Interfacial Aspects of Metal Matrix Composites Prepared from Liquid Metals and Aqueous Solutions: A Review
Abstract
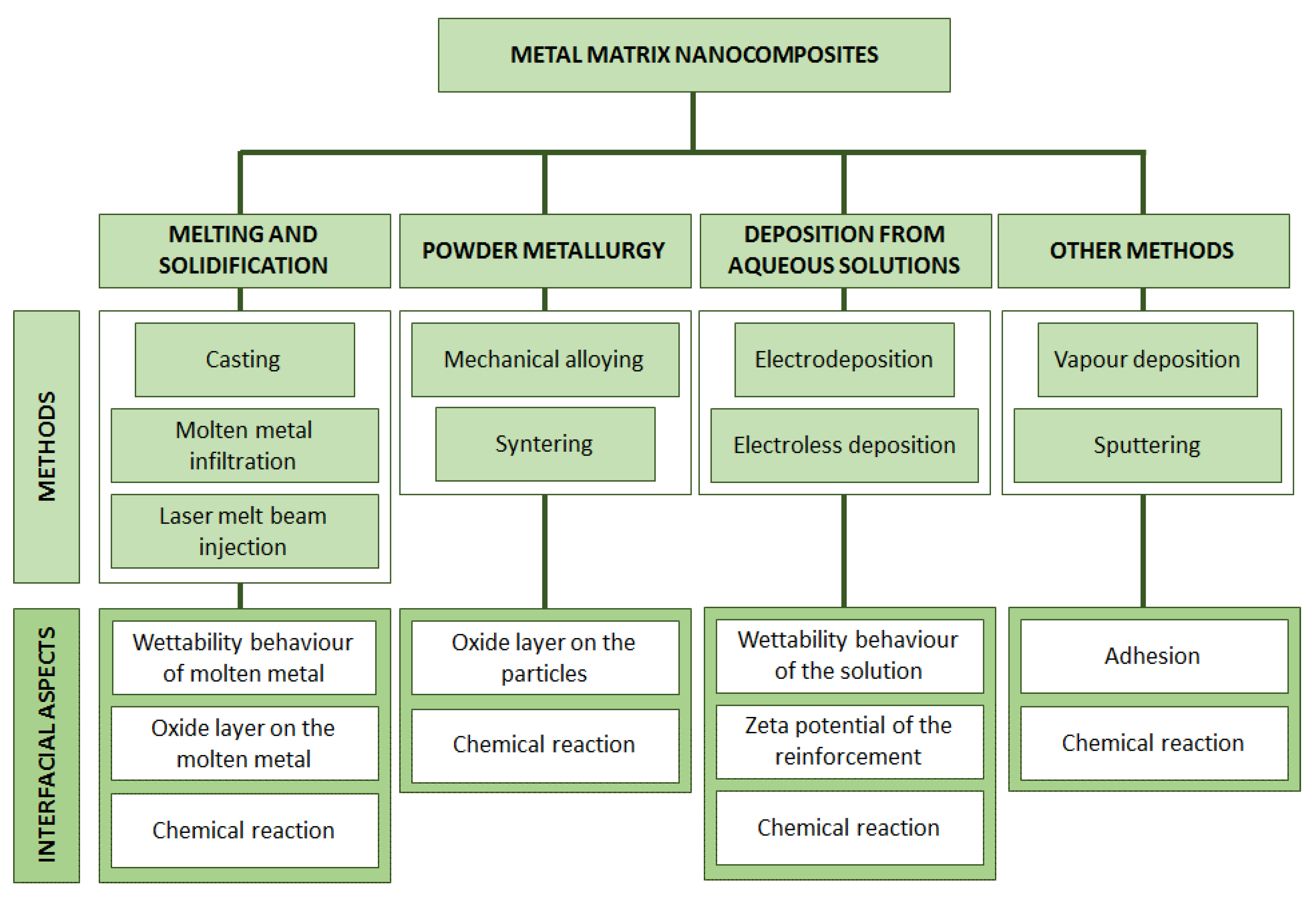
:1. Introduction
2. Using Melted Metal Matrix for the Preparation of the Composite
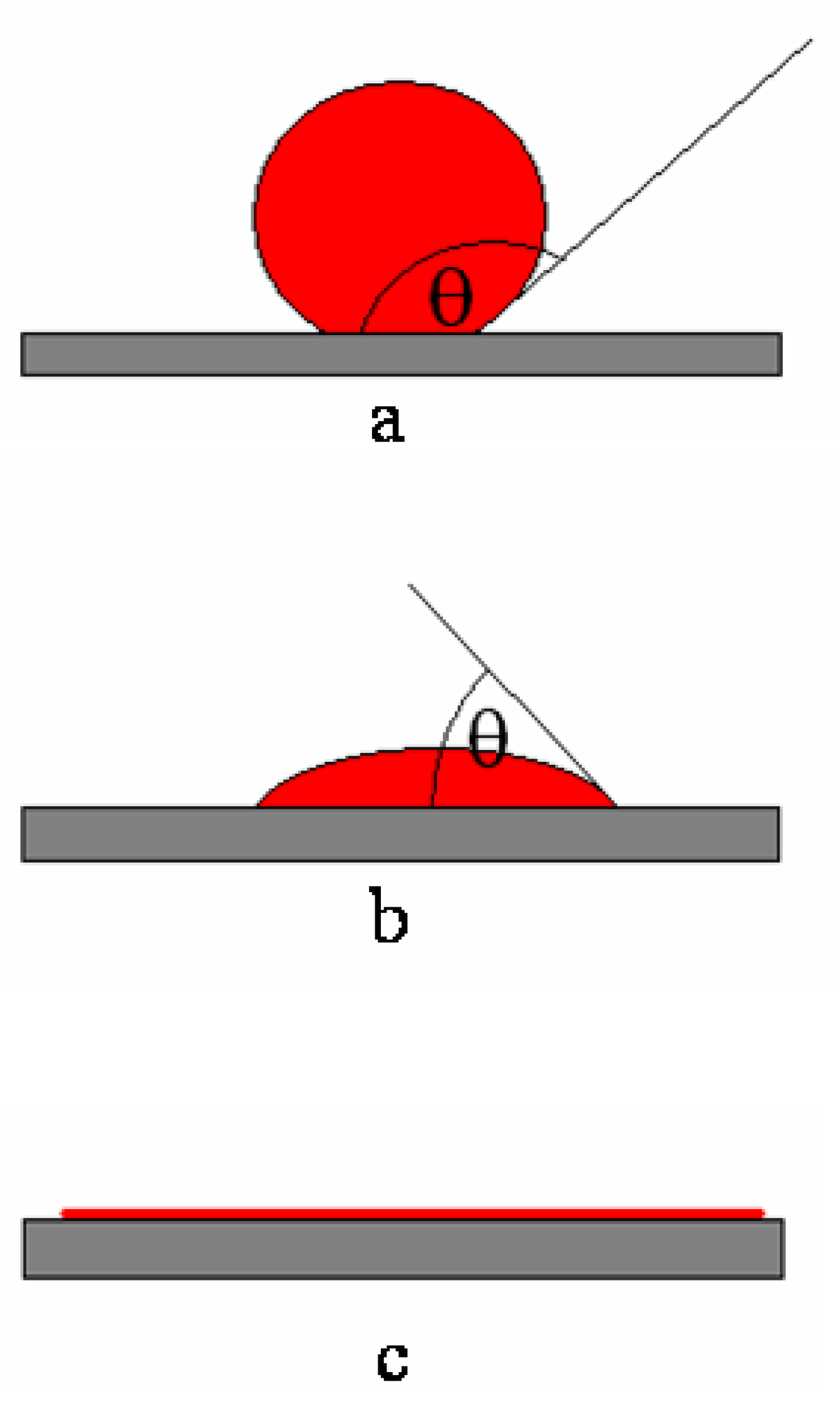
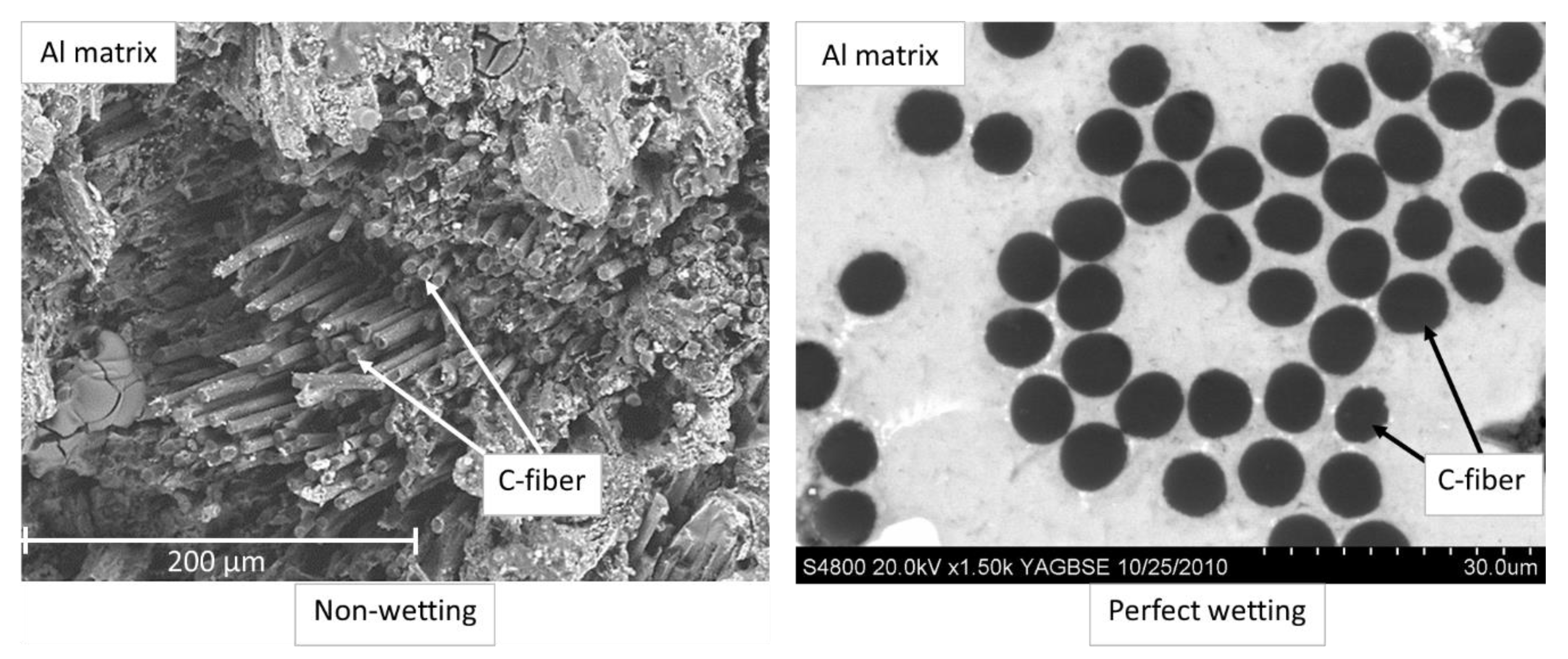
2.1. Role of the Wettability of the Reinforcement by Molten Metal
- —the surface energy between the ceramic and the vacuum, J/m2
- —the interfacial energy between the ceramic and the melt, J/m2
- —the surface tension of the melt, J/m2.
- (a)
- ≥90°: the melt does not wet the ceramic; the adhesion energy is less than the surface tension of the melt;
- (b)
- <90°: the melt wets the ceramic; the adhesion energy of the melt surface is greater than the surface tension of the melt (but less than twice the surface tension);
- (c)
- =0°: the melt completely wets the ceramic; the adhesion energy is at least twice the surface tension of the melt.
- -
- stirring does not promote the immersion of the particles in the matrix: the particles float on the surface of the molten metal, regardless of the speed of stirring;
- -
- upon stirring, the ceramic particles are incorporated into the solidifying metal, but during the remelting of the composite, when the metal is completely melted, the composite can be separated;
- -
- using magnesium improves wetting, but if the magnesium content of the aluminum melt exceeds 1 wt.%, the viscosity of the melt increases.
2.2. Improvement of the Wetting Behavior
2.2.1. Modification of the Surface of the Reinforcement
2.2.2. Molten Salt-Assisted Process
- The oxide layer is dissolved from the surface of molten aluminum due to the K2TiF6. In the molten salt, an oxo-fluoro complex compound is formed which prevents the further reaction of Ti ions.
- As the molten salt/aluminum ratio increases, the Ti content increases in the system (salt/aluminum/graphite). Due to higher Ti content, the free Ti ion number will be raised so the possibility of the exchange reaction also grows.
- At a critical Ti content and temperature (higher than 0.4 w% Ti in aluminum and above 750 °C), TiC can be formed at the Al/C interface. Due to the TiC nanolayer at the interface, the wettability of graphite by molten aluminum will be increased.
- During the cooling of the sample, an Al3Ti intermetallic phase will be created due to a decrease in the solubility of Ti.
