Stress–Corrosion Cracking of AISI 316L Stainless Steel in Seawater Environments: Effect of Surface Machining
Abstract
:1. Introduction
2. Experimental Methods
| SPT: | AISI 316L nuts machined by a single-point tool. |
| FT1: | AISI 316L nuts machined by a form tool of the first type. |
| FT2: | AISI 316L nuts machined by a form tool of the second type. |
3. Results
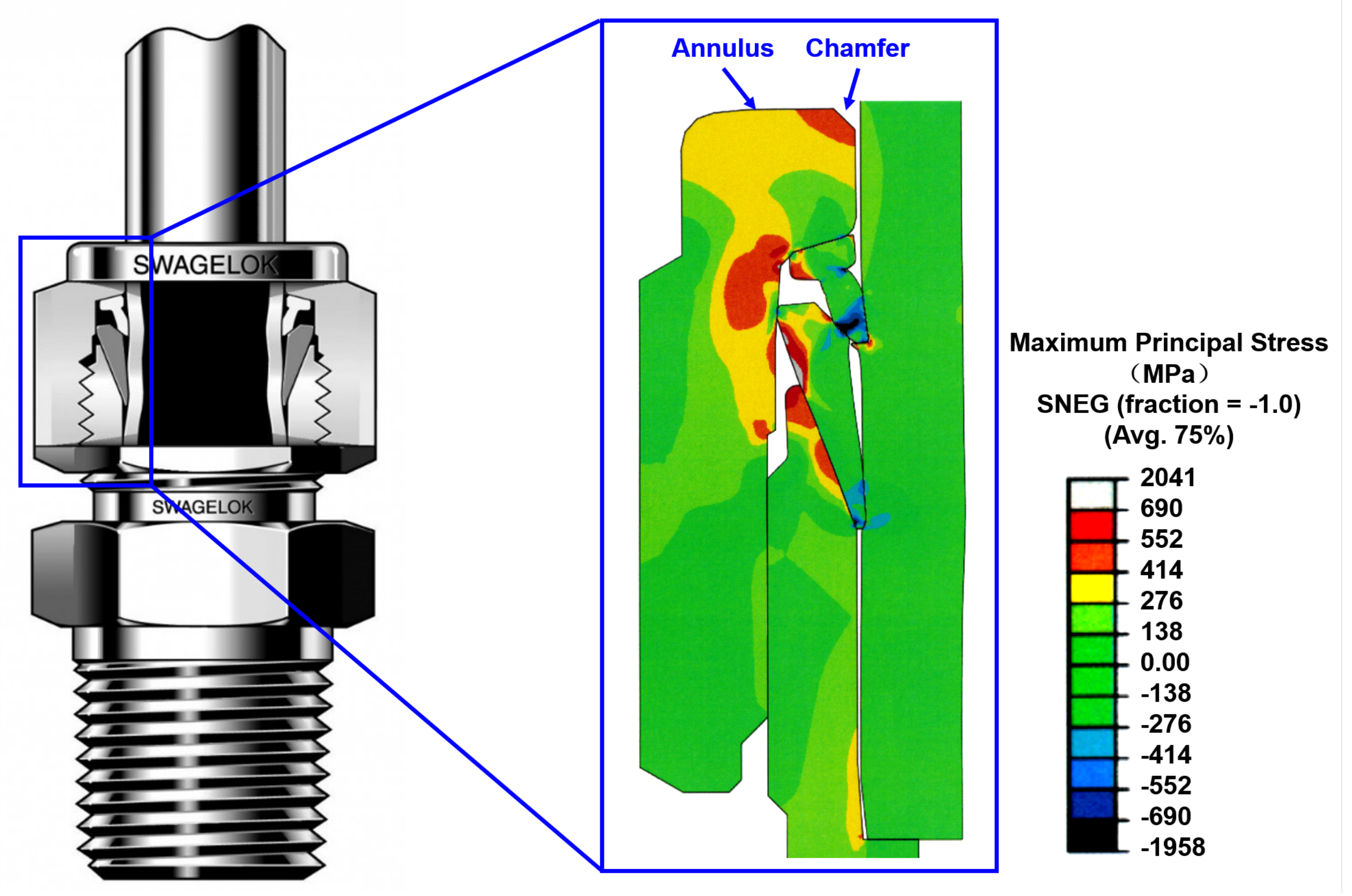
3.1. Stress Distribution
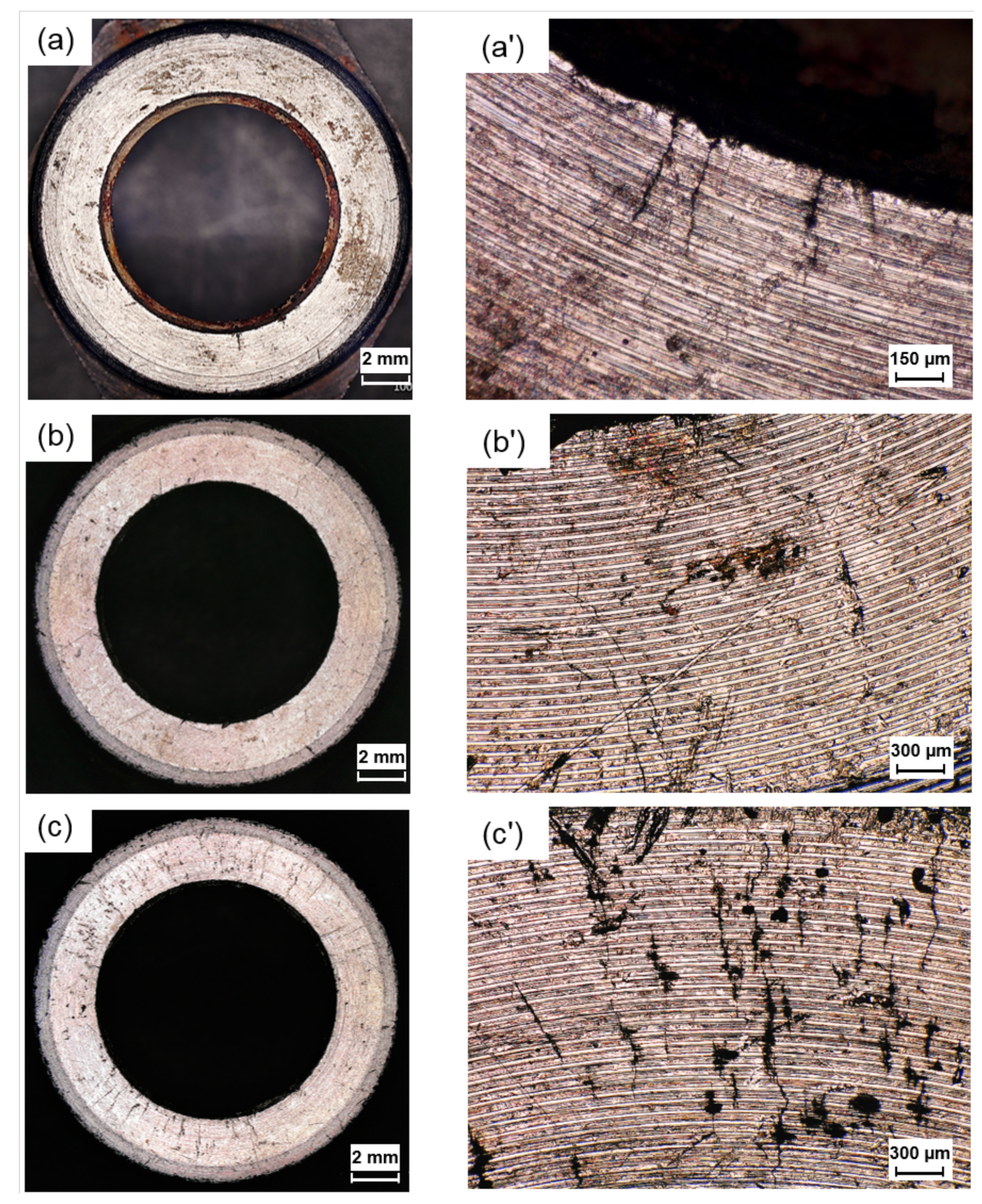
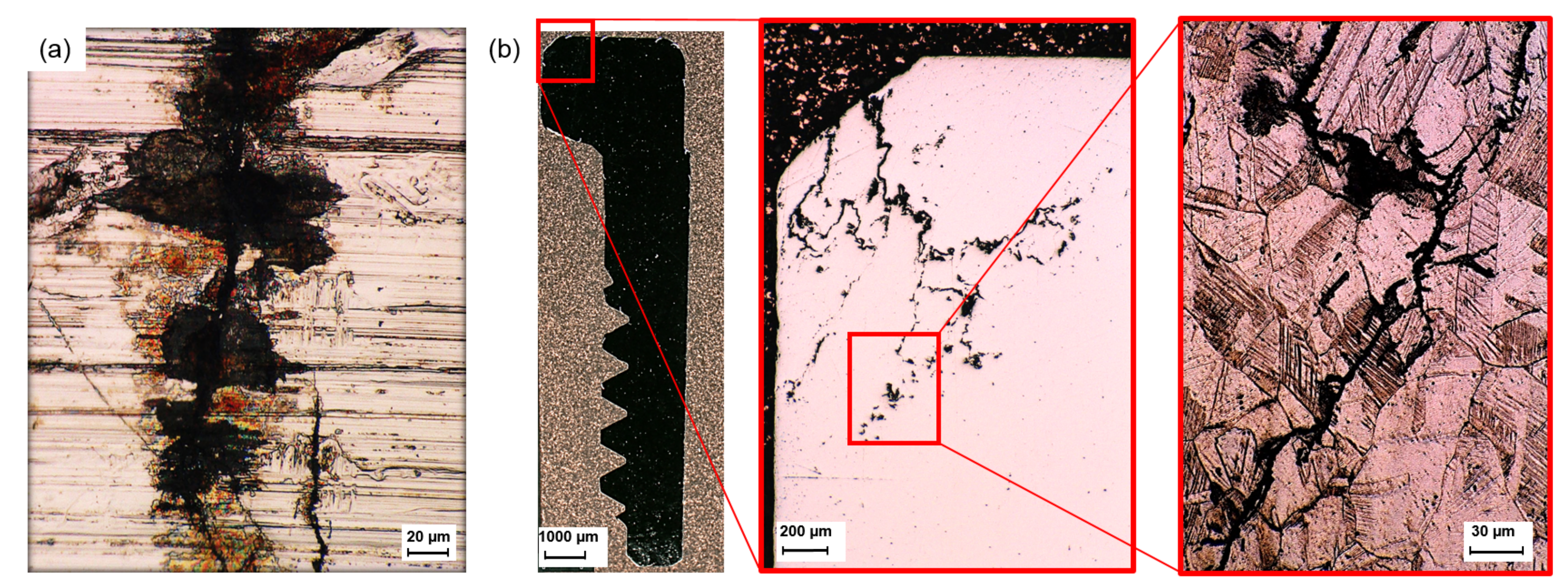
3.2. Crack Morphology
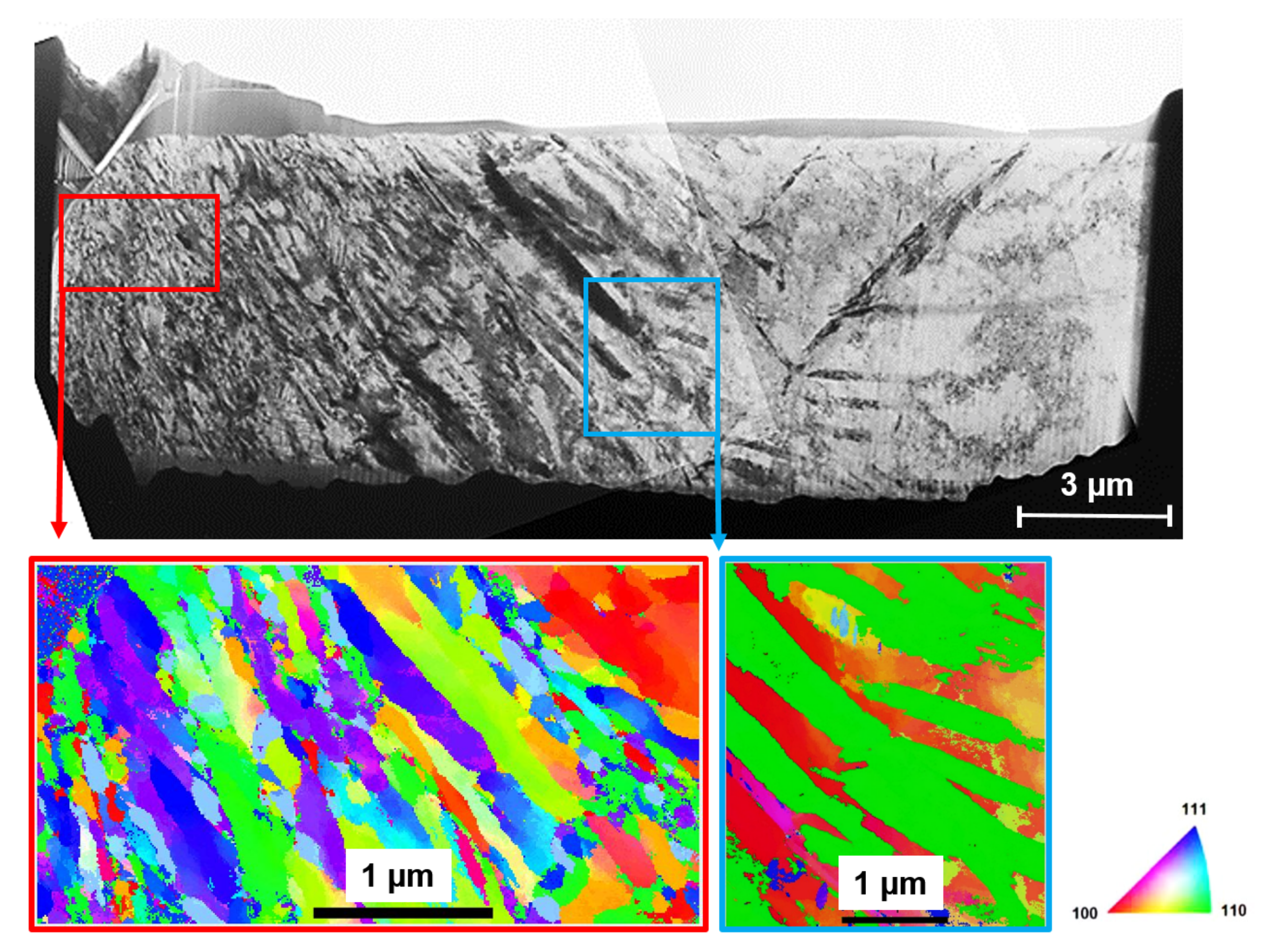
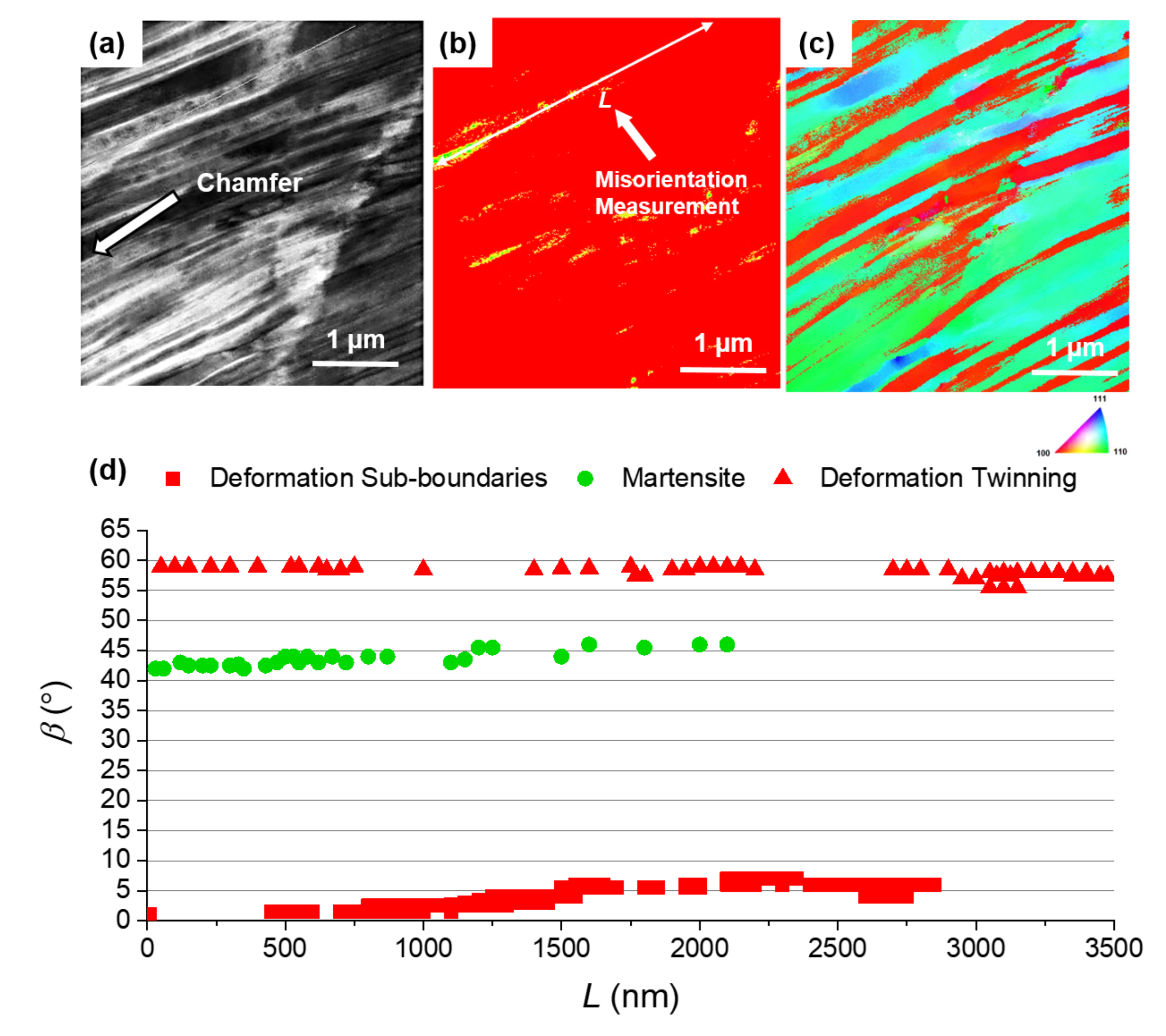
3.3. Microstructure Evolution
3.4. Phase Composition
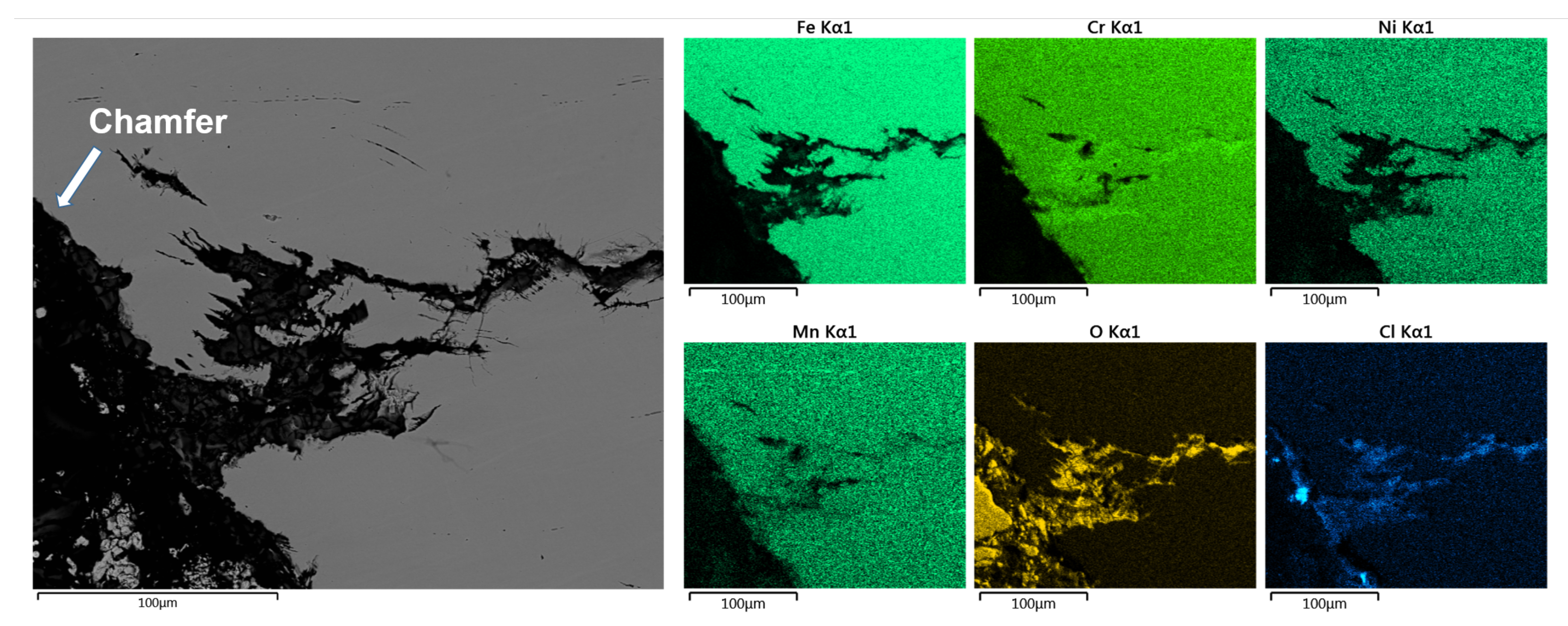
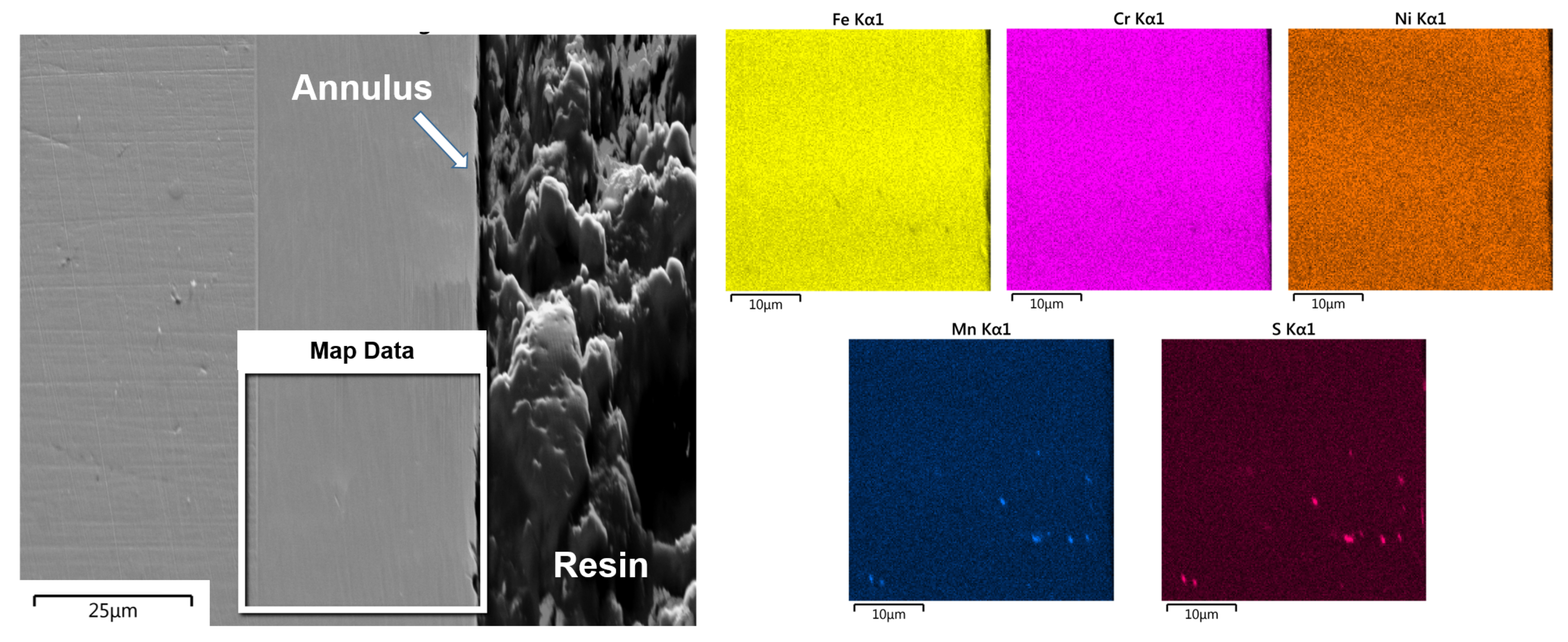
