The Mechanism for HAZ Liquation of Nickel-Based Alloy 617B During Gas Tungsten Arc Welding
Abstract
1. Introduction
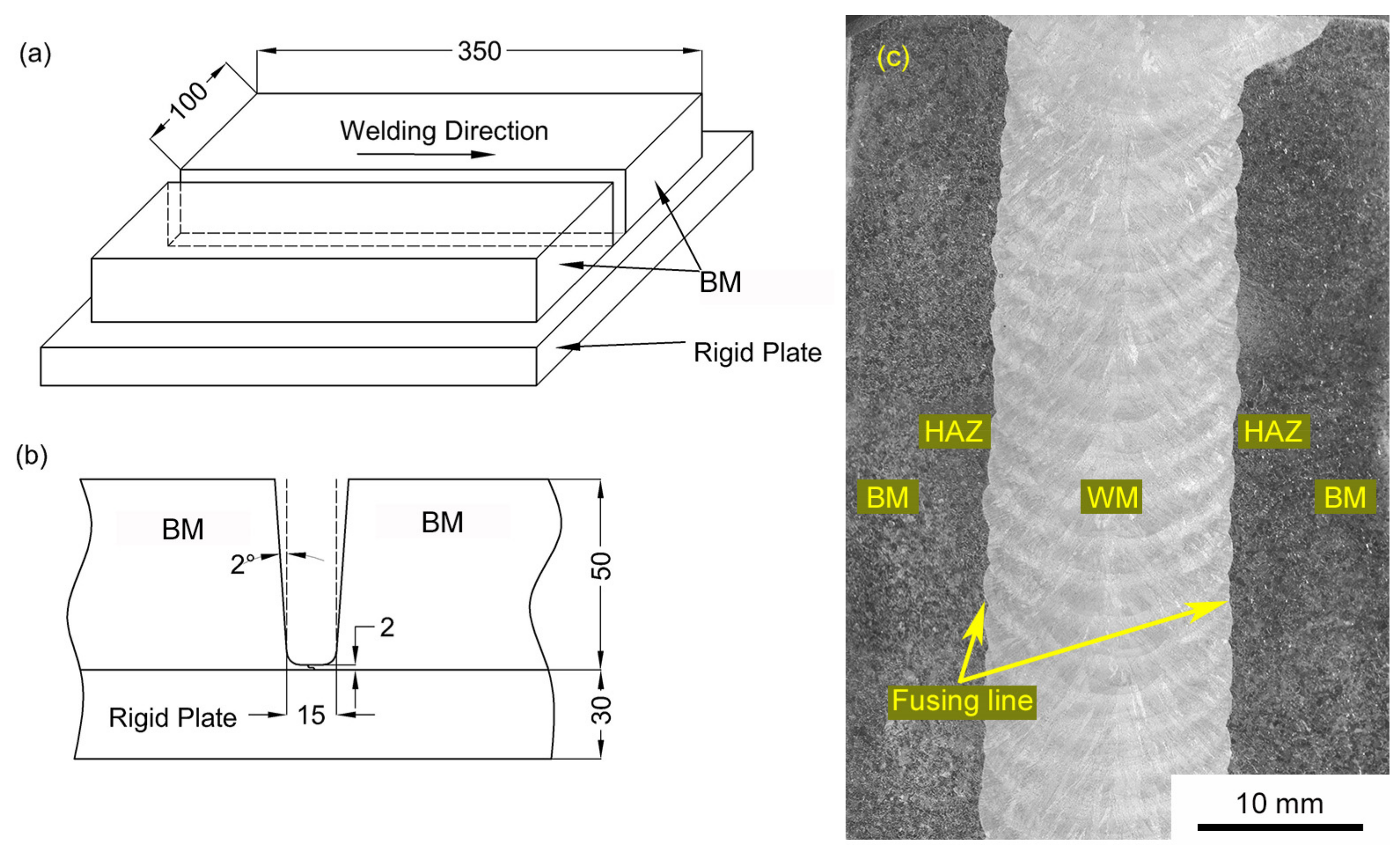
2. Materials and Methods
3. Results
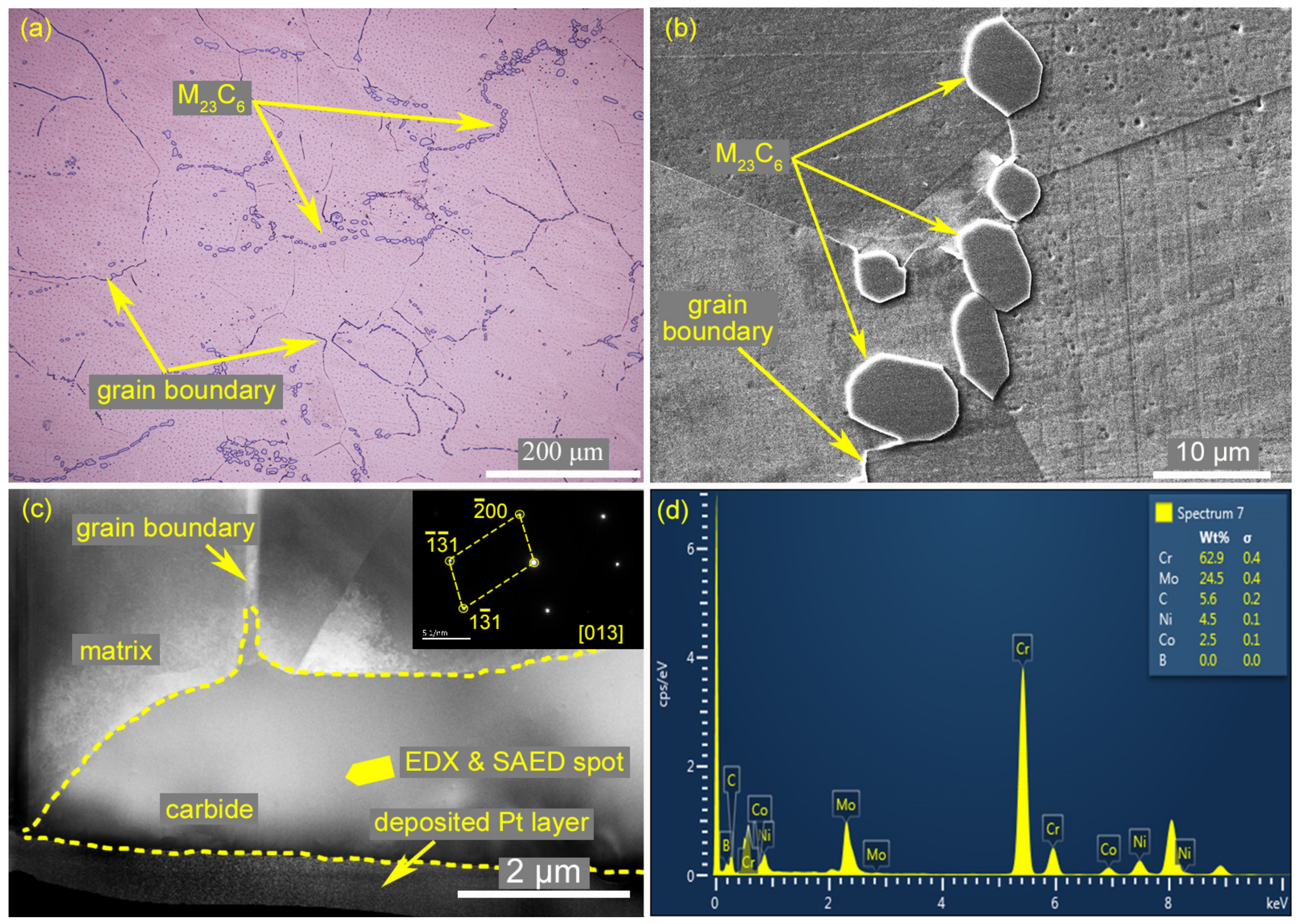
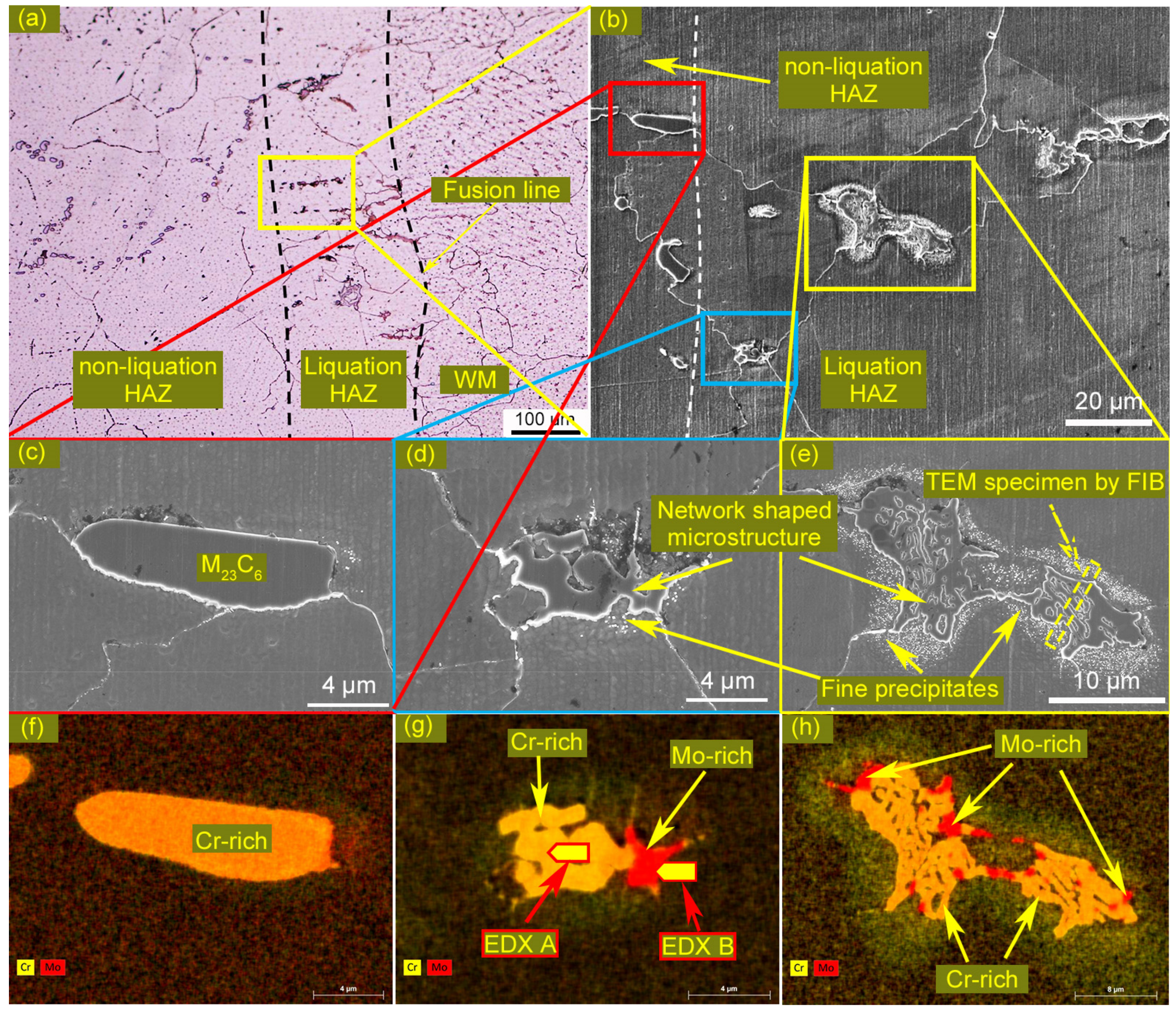
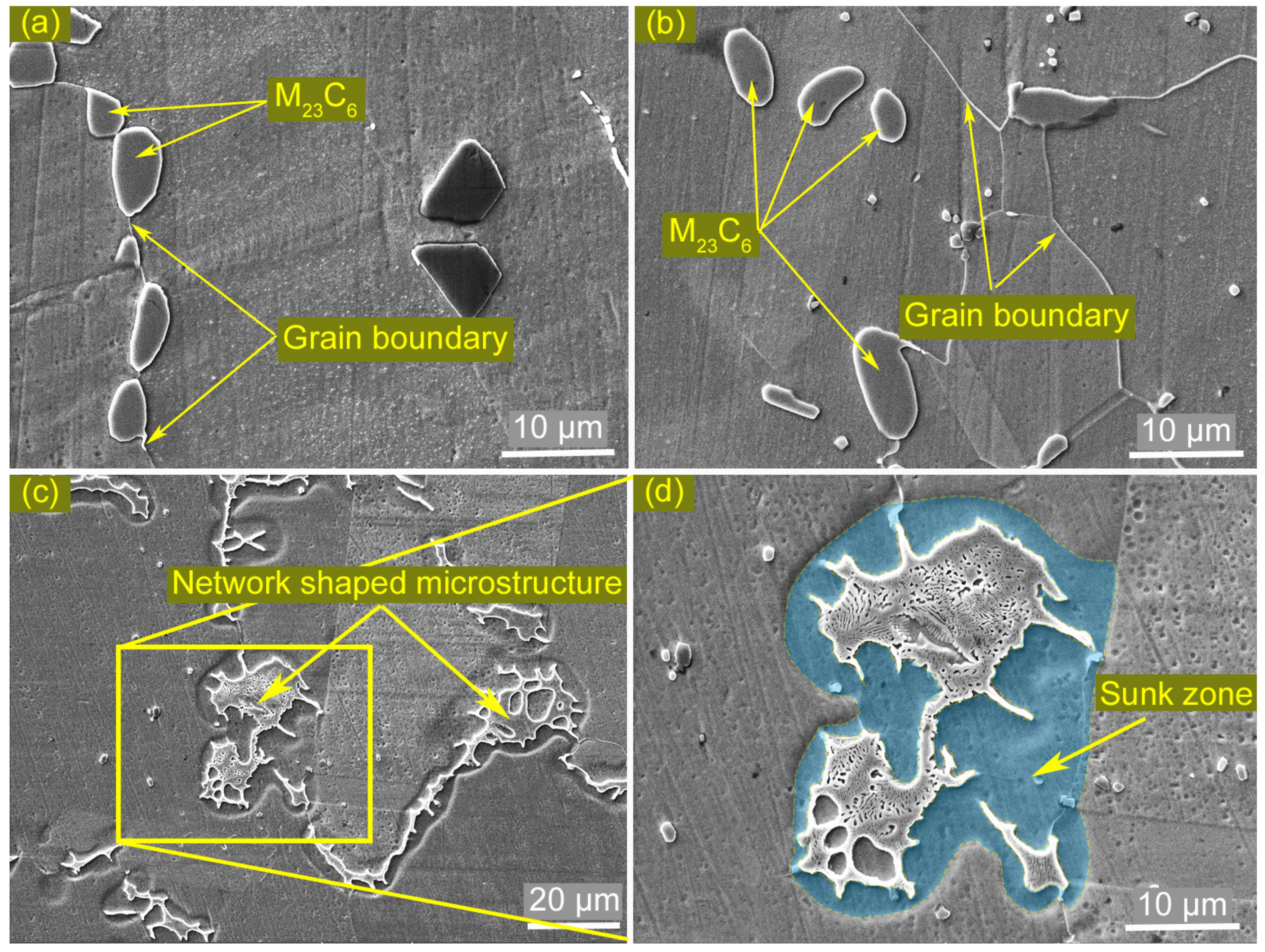
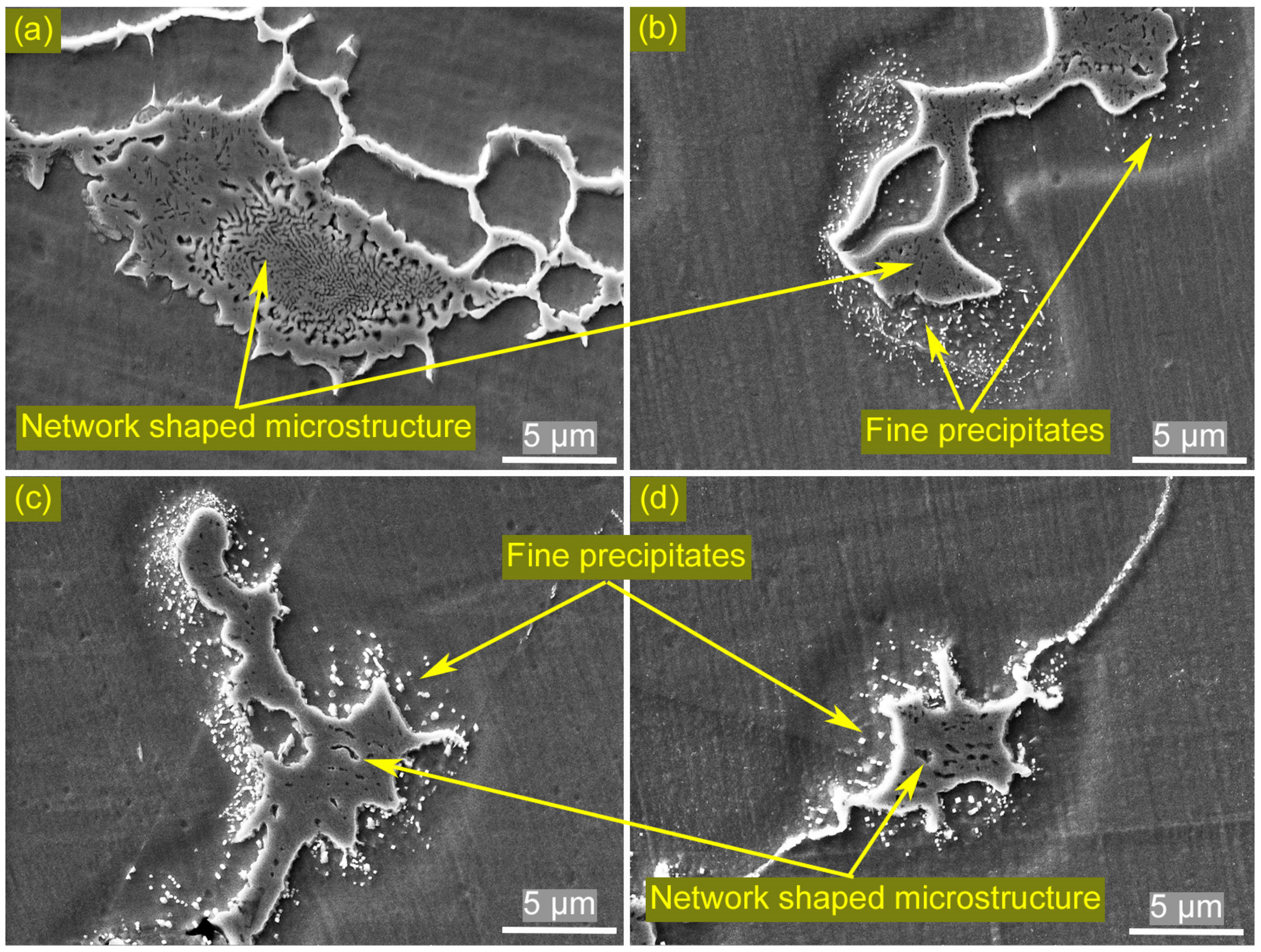
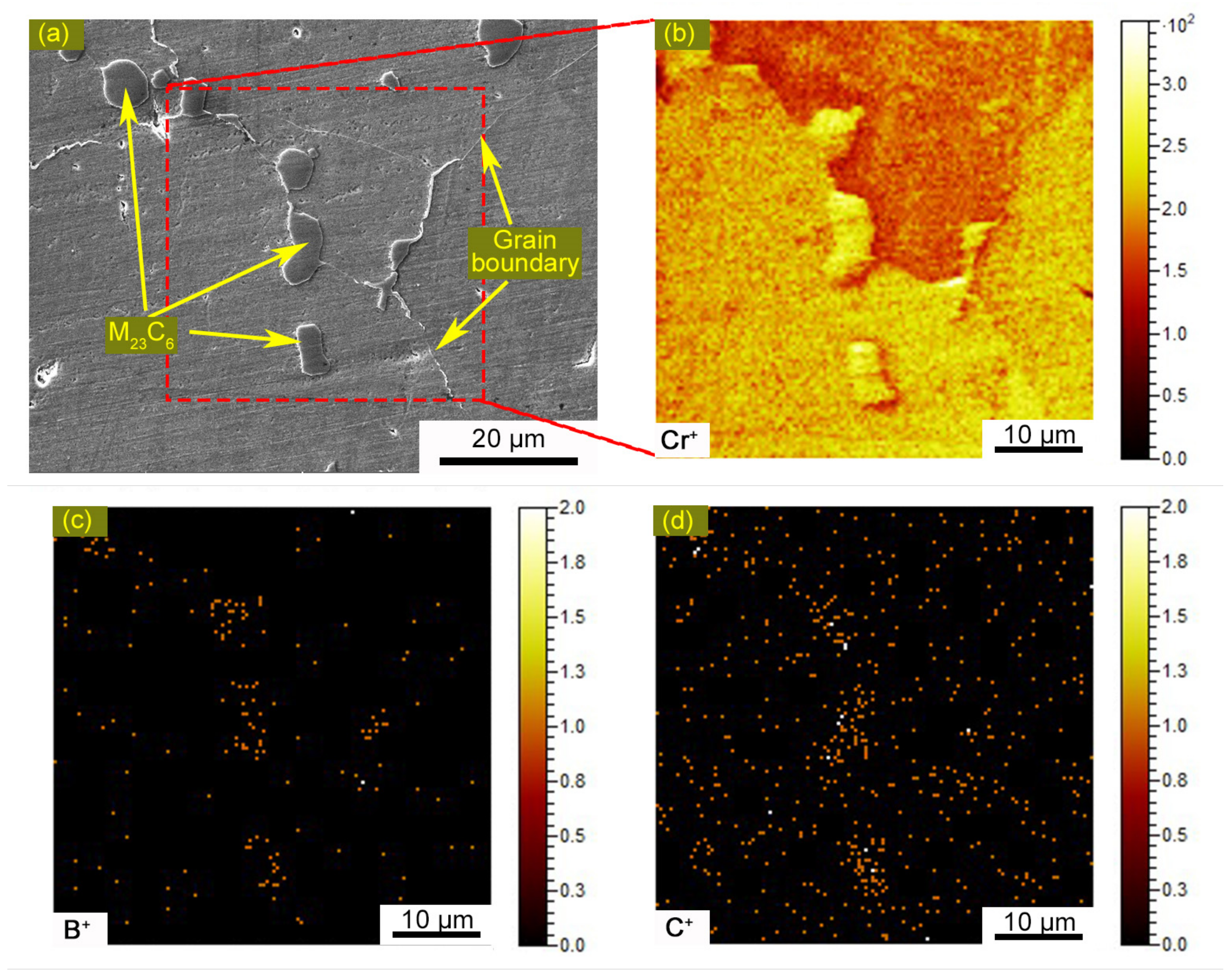
3.1. Microstructures of the Welded Joint
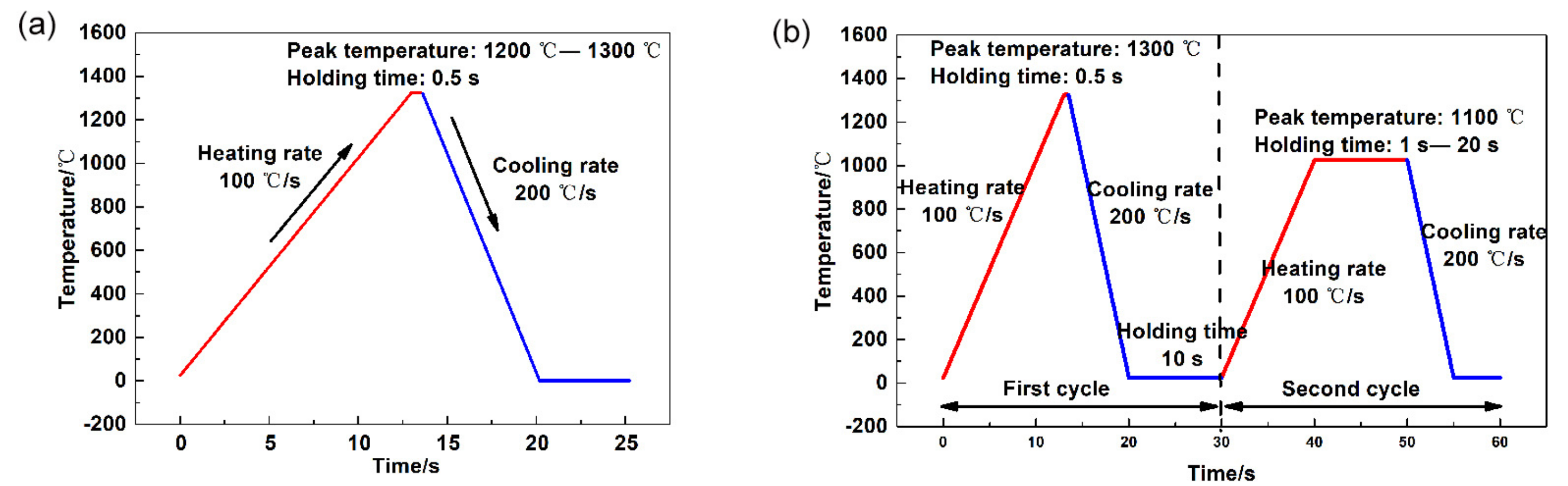
3.2. Thermal Simulation with One Thermal Cycle
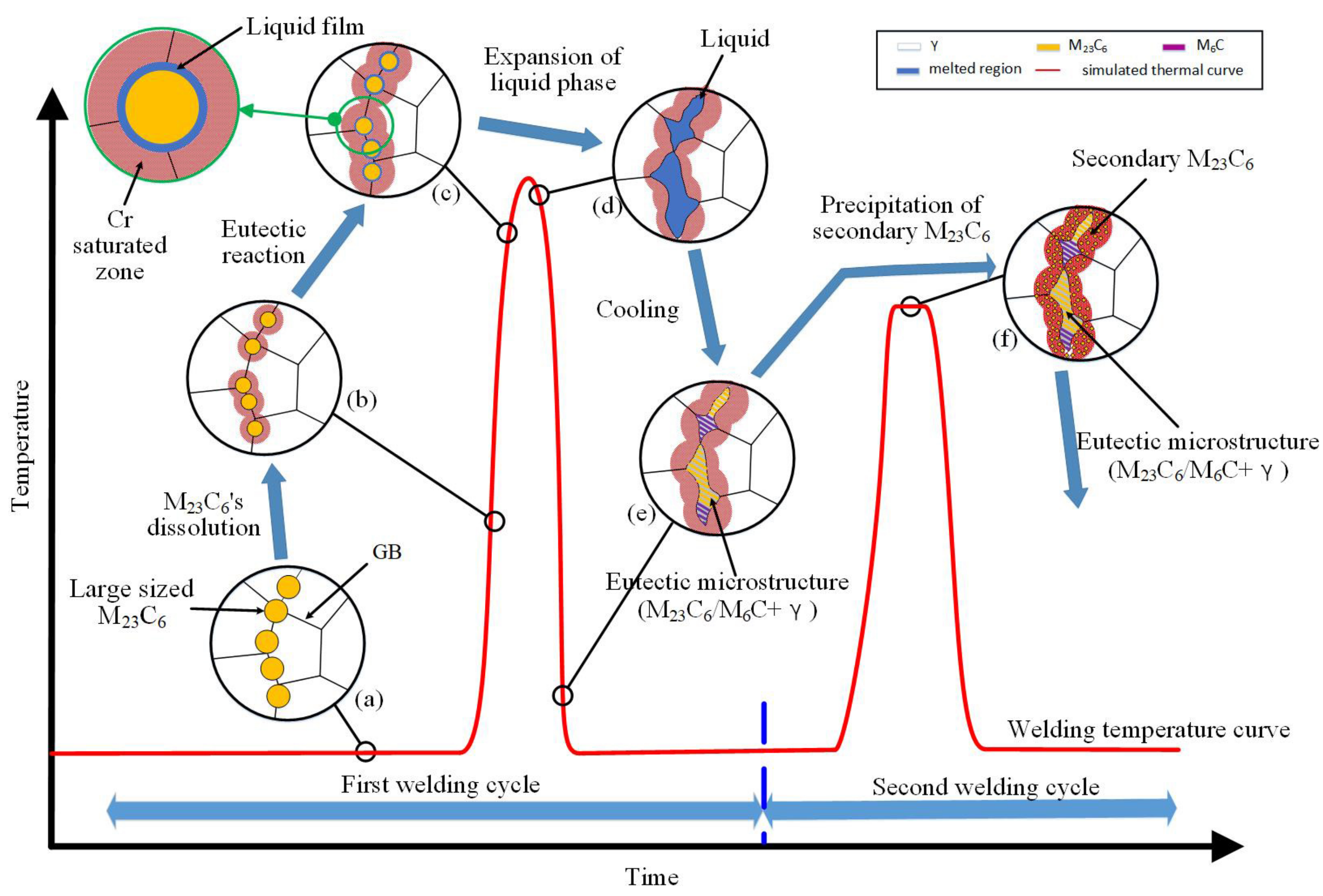
