Enhancing the Corrosion Resistance and Mechanical Properties of a High-Alloying Al-Zn-Mg-Cu-Zr Alloy by Ce Addition and Aging Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, J.C.; A Starke, E. Progress in structural materials for aerospace systems11The Golden Jubilee Issue—Selected topics in Materials Science and Engineering: Past, Present and Future, edited by S. Suresh. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, K.; Chen, S.; Zhou, L.; Chang, J.; Huang, L.; Yi, Y. Enhancing stress corrosion cracking resistance of low Cu-containing Al-Zn-Mg-Cu alloys by slow quench rate. Mater. Des. 2019, 164, 107558. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Y.; Li, X.; Wen, K.; Xiong, B.; Li, Z.; Yan, L.; Yan, H.; Liu, H.; Li, Y. Effect of recrystallization on plasticity, fracture toughness and stress corrosion cracking of a high-alloying Al-Zn-Mg-Cu alloy. Mater. Lett. 2020, 275, 128074. [Google Scholar] [CrossRef]
- Liu, J.-T.; Zhang, Y.-A.; Li, X.-W.; Li, Z.-H.; Xiong, B.Q.; Zhang, J.-S. Thermodynamic calculation of high zinc-containing Al-Zn-Mg-Cu alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 1481–1487. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Tang, J.; Zhang, J. Effect of gradient grain structures on corrosion resistance of extruded Al–Zn–Mg–Cu alloy. J. Alloys Compd. 2020, 832, 154911. [Google Scholar] [CrossRef]
- Chemingui, M.; Khitouni, M.; Jozwiak, K.; Mesmacque, G.; Kolsi, A. Characterization of the mechanical properties changes in an Al-Zn-Mg alloy after a two-step ageing treatment at 70 °C and 135 °C. Mater. Des. 2010, 31, 3134–3139. [Google Scholar] [CrossRef]
- Wang, D.; Ni, D.; Ma, Z. Effect of pre-strain and two-step aging on microstructure and stress corrosion cracking of 7050 alloy. Mater. Sci. Eng. A 2008, 494, 360–366. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Chen, S.; Fang, H. Influence of repetitious-RRA treatment on the strength and SCC resistance of Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2011, 528, 4014–4018. [Google Scholar] [CrossRef]
- Xiao, Y.-P.; Pan, Q.-L.; Li, W.-B.; Liu, X.-Y.; He, Y. Influence of retrogression and re-aging treatment on corrosion behaviour of an Al–Zn–Mg–Cu alloy. Mater. Des. 2011, 32, 2149–2156. [Google Scholar] [CrossRef]