3.5. Element-Distribution Analysis
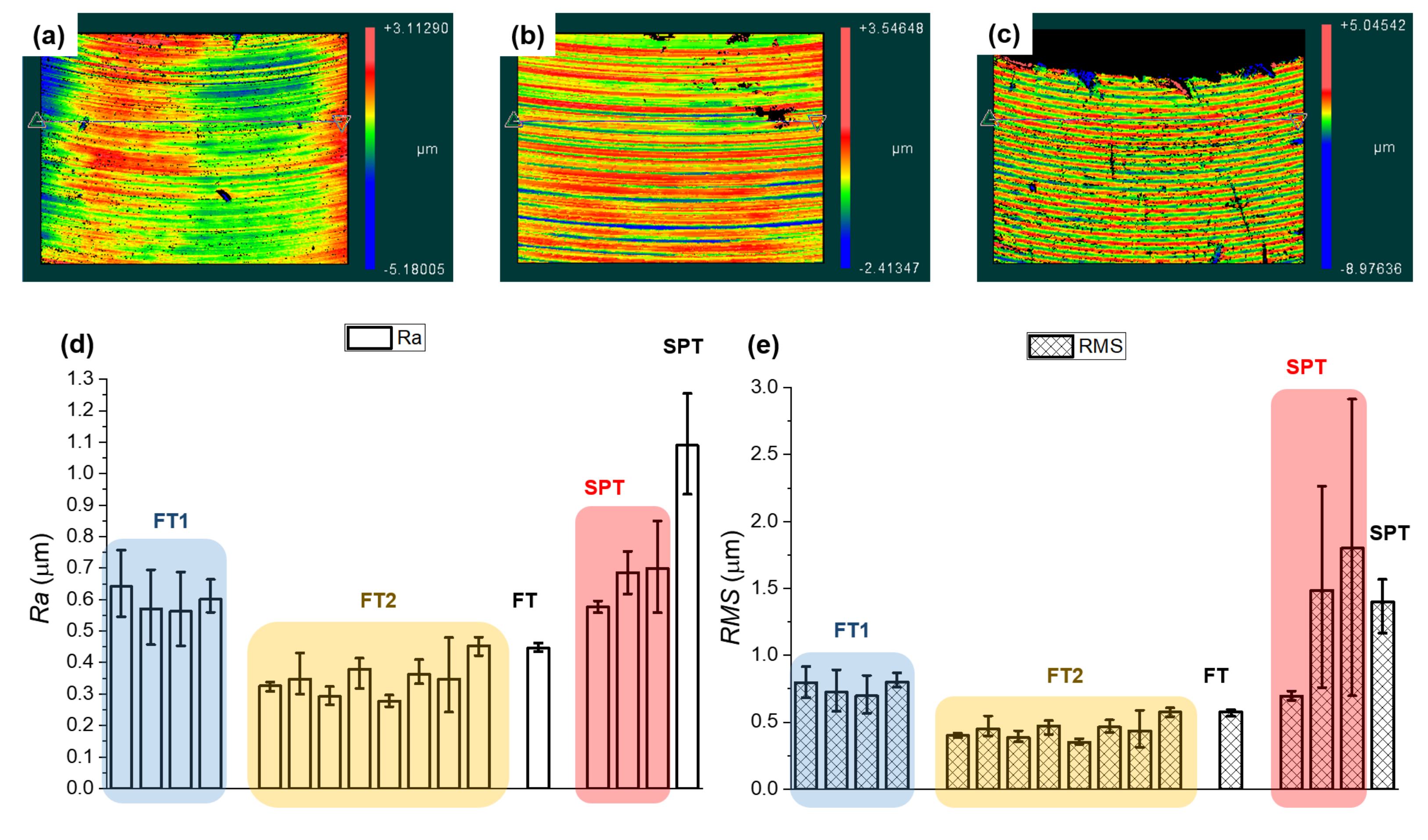
3.6. Surface Roughness
4. Discussion
4.1. Catastrophic Transgranular Cracking
4.2. Factors Impacting SCC Resistance
4.3. Element Distribution in Cracks
4.4. SCC Model
5. Conclusions
- The new seawater spray protocol we used, and especially its cyclic exposure to two distinct levels of relative humidity, allows seawater to