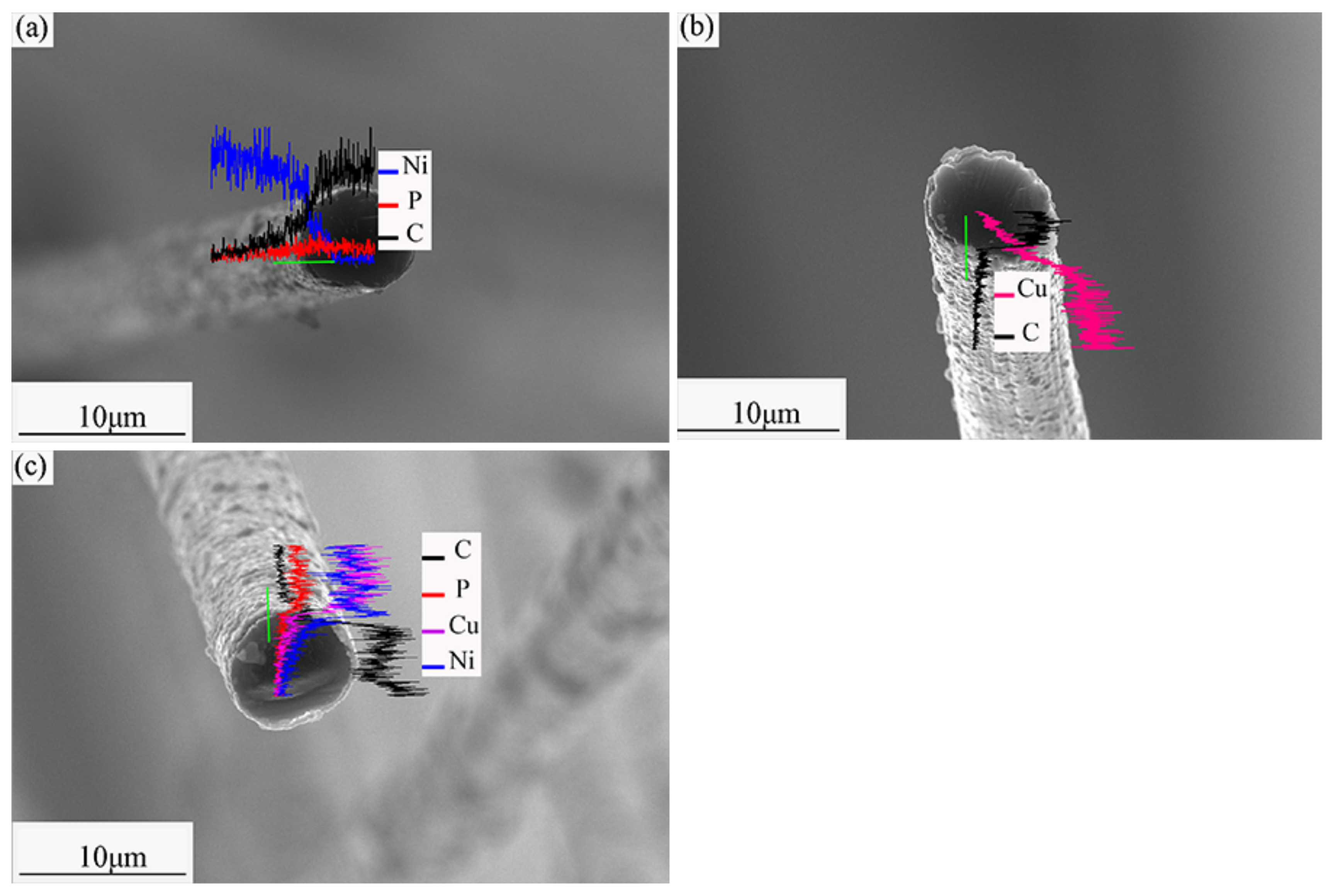
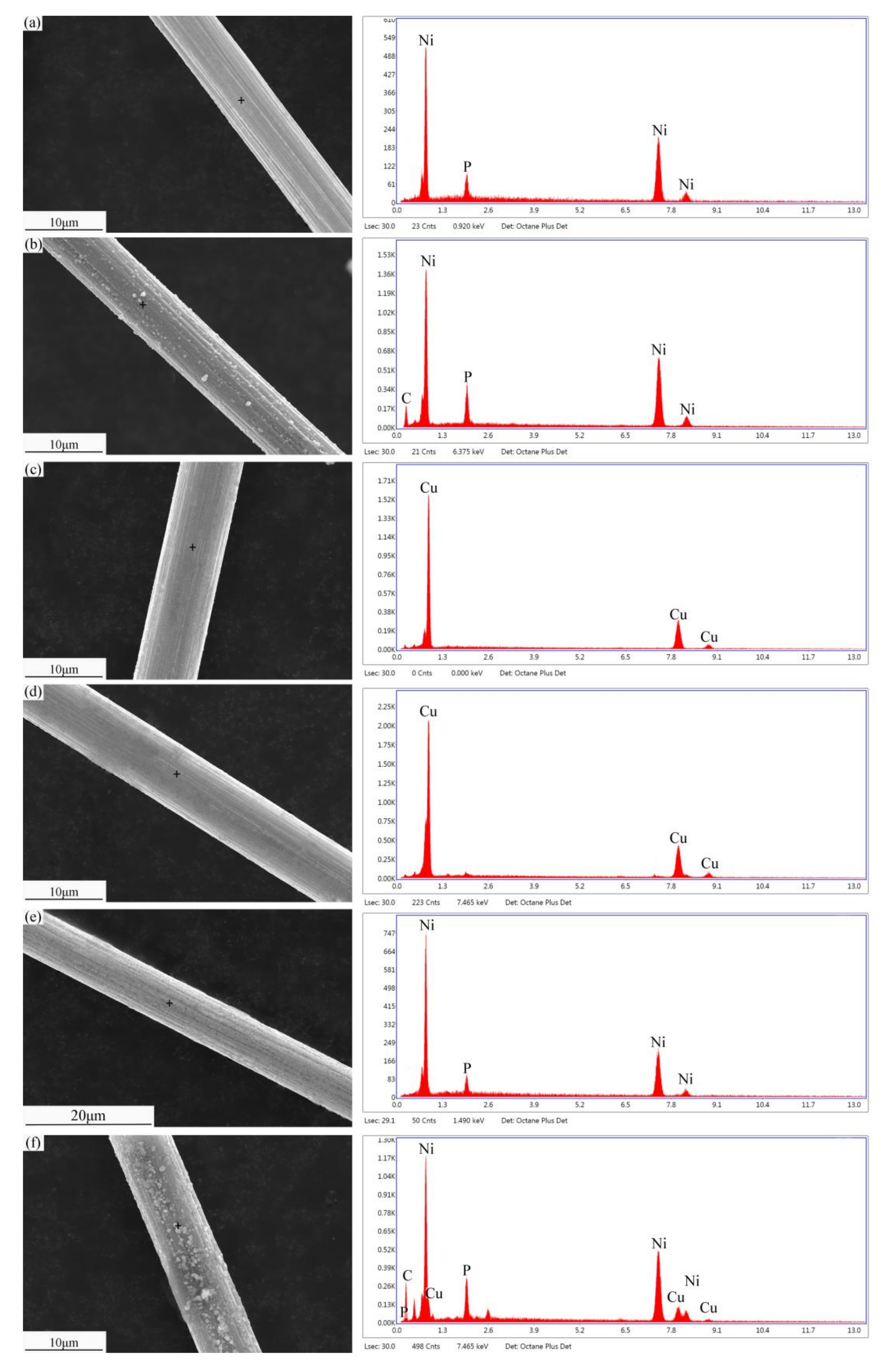
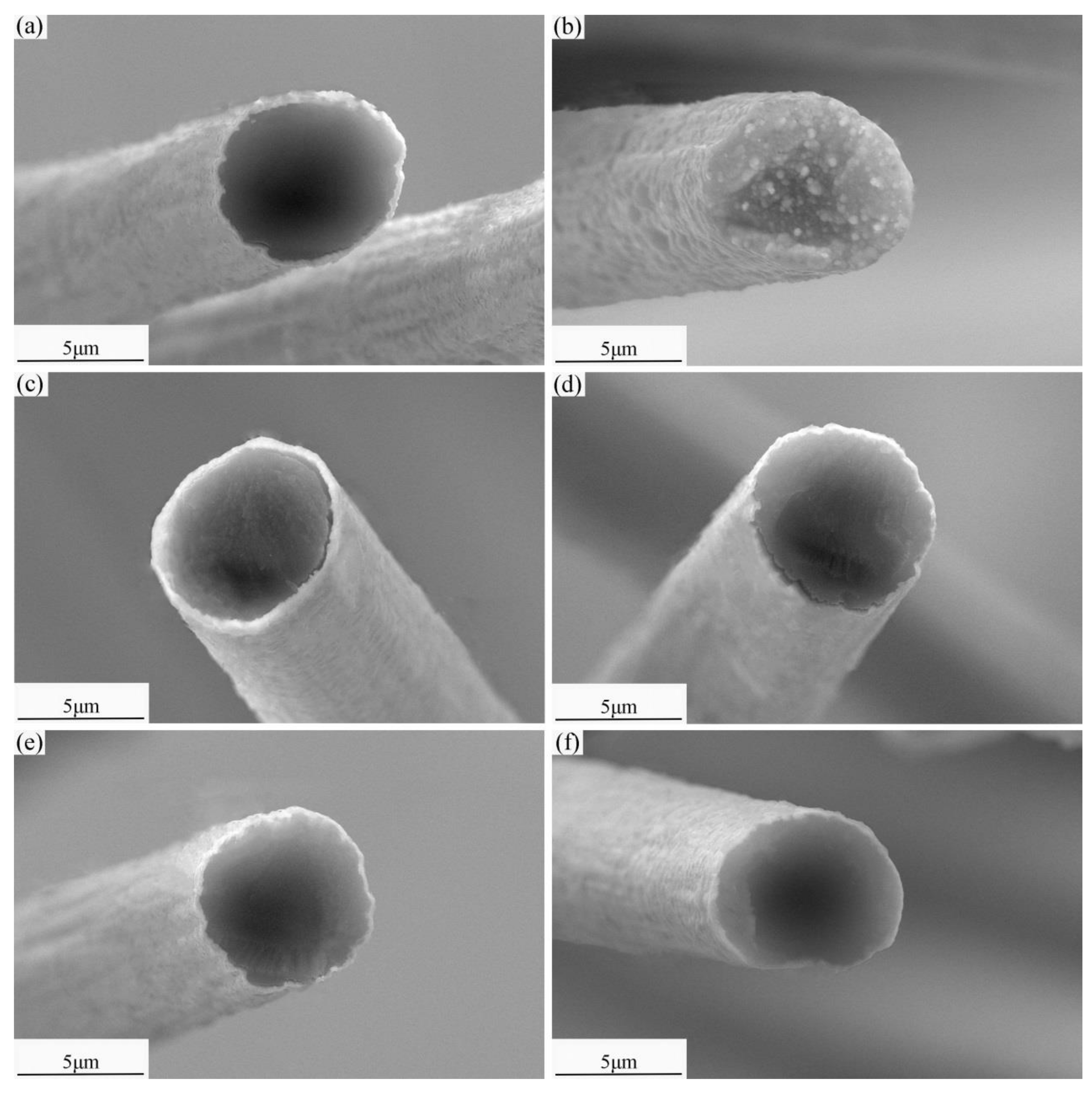
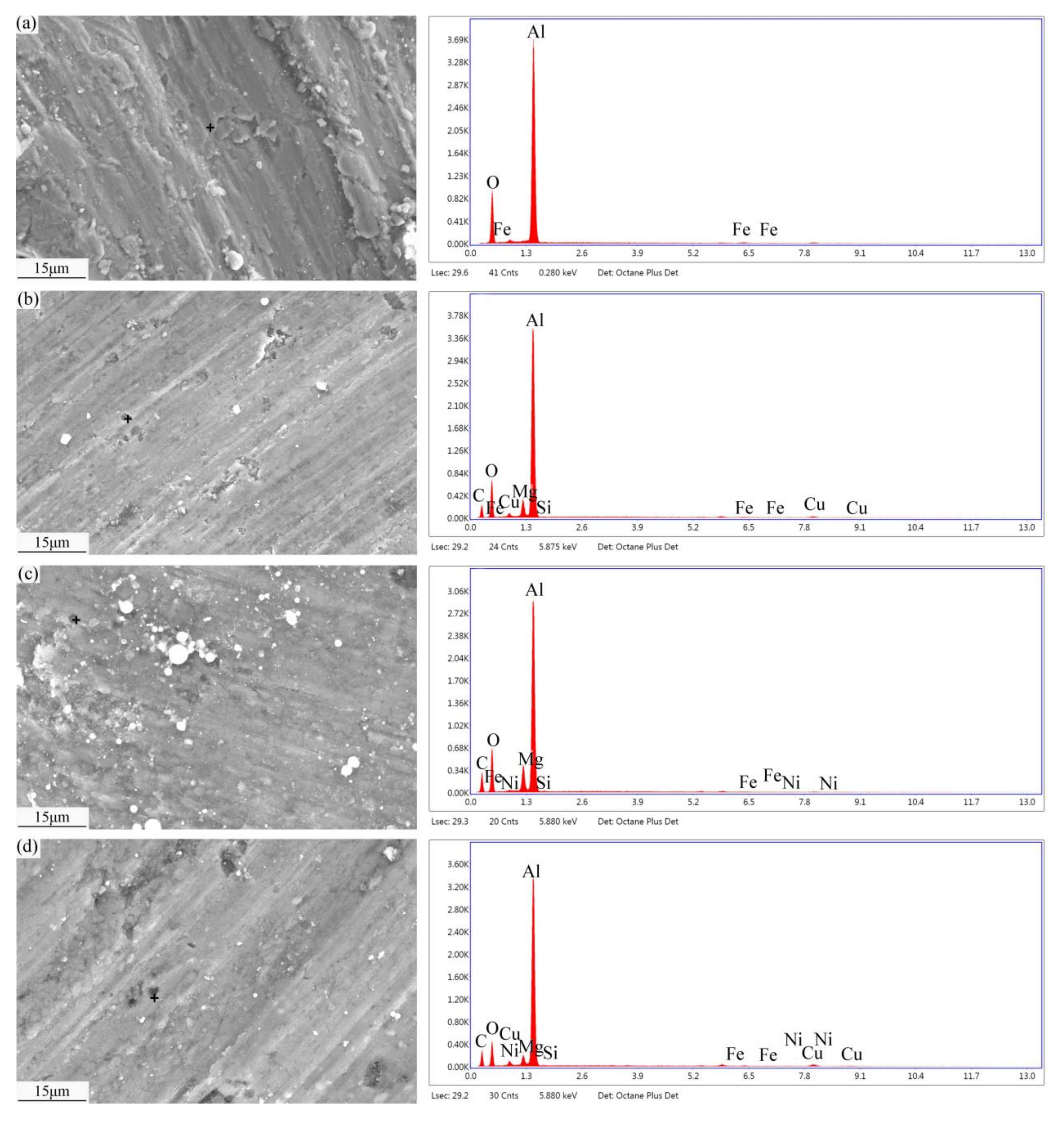
3.1. Microstructure of Modified SCFs
3.2. Heat Treatment of Modified SCFs.
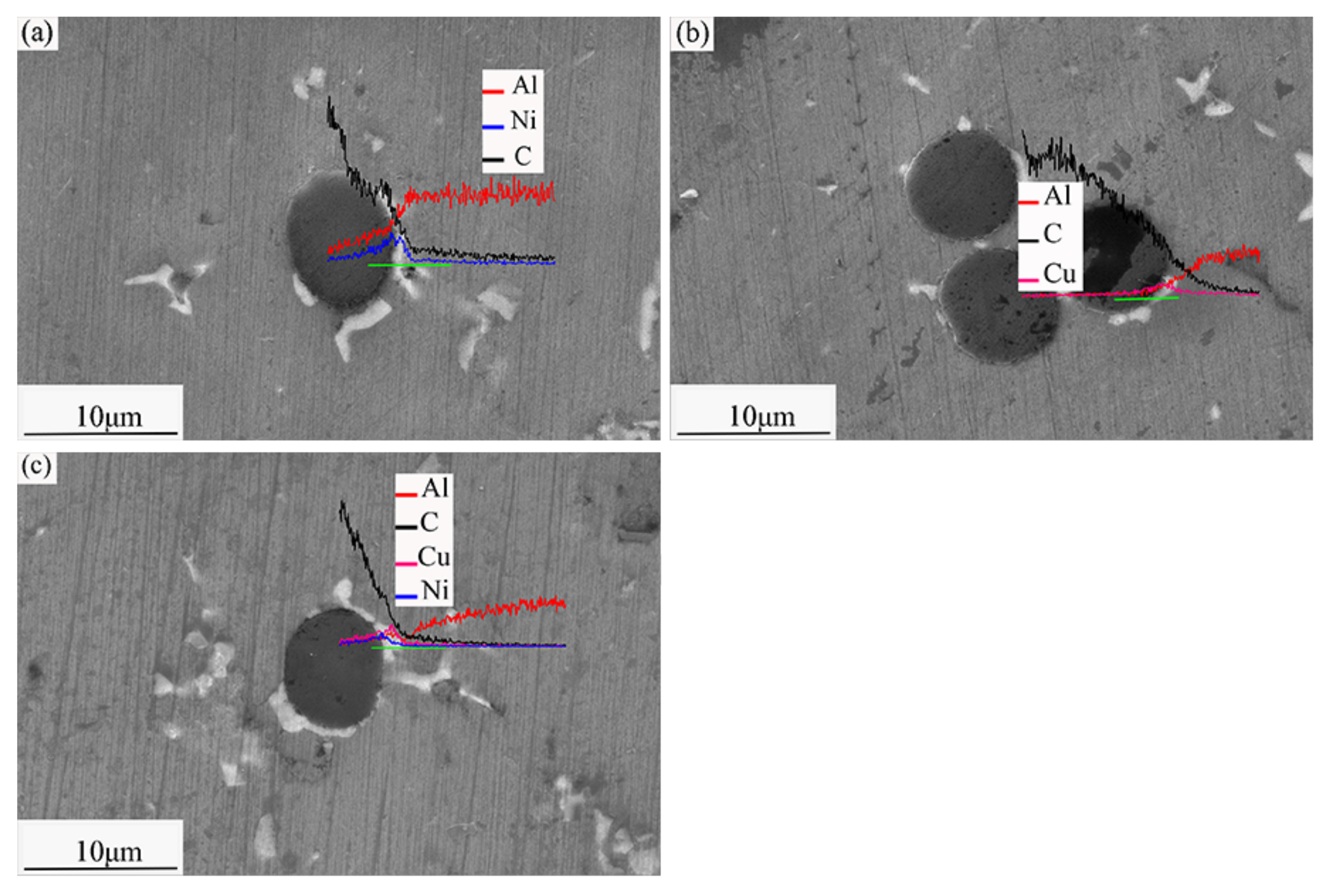
3.3. Composite Microstructures
3.4. Composite Hardness
- After the solid solution treatment, the high-concentration solid solution is transformed via the