- Reda, Y.; Abdel-Karim, R.; El Mahallawi, I. Improvements in mechanical and stress corrosion cracking properties in Al-alloy 7075 via retrogression and reaging. Mater. Sci. Eng. A 2008, 485, 468–475. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Y.; Liang, S.; Xie, W. The effects of non-isothermal aging on the strength and corrosion behavior of Al Zn Mg Cu alloy. J. Alloys Compd. 2016, 681, 57–65. [Google Scholar] [CrossRef]
- Huang, L.; Chen, K.; Li, S. Influence of grain-boundary pre-precipitation and corrosion characteristics of inter-granular phases on corrosion behaviors of an Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. B 2012, 177, 862–868. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.; Zhao, K.; Duan, J.; Hu, J.; He, Z. Effects of Sc and Zr microalloying additions and aging time at 120 °C on the corrosion behaviour of an Al–Zn–Mg alloy. Corros. Sci. 2012, 65, 288–298. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Q.; Li, M.; Huang, X.; Li, B. Effect of Sc and Zr additions on corrosion behaviour of Al–Zn–Mg–Cu alloys. J. Alloys Compd. 2014, 612, 42–50. [Google Scholar] [CrossRef]
- Meng, Q.; Frankel, G.S. Effect of Cu Content on Corrosion Behavior of 7xxx Series Aluminum Alloys. J. Electrochem. Soc. 2004, 151, B271. [Google Scholar] [CrossRef]
- Chen, S.; Chen, K.; Peng, G.; Jia, L.; Dong, P. Effect of heat treatment on strength, exfoliation corrosion and electrochemical behavior of 7085 aluminum alloy. Mater. Des. 2012, 35, 93–98. [Google Scholar] [CrossRef]
- Arnott, D.R.; Hinton, B.R.W.; Ryan, N.E. Cationic-Film-Forming Inhibitors for the Protection of the AA 7075 Aluminum Alloy Against Corrosion in Aqueous Chloride Solution. Corros. Sci. 1989, 45, 12–18. [Google Scholar] [CrossRef]
- Chaubey, A.K.; Mohapatra, S.; Jayasankar, K.; Pradhan, S.K.; Satpati, B.; Sahay, S.S.; Mishra, B.K.; Mukherjee, P.S. Effect of cerium addition on microstructure and mechanical properties of Al-Zn-Mg-Cu alloy. Trans. Indian Inst. Met. 2009, 62, 539–543. [Google Scholar] [CrossRef]
- Yu, X.; Dai, H.; Li, Z.; Sun, J.; Zhao, J.; Li, C.; Liu, W. Improved Recrystallization Resistance of Al–Cu–Li–Zr Alloy through Ce Addition. Metals 2018, 8, 1035. [Google Scholar] [CrossRef]
- Bo, H.; Jin, S.; Zhang, L.; Chen, X.; Chen, H.; Liu, L.; Zheng, F.; Jin, Z. Thermodynamic assessment of Al–Ce–Cu system. J. Alloys Compd. 2009, 484, 286–295. [Google Scholar] [CrossRef]