3. Composite Preparation from Aqueous Solutions
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Zou, C.; Chen, Z.; Li, M.; Wang, W.; Li, R.; Kang, H. In situ synthesis of TiB2 particulate reinforced copper matrix composite with a rotating magnetic field. Mater. Des. 2015, 65, 280–288. [Google Scholar] [CrossRef]
- Lekatou, A.; Karantzalis, A.E.; Evangelou, A.; Gousia, V.; Kaptay, G.; Gácsi, Z.; Baumli, P.; Simon, A. Aluminium reinforced by WC and TiC nanoparticles (ex-situ) and aluminide particles (in-situ): Microstructure, wear and corrosion behaviour. Mater. Des. 2015, 65. [Google Scholar] [CrossRef]
- Varol, T.; Canakci, A. The effect of flake microstructure on the preparation and properties of Cu-graphite sintered nanocomposites. Powder Metall. Met. Ceram. 2016, 55, 426–436. [Google Scholar] [CrossRef]
- Wen, X.; Joshi, R. 2D materials-based metal matrix composites. J. Phys. D Appl. Phys. 2020, 53, 423001. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, P.; Du, W.; Liu, B.; Pan, D.; Das, R.; Liu, C.; Guo, Z. An overview of graphene and its derivatives reinforced metal matrix composites: Preparation, properties and applications. Carbon N. Y. 2020, 170, 302–326. [Google Scholar] [CrossRef]
- Güler, Ö.; Bağci, N. A short review on mechanical properties of graphene reinforced metal matrix composites. J. Mater. Res. Technol. 2020, 9, 6808–6833. [Google Scholar] [CrossRef]
- Tabandeh-Khorshid, M.; Ajay, K.; Omrani, E.; Kim, C.; Rohatgi, P. Synthesis, characterization, and properties of graphene reinforced metal-matrix nanocomposites. Compos. Part B Eng. 2020, 183, 107664. [Google Scholar] [CrossRef]
- Saboori, A.; Pavese, M.; Badini, C.; Fino, P. Development of Al- and Cu-based nanocomposites reinforced by graphene nanoplatelets: Fabrication and characterization. Front. Mater. Sci. 2017, 11, 171–181. [Google Scholar] [CrossRef]
- Alipour, M.; Eslami-Farsani, R. Synthesis and characterization of graphene nanoplatelets reinforced AA7068 matrix nanocomposites produced by liquid metallurgy route. Mater. Sci. Eng. A 2017, 706, 71–82. [Google Scholar] [CrossRef]
- Shu, R.; Jiang, X.; Shao, Z.; Sun, D.; Zhu, D.; Luo, Z. Fabrication and mechanical properties of MWCNTs and graphene synergetically reinforced Cu–graphite matrix composites. Powder Technol. 2019, 349, 59–69. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Lahiri, D.; Agarwal, A. Carbon nanotube reinforced metal matrix composites—A review. Int. Mater. Rev. 2010, 55, 41–64. [Google Scholar] [CrossRef]
- Faria, B.; Guarda, C.; Silvestre, N.; Lopes, J.N.C. CNT-reinforced iron and titanium nanocomposites: Strength and deformation mechanisms. Compos. Part B Eng. 2020, 187, 107836. [Google Scholar] [CrossRef]
- Azarniya, A.; Safavi, M.; Sovizi, S.; Azarniya, A.; Chen, B.; Madaah Hosseini, H.; Ramakrishna, S. Metallurgical Challenges in Carbon Nanotube-Reinforced Metal Matrix Nanocomposites. Metals 2017, 7, 384. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, R.; Kumar, A.; Raj, H.; Kumar, S.; Tiwari, S.; Khan, S.; Sharma, V. Fabrication and characterization of stir cast Al2024/SiCp metal matrix composite. Mater. Today Proc. 2019, 26, 3316–3320. [Google Scholar] [CrossRef]
- Saxena, A.; Singh, N.; Kumar, D.; Gupta, P. Effect of Ceramic Reinforcement on the Properties of Metal Matrix Nanocomposites. Mater. Today Proc. 2017, 4, 5561–5570. [Google Scholar] [CrossRef]
- Taherzadeh Mousavian, R.; Azari Khosroshahi, R.; Yazdani, S.; Brabazon, D.; Boostani, A.F. Fabrication of aluminum matrix composites reinforced with nano- to micrometer-sized SiC particles. Mater. Des. 2016, 89, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Du, M.; Fan, K.; Ye, C.; Zhang, B. Fabrication and mechanical properties of network structured titanium alloy matrix composites reinforced with Ti2AlC particulates. Mater. Sci. Eng. A 2020, 776. [Google Scholar] [CrossRef]
- Zhang, W.W.; Hu, Y.; Wang, Z.; Yang, C.; Zhang, G.Q.; Prashanth, K.G.; Suryanarayana, C. A novel high-strength Al-based nanocomposite reinforced with Ti-based metallic glass nanoparticles produced by powder metallurgy. Mater. Sci. Eng. A 2018, 734, 34–41. [Google Scholar] [CrossRef]
- Janovszky, D.; Kristaly, F.; Miko, T.; Sveda, M.; Sycheva, A. Development of novel Ultrafine Grain Cu metal matrix composites reinforced with Ti-Cu-Co-M (M: Ni, Zr) amorphous-nanocrystalline powder. J. Min. Metall. Sect. B Metall. 2018, 54, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Czel, G.; Tomolya, K.; Sveda, M.; Sycheva, A.; Kristaly, F.; Roosz, A.; Janovszky, D. Synthesis and characterization of Zr-based in situ crystal precipitated and liquid phase separated bulk metallic glass composite. J. Non. Cryst. Solids 2017, 458, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, I.; Ranganathan, S.; Gopalakannan, S.; Suresh, S.; Nagakarthigan, K.; Jubendradass, R. Tribological and surface behavior of silicon carbide reinforced aluminum matrix nanocomposite. Surf. Interfaces 2017, 8, 127–136. [Google Scholar] [CrossRef]
- Maleki, A.; Taherizadeh, A.R.; Issa, H.K.; Niroumand, B.; Allafchian, A.R.; Ghaei, A. Development of a new magnetic aluminum matrix nanocomposite. Ceram. Int. 2018, 44, 15079–15085. [Google Scholar] [CrossRef]
- Jabbari, A.H.; Delavar, H.; Sedighi, M. High cycle fatigue behavior of magnesium matrix nanocomposite at elevated temperatures. Mech. Mater. 2020, 142, 103278. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Yang, J.M. Nanocrystalline TiC-reinforced H13 steel matrix nanocomposites fabricated by selective laser melting. Mater. Des. 2016, 96, 150–161. [Google Scholar] [CrossRef]