rapid cooling process to a supersaturated solid solution, yielding the solid solution strengthening effect. After the aging treatment, the phases of β and α form a common lattice strain region in the matrix, which impedes dislocation movement and improves the deformation resistance of the material.
- Because of the difference in the thermal expansion coefficient between the SCF and the matrix, the high-density dislocations generated at the interface are favorable for the heterogeneous nucleation of the precipitated strengthening phase, and act as short-circuit diffusion channels to improve the diffusion speed of solute atoms and promote the nucleation and growth of the precipitated phase. The interaction of these two aspects is shown as the acceleration of precipitation strengthening kinetics on the microscale level and the acceleration of hardening on the macroscale level.
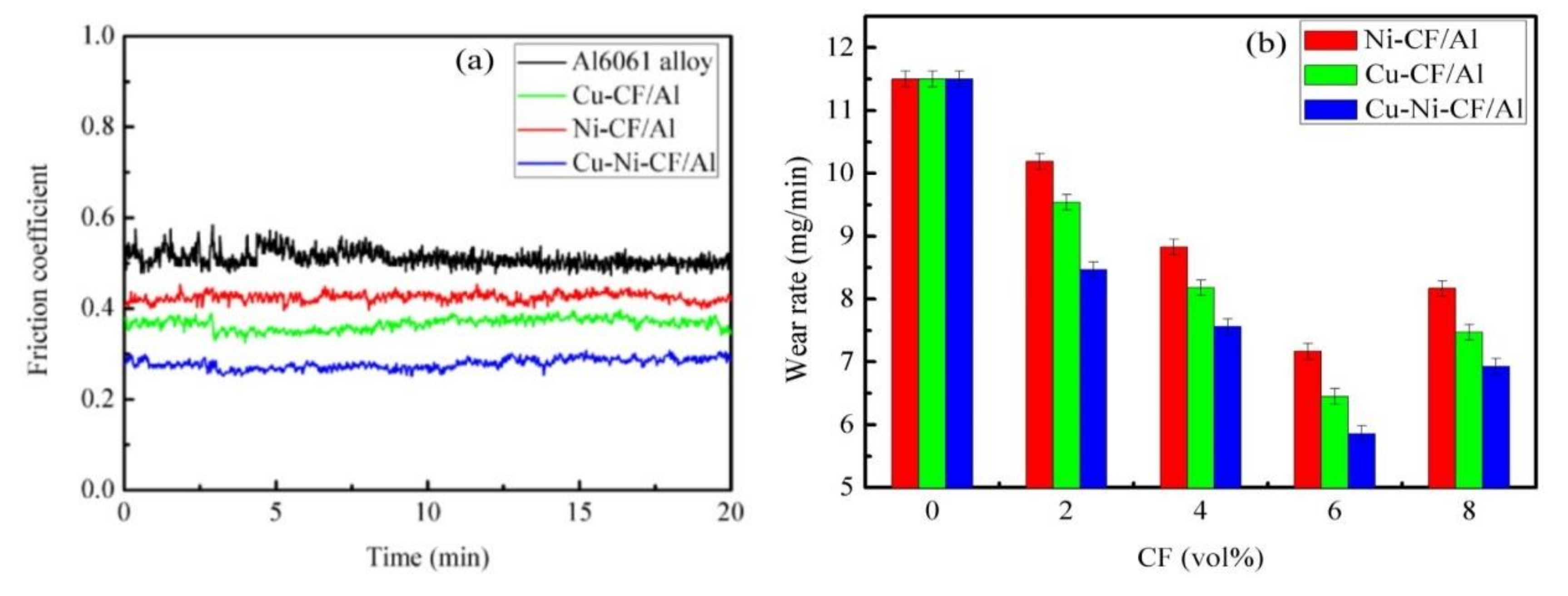
- Influence of the composite hardness: Improved composite hardness can enhance wear resistance and prevent the serious wear caused by plastic deformation. The hardness of the composites proceeds in the order Cu-Ni-SCF/Al > Cu-SCF/Al > Ni-SCF/Al > Al6061 alloy. Therefore, the abrasive resistance of the Cu-Ni-SCF/Al composite is the highest and its friction coefficient is the lowest.