- Fang, H.; Chao, H.; Chen, K. Effect of recrystallization on intergranular fracture and corrosion of Al–Zn–Mg–Cu–Zr alloy. J. Alloys Compd. 2015, 622, 166–173. [Google Scholar] [CrossRef]
- Yu, X.; Sun, J.; Li, Z.; Dai, H.; Fang, H.-J.; Zhao, J.; Yin, D.-F. Solidification behavior and elimination of undissolved Al2CuMg phase during homogenization in Ce-modified Al–Zn–Mg–Cu alloy. Rare Met. 2018, 39, 1279. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Shi, D.; Dai, H.; Sun, J.; Dong, X. Enhanced High-Temperature Mechanical Properties of Al⁻Cu⁻Li Alloy through T1 Coarsening Inhibition and Ce-Containing Intermetallic Refinement. Materials 2019, 12, 1521. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yin, Z.; Cong, F. Intermetallic phase evolution of 7050 aluminum alloy during homogenization. Intermetallics 2012, 26, 114–121. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.; Duan, J.; Zhao, K.; Tang, B.; He, Z. Evolution of microstructure and properties in a new type 2mm Al–Zn–Mg–Sc–Zr alloy sheet. J. Alloys Compd. 2012, 517, 118–126. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, B.; Zhang, Y.; Zhu, B.; Wang, F.; Liu, H. Investigation of microstructural evolution and mechanical properties during two-step ageing treatment at 115 and 160 °C in an Al–Zn–Mg–Cu alloy pre-stretched thick plate. Mater. Charact. 2008, 59, 278–282. [Google Scholar] [CrossRef]
- Wen, K.; Fan, Y.; Wang, G.; Jin, L.; Li, X.; Li, Z.; Zhang, Y.; Xiong, B. Aging behavior and precipitate characterization of a high Zn-containing Al-Zn-Mg-Cu alloy with various tempers. Mater. Des. 2016, 101, 16–23. [Google Scholar] [CrossRef]
- Kairy, S.K.; Turk, S.; Birbilis, N.; Shekhter, A. The role of microstructure and microchemistry on intergranular corrosion of aluminium alloy AA7085-T7452. Corros. Sci. 2018, 143, 414–427. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminium alloys: An experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140. [Google Scholar] [CrossRef]
- Ramgopal, T.; Schmutz, P.; Frankel, G.S. Electrochemical behaviour of thinfilm analogs of Mg (Zn,Cu,Al)2. J. Electrochem. Soc. 2001, 148, B348. [Google Scholar] [CrossRef]

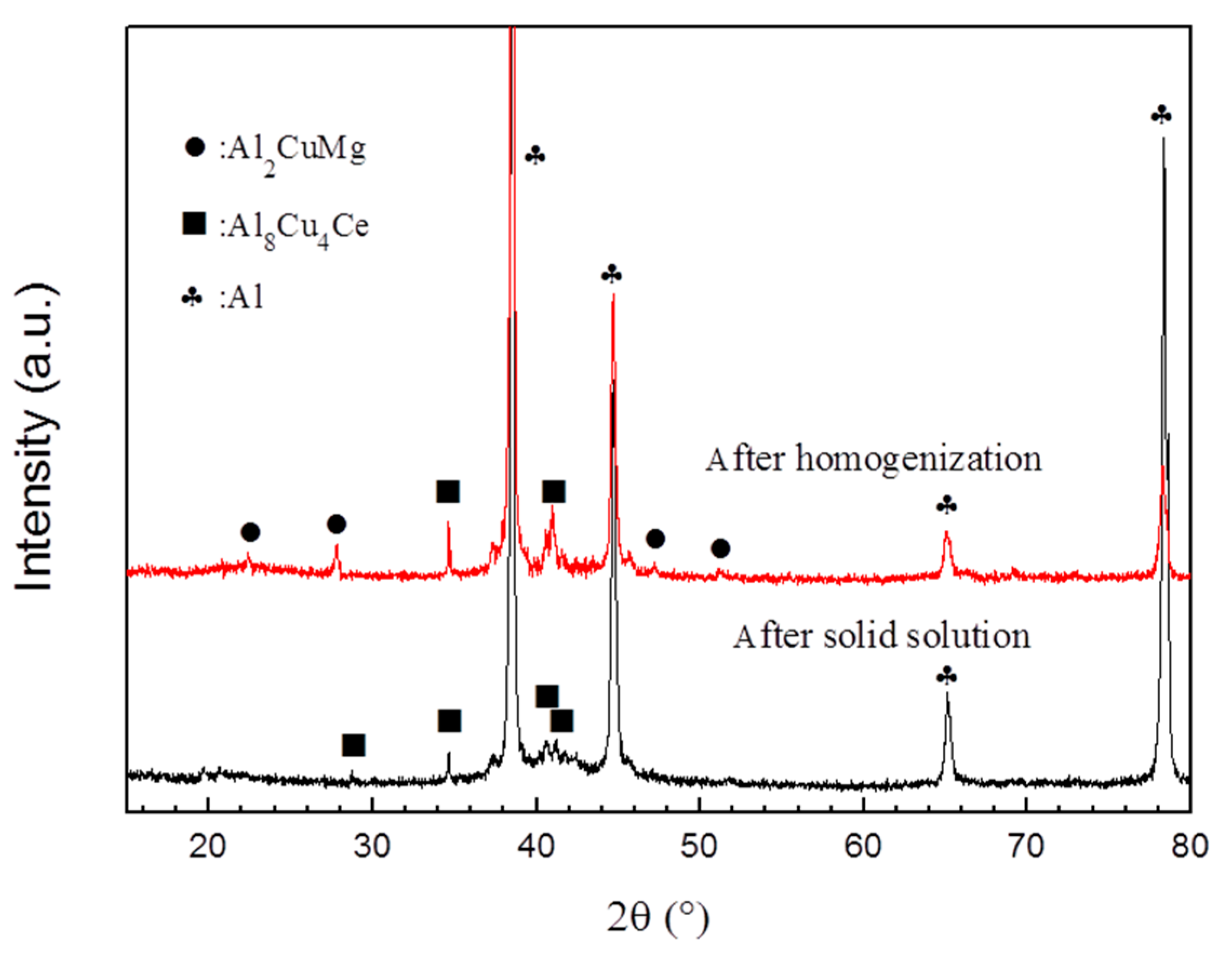
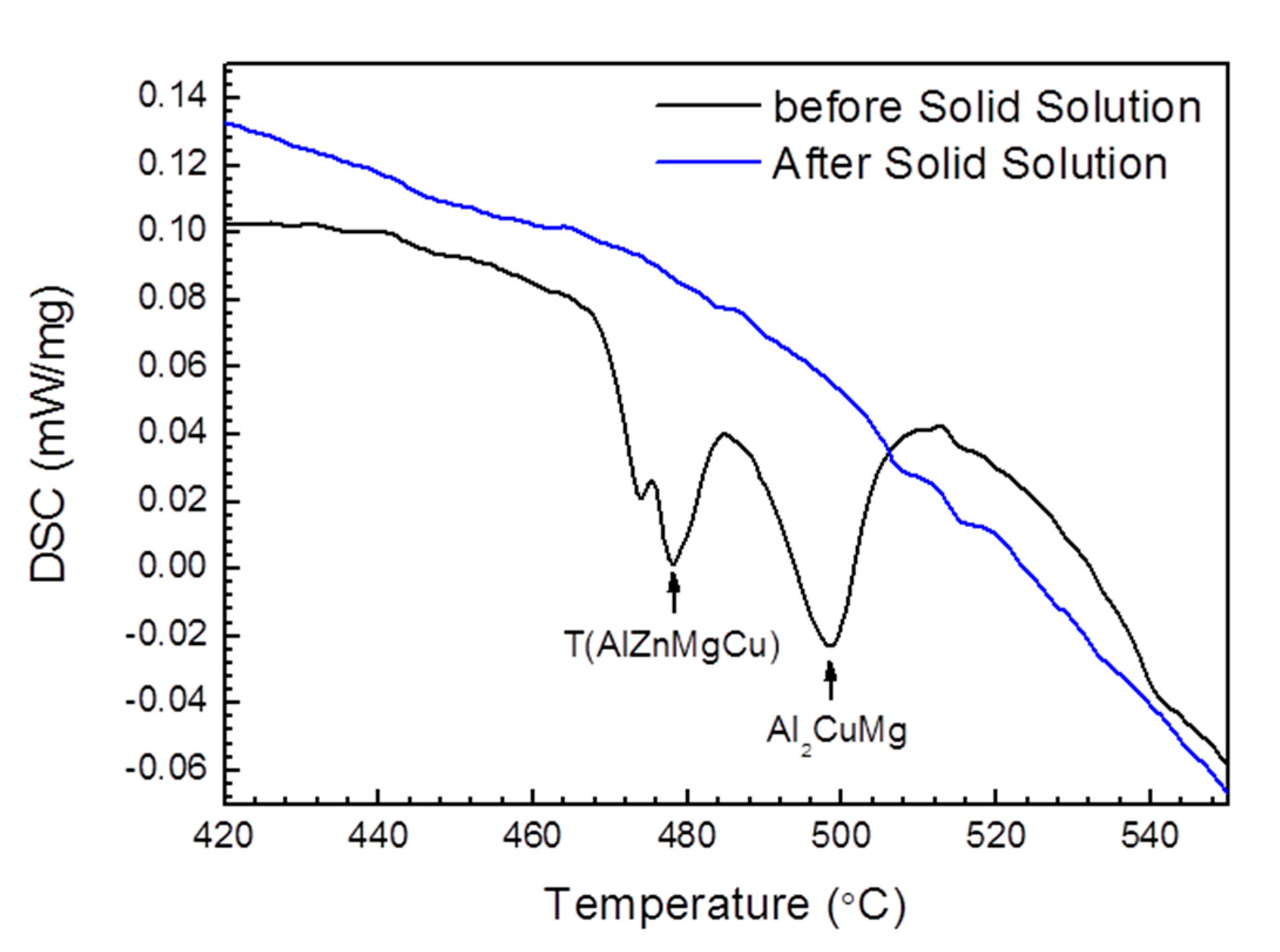
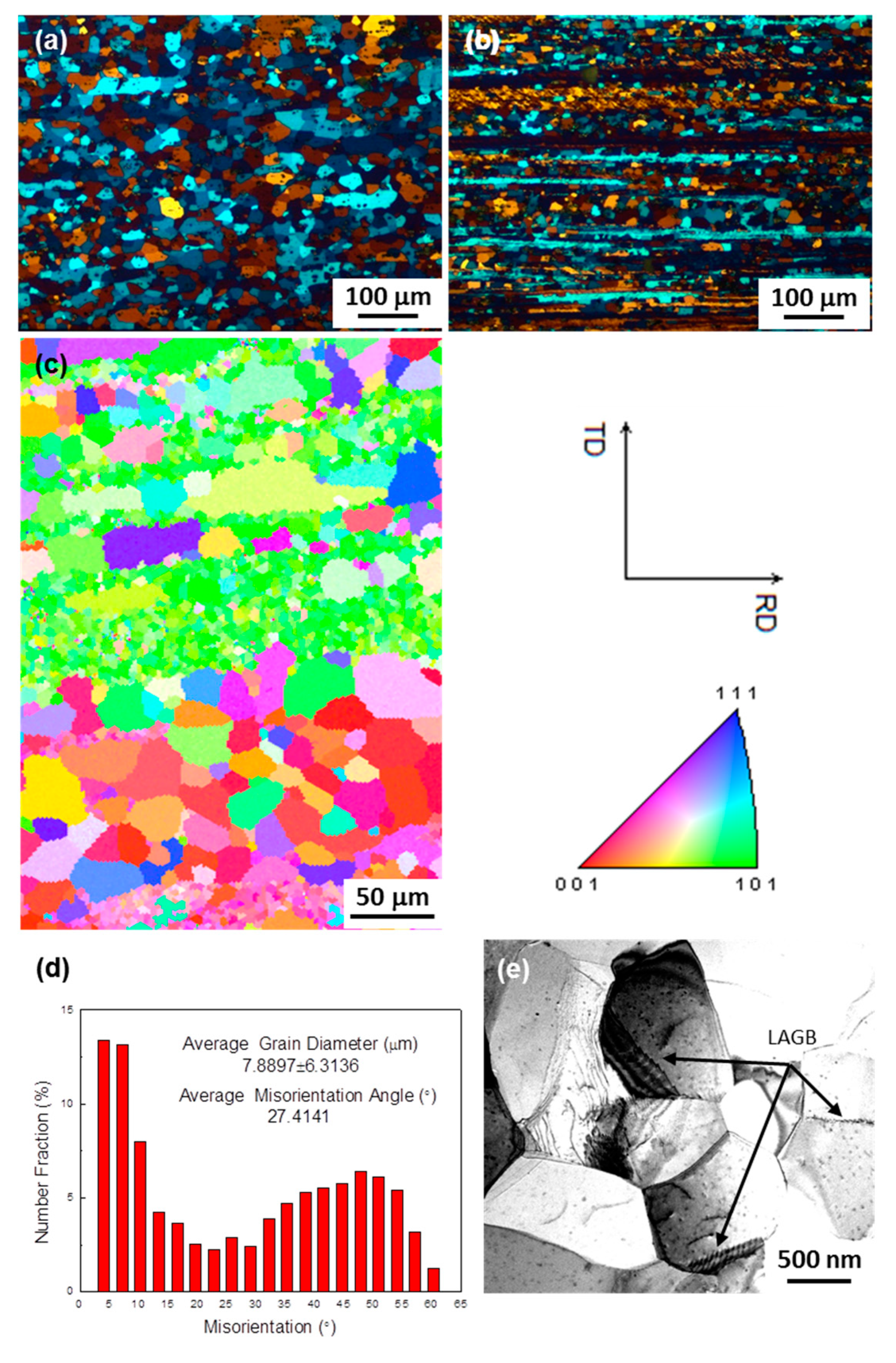