- Fathy, A.; Elkady, O.; Abu-Oqail, A. Microstructure, mechanical and wear properties of Cu–ZrO2 nanocomposites. Mater. Sci. Technol. 2017, 33, 2138–2146. [Google Scholar] [CrossRef]
- Chawla, K.K. Composite Materials: Science and Engineering, 3rd ed.; Springer: New York, NY, USA, 2012; ISBN 9780387743653. [Google Scholar]
- Sharma, D.K.; Mahant, D.; Upadhyay, G. Manufacturing of metal matrix composites: A state of review. Mater. Today Proc. 2019, 26, 506–519. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bhandari, R.; Aherwar, A.; Pinca-Bretotean, C. A study of fabrication methods of aluminum based composites focused on stir casting process. Mater. Today Proc. 2020, 27, 1608–1612. [Google Scholar] [CrossRef]
- Guan, Z.; Hwang, I.; Li, X. Highly Concentrated WC Reinforced Ag Matrix Nanocomposite Manufactured by Molten Salt Assisted Stir Casting. Proc. Manuf. 2018, 26, 146–151. [Google Scholar] [CrossRef]
- Chandra Kandpal, B.; Kumar, J.; Singh, H. Manufacturing and technological challenges in Stir casting of metal matrix composites—A Review. Mater. Today Proc. 2018, 5, 5–10. [Google Scholar] [CrossRef]
- Kandpal, B.C.; Kumar, J.; Singh, H. Fabrication and characterisation of Al2O3/aluminium alloy 6061 composites fabricated by Stir casting. Mater. Today Proc. 2017, 4, 2783–2792. [Google Scholar] [CrossRef]
- Kumar Koli, D.; Agnihotri Professor, G.; Professor, A. Properties and Characterization of Al-Al2O3 Composites Processed by Casting and Powder Metallurgy Routes (Review) Rajesh Purohit. Int. J. Latest Trends Eng. Technol. 2013, 2, 486–496. [Google Scholar]
- Prasad Reddy, A.; Vamsi Krishna, P.; Rao, R.N. Mechanical and Wear Properties of Aluminum-Based Nanocomposites Fabricated through Ultrasonic Assisted Stir Casting. J. Test. Eval. 2020, 48, 20170560. [Google Scholar] [CrossRef]
- Etemadi, R.; Wang, B.; Pillai, K.M.; Niroumand, B.; Omrani, E.; Rohatgi, P. Pressure infiltration processes to synthesize metal matrix composites–A review of metal matrix composites, the technology and process simulation. Mater. Manuf. Process. 2018, 33, 1261–1290. [Google Scholar] [CrossRef]
- Orbulov, I.N.; Németh, Á.; Dobránszky, J. Composite production by pressure infiltration. Mater. Sci. Forum 2008, 589, 137–142. [Google Scholar] [CrossRef]
- Blucher, J.T.; Dobranszky, J.; Narusawa, U. Aluminium double composite structures reinforced with composite wires. Mater. Sci. Eng. A 2004, 387–389, 867–872. [Google Scholar] [CrossRef]
- Blucher, J.T.; Narusawa, U.; Katsumata, M.; Nemeth, A. Continuous manufacturing of fiber-reinforced metal matrix composite wires—Technology and product characteristics. Compos. Part A Appl. Sci. Manuf. 2001, 32, 1759–1766. [Google Scholar] [CrossRef]
- Cramer, C.L.; Edwards, M.S.; McMurray, J.W.; Elliott, A.M.; Lowden, R.A. Lightweight TiC–(Fe–Al) ceramic–metal composites made in situ by pressureless melt infiltration. J. Mater. Sci. 2019, 54, 12573–12581. [Google Scholar] [CrossRef]
- Yu, W.H.; Sing, S.L.; Chua, C.K.; Kuo, C.N.; Tian, X.L. Particle-reinforced metal matrix nanocomposites fabricated by selective laser melting: A state of the art review. Prog. Mater. Sci. 2019, 104, 330–379. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Zhang, W.; Huan, Y. Preparation of Ni-W/SiC nanocomposite coatings by electrochemical deposition. J. Alloys Compd. 2017, 702, 38–50. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Hassan, M.K.; Rasool, K.; Mahmoud, K.A.; Mohamed, A.M.A.; Jarjoura, G.; Farhat, Z.; Abdullah, A.M. Novel electroless deposited corrosion—Resistant and anti-bacterial NiP–TiNi nanocomposite coatings. Surf. Coat. Technol. 2019, 369, 323–333. [Google Scholar] [CrossRef]
- Farzaneh, A.; Ehteshamzadeh, M.; Can, M.; Mermer, O.; Okur, S. Effects of SiC Particles Size on Electrochemical Properties of Electroless Ni-P-SiC Nanocomposite Coatings 1. Prot. Met. Phys. Chem. Surf. 2016, 52, 632–636. [Google Scholar] [CrossRef]
- Czagány, M.; Baumli, P. Effect of surfactants on the behavior of the Ni-P bath and on the formation of electroless Ni-P-TiC composite coatings. Surf. Coat. Technol. 2019, 361, 42–49. [Google Scholar] [CrossRef]
- Canakci, A.; Varol, T.; Erdemir, F.; Canakci, B.A. The Effect of Flake Powder Metallurgy on the Microstructure and Densification Behavior of B4C Nanoparticle-Reinforced Al-Cu-Mg Alloy Matrix Nanocomposites. Arab. J. Sci. Eng. 2016, 41, 1781–1796. [Google Scholar] [CrossRef]
- Ramachandra, M.; Abhishek, A.; Siddeshwar, P.; Bharathi, V. Hardness and Wear Resistance of ZrO2 Nano Particle Reinforced Al Nanocomposites Produced by Powder Metallurgy. Procedia Mater. Sci. 2015, 10, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Casati, R.; Bonollo, F.; Dellasega, D.; Fabrizi, A.; Timelli, G.; Tuissi, A.; Vedani, M. Ex situ Al-Al2O3 ultrafine grained nanocomposites produced via powder metallurgy. J. Alloys Compd. 2015, 615, S386–S388. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.; Ostovan, F.; Abdul Aziz, S.; Mamat, M. Effect of Milling Time on the Microstructure, Physical and Mechanical Properties of Al-Al2O3 Nanocomposite Synthesized by Ball Milling and Powder Metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarpour, M.R.; Pouresmaeil, A. The influence of CNTs on the microstructure and strength of Al-CNT composites produced by flake powder metallurgy and hot pressing method. Diam. Relat. Mater. 2018, 88, 6–11. [Google Scholar] [CrossRef]
- Akbarpour, M.R. Analysis of Load Transfer Mechanism in Cu Reinforced with Carbon Nanotubes Fabricated by Powder Metallurgy Route. J. Mater. Eng. Perform. 2016, 25, 1749–1756. [Google Scholar] [CrossRef]
- Sadooghi, A.; Hashemi, S.J. Investigating the influence of ZnO, CuO, Al2O3 reinforcing nanoparticles on strength and wearing properties of aluminum matrix nanocomposites produced by powder metallurgy process. Mater. Res. Express 2019, 6, 1–19. [Google Scholar] [CrossRef]