- Good adhesion between the coated SCFs and the Al6061 alloy matrix: The composites show higher interfacial bonding strengths, load-carrying capacities, and wear resistances [26]. The interfacial bonding strengths of the composites increase in the order Ni-SCF < Cu-SCF/Al < Cu-Ni-SCF/Al. After heat treatment, Ni diffusion in the Ni-SCF causes fiber damage that destroys the SCF structures and weakens the strengthening effect of CF on composites. The interfaces in the Cu-SCF/Al composite are weakly mechanically bonded. However, in Cu-Ni-SCF, Cu as an intermediate transition layer hinders Ni diffusion and forms a diffusion-bonded interface. The interfacial combination between the matrix and reinforcement is better and thus the wear resistance of the MMC is better [27,28]. With a higher interfacial bonding strength, the SCFs are more difficult to remove from the Al matrix and the resistance to shear friction, deformation, and fracture are improved.
- Effect of mechanical mixing friction layers: The significant improvement in the abrasion resistance of the composite is due to the formation of mechanical mixing friction layers (MMLs) comprising fine mixtures of hard intermetallic compounds, metallic oxides, and carbon fragments. Various metal oxides and SCF fragments are mixed in MMLs on the composite surfaces, thus enhancing the composite wear resistance [29]. During the wear tests, the materials are softened as the surface temperatures are increased. However, C and Cu have good thermal conductivity and reduce the damage caused by the softening effect. Therefore, the wear resistances of the Cu-Ni-SCF/Al and Cu-SCF/Al composites are better than that of the Ni-SCF/Al composite.
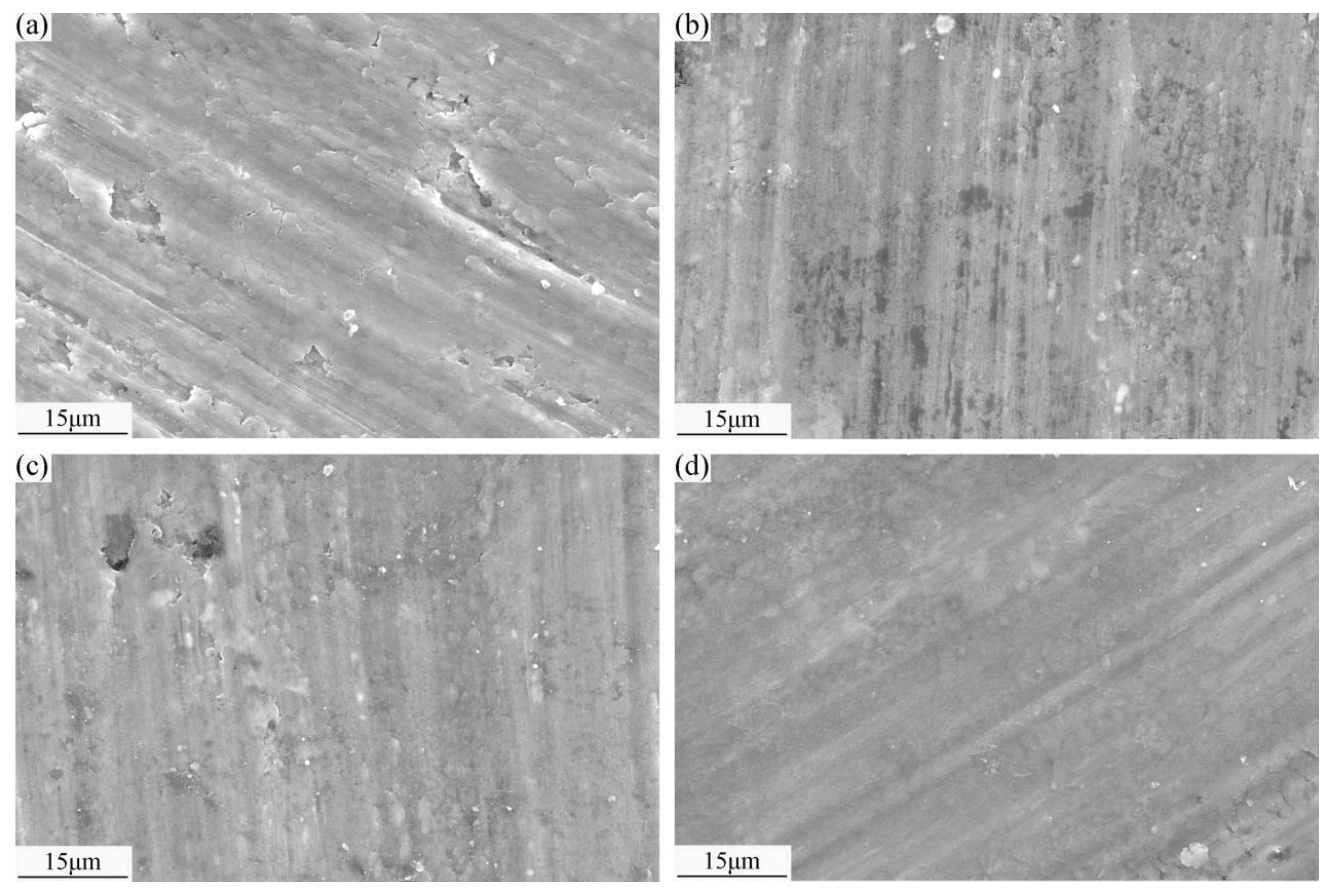
3.5. Wear Mechanisms
- Under the action of external force, SCFs are extruded and ground into fine particles that form a lubricating carbon film on the worn surface. The pinning effect of the SCFs restricts matrix deformation and improves the deformation resistance of the composite, thus reducing crack generation.
