Microstructural Characteristics of AlSi9Cu3(Fe) Alloy with High Melting Point Elements
Abstract
1. Introduction
2. Materials and Methods
3. Results
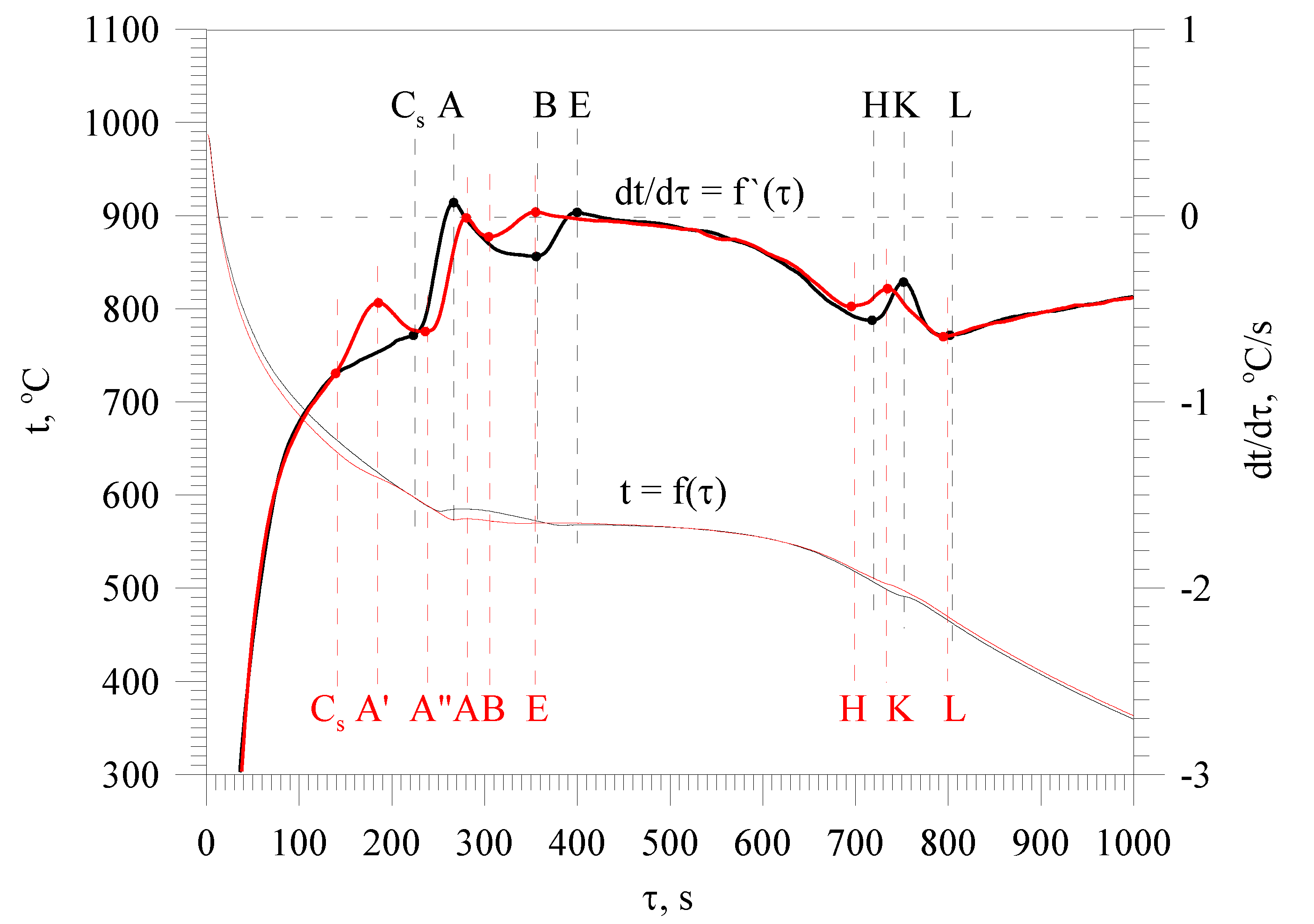
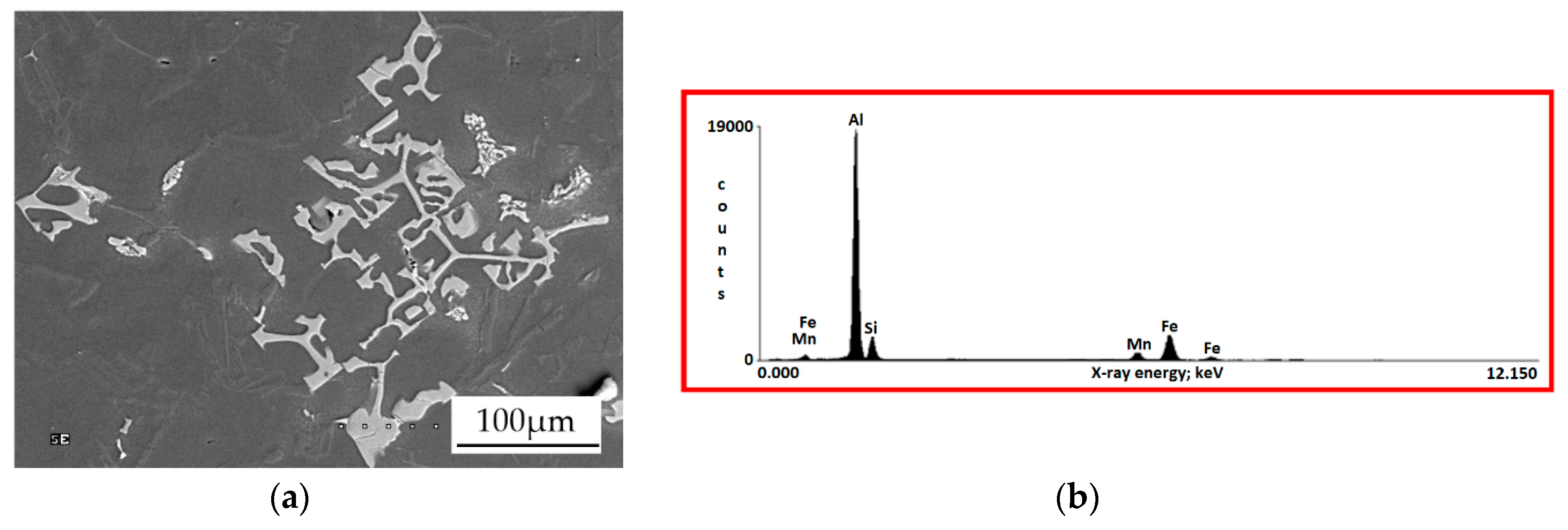
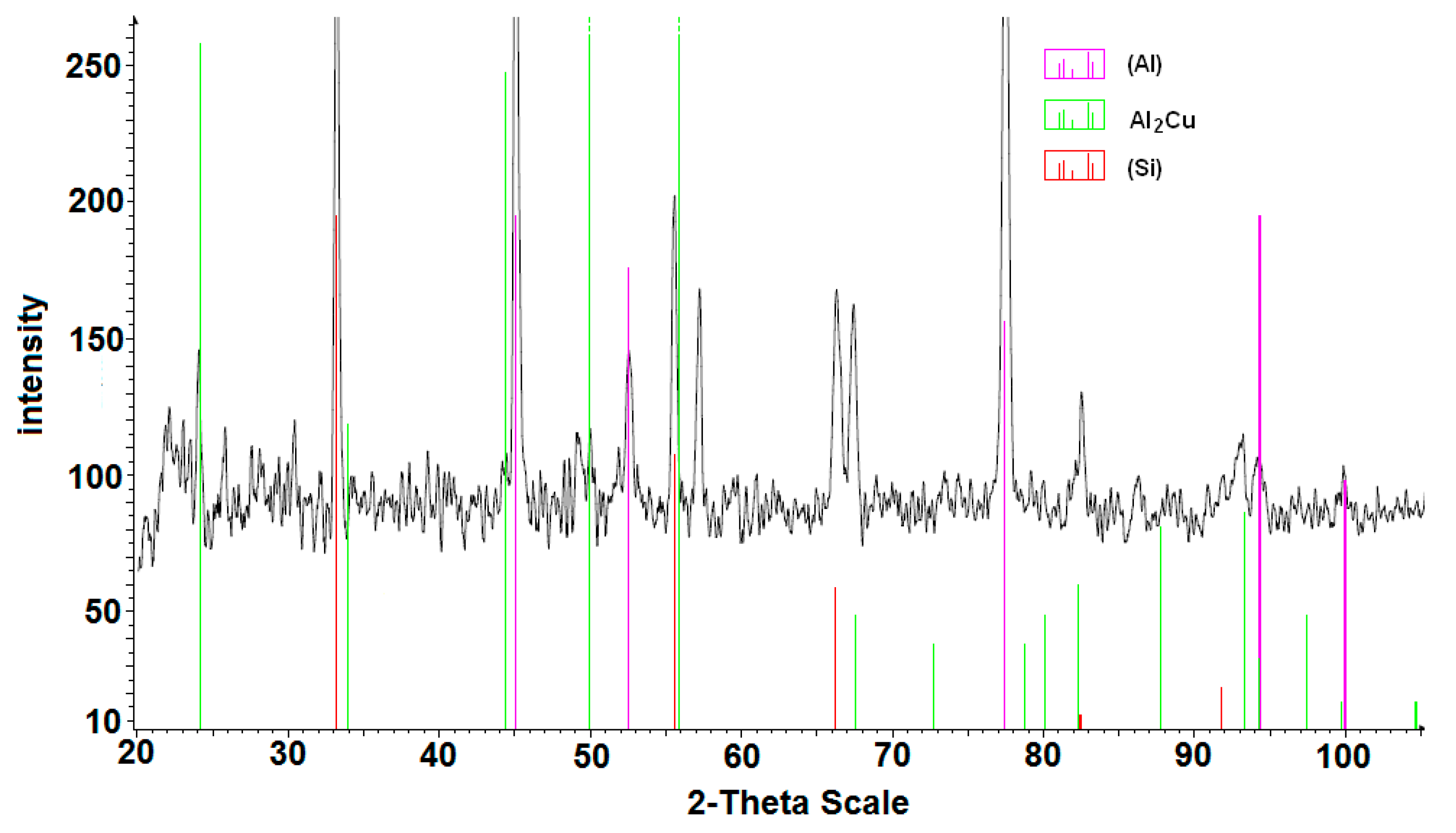
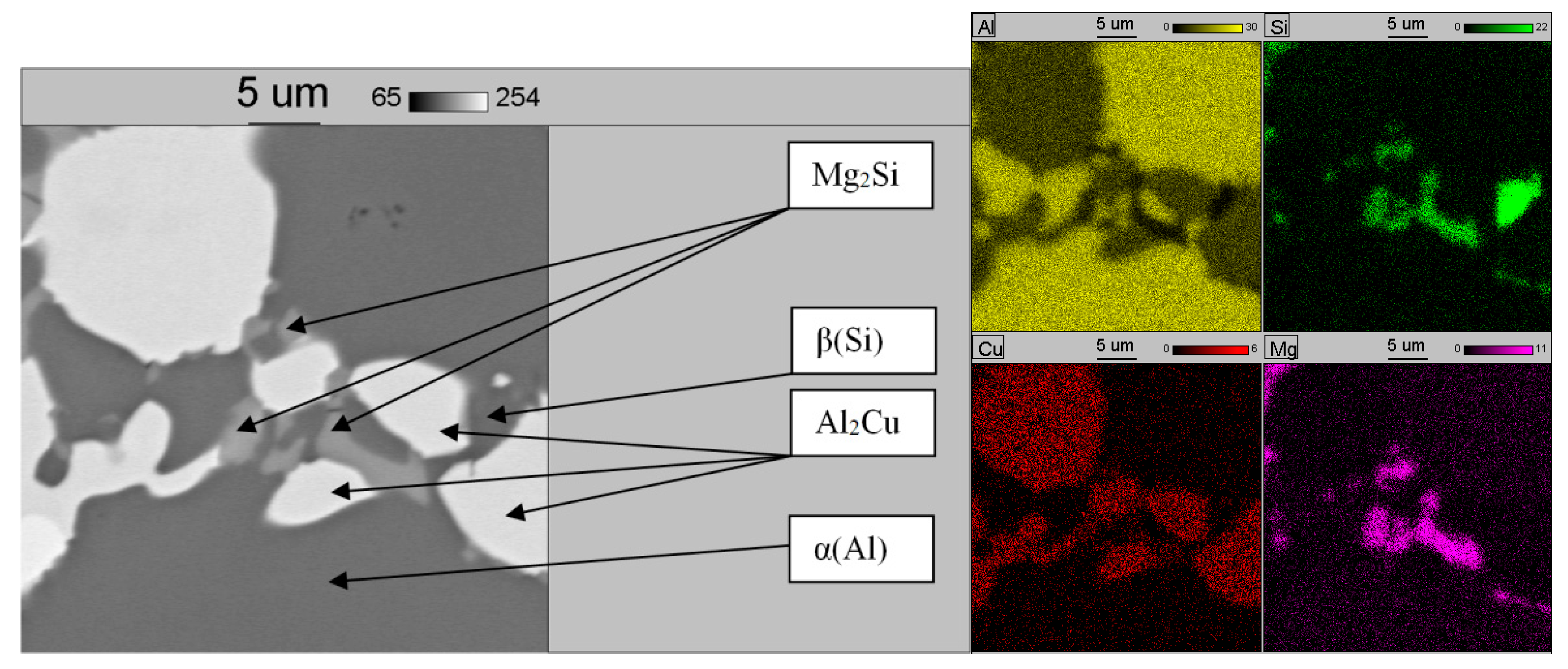
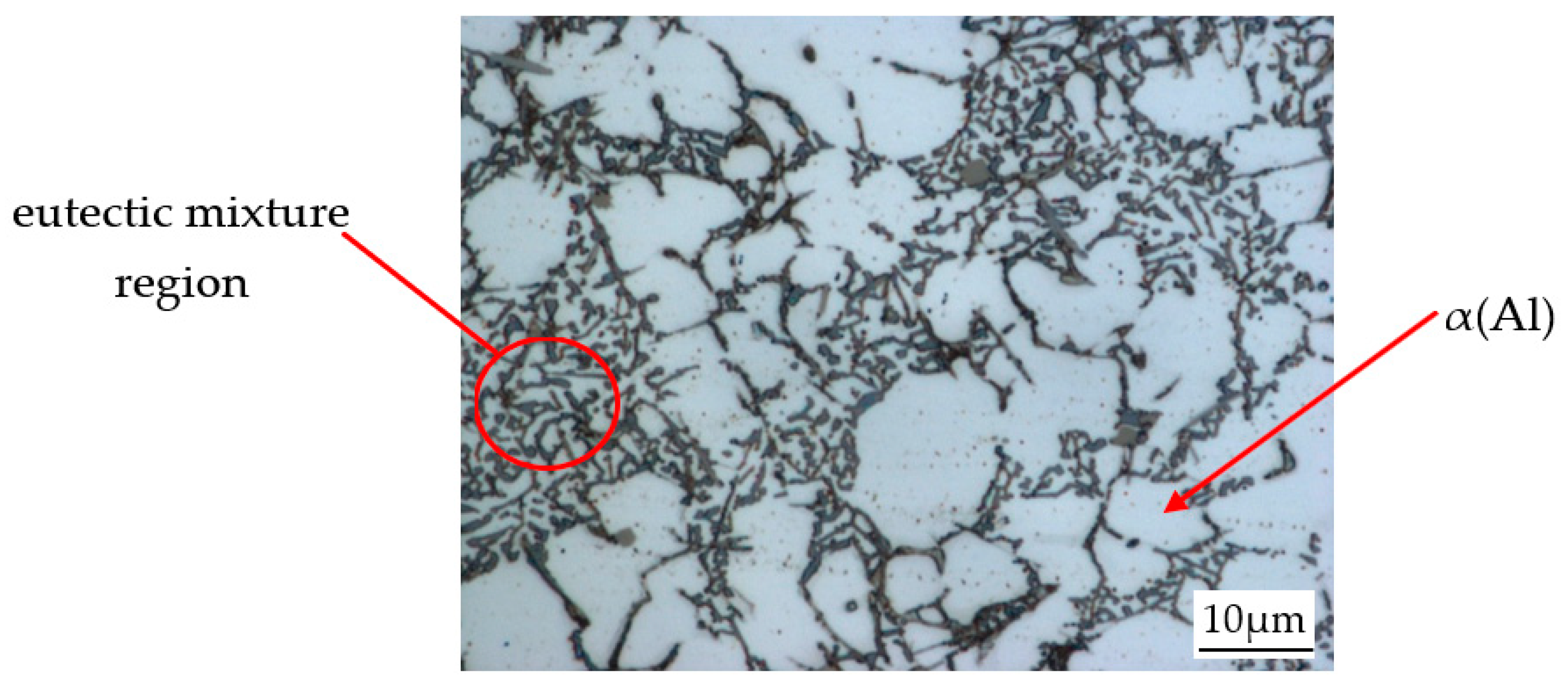
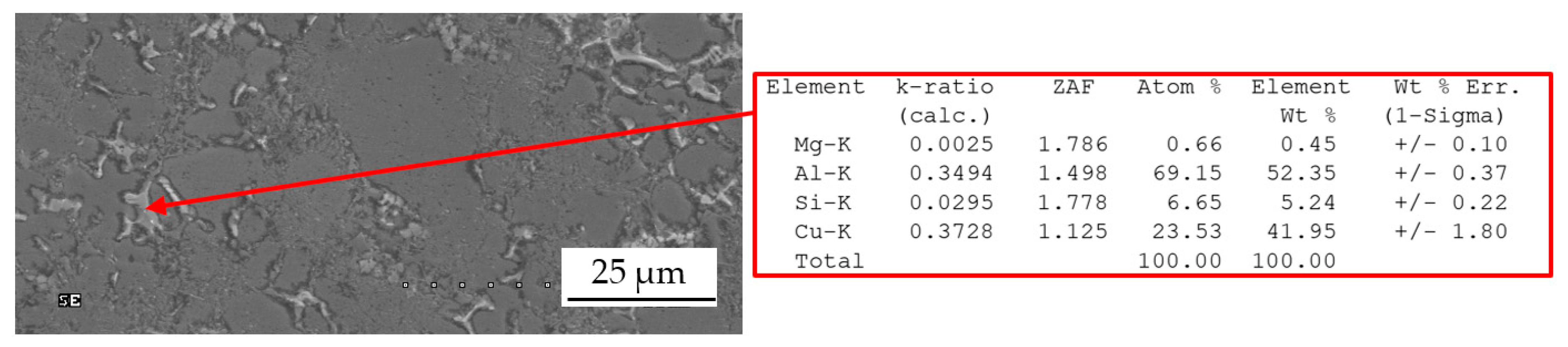
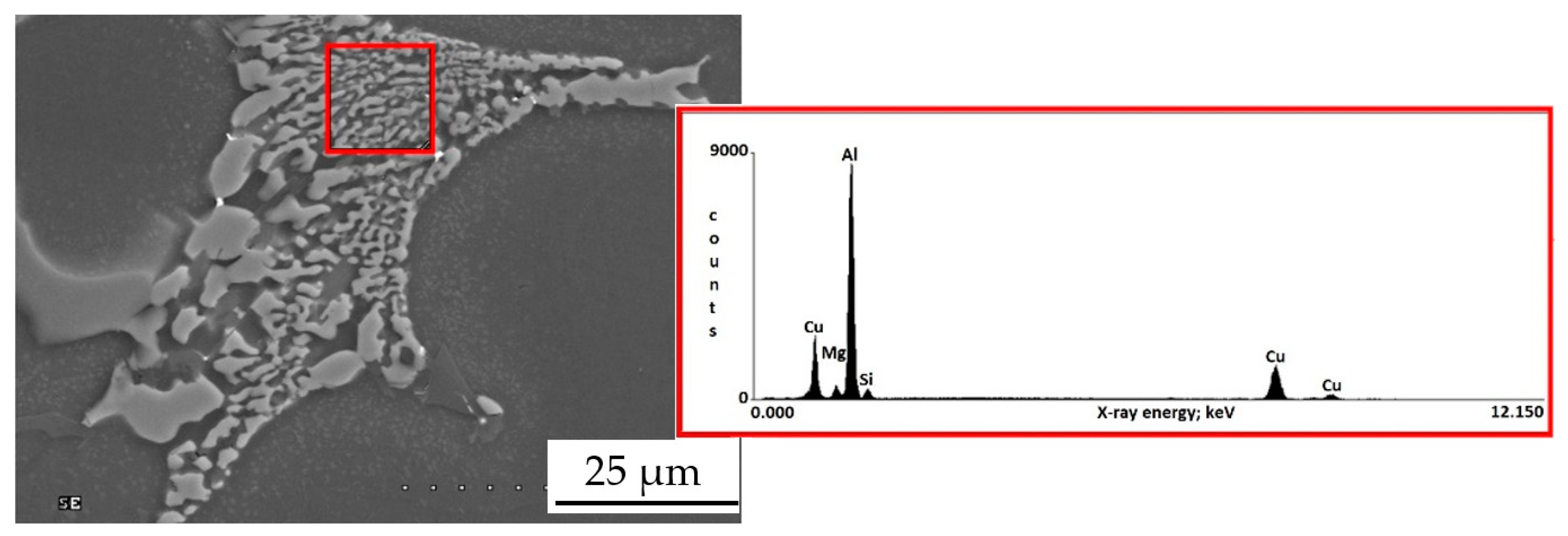
3.1. Base Alloy
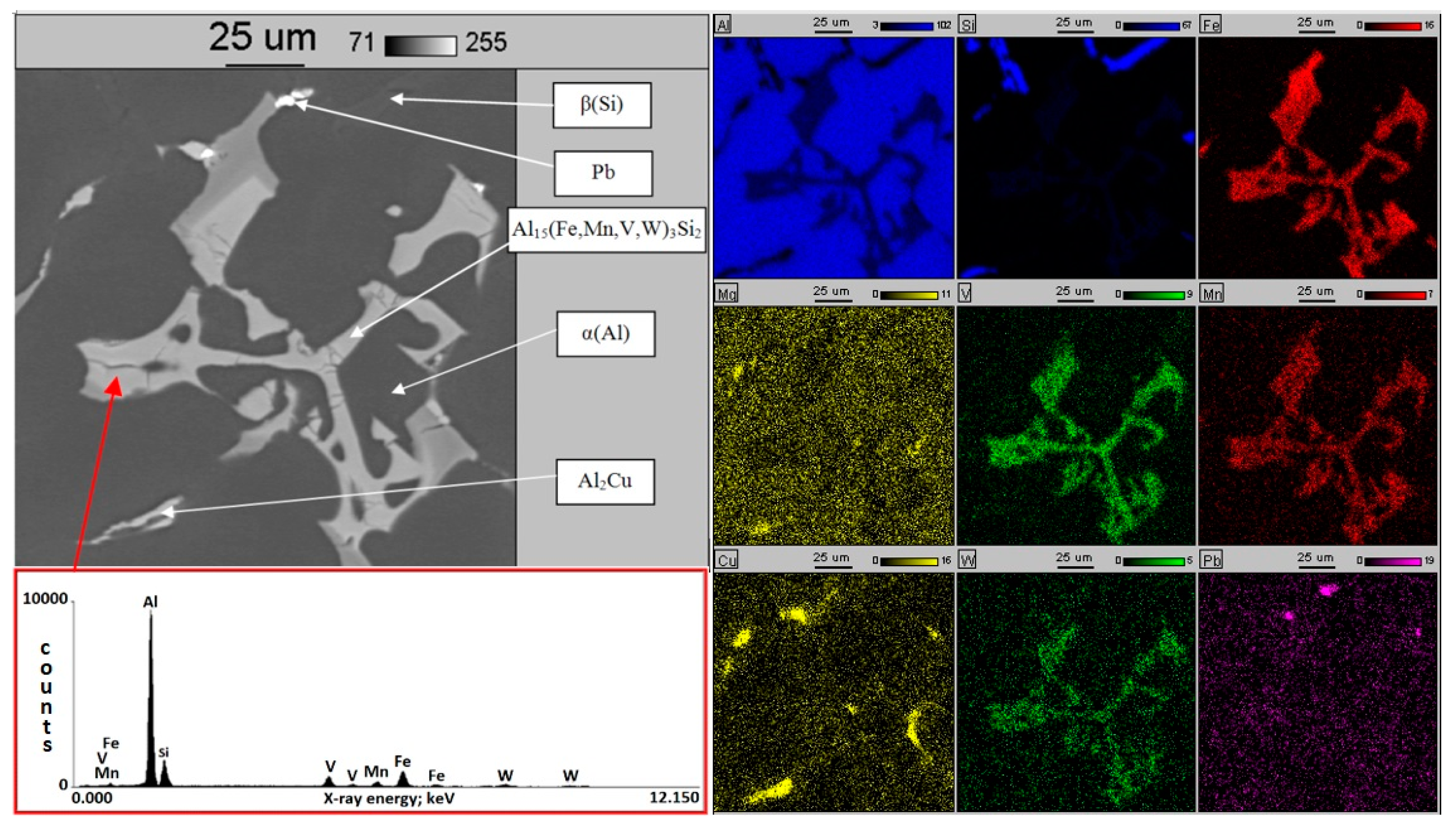
3.2. Al-Si Alloys with High Melting Point Elements from the Shell Mold
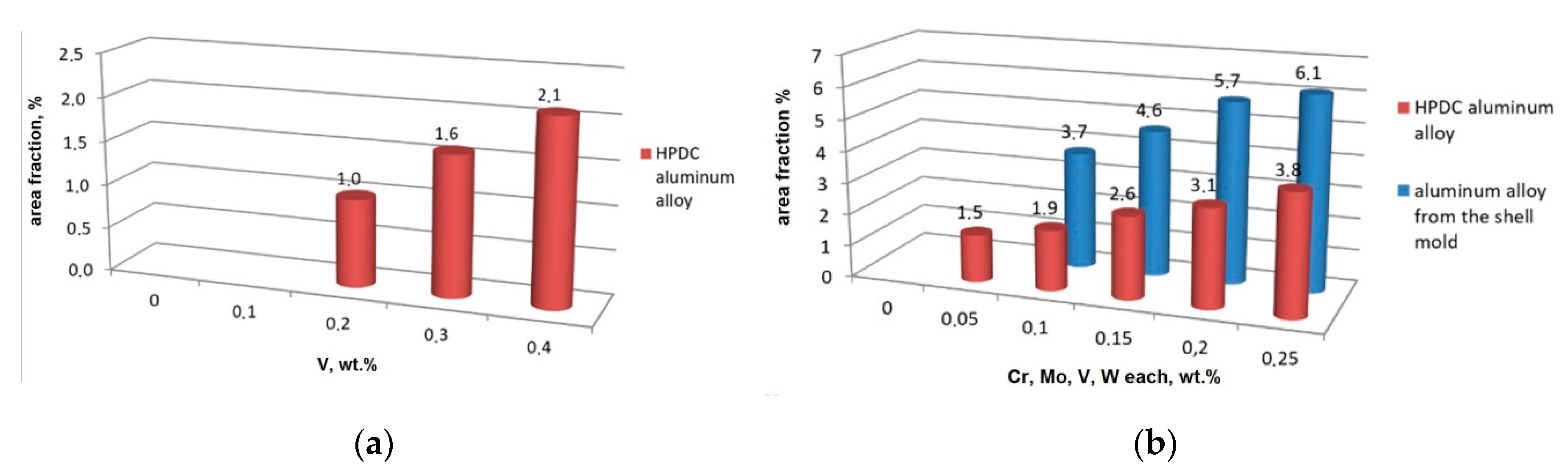