- Kaptay, G. Interfacial Forces in Dispersion Science and Technology. J. Dispers. Sci. Technol. 2012, 33, 130–140. [Google Scholar] [CrossRef]
- Kaptay, G. Classification and general derivation of interfacial forces, acting on phases, situated in the bulk, or at the interface of other phases. J. Mater. Sci. 2005, 40, 2125–2131. [Google Scholar] [CrossRef]
- Kaptay, G. A coherent set of model equations for various surface and interface energies in systems with liquid and solid metals and alloys. Adv. Colloid Interface Sci. 2020, 283, 102212. [Google Scholar] [CrossRef] [PubMed]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B.B. Wettability at High Temperatures; Elsevier: Amsterdam, The Netherlands, 1999; Volume 3, ISBN 0080421466. [Google Scholar]
- Delannay, F.; Froyen, L.; Deruyttere, A. The wetting of solids by molten metals and its relation to the preparation of metal-matrix composites. J. Mater. Sci. 1987, 22, 1–16. [Google Scholar] [CrossRef]
- Kaptay, G. Interfacial phenomena during melt processing of ceramic particle-reinforced metal matrix composites part I. Introduction (incorporation) of solid particles into melts. Mater. Sci. Forum 1996, 215–216, 459–466. [Google Scholar] [CrossRef]
- Asthana, R.; Tewari, S.N. Interfacial and capillary phenomena in solidification processing of metal-matrix composites. Compos. Manuf. 1993, 4, 3–25. [Google Scholar] [CrossRef]
- Kaptay, G. Interfacial Aspects to Produce Particulate Reinforced Metal Matrix Composites. In Affordable Metal-Matrix Composites for High Performance Applications II; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 71–99. [Google Scholar]
- Hashim, J.; Looney, L.; Hashmi, M.S.J. Metal matrix composites: Production by the stir casting method. J. Mater. Process. Technol. 1999, 92–93, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Cheng, X. Ultrasonic-assisted fabrication of metal matrix nanocomposites. J. Mater. Sci. 2004, 39, 3211–3212. [Google Scholar] [CrossRef]
- Rajan, T.P.D.; Pillai, R.M.; Pai, B.C. Reinforcement coatings and interfaces in aluminium metal matrix composites. J. Mater. Sci. 1998, 33, 3491–3503. [Google Scholar] [CrossRef]
- Takács, D.; Sziráki, L.; Török, T.I.; Sólyom, J.; Gácsi, Z.; Gál-Solymos, K. Effects of pre-treatments on the corrosion properties of electroless Ni-P layers deposited on AlMg2 alloy. Surf. Coat. Technol. 2007, 201, 4526–4535. [Google Scholar] [CrossRef]
- Czagány, M.; Baumli, P.; Kaptay, G. The influence of the phosphorous content and heat treatment on the nano-micro-structure, thickness and micro-hardness of electroless Ni-P coatings on steel. Appl. Surf. Sci. 2017, 423. [Google Scholar] [CrossRef]
- Xia, Z.; Zhou, Y.; Mao, Z.; Shang, B. Fabrication of fiber-reinforced metal-matrix composites by variable pressure infiltration. Metall. Trans. B 1992, 23, 295–302. [Google Scholar] [CrossRef]
- Rams, J.; Ureña, A.; Escalera, M.D.; Sánchez, M. Electroless nickel coated short carbon fibres in aluminium matrix composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 566–575. [Google Scholar] [CrossRef]
- Ureña, A.; Rams, J.; Escalera, M.D.; Sánchez, M. Effect of copper electroless coatings on the interaction between a molten Al-Si-Mg alloy and coated short carbon fibres. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1947–1956. [Google Scholar] [CrossRef]
- Alten, A.; Erzi, E.; Gürsoy, Ö.; Hapçı Ağaoğlu, G.; Dispinar, D.; Orhan, G. Production and mechanical characterization of Ni-coated carbon fibers reinforced Al-6063 alloy matrix composites. J. Alloys Compd. 2019, 787, 543–550. [Google Scholar] [CrossRef]
- León, C.A.; Drew, R.A.L. The influence of nickel coating on the wettability of aluminum on ceramics. In Composites Part A: Applied Science and Manufacturing; Elsevier: Amsterdam, The Netherlands, 2002; Volume 33, pp. 1429–1432. [Google Scholar]
- Kretz, F.; Gácsi, Z.; Kovács, J.; Pieczonka, T. The electroless deposition of nickel on SiC particles for aluminum matrix composites. Surf. Coat. Technol. 2004, 180–181, 575–579. [Google Scholar] [CrossRef]
- Trespaillé-Barrau, P.; Suéry, M. Microstructural and mechanical characterisation of aluminium matrix composites reinforced with Ni and NiP coated SiC particles via liquid processing. Mater. Sci. Technol. 1994, 10, 497–504. [Google Scholar] [CrossRef]
- Huang, C.W.; Huang, Y.F.; Aoh, J.N. Strengthening mechanisms of aluminum matrix composite containing Cu-coated SiC particles produced by friction stir processing. J. Chinese Inst. Eng. Trans. Chin. Inst. Eng. A 2019, 42, 653–663. [Google Scholar] [CrossRef]
- Huang, C.-W.; Aoh, J.-N. Friction Stir Processing of Copper-Coated SiC Particulate-Reinforced Aluminum Matrix Composite. Materials 2018, 11, 599. [Google Scholar] [CrossRef] [Green Version]
- Abolkassem, S.A.; Elkady, O.A.; Elsayed, A.H.; Hussein, W.A.; Yehya, H.M. Effect of consolidation techniques on the properties of Al matrix composite reinforced with nano Ni-coated SiC. Results Phys. 2018, 9, 1102–1111. [Google Scholar] [CrossRef]
- Zou, G.; Cao, M.; Lin, H.; Jin, H.; Kang, Y.; Chen, Y. Nickel layer deposition on SiC nanoparticles by simple electroless plating and its dielectric behaviors. Powder Technol. 2006, 168, 84–88. [Google Scholar] [CrossRef]
- Körner, C.; Schäff, W.; Ottmüller, M.; Singer, R.F. Carbon Long Fiber Reinforced Magnesium Alloys. Adv. Eng. Mater. 2000, 2, 327–337. [Google Scholar] [CrossRef]
- Xu, Q.G.; Guo, L.W.; Zhang, L.; Liu, H. Wettability of zirconium-coated alumina by molten aluminum. Surf. Coat. Technol. 2016, 302, 150–157. [Google Scholar] [CrossRef]