concentrate on stressed alloy parts, temporarily generating a solution of mobile Cl- ions with concentrations considerably higher than in the original (simulated) seawater. These conditions of concentrated salt solution are particularly detrimental to alloy parts prone to SCC.
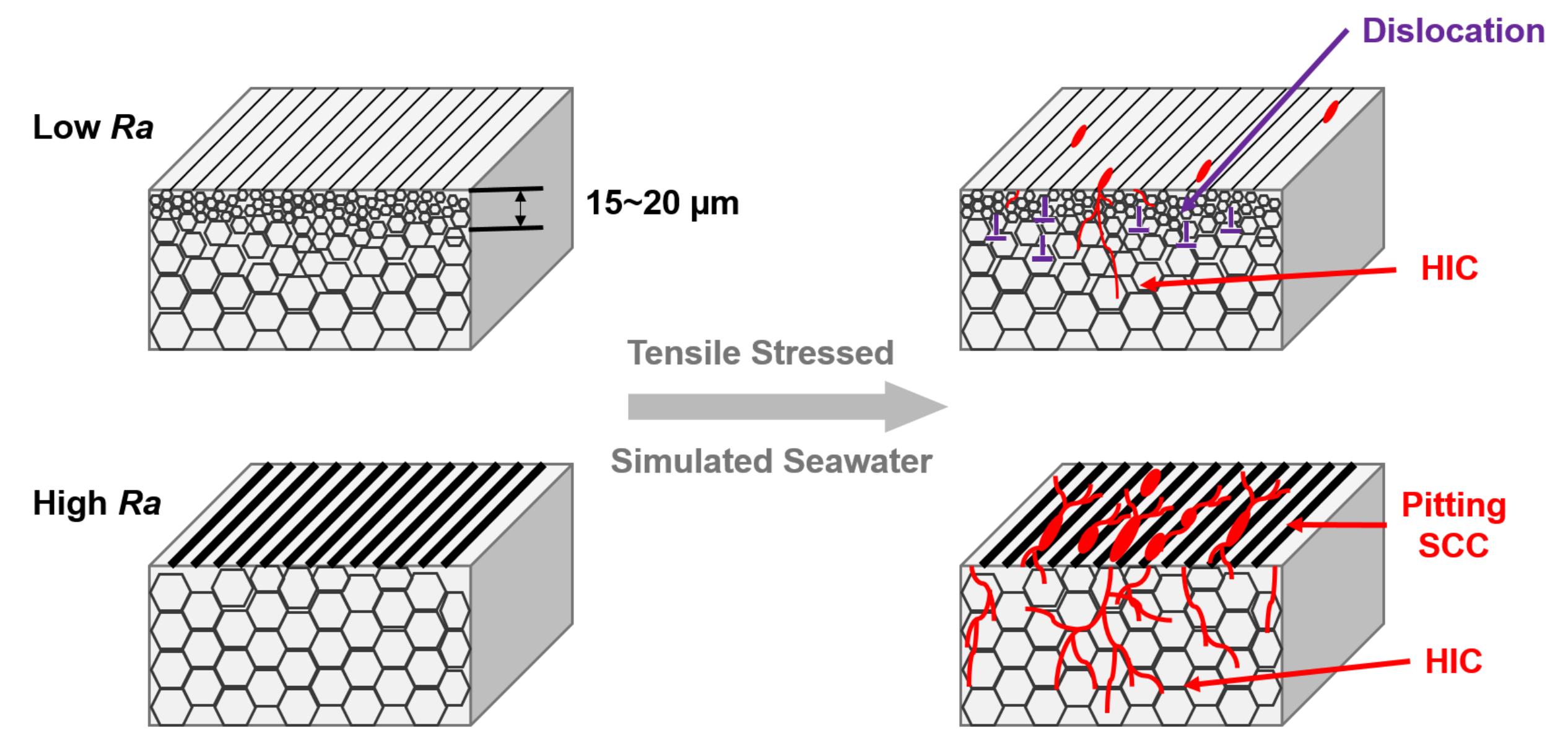
- Different methods of alloy surface machining can change the susceptibility to SCC—inferred from the crack density (crack length per area)—by at least one order of magnitude. Particularly, the use of a single-point tool dramatically increases susceptibility to SCC compared to using form tools. This can be explained by differences in the resulting immediate-subsurface microstructure. Specifically, a plastically deformed subsurface zone with refined grain size, reaching to a depth of 15 to 20, as introduced by form tools, increases the resistance to SCC. This can be explained by increased crack deflection along grain boundaries between refined (nanometer-sized) grains, thus increased fracture energy because of increased fractured surfaces. Obviously, this mechanism does not depend on the formation of deformation-induced martensite.