- The MML formed on the worn surface includes hard intermetallic compounds, such as Al3Ni and CuAl2, and metal oxides, such as CuO and Al2O3, which improve wear resistance. The C and Cu improve the thermal conductivity and thereby reduce the softening effect caused by temperature increases. Therefore, the abrasive particles of the composites are small and the wear marks are shallow.
- The SCFs randomly distributed in the matrix prevent the nucleation, deflection, and expansion of microcracks at the interface, release concentrated stress, and prevent material damage; therefore, the wear resistance of the composites is better than that of the Al6061 alloy.
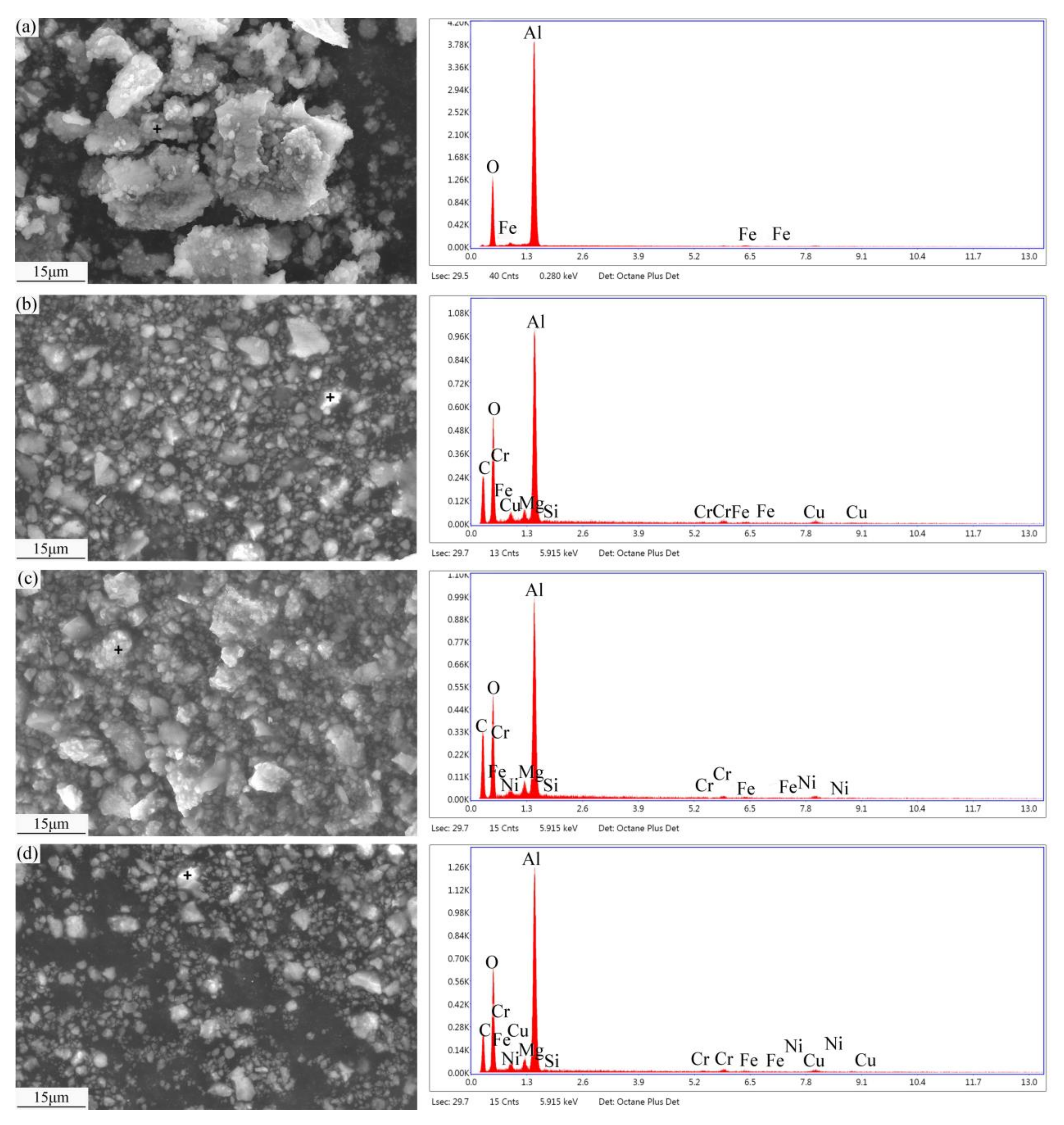
3.6. Wear Debris
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, Q.; Cao, X.; Li, J.; Chen, G.; Liu, Q. Improved mechanical properties in friction stir processed carbon fiber reinforced aluminum composites. J. Tsinghua Univ. 2017, 57, 792–797. [Google Scholar] [CrossRef]
- Harris, S.J. Cast metal matrix composites. Mater. Sci. Technol. 2013, 4, 231–239. [Google Scholar] [CrossRef]
- Yao, J.J.; Chu, D.; Han, Y.Q.; Ben, L.H.; Wu, C.J. Continuous Carbon Fiber Reinforced Aluminum Matrix Composites—A Review. Adv. Mater. Res. 2013, 850, 173–176. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Ramesh, C.; Adarsha, H.; Pramod, S.; Khan, Z. Tribological characteristics of innovative Al6061–carbon fiber rod metal matrix composites. Mater. Des. 2013, 50, 597–605. [Google Scholar] [CrossRef]
- Xia, L.; Jia, B.; Zeng, J.; Xu, J. Wear and mechanical properties of carbon fiber reinforced copper alloy composites. Mater. Charact. 2009, 60, 363–369. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, J.; Hua, W.; Xia, L.; Deng, X.; Wang, S.; Tao, P.; Ma, X.; Yao, J.; Jiang, C.; et al. Wear performance of the lead free tin bronze matrix composite reinforced by short carbon fibers. Appl. Surf. Sci. 2009, 255, 6647–6651. [Google Scholar] [CrossRef]
- Lancin, M.; Marhic, C. TEM study of carbon fibre reinforced aluminium matrix composites: Influence of brittle phases and interface on mechanical properties. J. Eur. Ceram. Soc. 2000, 20, 1493–1503. [Google Scholar] [CrossRef]
- Lalet, G.; Kurita, H.; Miyazaki, T.; Kawasaki, A.; Silvain, J.-F. Microstructure of a carbon fiber-reinforced aluminum matrix composite fabricated by spark plasma sintering in various pulse conditions. J. Mater. Sci. 2014, 49, 3268–3275. [Google Scholar] [CrossRef]
- Rams, J.; Ureña, A.; Escalera, M.; Sánchez, M. Electroless nickel coated short carbon fibres in aluminium matrix composites. Compos. Part A: Appl. Sci. Manuf. 2007, 38, 566–575. [Google Scholar] [CrossRef]
- Singh, B.B.; Balasubramanian, M. Processing and properties of copper-coated carbon fibre reinforced aluminium alloy composites. J. Mater. Process. Technol. 2009, 209, 2104–2110. [Google Scholar] [CrossRef]