3.3. High Pressure Die Casting (HPDC) Al-Si Alloys with High Melting Point Elements
4. Discussion
5. Conclusions
- The addition of a small amount of high-melting point elements to the alloy causes their attachment to the constituent phases of the base alloy.
- Exceeding a certain limit content of high melting point elements causes the crystallization of Al15(FeMnM)3Si2 phase at a temperature higher than the crystallization temperature of the primary α(Al) phase. Further increase in the content of high-melting-point elements causes an increase in the area fraction of the Al15(FeMnM)3Si2 phase in the alloy.
- High-melting-point elements in the range of content that does not cause formation of the primary Al15(FeMnM)3Si2 phase result in an increase in their concentration before the α(Al) dendrite crystallization front.
- Primary crystallization of Al15(FeMnM)3Si2 at a temperature higher than the crystallization temperature of α(Al) dendrites, absorbs a significant amount of high melting point elements, thus reducing their concentration in front of α(Al) dendrite crystallization front.
- The greatest possibility of effective supersaturation of α(Al) dendrites with high-melting-point elements is created by their possibly high content in the alloy, which does not, however, cause the primary crystallization of the Al15(FeMnM)3Si2 phase.
Author Contributions
Funding
Conflicts of Interest
References
- Kahler, G.A.; Fisher, F.M.; Sass, R.L. The chemical composition and mechanical properties of the hinge ligament in bivalve molluscs. Biol. Bull. 1976, 151, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A. Iron-Containing Intermetallic Phases in Al-Si Based Casting. In Procedia Materials Science, Proceeding of the 11th International Congress on Metallurgy & Materials SAM/CONAMET, Rosario, Argentina, 18–21 October 2011; pp. 19–33. Available online: https://www.sciencedirect.com/science/article/pii/S2211812812000053 (accessed on 22 September 2020).