- Kaptay, G.; Báder, E.; Bolyán, L. Interfacial forces and energies relevant to production of metal matrix composites. Mater. Sci. Forum 2000, 329, 151–156. [Google Scholar] [CrossRef]
- Ip, S.W.; Sridhar, R.; Toguri, J.M.; Stephenson, T.F.; Warner, A.E.M. Wettability of nickel coated graphite by aluminum. Mater. Sci. Eng. A 1998, 244, 31–38. [Google Scholar] [CrossRef]
- Silva, V.L.; Fernandes, C.M.; Senos, A.M.R. Copper wettability on tungsten carbide surfaces. Ceram. Int. 2016, 42, 1191–1196. [Google Scholar] [CrossRef]
- Kong, B.; Fan, T.; Ru, J. Improved wetting and thermal properties of graphite-Cu composite by Cr-solution immersion method. Diam. Relat. Mater. 2016, 65, 191–197. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Zhou, Y.; Shen, P. Reactive wetting and infiltration of polycrystalline WC by molten Zr2Cu alloy. Scr. Mater. 2011, 64, 229–232. [Google Scholar] [CrossRef]
- Lin, Q.; Sui, R. Wetting of carbide ceramics (B4C, SiC, TiC and ZrC) by molten Ni at 1753 K. J. Alloys Compd. 2015, 649, 505–514. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Meng, J.; Yang, Y.; Zhou, Y. Wettability and interfacial reactions of a low Hf-containing nickel-based superalloy on Al2O3-based, SiO2-based, ZrSiO4, and CoAl2O4 substrates. Ceram. Int. 2020, 46, 22057–22066. [Google Scholar] [CrossRef]
- Roy, R.R.; Sahai, Y. Coalescence behavior of aluminum alloy drops in molten salts. Mater. Trans. JIM 1997, 38, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Juhasz, K.L.; Baumli, P.; Kaptay, G. Fabrication of carbon fibre reinforced, aluminium matrix composite by potassium iodide (KI)—Potassium hexafluoro-titanate (K2TiF6) flux. Materwiss. Werksttech. 2012, 43. [Google Scholar] [CrossRef]
- Baumli, P.; Sychev, J.; Budai, I.; Szabo, J.T.; Kaptay, G. Fabrication of carbon fiber reinforced aluminum matrix composites via a titanium-ion containing flux. Compos. Part A Appl. Sci. Manuf. 2013, 44, 47–50. [Google Scholar] [CrossRef]
- López, V.H.; Kennedy, A.R. Flux-assisted wetting and spreading of Al on TiC. J. Colloid Interface Sci. 2006, 298, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Karantzalis, A.E. The incorporation of ceramic particles in molten aluminium and the relationship to contact angle data. Mater. Sci. Eng. A 1999, 264, 122–129. [Google Scholar] [CrossRef]
- Rocher, J.P.; Quenisset, J.M.; Naslain, R. Wetting improvement of carbon or silicon carbide by aluminium alloys based on a K2ZrF6 surface treatment: Application to composite material casting. J. Mater. Sci. 1989, 24, 2697–2703. [Google Scholar] [CrossRef]
- Lekatou, A.; Gkikas, N.; Karantzalis, A.E.; Kaptay, G.; Gacsi, Z.; Baumli, P.; Simon, A. Effect of Wetting Agent and Carbide Volume Fraction on the Wear Response of Aluminum Matrix Composites Reinforced by WC Nanoparticles and Aluminide Particles. Arch. Metall. Mater. 2017, 62. [Google Scholar] [CrossRef] [Green Version]
- Mohan, P.; Azhagesan, N.; Sivapragash, M. The preparation and mechanical properties of Al metal matrix composites by in-situ method. Int. J. Comput. Aided Eng. Technol. 2018, 10, 35–41. [Google Scholar] [CrossRef]
- Birol, Y. In situ synthesis of Al-TiCp composites by reacting K2TiF6 and particulate graphite in molten aluminium. J. Alloys Compd. 2008, 454, 110–117. [Google Scholar] [CrossRef]
- Mahamani, A.; Jayasree, A.; Mounika, K.; Prasad, K.R.; Sakthivelan, N. Evaluation of mechanical properties of AA6061-TiB2/ZrB2 in-situ metal matrix composites fabricated by K2TiF6-KBF4-K2ZrF6 reaction system. Int. J. Microstruct. Mater. Prop. 2015, 10, 185–200. [Google Scholar] [CrossRef]
- Mallikarjuna, C.; Shashidhara, S.M. The Precipitation Of TiB2 in Aluminum Alloy Melts from the Exothermic Reaction of K2TiF6 and KBF4 Halide Salts and Evaluation of Its Mechanical Properties. In Proceedings of the World Congress on Engineering and Computer Science 2007; Newswood Limited: San Francisco, CA, USA, 2007; pp. 189–194. ISBN 9789889867164. [Google Scholar]
- Kim, D.Y.; Lee, Y.J.; Lee, T.H.; Lee, K.H.; Nersisyan, H.H.; Han, M.H.; Jeong, S.U.; Kang, K.S.; Bae, K.K.; Lee, J.H. Aluminothermic reduction of K2TiF6 to prepare TiC, TiB2, and TiN nanoparticles. Combust. Sci. Technol. 2014, 186, 90–101. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhari, G.P.; Daniel, B.S.S. Strengthening mechanisms in ultrasonically processed aluminium matrix composite with in-situ Al3Ti by salt addition. Compos. Part B Eng. 2018, 140, 27–34. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Du, S.; Zhang, W. Synthesis of a novel Ni–B/YSZ metal-ceramic composite coating via single-step electrodeposition at different current density. Ceram. Int. 2019, 45, 24884–24893. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Huan, Y.; Dong, J. Synthesis and characterization of Ni-B/Al2O3 nanocomposite coating by electrodeposition using trimethylamine borane as boron precursor. Surf. Coat. Technol. 2018, 337, 186–197. [Google Scholar] [CrossRef]
- Ratajski, T.; Kalemba-Rec, I.; Indyka, P.; Ledwig, P.; Szczerba, M.J.; Dubiel, B. Effect of PDDA surfactant on the microstructure and properties of electrodeposited SiO2/Ni nanocomposites. Mater. Charact. 2020, 163, 110229. [Google Scholar] [CrossRef]
- Qu, N.S.; Zhu, D.; Chan, K.C. Fabrication of Ni-CeO2 nanocomposite by electrodeposition. Scr. Mater. 2006, 54, 1421–1425. [Google Scholar] [CrossRef]