3.3. Thermal Simulation with Two Thermal Cycles
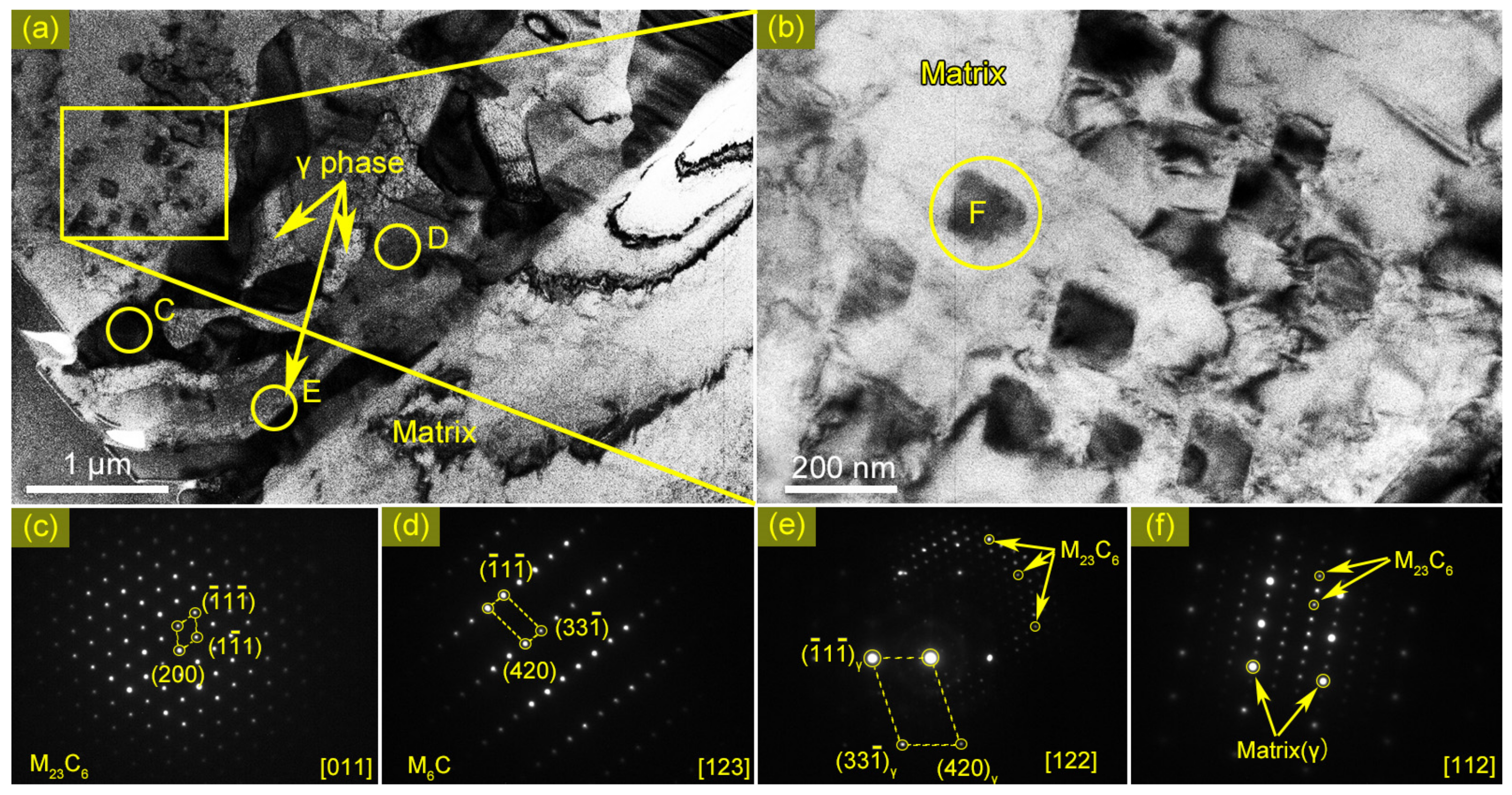
3.4. Identification of the Eutectic Microstructure in HAZ
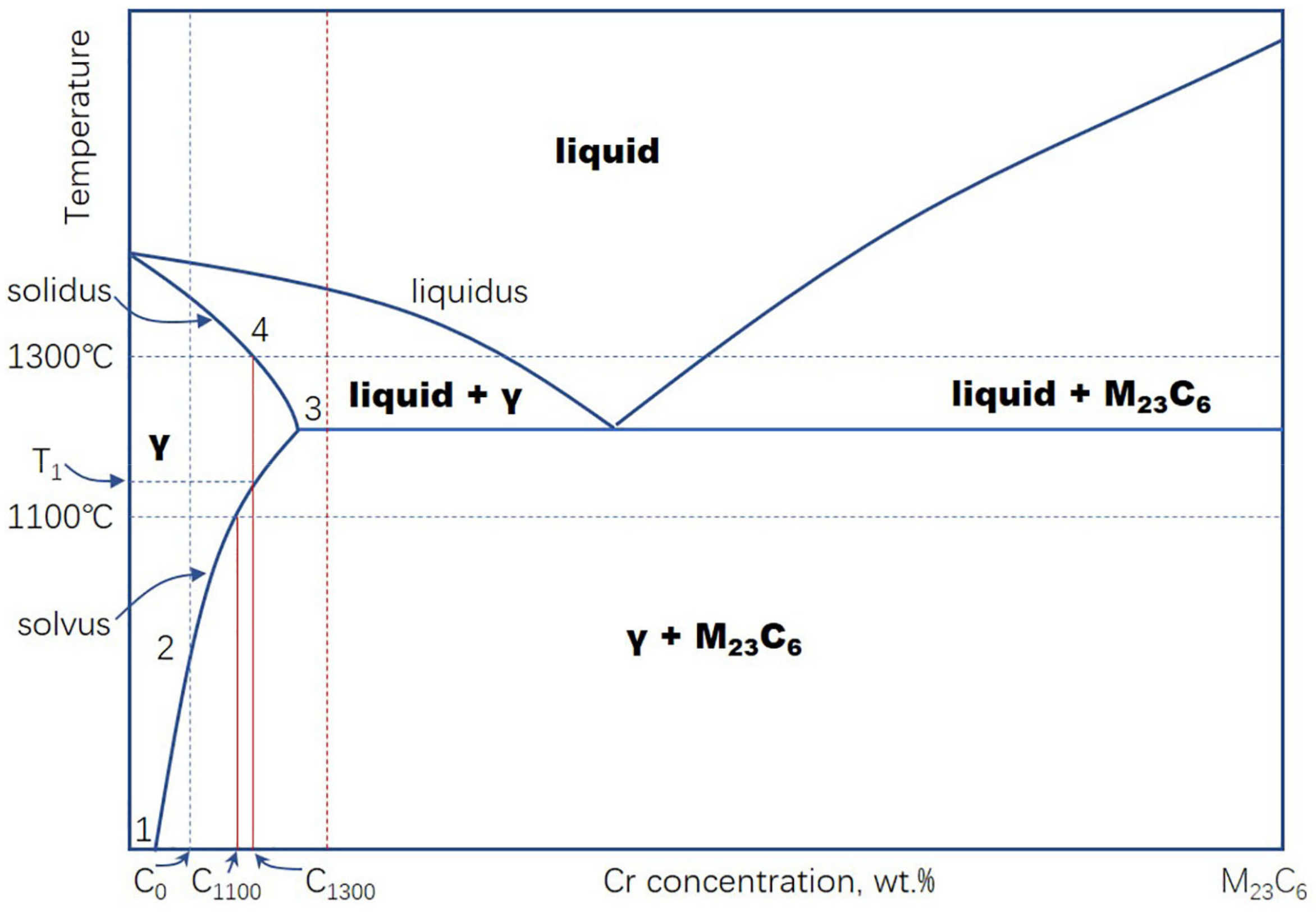
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Viswanathan, R.; Henry, J.F.; Tanzosh, J.; Stanko, G.; Shingledecker, J.; Vitalis, B.; Purgert, R.U.S. Program on materials technology for ultra-supercritical coal power plants. J. Mater. Eng. Perform. 2005, 14, 281–292. [Google Scholar] [CrossRef]
- Klöwer, J.; Husemann, R.U.; Bader, M. Development of nickel alloys based on alloy 617 for components in 700 °C power plants. Procedia Eng. 2013, 55, 226–231. [Google Scholar] [CrossRef]