- Szymczak, T.; Gumienny, G.; Kurowska, B.; Tadeusz, P. Hypoeutectic Al-Si Alloy doped with Chromium, Tungsten and Molybdenum designated for pressure die casting. Arch. Met. Mater. 2017, 62, 1629–1635. [Google Scholar] [CrossRef]
- Dinnis, C.M.; Taylor, J.A.; Dahle, A.K. Porosity formation and eutectic growth in Al-Si-Cu-Mg alloys containing Iron and Manganese. In Proceedings of the 9th International Conference on Aluminium Alloys (ICAA9), Brisbane, Australia, 2–5 August 2004; pp. 1016–1021. [Google Scholar]
- Timelli, G.; Bonollo, F. The influence of Cr content on the microstructure and mechanical properties of AlSi9Cu3(Fe) die-casting alloys. Mater. Sci. Eng. A 2010, 528, 273–282. [Google Scholar] [CrossRef]
- Outmani, I.; Fouilland-Paille, L.; Isselin, J.; El Mansori, M. Effect of Si, Cu and processing parameters on Al-Si-Cu HPDC castings. J. Mater. Process. Technol. 2017, 249, 559–569. [Google Scholar] [CrossRef]
- Dos Santos, S.L.; Antunes, R.A.; Santos, S.F. Influence of injection temperature and pressure on the microstructure, mechanical and corrosion properties of a AlSiCu alloy processed by HPDC. Mater. Des. 2015, 88, 1071–1081. [Google Scholar] [CrossRef]
- Makhlouf, M.M.; Apelian, D. Casting Characteristics of Aluminum Die Casting Alloys; Advanced Casting Research Center Worcester Polytechnic Institute: Worcester, MA, USA, 2002. [Google Scholar]
- Han, Q.; Viswanathan, S. Analysis of the mechanism of die soldering in Aluminum die casting. Met. Mater. Trans. A 2003, 34, 139–146. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Chen, X.G.; Pekguleryuz, M. Dispersoid strengthening of a high temperature Al–Si–Cu–Mg alloy via Mo addition. Mater. Sci. Eng. A 2015, 620, 181–189. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Wu, Y.; Wei, Z.; Liu, X. Supportive strengthening role of Cr-rich phase on Al–Si multicomponent piston alloy at elevated temperature. Mater. Sci. Eng. A 2011, 528, 4427–4430. [Google Scholar] [CrossRef]
- Kim, H.Y.; Han, S.W.; Lee, H.M. The influence of Mn and Cr on the tensile properties of A356–0.20Fe alloy. Mater. Lett. 2006, 60, 1880–1883. [Google Scholar] [CrossRef]
- Elhadari, H.; Patel, H.; Chen, D.; Kasprzak, W. Tensile and fatigue properties of a cast aluminum alloy with Ti, Zr and V additions. Mater. Sci. Eng. A 2011, 528, 8128–8138. [Google Scholar] [CrossRef]
- Mbuya, T.O.; Odera, B.O.; Ng’Ang’A, S.P. Influence of iron on castability and properties of aluminium silicon alloys: Literature review. Int. J. Cast Met. Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; Xu, R.; Xiao, H.; Zhang, W.; Li, S. Effects of Vanadium on Modification of Iron-Rich Intermetallics and Mechanical Properties in A356 Cast Alloys with 1.5 wt.% Fe. J. Mater. Eng. Perform. 2018, 28, 475–484. [Google Scholar] [CrossRef]
- Bolibruchova, D.; Žihalová, M. Vanadium Influence on Iron Based Intermetallic Phases in AlSi6Cu4 Alloy. Arch. Met. Mater. 2014, 59, 1029–1032. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Mo (Aluminum-Molybdenum). J. Phase Equilibria Diffus. 2010, 31, 492–493. [Google Scholar] [CrossRef]