- In addition to the subsurface microstructure, machining-induced surface roughness has a significant negative effect on susceptibility to SCC. High surface roughness increases susceptibility to SCC. The reason is that increased surface roughness increases surface-relief-based stress concentration and promotes nucleation of pitting corrosion by providing pre-formed pits and corresponding attracting interaction with surface-attacking solutions.
- Cracks with dissimilar morphology at- and underneath the surface indicate two distinct failure mechanisms. At the alloy surface, cracks form connected with pitting corrosion sites. They propagate perpendicular to the main direction of tensile stress. These are stress–corrosion cracks. Under the surface, in contrast, catastrophic cracks form with lengths of several millimeters. These cracks exhibit pronounced branching, deflection, and transgranular propagation. Apparently, these cracks are induced by hydrogen, released by chemical reactions between the alloy, the solution and the chloride-concentrating humid atmosphere.
- The behavior observed here for surface-machined AISI 316L nuts is generally expected for other AISI 316L parts and components exposed to tensile stress in seawater environments.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.L.; Xia, S.; Bai, Q.; Liu, T.G.; Li, H.; Zhou, B.X.; Wang, L.J.; Ma, W.J. Effects of 3-D grain boundary geometrical angles and the net normal stress on intergranular stress corrosion cracking initiation in a 316 stainless steel. Mater. Sci. Eng. 2019, 765, 138277. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Effect of surface machining and cold working on the ambient temperature chloride stress corrosion cracking susceptibility of AISI 304L stainless steel. Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2010, 527, 679–683. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Fang, K.W.; Hu, Y.J.; Wang, S.Y.; Wang, X.L. Effect of machining-induced surface residual stress on initiation of stress corrosion cracking in 316 austenitic stainless steel. Corros. Sci. 2016, 108, 173–184. [Google Scholar] [CrossRef]
- Atashin, S.; Pakshir, M.; Yazdani, A. Synergistic investigation into the marine parameters’ effect on the corrosion rate of AISI 316 stainless steel. Mater. Des. 2011, 32, 1315–1324. [Google Scholar] [CrossRef]
- Wyllie, W.E.; Duquette, D.J. Effect of dissolved ozone on corrosion behavior of stainless steels in artificial seawater. Corrosion 1998, 54, 781–799. [Google Scholar] [CrossRef]
- Malik, A.U.; Ahmad, S.; Andijani, I.; Al-Fouzan, S. Corrosion behavior of steels in Gulf seawater environment. Desalination 1999, 123, 205–213. [Google Scholar] [CrossRef]
- Sridhar, N.; Brossia, C.S.; Dunn, D.S.; Anderko, A. Predicting localized corrosion in seawater. Corrosion 2004, 60, 915–936. [Google Scholar] [CrossRef]
- Malik, A.U.; Siddiqi, N.A.; Ahmad, S.; Andijani, I.N. The Effect of Dominant Alloy Additions on the Corrosion Behavior of Some Conventional and High-Alloy Stainless-Steels in Seawater. Corros. Sci. 1995, 37, 1521–1535. [Google Scholar] [CrossRef]
- Zhang, C.H.; Rong, H.; Song, G.D.; Hu, K. Effect of Surface Roughness by Shot Peening on Stress Corrosion Cracking Behavior of Pure Titanium Welded Joints in HCl Solution. Acta Metall. Sin. 2019, 55, 1282–1290. [Google Scholar]
- Zeman, A.; Novotny, R.; Uca, O.; Krsjak, V.; Macak, J.; Debarberis, L. Behavior of cold-worked AISI-304 steel in stress-corrosion cracking process: Microstructural aspects. Appl. Surf. Sci. 2008, 255, 160–163. [Google Scholar] [CrossRef]
- Scatigno, G.G.; Ryan, M.P.; Giuliani, F.; Wenman, M.R. The effect of prior cold work on the chloride stress corrosion cracking of 304L austenitic stainless steel under atmospheric conditions. Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2016, 668, 20–29. [Google Scholar] [CrossRef]
- Sundar, R.; Ganesh, P.; Kumar, B.S.; Gupta, R.K.; Nagpure, D.C.; Kaul, R.; Ranganathan, K.; Bindra, K.S.; Kain, V.; Oak, S.M.; et al. Mitigation of Stress Corrosion Cracking Susceptibility of Machined 304L Stainless Steel Through Laser Peening. J. Mater. Eng. Perform. 2016, 25, 3710–3724. [Google Scholar] [CrossRef]
- Rajaguru, J.; Arunachalam, N. Investigation on machining induced surface and subsurface modifications on the stress corrosion crack growth behaviour of super duplex stainless steel. Corros. Sci. 2018, 141, 230–242. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Bali, S.C.; Shoji, T. Effects of dissolved hydrogen and surface condition on the intergranular stress corrosion cracking initiation and short crack growth behavior of non-sensitized 316 stainless steel in simulated PWR primary water. Corros. Sci. 2017, 118, 143–157. [Google Scholar] [CrossRef]