- Ramesh, C.S.; Prasad, T.B. Friction and Wear Behavior of Graphite-Carbon Short Fiber Reinforced Al–17%Si Alloy Hybrid Composites. J. Tribol. 2008, 131, 014501. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Tang, Y.; Shen, B.; Hu, W. Friction and wear properties of short carbon fiber reinforced aluminum matrix composites. Wear 2009, 266, 733–738. [Google Scholar] [CrossRef]
- Gupta, N.; Nguyen, N.Q.; Rohatgi, P.K. Analysis of active cooling through nickel coated carbon fibers in the solidification processing of aluminum matrix composites. Compos. Part B Eng. 2011, 42, 916–925. [Google Scholar] [CrossRef]
- Seong, H.; López, H.; Rohatgi, P. Microsegregation during Solidification of Graphitic Fiber-Reinforced Aluminum Alloys under External Heat Sinks. Met. Mater. Trans. A 2007, 38, 138–149. [Google Scholar] [CrossRef]
- Ureña, A.; Rams, J.; Escalera, M.; Sánchez, M. Characterization of interfacial mechanical properties in carbon fiber/aluminium matrix composites by the nanoindentation technique. Compos. Sci. Technol. 2005, 65, 2025–2038. [Google Scholar] [CrossRef]
- Ureña, A.; Rams, J.; Escalera, M.; Sánchez, M. Effect of copper electroless coatings on the interaction between a molten Al–Si–Mg alloy and coated short carbon fibres. Compos. Part A: Appl. Sci. Manuf. 2007, 38, 1947–1956. [Google Scholar] [CrossRef]
- Tzeng, S.-S. Catalytic graphitization of electroless Ni–P coated PAN-based carbon fibers. Carbon 2006, 44, 1986–1993. [Google Scholar] [CrossRef]
- Sui, X.M.; Xu, X.L.; Zheng, X.M. Preparation and Characterization of Carbon Fibre Reinforced Aluminium Matrix Composite. Materials Science Forum 2011, 686, 758–764. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xu, Z.F.; Yu, H.; Yan, Q.-S.; Xiong, B.W. Fabrication of Continuous Nickel-Coated Carbon Fiber Reinforced Aluminum Matrix Composites Using Low Gas Pressure Infiltration Method. Adv. Mater. Res. 2013, 634, 1914–1917. [Google Scholar] [CrossRef]
- Hajjari, E.; Divandari, M.; Arabi, H. Effect of Applied Pressure and Nickel Coating on Microstructural Development in Continuous Carbon Fiber-Reinforced Aluminum Composites Fabricated by Squeeze Casting. Mater. Manuf. Process. 2011, 26, 599–603. [Google Scholar] [CrossRef]
- Ureña, A.; Rams, J.; Campo, M.; Sánchez, M. Effect of reinforcement coatings on the dry sliding wear behaviour of aluminium/SiC particles/carbon fibres hybrid composites. Wear 2009, 266, 1128–1136. [Google Scholar] [CrossRef]
- Deng, X.Y.; Zhang, G.H.; Qiang, C.W.; Cairang, Z.M.; Wang, T. Friction and Wear Properties of Aluminum Matrix Composites Reinforced by Coated Carbon Fibers. Adv. Mater. Res. 2013, 684, 342–346. [Google Scholar] [CrossRef]
- Härtl, J.; Ooi, J.Y. Experiments and simulations of direct shear tests: Porosity, contact friction and bulk friction. Granular. Matter. 2008, 10, 263–271. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Ge, Y.; Ran, L.; Peng, K.; Yi, M. Surface structures of PAN-based carbon fibers and their influences on the interface formation and mechanical properties of carbon-carbon composites. Compos. Part A: Appl. Sci. Manuf. 2016, 90, 480–488. [Google Scholar] [CrossRef]