- Wood, D.M. Coherent epitaxy, surface effects, and semiconductor alloy phase diagrams. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1992, 10, 1675. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Cr (Aluminum-Chromium). J. Phase Equilibria Diffus. 2007, 29, 112–113. [Google Scholar] [CrossRef]
- Okamoto, H. Mo-Si (Molybdenum-Silicon). J. Phase Equilibria Diffus. 2011, 32, 176. [Google Scholar] [CrossRef]
- Smith, J.F. The Si-V (Silicon-Vanadium) system: Addendum. Bull. Alloy. Phase Diagr. 1985, 6, 266–271. [Google Scholar] [CrossRef]
- Guo, Z.; Yuan, W.; Sun, Y.; Cai, Z.; Qiao, Z. Thermodynamic Assessment of the Si-Ta and Si-W Systems. J. Phase Equilibria Diffus. 2009, 30, 564–570. [Google Scholar] [CrossRef]
- Venkatraman, M.; Neumann, J.P. The Cr−Mo (Chromium-Molybdenum) system. Bull. Alloy Phase Diagr. 1987, 8, 216–220. [Google Scholar] [CrossRef]
- Smith, J.F.; Bailey, D.M.; Carlson, O.N. The Cr-V (Chromium-Vanadium) System. Bull. Alloy Phase Diagr. 1982, 2, 469–473. [Google Scholar] [CrossRef]
- Nagender Naidu, S.V.; Sriramamurthy, A.M.; Rama Rao, P. The Cr-W (Chromium-Tungsten) System. Bull. Alloy Phase Diagr. 1984, 5, 289–292. [Google Scholar] [CrossRef]
- Zheng, F.; Argent, B.B.; Smith, J.F. Thermodynamic computation of the Mo-V binary phase diagram. J. Phase Equilibria Diffus. 1999, 20, 370–372. [Google Scholar] [CrossRef]
- Okamoto, H. V-W (Vanadium-Tungsten). J. Phase Equilibria Diffus. 2010, 31, 324. [Google Scholar] [CrossRef]
- Szymczak, T.; Szymszal, J.; Gumienny, G. Statistical Methods Used in the Assessment of the Influence of the Al-Si Alloy’s Chemical Composition on its Properties. Arch. Foundry Eng. 2018, 18, 203–211. [Google Scholar]
- Szymczak, T.; Szymszal, J.; Gumienny, G. Evaluation of the Effect of the Cr, Mo, V and W Content in an Al-Si Alloy Used for Pressure Casting on its Proof Stress. Arch. Foundry Eng. 2018, 18, 105–111. [Google Scholar]
- Szymczak, T.; Szymszal, J.; Gumienny, G. Evaluation of the Effect of Cr, Mo, V and W on the Selected Properties of Silumins. Arch. Foundry Eng. 2018, 18, 77–82. [Google Scholar]
- Nývlt, J. Kinetics of nucleation in solutions. J. Cryst. Growth 1968, 3-4, 377–383. [Google Scholar] [CrossRef]
- Nemdili, L.; Koutchoukali, O.; Mameri, F.; Gouaou, I.; Koutchoukali, M.; Ulrich, J. Crystallization study of potassium sulfate-water system, metastable zone width and induction time measurements using ultrasonic, turbidity and 3D-ORM techniques. J. Cryst. Growth 2018, 500, 44–51. [Google Scholar] [CrossRef]
- Chai, G.; Bäckerud, L.; Rolland, T.; Arnberg, L. Dendrite coherency during equiaxed solidification in binary aluminum alloys. Met. Mater. Trans. A 1995, 26, 965–970. [Google Scholar] [CrossRef]
- Gumienny, G.; Kacprzyk, B.; Gawroński, J. Effect of Copper on the Crystallization Process, Microstructure and Selected Properties of CGI. Arch. Foundry Eng. 2017, 17, 51–56. [Google Scholar] [CrossRef]