- Ren, W.J.; Lu, F.G.; Yang, R.J.; Liu, X.; Li, Z.G. Liquation cracking in fiber laser welded joints of inconel 617. J. Mater. Process. Technol. 2015, 226, 214–220. [Google Scholar] [CrossRef]
- Ojo, O.A.; Richards, N.L.; Chaturvedi, M.C. Contribution of constitutional liquation of gamma prime precipitate to weld HAZ cracking of cast Inconel 738 superalloy. Scr. Mater. 2004, 50, 641–646. [Google Scholar] [CrossRef]
- Qian, M.; Lippold, J.C. The effect of annealing twin-generated special grain boundaries on HAZ liquation cracking of nickel-base superalloys. Acta Mater. 2003, 51, 3351–3361. [Google Scholar] [CrossRef]
- Cieslak, M.J.; Stephens, J.J.; Carr, M.J. A study of the weldability and weld related microstructure of cabot alloy 214. Metall. Trans. A 1988, 19, 657–667. [Google Scholar] [CrossRef]
- Cieslak, M.; Headley, T.; Romig, A. The welding metallurgy of HASTELLOY alloys C-4, C-22, and C-276. Metall. Trans. A 1986, 17, 2035–2047. [Google Scholar] [CrossRef]
- Taheri, M.; Halvaee, A.; Kashani-Bozorg, S.F. Effect of Nd: YAG pulsed-laser welding parameters on microstructure and mechanical