- Li, S.-H.; Chao, C.-G. Effects of carbon fiber/Al interface on mechanical properties of carbon-fiber-reinforced aluminum-matrix composites. Met. Mater. Trans. A 2004, 35, 2153–2160. [Google Scholar] [CrossRef]
- Moustafa, S.; El-Badry, S.; Sanad, A.; Kieback, B. Friction and wear of copper–graphite composites made with Cu-coated and uncoated graphite powders. Wear 2002, 253, 699–710. [Google Scholar] [CrossRef]
- Thakur, S.K.; Dhindaw, B.K. The influence of interfacial characteristics between SiCp and Mg/Al metal matrix on wear, coefficient of friction and microhardness. Wear 2001, 247, 191–201. [Google Scholar] [CrossRef]
- Ramesh, C.; Keshavamurthy, R.; Channabasappa, B.; Ahmed, A. Microstructure and mechanical properties of Ni–P coated Si3N4 reinforced Al6061 composites. Mater. Sci. Eng. A 2009, 502, 99–106. [Google Scholar] [CrossRef]




| Element | Si | Fe | Cu | Mg | Mn | Zn | Cr | Sn | Ti | Ni | Pb | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage (wt.%) | 0.5 | 0.7 | 0.2 | 0.8 | 0.15 | 0.01 | 0.18 | 0.001 | 0.02 | <0.05 | 0.02 | Balance |
| C/(%) | σb/(MPa) | E/(GPa) | D/(μm) | Ρ/(g·cm−3) |
|---|---|---|---|---|
| 98.5 | 3280.5 | 201.1 | 6.9 | 1.76 |
| Stage and Conditions | Concentration of Chemicals |
|---|---|
| Metallization pH (8–9) Temperature (65–70 °C) Time (5 min) | 25 g/L NiSO4 × 2H2O 30 g/L NH4Cl 25 g/L NaH2PO2 × H2O 25 g/L Na3C6H5O7 × 2H2O 1 mg/L CH4N2S NH3·H2O to control the pH |
| Stage and Conditions | Concentration of Chemicals |
|---|---|
| Metallization pH (12–13) Temperature (55–60 °C) Time (5 min) | 25 g/L CuSO4 × 5H2O 20 g/L EDTA 20 g/L NaOH 15 mg/L K4Fe(CN)6 × 3H2O 20 mg/L C10H8N2 15 mL/L HCHO |
| Stage and Conditions | Concentration of Chemicals |
|---|---|
| Metallization pH (9) Temperature (65–70 °C) Time (10 min) | 10 g/L CuSO4 × 5H2O 2–3 g/L NiSO4 × 2H2O 30 g/L H3BO3 10 mg/L C10H8N2 50 mg/L C6H5SO2Na 25 g/L Na3C6H5O7 × 2H2O 30 g/L NaH2PO2 × H2O 6.5 mg/L C14H14N3SO3Na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Yuan, H.; Cheng, X.; Zhao, X.; Yang, M.; Fan, Y.; Cao, X. Improved Friction and Wear Properties of Al6061-Matrix Composites Reinforced by Cu-Ni Double-Layer-Coated Carbon Fibers. Metals 2020, 10, 1542. https://doi.org/10.3390/met10111542
Dong S, Yuan H, Cheng X, Zhao X, Yang M, Fan Y, Cao X. Improved Friction and Wear Properties of Al6061-Matrix Composites Reinforced by Cu-Ni Double-Layer-Coated Carbon Fibers. Metals. 2020; 10(11):1542. https://doi.org/10.3390/met10111542
Chicago/Turabian StyleDong, Shuhan, Huiyong Yuan, Xiaochao Cheng, Xue Zhao, Mingxu Yang, Yongzhe Fan, and Xiaoming Cao. 2020. "Improved Friction and Wear Properties of Al6061-Matrix Composites Reinforced by Cu-Ni Double-Layer-Coated Carbon Fibers" Metals 10, no. 11: 1542. https://doi.org/10.3390/met10111542
APA StyleDong, S., Yuan, H., Cheng, X., Zhao, X., Yang, M., Fan, Y., & Cao, X. (2020). Improved Friction and Wear Properties of Al6061-Matrix Composites Reinforced by Cu-Ni Double-Layer-Coated Carbon Fibers. Metals, 10(11), 1542. https://doi.org/10.3390/met10111542