- Pezda, J. Effect of The T6 Heat Treatment On Change Of Mechanical Properties Of The AlSi12CuNiMg Alloy Modified With Strontium. Arch. Met. Mater. 2015, 60, 627–632. [Google Scholar] [CrossRef]
- Władysiak, R.; Kozuń, A.; Dębowska, K.; Tadeusz, P. Analysis of Crystallization Process of Intensive Cooled AlSi20CuNiCoMg Alloy. Arch. Foundry Eng. 2017, 17, 137–144. [Google Scholar] [CrossRef]
- Pisarek, B. Model of Cu-Al-Fe-Ni Bronze Crystallization. Arch. Foundry Eng. 2013, 13, 72–79. [Google Scholar] [CrossRef]
- Rapiejko, C.; Pisarek, B.; Tadeusz, P. Effect of Cr and V Alloy Additions on the Microstructure and Mechanical Properties of Am60 Magnesium Alloy. Arch. Met. Mater. 2014, 59, 761–765. [Google Scholar] [CrossRef]
- Kacprzyk, B.; Szymczak, T.; Gumienny, G.; Klimek, L. Effect of the Remelting on Transformations in Co-Cr-Mo Prosthetics Alloy. Arch. Foundry Eng. 2013, 13, 47–50. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Multicomponent Phase Diagrams: Applications for Commercial Aluminum Alloys; Elsevier: London, UK, 2005. [Google Scholar]
- Glazoff, M.V.; Khvan, A.V.; Zolotorevsky, V.S.; Belov, N.A.; Dinsdale, A.T. Casting Aluminum Alloys; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Pietrowski, S. Al-Si Alloys; Lodz University of Technology Publishing House: Lodz, Poland, 2019. [Google Scholar]
- Hurtalová, L.; Tillová, E.; Chalupová, M.; Ďuriníková, E. Effect of Chemical Composition of Secondary Al-Si Cast Alloy on Intermetallic Phases. In Machines, Technologies, Materials, Proceedings of the 9th International Congress, Varna, Bulgaria, 19–21 September 2012; pp. 23–26. Available online: https://scholar.google.com.hk/scholar?hl=en&as_sdt=0%2C5&q=Effect+of+Chemical+Composition+of+Secondary+Al-Si+Cast+Alloy+on+Intermetallic+Phases&btnG= (accessed on 22 September 2020).
- Tillová, E.; Závodská, D.; Kuchariková, L.; Chalupová, M.; Belan, J. Study of Bending Fatigue Properties of Al-Si Cast Alloy. Arch. Met. Mater. 2017, 62, 1591–1596. [Google Scholar] [CrossRef][Green Version]
- Kuchariková, L.; Tillová, E.; Matvija, M.; Belan, J.; Chalupová, M. Study of the precipitation hardening process in recycled Al-Si-Cu cast alloys. Arch. Met. Mater. 2017, 62, 397–403. [Google Scholar] [CrossRef]

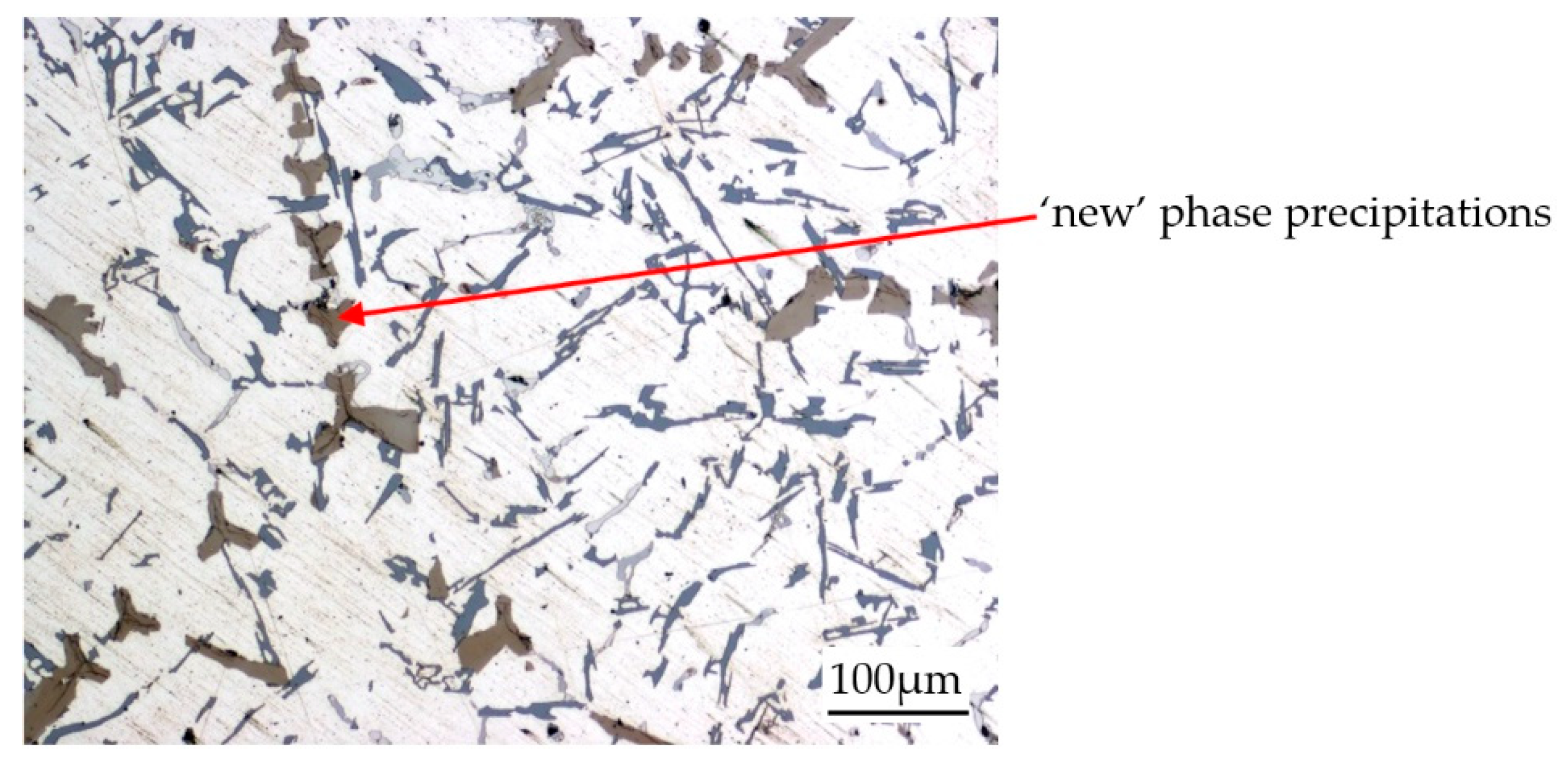
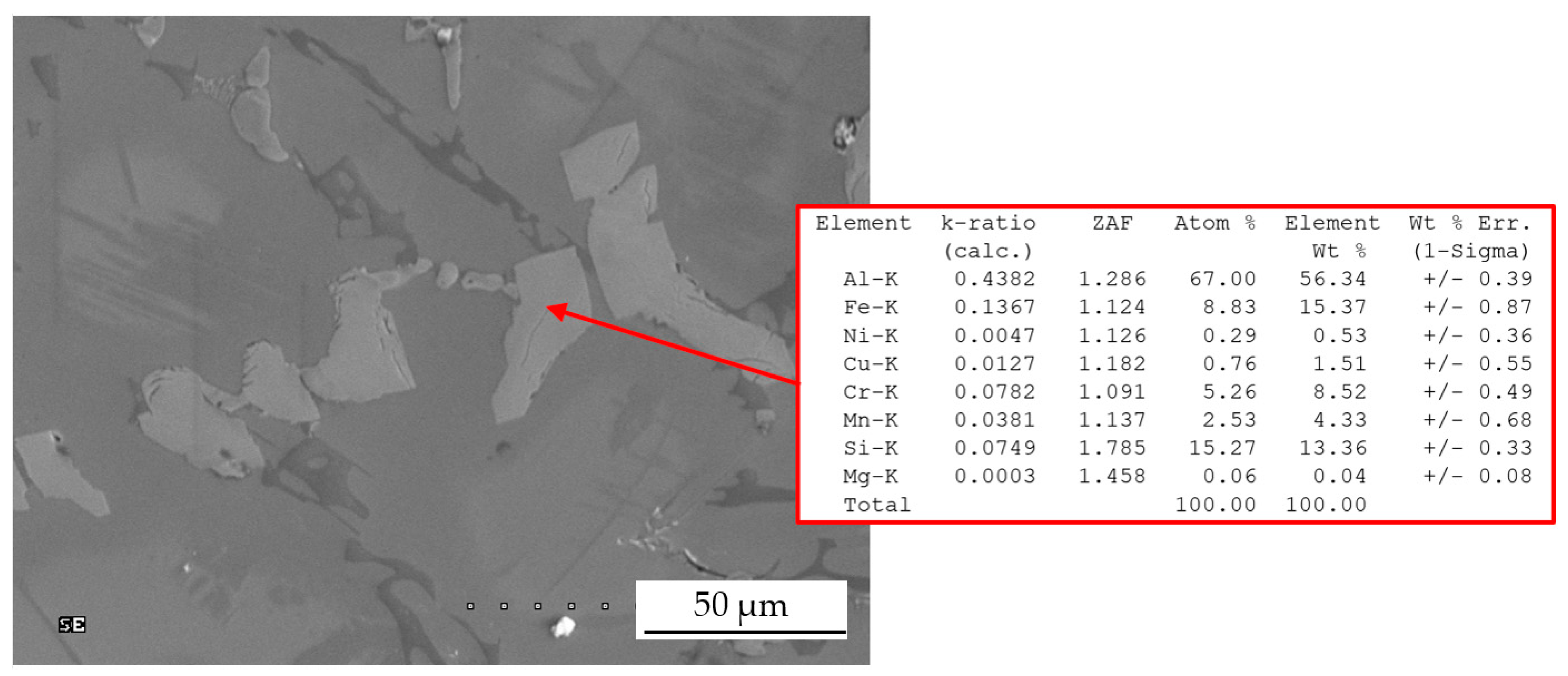
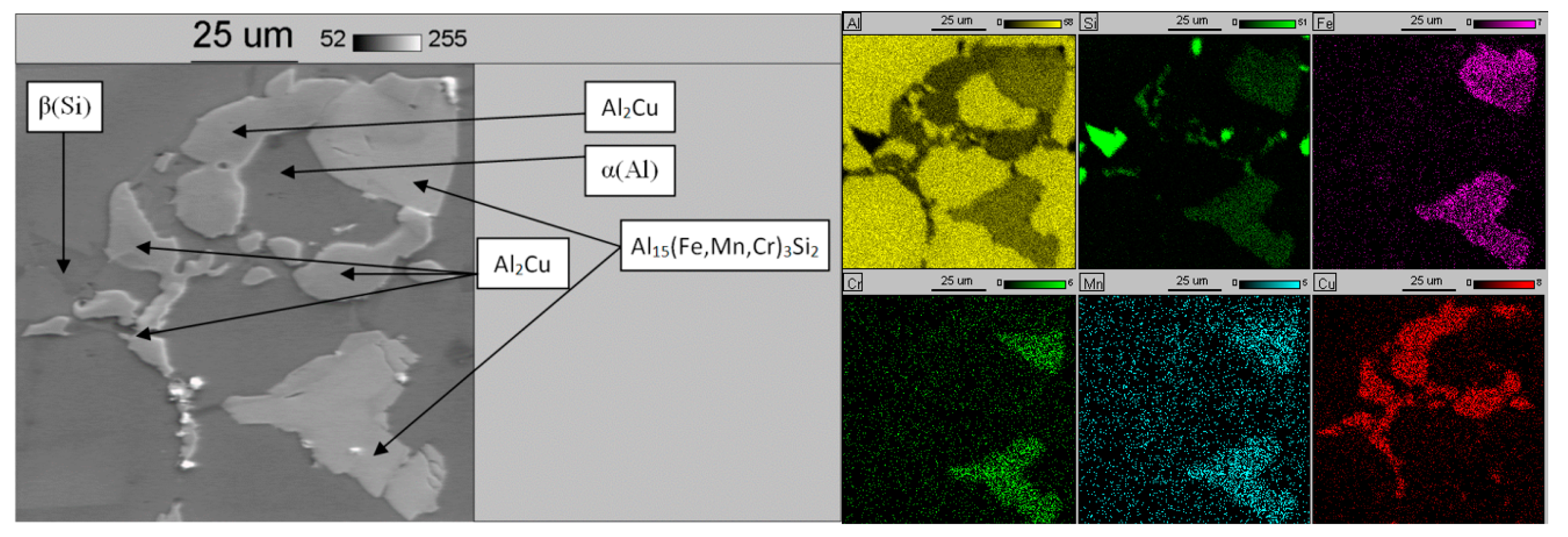
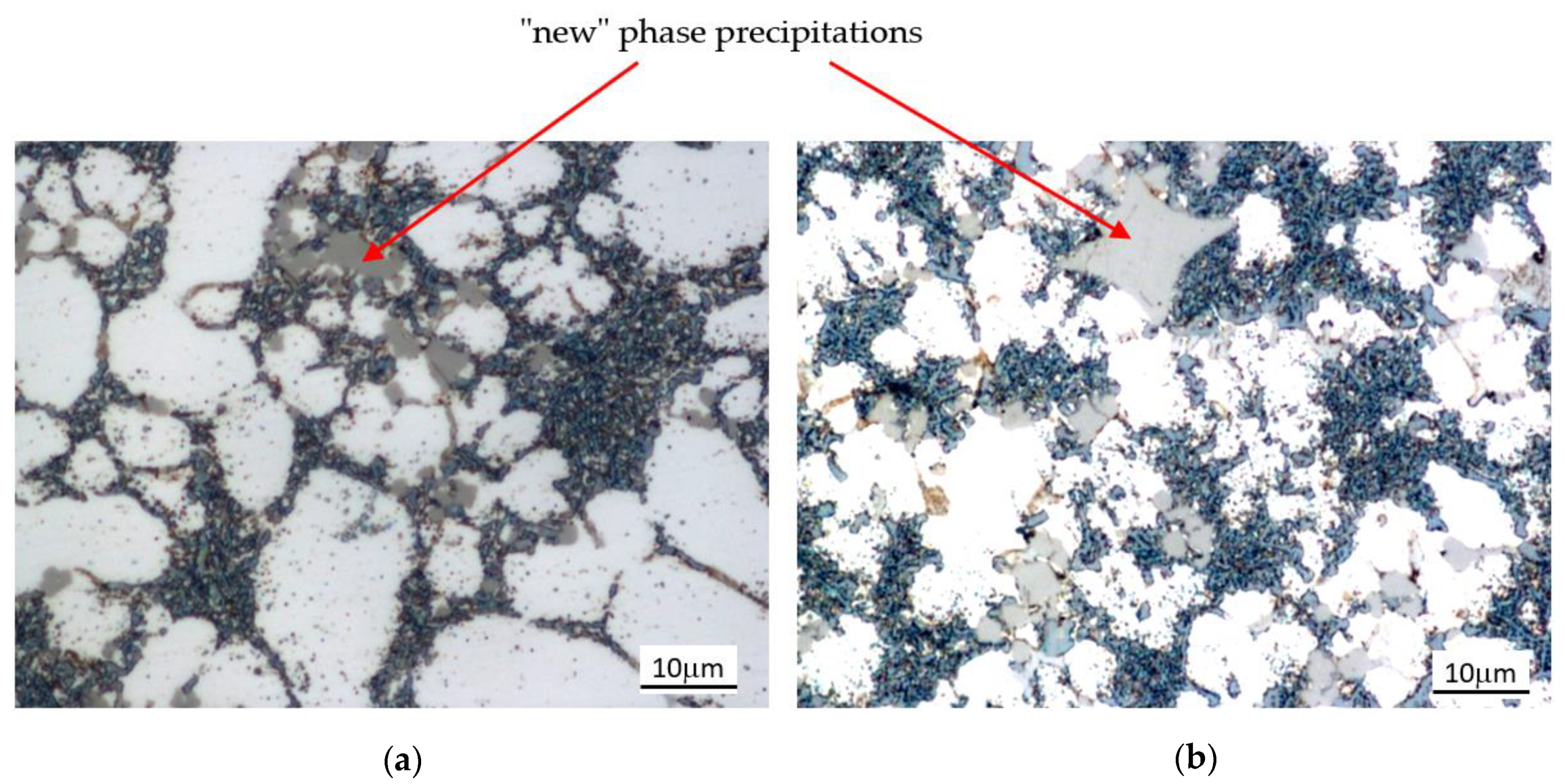


| Chemical Composition, wt % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Si | Cu | Zn | Fe | Mg | Mn | Ni | Ti | Al |
| 8.69 ÷ 9.35 | 2.09 ÷ 2.43 | 0.90 ÷ 1.07 | 0.82 ÷ 0.97 | 0.21 ÷ 0.32 | 0.18 ÷ 0.25 | 0.05 ÷ 0.13 | 0.042 ÷ 0.049 | al. |
| Combinations of High Melting Point Elements | Content Range, wt % | Step, wt % | |
|---|---|---|---|
| Single elements | Cr | 0.0 ÷ 0.5 | 0.1 |
| Mo | |||
| V | |||
| W | |||
| Double combinations | Cr, Mo | 0.0 ÷ 0.4 each | |
| Cr, V | |||
| Cr, W | |||
| Mo, V | |||
| Mo, W | |||
| V, W | |||
| Triple combinations | Cr, Mo, V | 0.00 ÷ 0.25 each | 0.05 |
| Mo, V, W | |||
| Cr, V, W | |||