properties of GTD-111 superalloy joint. Mater. Res. Express 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Zhan, X.; Li, D.; Li, Y.; Lu, S. The influence of niobium on the plastic deformation behaviors of 310s austenitic stainless steel weld metals at different temperatures. Mater. Sci. and Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 743, 648–655. [Google Scholar] [CrossRef]
- Robino, C.; Cieslak, J. Solidification and welding metallurgy of thermo-span alloy. Sci. Technol. Weld. Join. 1997, 2, 220–230. [Google Scholar] [CrossRef]
- Lippold, J. An investigation of weld cracking in alloy 800. Weld. J. 1983, 63, 91–103. [Google Scholar]
- Lippold, J.; Baeslack, W.; Varol, I. Heat-affected zone liquation cracking in austenitic and duplex stainless steels. Weld. J. (USA) 1988, 71, 1–14. [Google Scholar]
- Klöwer, J. Alloy 617 and derivatives. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Woodhead Publishing: Cambridge, UK, 2017; pp. 547–570. [Google Scholar]
- Vishwakarma, K.R.; Chaturvedi, M.C. Effect of boron and phosphorus on HAZ microfissuring of Allvac 718 Plus superalloy. Mater. Sci. Technol. 2009, 25, 351–360. [Google Scholar] [CrossRef]