- Toppo, A.; Pujar, M.G.; Sreevidya, N.; Philip, J. Pitting and stress corrosion cracking studies on AISI type 316N stainless steel weldments. Def. Technol. 2018, 14, 226–237. [Google Scholar] [CrossRef]
- Veleva, L.; Alpuche-Aviles, M.A.; Graves-Brook, M.K.; Wipf, D.O. Voltammetry and surface analysis of AISI 316 stainless steel in chloride-containing simulated concrete pore environment. J. Electroanal. Chem. 2005, 578, 45–53. [Google Scholar] [CrossRef]
- Odnobokova, M.; Belyakov, A.; Kaibyshev, R. Development of Nanocrystalline 304L Stainless Steel by Large Strain Cold Working. Metals 2015, 5, 656–668. [Google Scholar] [CrossRef]
- Shakhova, I.; Dudko, V.; Belyakov, A.; Tsuzaki, K.; Kaibyshev, R. Effect of large strain cold rolling and subsequent annealing on microstructure and mechanical properties of an austenitic stainless steel. Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2012, 545, 176–186. [Google Scholar] [CrossRef]
- Sakai, T.; Belyakov, A.; Kaibyshev, R.; Miura, H.; Jonas, J.J. Dynamic and post-dynamic recrystallization under hot, cold and severe plastic deformation conditions. Prog. Mater. Sci. 2014, 60, 130–207. [Google Scholar] [CrossRef] [Green Version]
- Wu, H. Understanding residual stresses and fracture toughness in ceramic nanocomposites. In Residual Stresses in Composite Materials; Woodhead Publishing: Cambridge, UK, 2014; pp. 256–292. [Google Scholar] [CrossRef]
- Alyousif, O.; Nishimura, R. On the Stress Corrosion Cracking and Hydrogen Embrittlement Behavior of Austenitic Stainless Steels in Boiling Saturated Magnesium Chloride Solutions. Int. J. Corros. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ornek, C. Stress Corrosion Cracking and Hydrogen Embrittlement of Type 316L Austenitic Stainless Steel Beneath MgCl2 and MgCl2: FeCl3 Droplets. Corrosion 2019, 75, 657–667. [Google Scholar] [CrossRef]
- Eliezer, D.; Chakrapani, D.G.; Altstetter, C.J.; Pugh, E.N. Influence of Austenite Stability on the Hydrogen Embrittlement and Stress-Corrosion Cracking of Stainless-Steel. Metall. Trans. Phys. Metall. Mater. Sci. 1979, 10, 935–941. [Google Scholar] [CrossRef]
- Li, W.J.; Young, M.C.; Lai, C.L.; Kai, W.; Tsay, L.W. The effects of rolling and sensitization treatments on the stress corrosion cracking of 304L stainless steel in salt-spray environment. Corros. Sci. 2013, 68, 25–33. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Microstructural changes in AISI 304L stainless steel due to surface machining: Effect on its susceptibility to chloride stress corrosion cracking. J. Nucl. Mater. 2010, 403, 62–67. [Google Scholar] [CrossRef]
- Kubota, M.; Macadre, A.; Mori, K.; Mori, R. Fatigue Properties of Ultra-Fine Grain Austenitic Stainless Steel and the Effect of Hydrogen. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018. [Google Scholar] [CrossRef]
- Kentish, P. Stress corrosion cracking of gas pipelines—Effect of surface roughness, orientations and flattening. Corros. Sci. 2007, 49, 2521–2533. [Google Scholar] [CrossRef]
- Lillard, R.S.; Kashfipour, M.A.; Niu, W. Pit Propagation at the Boundary between Manganese Sulfide Inclusions and Austenitic Stainless Steel 303 and the Role of Copper. J. Electrochem. Soc. 2016, 163, C440–C451. [Google Scholar] [CrossRef]
- Hara, N.; Hirabayashi, K.; Sugawara, Y.; Muto, I. Improvement of Pitting Corrosion Resistance of Type 316L Stainless Steel by Potentiostatic Removal of Surface MnS Inclusions. Int. J. Corros. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Wijesinghe, T.L.S.L.; Blackwood, D.J. Real time pit initiation studies on stainless steels: The effect of sulphide inclusions. Corros. Sci. 2007, 49, 1755–1764. [Google Scholar] [CrossRef]
- Roffey, P.; Davies, E.H. The generation of corrosion under insulation and stress corrosion cracking due to sulphide stress cracking in an austenitic stainless steel hydrocarbon gas pipeline. Eng. Fail. Anal. 2014, 44, 148–157. [Google Scholar] [CrossRef]



| Atom Fractions | |||||||
| Tool | ||
|---|---|---|
| FT1 | ||
| FT2 | ||
| SPT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Ernst, F. Stress–Corrosion Cracking of AISI 316L Stainless Steel in Seawater Environments: Effect of Surface Machining. Metals 2020, 10, 1324. https://doi.org/10.3390/met10101324
Ren Z, Ernst F. Stress–Corrosion Cracking of AISI 316L Stainless Steel in Seawater Environments: Effect of Surface Machining. Metals. 2020; 10(10):1324. https://doi.org/10.3390/met10101324
Chicago/Turabian StyleRen, Zhe, and Frank Ernst. 2020. "Stress–Corrosion Cracking of AISI 316L Stainless Steel in Seawater Environments: Effect of Surface Machining" Metals 10, no. 10: 1324. https://doi.org/10.3390/met10101324
APA StyleRen, Z., & Ernst, F. (2020). Stress–Corrosion Cracking of AISI 316L Stainless Steel in Seawater Environments: Effect of Surface Machining. Metals, 10(10), 1324. https://doi.org/10.3390/met10101324