| Cr, Mo, W | |||
| Simultaneously | Cr, Mo, V, W | ||
| Combinations of High Melting Point Elements | Content of High Melting Point Elements, at which the Primary Crystallization of the Al15(Fe,Mn,M)3Si2 Phase Was Observed; wt % | |
|---|---|---|
| Single elements | Cr | 0.2 |
| Mo | 0.5 | |
| V | - * | |
| W | 0.5 | |
| Double combinations | Cr, Mo | 0.1 each |
| Cr, V | 0.1 each | |
| Cr, W | 0.1 each | |
| Mo, V | 0.4 each | |
| Mo, W | 0.3 each | |
| V, W | - * | |
| Triple combinations | Cr, Mo, V | 0.25 each |
| Cr, Mo, W | 0.25 each | |
| Cr, V, W | 0.20 each | |
| Mo, V, W | 0.25 each | |
| Simultaneously | Cr, Mo, V, W | 0.10 each |
| Combinations of High Melting Point Elements | The Content of High Melting Point Elements, at which the Primary Al15(Fe,Mn,M)3Si2 Phase Was Observed; wt % | |
|---|---|---|
| Single elements | Cr | 0.2 |
| Mo | 0.2 | |
| V | 0.2 | |
| W | 0.2 | |
| Double combinations | Cr, Mo | 0.2 each |
| Cr, V | 0.2 each | |
| Cr, W | 0.2 each | |
| Mo, V | 0.2 each | |
| Mo, W | 0.2 each | |
| V, W | 0.2 each | |
| Triple combinations | Cr, Mo, V | 0.2 each |
| Cr, Mo, W | 0.2 each | |
| Cr, V, W | 0.2 each | |
| Mo, V, W | 0.2 each | |
| Simultaneously | Cr, Mo, V, W | 0.05 each |
| Phase No. | Concentration, wt % | |||||||
|---|---|---|---|---|---|---|---|---|
| Al | Si | Fe | Mn | Cr | Mo | V | W | |
| 1 | 56.04 | 9.50 | 15.56 | 4.25 | 6.40 | 5.10 | 2.64 | 0.52 |
| 2 | 56.28 | 9.28 | 19.73 | 4.82 | 6.56 | 3.30 | 2.74 | 0.29 |
| 3 | 56.47 | 8.86 | 16.27 | 5.38 | 5.29 | 3.80 | 3.08 | 0.86 |
| 4 | 58.81 | 12.33 | 15.03 | 4.69 | 4.20 | 2.57 | 2.12 | 0.25 |
| 5 | 56.86 | 9.10 | 15.78 | 4.17 | 6.44 | 3.72 | 2.96 | 0.99 |
| 6 | 56.78 | 9.05 | 15.59 | 4.15 | 6.03 | 4.17 | 2.30 | 1.93 |
| 7 | 69.73 | 6.40 | 9.57 | 3.33 | 3.41 | 2.58 | 1.27 | 3.71 |
| 8 | 57.00 | 8.91 | 16.31 | 5.66 | 5.23 | 3.76 | 2.83 | 0.40 |
| 9 | 59.75 | 9.10 | 15.62 | 4.91 | 5.31 | 2.78 | 2.12 | 0.40 |
| 10 | 56.46 | 9.00 | 17.12 | 4.08 | 6.10 | 3.70 | 2.55 | 0.99 |
| Average | 58.42 | 9.15 | 15.66 | 4.54 | 5.50 | 3.55 | 2.46 | 1.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, T.; Gumienny, G.; Klimek, L.; Goły, M.; Pacyniak, T. Microstructural Characteristics of AlSi9Cu3(Fe) Alloy with High Melting Point Elements. Metals 2020, 10, 1278. https://doi.org/10.3390/met10101278
Szymczak T, Gumienny G, Klimek L, Goły M, Pacyniak T. Microstructural Characteristics of AlSi9Cu3(Fe) Alloy with High Melting Point Elements. Metals. 2020; 10(10):1278. https://doi.org/10.3390/met10101278
Chicago/Turabian StyleSzymczak, Tomasz, Grzegorz Gumienny, Leszek Klimek, Marcin Goły, and Tadeusz Pacyniak. 2020. "Microstructural Characteristics of AlSi9Cu3(Fe) Alloy with High Melting Point Elements" Metals 10, no. 10: 1278. https://doi.org/10.3390/met10101278
APA StyleSzymczak, T., Gumienny, G., Klimek, L., Goły, M., & Pacyniak, T. (2020). Microstructural Characteristics of AlSi9Cu3(Fe) Alloy with High Melting Point Elements. Metals, 10(10), 1278. https://doi.org/10.3390/met10101278