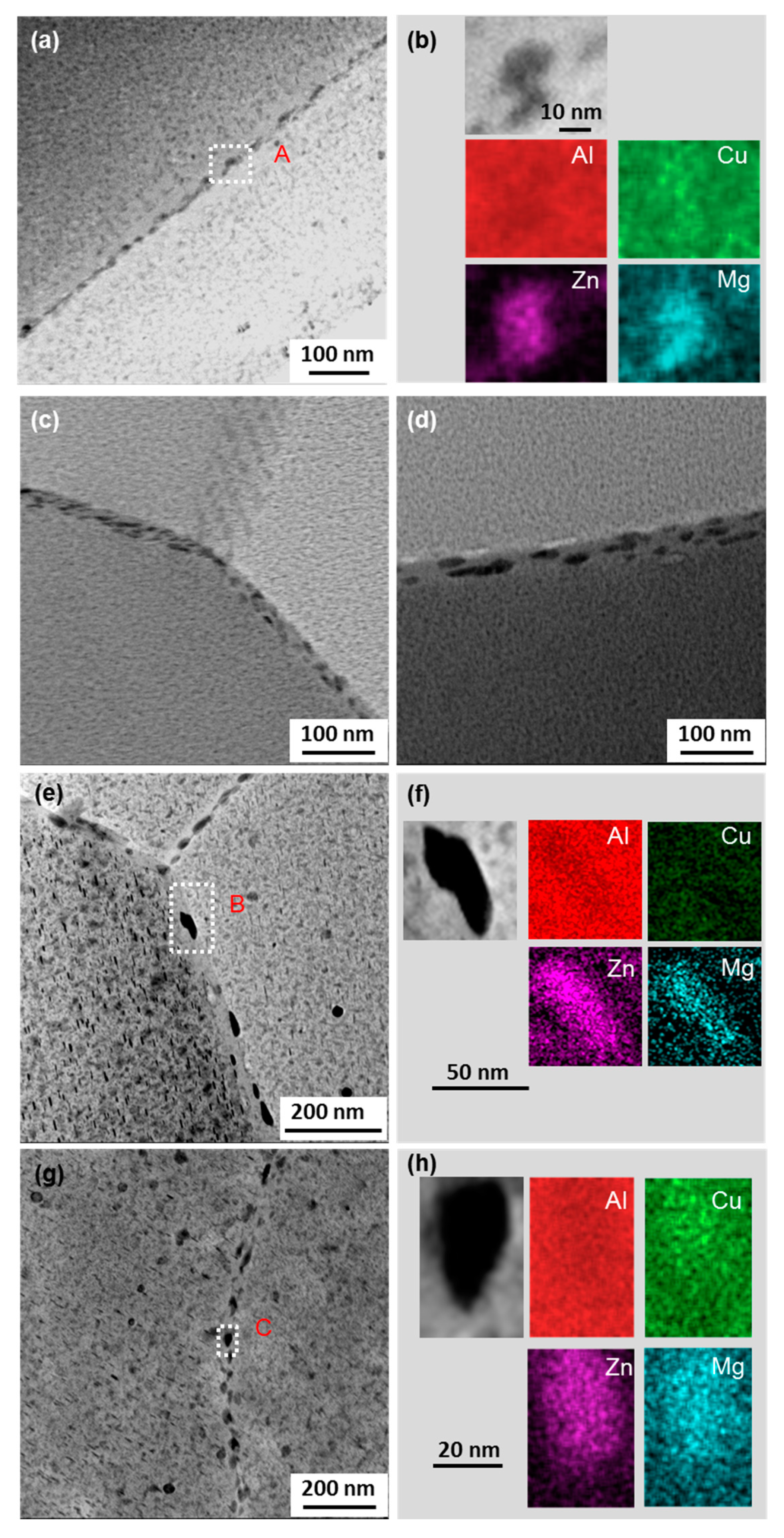

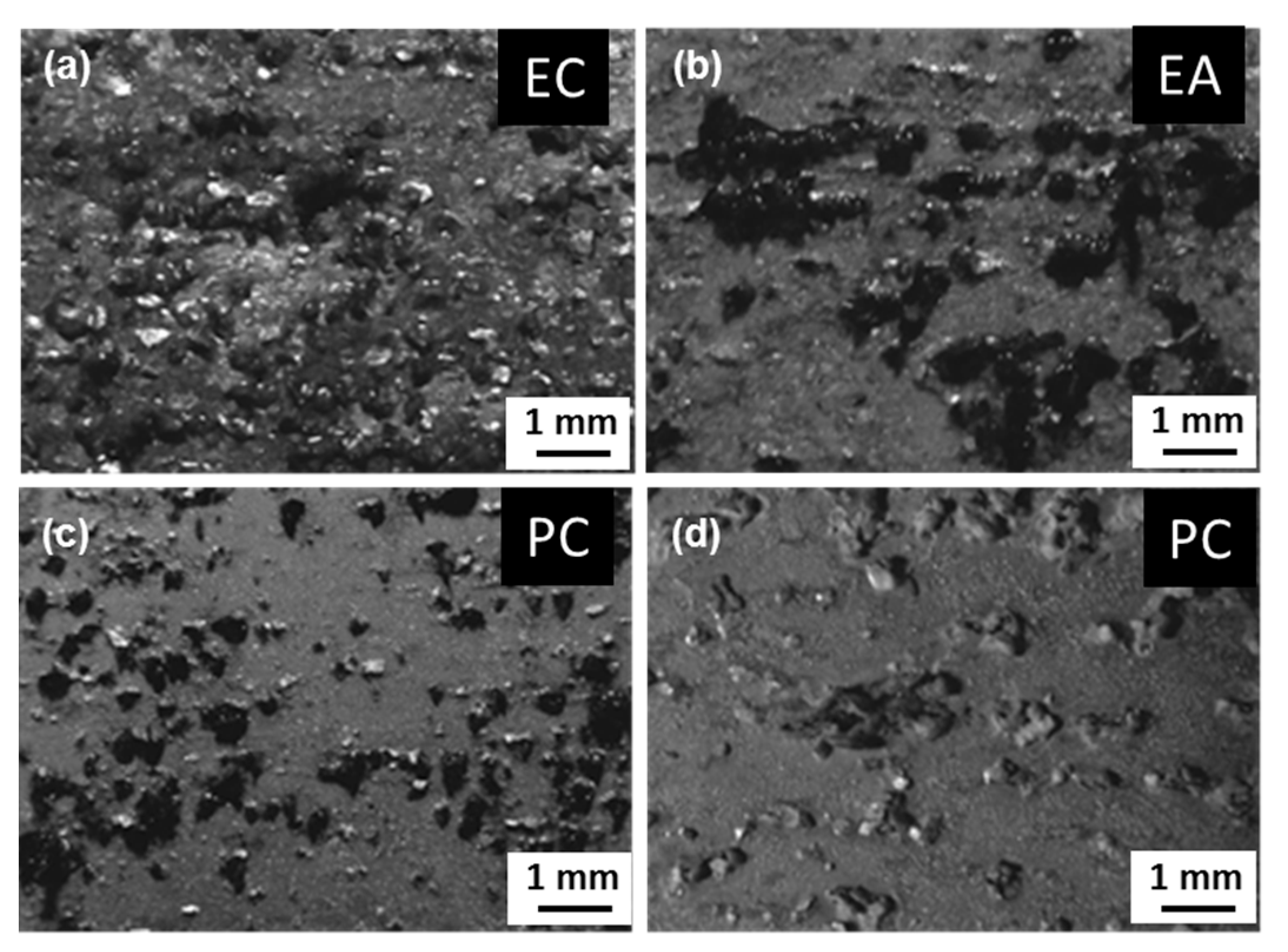
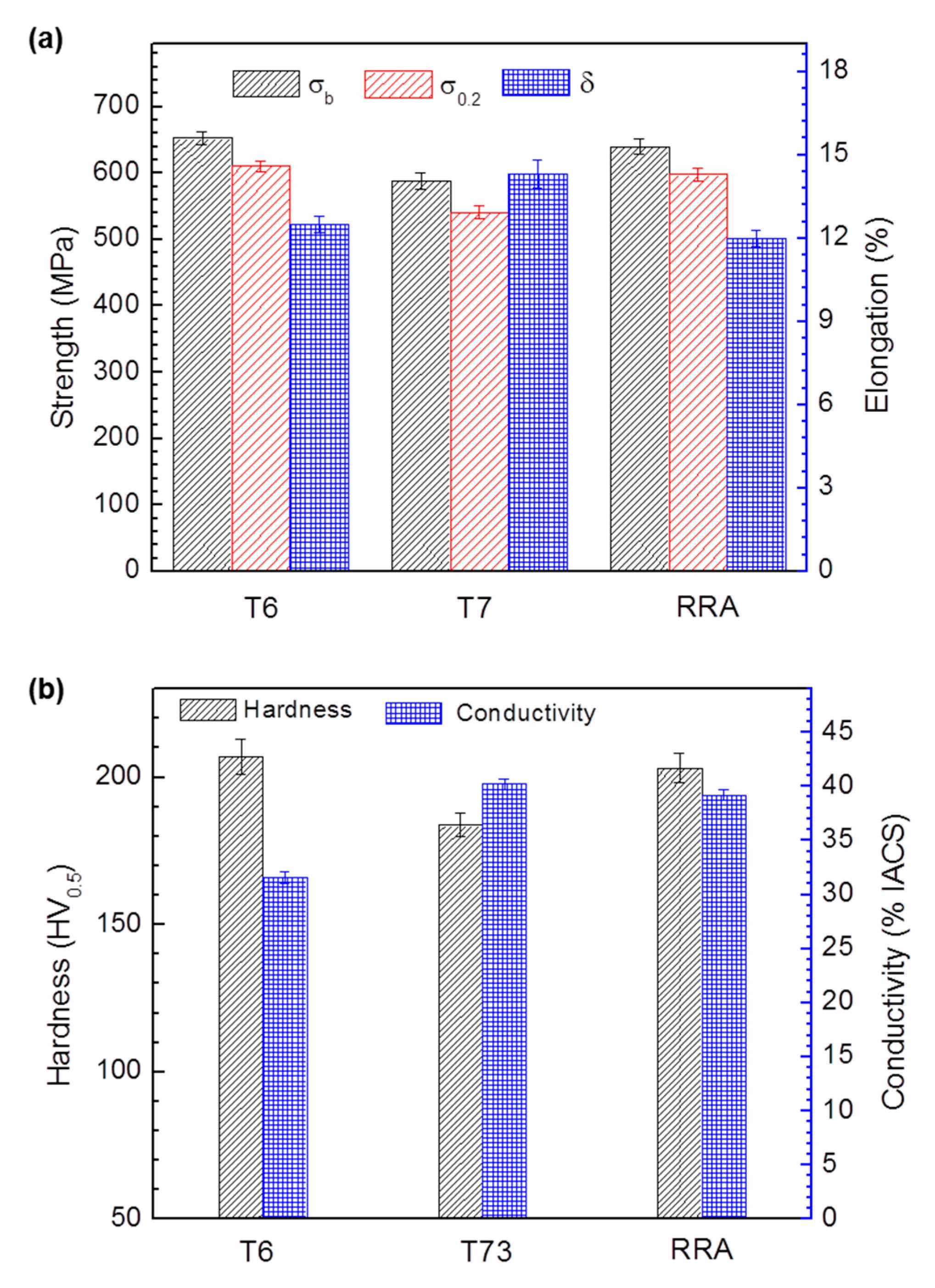

| Alloy | Zn | Mg | Cu | Zr | Fe | Si | Ce | Al |
|---|---|---|---|---|---|---|---|---|
| Ce-free | 3.91 | 2.43 | 0.97 | 0.03 | 0.03 | 0.04 | - | Bal. |
| Ce-containing | 3.90 | 2.41 | 0.98 | 0.03 | 0.03 | 0.09 | 0.02 | Bal. |
| Heat Treatment Condition | Procedures | |
|---|---|---|
| H | Homogenization | 435 °C/8 h + 470 °C/32 h |
| SS | Solid solution | 450 °C/50 min + 485 °C/30 min |
| T6 | Aging | 120 °C/24 h |
| T7 | 120 °C/24 h + 160°C/8 h | |
| RRA | 120 °C/24 h + 190 °C/1 h + 120 °C/24 h | |
| Point | Al | Zn | Mg | Cu | Ce | Phases |
|---|---|---|---|---|---|---|
| A | 56.8445 | 8.7635 | 1.2425 | 25.1083 | 6.5830 | Al8Cu4(Ce, Zn) |
| B | 51.1837 | 1.2160 | 25.6502 | 21.9270 | 0.0230 | Al2CuMg |
| C | 94.5761 | 3.0612 | 1.9676 | 0.3801 | 0.0001 | Matrix |
| Point | Al | Cu | Zn | Mg | Phase |
|---|---|---|---|---|---|
| A | 56.1 | 9.7 | 20.7 | 13.5 | η (Mg(Zn,Cu)2) |
| B | 64.3 | 4.0 | 19.0 | 12.6 | η (Mg(Zn,Cu)2) |