- Rasooli, A.; Safavi, M.S.; Babaei, F.; Ansarian, A. Electrodeposited Ni–Fe–Cr2O3 nanocomposite coatings: A survey of influences of Cr2O3 nanoparticles loadings in the electrolyte. J. Alloys Compd. 2020, 822, 153725. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Mohamed, A.M.A.; Jarjoura, G.; Farhat, Z.; Hassan, M.K. Effect of electroless bath composition on the mechanical, chemical, and electrochemical properties of new NiP–C3N4 nanocomposite coatings. Surf. Coat. Technol. 2019, 362, 239–251. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Gyawali, G.; Kshetri, Y.K.; Choi, J.H.; Lee, S.W. Microstructural and electrochemical corrosion properties of electroless Ni-P-TaC composite coating. Surf. Coat. Technol. 2020, 381. [Google Scholar] [CrossRef]
- Hanachi, M.; Seyedraoufi, Z.S.; Abouei, V. Advanced Ceramics Progress Investigation of Microstructure, Hardness, and Corrosion Resistance of Ni-P-GO Electroless Nanocomposite Coating on AZ31D Alloy Surface. Adv. Ceram. Prog. 2020, 6, 55–62. [Google Scholar] [CrossRef]
- Gholizadeh-Gheshlaghi, M.; Seifzadeh, D.; Shoghi, P.; Habibi-Yangjeh, A. Electroless Ni-P/nano-WO3 coating and its mechanical and corrosion protection properties. J. Alloys Compd. 2018, 769, 149–160. [Google Scholar] [CrossRef]
- Akyol, A.; Algul, H.; Uysal, M.; Akbulut, H.; Alp, A. A novel approach for wear and corrosion resistance in the electroless Ni-P-W alloy with CNFs co-depositions. Appl. Surf. Sci. 2018, 453, 482–492. [Google Scholar] [CrossRef]
- MacLean, M.; Farhat, Z.; Jarjoura, G.; Fayyad, E.; Abdullah, A.; Hassan, M. Fabrication and investigation of the scratch and indentation behaviour of new generation Ni-P-nano-NiTi composite coating for oil and gas pipelines. Wear 2019, 426–427, 265–276. [Google Scholar] [CrossRef]
- León-Patiño, C.A.; García-Guerra, J.; Aguilar-Reyes, E.A. Tribological characterization of heat-treated Ni-P and Ni-P-Al2O3 composite coatings by reciprocating sliding tests. Wear 2019, 426–427, 330–340. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Ashrafi, A. Characterisations of low phosphorus electroless Ni and composite electroless Ni-P-SiC coatings on A356 aluminium alloy. Trans. Inst. Met. Finish. 2018, 96, 52–56. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef] [Green Version]
- Kaya, B.; Gulmez, T.; Demirkol, M. Study on the electroless Ni-B nano-composite coatings. AIP 2009, 1127, 62–73. [Google Scholar]
- Wang, L.Y.; Tu, J.P.; Chen, W.X.; Wang, Y.C.; Liu, X.K.; Olk, C.; Cheng, D.H.; Zhang, X.B. Friction and wear behavior of electroless Ni-based CNT composite coatings. Wear 2003, 254, 1289–1293. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Z.; Li, M.K.; Shi, Y.L.; Huang, Y.; Li, H.L. Synthesis and properties of electroless Ni-P-Nanometer Diamond composite coatings. Surf. Coat. Technol. 2005, 191, 161–165. [Google Scholar] [CrossRef]
- Chintada, V.B.; Koona, R. Preparation and properties of composite electroless Ni-P-ZnO coatings. Mater. Res. Innov. 2020, 24, 67–74. [Google Scholar] [CrossRef]
- Franco, M.; Sha, W.; Aldic, G.; Malinov, S.; Çimenoğlu, H. Effect of Reinforcement and Heat Treatment on Elevated Temperature Sliding of Electroless Ni–P/SiC Composite Coatings; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Dong, D.; Chen, X.H.; Xiao, W.T.; Yang, G.B.; Zhang, P.Y. Preparation and properties of electroless Ni-P-SiO2 composite coatings. Appl. Surf. Sci. 2009, 255, 7051–7055. [Google Scholar] [CrossRef]
- Gadhari, P.; Sahoo, P. Optimization of Coating Process Parameters to Improve Microhardness of Ni-P-TiO2 Composite Coatings. Mater. Today Proc. 2015, 2, 2367–2374. [Google Scholar] [CrossRef]
- Zielińska, K.; Stankiewicz, A.; Szczygieł, I. Electroless deposition of Ni-P-nano-ZrO2 composite coatings in the presence of various types of surfactants. J. Colloid Interface Sci. 2012, 377, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Z.A.; El Badry, S.A.; Aal, A.A. Electroless deposition and characterization of Ni-P-WC composite alloys. Surf. Coat. Technol. 2007, 201, 5948–5953. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Gyawali, G.; Kshetri, Y.K.; Choi, J.-H.; Lee, S.W. Influence of SiC and TiC nanoparticles reinforcement on the microstructure, tribological, and scratch resistance behavior of electroless Ni-P coatings. Nanotechnology 2020, 31, 104001. [Google Scholar] [CrossRef]
- Rana, A.R.K.; Farhat, Z. Preparation and tribological characterization of graphene incorporated electroless Ni-P composite coatings. Surf. Coat. Technol. 2019, 369, 334–346. [Google Scholar] [CrossRef]
- Zadeh, K.M.; Shakoor, R.A.; Radwan, A.B. Structural and Electrochemical Properties of Electrodeposited Ni-P nanocomposite Coatings Containing Mixed Ceramic Oxide Particles. Int. J. Electrochem. Sci. 2016, 11, 7020–7030. [Google Scholar] [CrossRef]
- Salari Mehr, M.; Akbari, A.; Damerchi, E. Electrodeposited Ni-B/SiC micro- and nano-composite coatings: A comparative study. J. Alloys Compd. 2019, 782, 477–487. [Google Scholar] [CrossRef]
- Xiang, L.; Shen, Q.; Zhang, Y.; Bai, W.; Nie, C. One-step electrodeposited Ni-graphene composite coating with excellent tribological properties. Surf. Coat. Technol. 2019, 373, 38–46. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Bathini, L.; Sundararajan, G. Tribological Behavior of Pulsed Electrodeposited Ni-W/SiC Nanocomposites. J. Mater. Eng. Perform. 2018, 27, 5236–5245. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W. Microstructural, surface and electrochemical properties of pulse electrodeposited Ni–W/Si3N4 nanocomposite coating. Ceram. Int. 2018, 44, 19907–19918. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Sun, W.; Reed, P.A.S.; Walsh, F.C. Synthesis and properties of electrodeposited Ni-Co/WS2 nanocomposite coatings. Coatings 2019, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wei, D.; Xu, R.; Mai, Y.; Zhang, L.; Jie, X. Electroplated Co-Ni/WS2 Composite Coating with Excellent Tribological and Anticorrosion Performance. Tribol. Trans. 2020, 1–12. [Google Scholar] [CrossRef]