| C | 59.6 | 7.8 | 19.6 | 13.0 | η (Mg(Zn,Cu)2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhao, Z.; Shi, D.; Dong, X.; Shi, X.; Li, C.; Zhao, J.; Dai, H. Enhancing the Corrosion Resistance and Mechanical Properties of a High-Alloying Al-Zn-Mg-Cu-Zr Alloy by Ce Addition and Aging Treatment. Metals 2020, 10, 1318. https://doi.org/10.3390/met10101318
Yu X, Zhao Z, Shi D, Dong X, Shi X, Li C, Zhao J, Dai H. Enhancing the Corrosion Resistance and Mechanical Properties of a High-Alloying Al-Zn-Mg-Cu-Zr Alloy by Ce Addition and Aging Treatment. Metals. 2020; 10(10):1318. https://doi.org/10.3390/met10101318
Chicago/Turabian StyleYu, Xinxiang, Zhiguo Zhao, Dandan Shi, Xiaoyan Dong, Xianli Shi, Caiqiong Li, Junfeng Zhao, and Han Dai. 2020. "Enhancing the Corrosion Resistance and Mechanical Properties of a High-Alloying Al-Zn-Mg-Cu-Zr Alloy by Ce Addition and Aging Treatment" Metals 10, no. 10: 1318. https://doi.org/10.3390/met10101318
APA StyleYu, X., Zhao, Z., Shi, D., Dong, X., Shi, X., Li, C., Zhao, J., & Dai, H. (2020). Enhancing the Corrosion Resistance and Mechanical Properties of a High-Alloying Al-Zn-Mg-Cu-Zr Alloy by Ce Addition and Aging Treatment. Metals, 10(10), 1318. https://doi.org/10.3390/met10101318