- Góral, A. Nanoscale structural defects in electrodeposited Ni/Al2O3 composite coatings. Surf. Coat. Technol. 2017, 319, 23–32. [Google Scholar] [CrossRef]
- Pavlatou, E.A.; Stroumbouli, M.; Gyftou, P.; Spyrellis, N. Hardening effect induced by incorporation of SiC particles in nickel electrodeposits. J. Appl. Electrochem. 2006, 36, 385–394. [Google Scholar] [CrossRef]
- Mahalingam, T.; Raja, M.; Thanikaikarasan, S.; Sanjeeviraja, C.; Velumani, S.; Moon, H.; Kim, Y.D. Electrochemical deposition and characterization of Ni-P alloy thin films. Mater. Charact. 2007, 58, 800–804. [Google Scholar] [CrossRef]
- Gwon, Y.G.; Lee, J.W.; Shin, H.C. Template-free, single-step electrochemical synthesis of nickel phosphide thin films with one-dimensional nano-pore channels. J. Appl. Electrochem. 2020, 50, 139–147. [Google Scholar] [CrossRef]
- He, Y.D.; Fu, H.F.; Li, X.G.; Gao, W. Microstructure and properties of mechanical attrition enhanced electroless Ni-P plating on magnesium alloy. Scr. Mater. 2008, 58, 504–507. [Google Scholar] [CrossRef]
- Cheong, W.J.; Luan, B.L.; Shoesmith, D.W. Protective coating on Mg AZ91D alloy—The effect of electroless nickel (EN) bath stabilizers on corrosion behaviour of Ni-P deposit. Corros. Sci. 2007, 49, 1777–1798. [Google Scholar] [CrossRef] [Green Version]
- Cheong, W.J.; Luan, B.L.; Shoesmith, D.W. The effects of stabilizers on the bath stability of electroless Ni deposition and the deposit. Appl. Surf. Sci. 2004, 229, 282–300. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Electroless Ni-P composite coatings. J. Appl. Electrochem. 2003, 33, 807–816. [Google Scholar] [CrossRef]
- Liu, D.; Yan, Y.; Lee, K.; Yu, J. Effect of surfactant on the alumina dispersion and corrosion behavior of electroless Ni-P-Al2O3 composite coatings. Mater. Corros. 2009, 60, 690–694. [Google Scholar] [CrossRef]
- Tamilarasan, T.R.; Rajendran, R.; Rajagopal, G.; Sudagar, J. Effect of surfactants on the coating properties and corrosion behaviour of Ni-P-nano-TiO2 coatings. Surf. Coat. Technol. 2015, 276, 320–326. [Google Scholar] [CrossRef]
- Shahbazi, H.; Mahdavi, M.; Alirezaei, S.; Tabatabaei, F. Corrosion behavior of Ni-P-Ag and Ni-P-Al2O3 composite coatings. Mater. Res. Express 2019, 6, 085076. [Google Scholar] [CrossRef]
- Necula, B.S.; Apachitei, I.; Fratila-Apachitei, L.E.; Teodosiu, C.; Duszczyk, J. Stability of nano-/microsized particles in deionized water and electroless nickel solutions. J. Colloid Interface Sci. 2007, 314, 514–522. [Google Scholar] [CrossRef]
- Afroukhteh, S.; Dehghanian, C.; Emamy, M. Corrosion behavior of Ni–P/nano-TiC composite coating prepared in electroless baths containing different types of surfactant. Prog. Nat. Sci. Mater. Int. 2012, 22, 480–487. [Google Scholar] [CrossRef]
- Afroukhteh, S.; Dehghanian, C.; Emamy, M. Preparation of the Ni-P composite coating co-deposited by nano TiC particles and evaluation of it’s corrosion property. Appl. Surf. Sci. 2012, 258, 2597–2601. [Google Scholar] [CrossRef]
- Chen, B.H.; Hong, L.; Ma, Y.; Ko, T.M. Effects of surfactants in an electroless nickel-plating bath on the properties of Ni-P alloy deposits. Ind. Eng. Chem. Res. 2002, 41, 2668–2678. [Google Scholar] [CrossRef]
- Amaral, M.; Lopes, M.A.; Santos, J.D.; Silva, R.F. Wettability and surface charge of Si3N4-bioglass composites in contact with simulated physiological liquids. Biomaterials 2002, 23, 4123–4129. [Google Scholar] [CrossRef]
- Nishizawa, K.; Toriyama, M.; Suzuki, T.; Kawamoto, Y.; Yokogawa, Y.; Nagae, H. Effects of the surface wettability and zeta potential of bioceramics on the adhesiveness of anchorage-dependent animal cells. J. Ferment. Bioeng. 1993, 75, 435–437. [Google Scholar] [CrossRef]
| Liquid Phase | Solid Phase | Temperature | Contact Angle | Reference |
|---|---|---|---|---|
| Al | Al2O3 | 700 °C | 140° | [76] |
| Al (oxide layer free) | Al2O3 | 710 °C | 63° | [77] |
| Al | Zr | 700 °C | ~10° | [76] |
| Al | 670 nm thick Zr-coated alumina | 700 °C | 20° | [76] |
| Al (oxide layer free) | SiO2 | 710 °C | 23° | [77] |
| Al (oxide layer free) | TiB2 | 710 °C | 0° | [77] |
| Al (oxide layer free) | TiB2 | 710 °C | 10° | [77] |
| Al | Graphite | 740 °C | 140° | [78] |
| Al | Ni | 740 °C | 45° | [78] |
| Al | Ni-coated graphite | 740 °C | 27°–45° | [78] |
| Cu | WC | 1080 °C | 25° | [79] |
| Cu | WC | 1133 °C | 0° | [77] |
| Cu | WC-Co | 1080 °C | 6° | [79] |
| Cu | Graphite | 1227 °C | 140° | [80] |
| Cu | Cr3C2 | 1227 °C | 0° | [80] |
| Zr2Cu | WC | 1150 °C | 28° | [81] |
| Ni | B4C | 1480 °C | 102° | [82] |
| Ni | ZrC | 1480 °C | 73° | [82] |
| Ni | TiC | 1480 °C | 25.5° | [82] |
| Ni | SiC | 1480 °C | 104° | [82] |
| Ni-based superalloy | Al2O3-based | 1500 °C | 141° | [83] |
| Ni-based superalloy | SiO2-based | 1500 °C | 143° | [83] |
| Ni-based superalloy | ZrSiO4 | 1500 °C | 136° | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumli, P. Interfacial Aspects of Metal Matrix Composites Prepared from Liquid Metals and Aqueous Solutions: A Review. Metals 2020, 10, 1400. https://doi.org/10.3390/met10101400
Baumli P. Interfacial Aspects of Metal Matrix Composites Prepared from Liquid Metals and Aqueous Solutions: A Review. Metals. 2020; 10(10):1400. https://doi.org/10.3390/met10101400
Chicago/Turabian StyleBaumli, Peter. 2020. "Interfacial Aspects of Metal Matrix Composites Prepared from Liquid Metals and Aqueous Solutions: A Review" Metals 10, no. 10: 1400. https://doi.org/10.3390/met10101400
APA StyleBaumli, P. (2020). Interfacial Aspects of Metal Matrix Composites Prepared from Liquid Metals and Aqueous Solutions: A Review. Metals, 10(10), 1400. https://doi.org/10.3390/met10101400