- Van, G.T.; Carron, D.; Le Masson, P.; Robin, V.; Andrieu, A.; Stodolna, J. Effect of boron content on hot ductility and hot cracking susceptibility in 316L austenitic stainless steel for welding components. J. Mater. Eng. Perform. 2018, 27, 5114–5123. [Google Scholar] [CrossRef]
- Li, Q.; Lin, X.; Wang, X.; Yang, H.; Song, M.; Huang, W. Research on the grain boundary liquation mechanism in heat affected zones of laser forming repaired K465 nickel-based superalloy. Metals 2016, 6, 64. [Google Scholar] [CrossRef]
- Pepe, J. Effects of constitutional liquation in 18-Ni maraging steel weldments. Weld. J. 1967, 46, 411–422. [Google Scholar]
- Duvall, D. Further heat-affected-zone studies in heat-resistant nickel alloys. Weld. J. 1967, 46, 423–432. [Google Scholar]
- Owczarski, W.; Duvall, D.; Sullivan, C. A model for heat affected zone cracking in nickel-based alloys. Weld. J. 1966, 45, 145. [Google Scholar]
- Thompson, R. The relationship between grain size and microfissuring in alloy 718. Weld. J. 1985, 64, 91s–96s. [Google Scholar]
- Baeslack, W.A.; Nelson, D. Morphology of weld heat-affected zone liquation in cast alloy 718. Metallography 1986, 19, 371–379. [Google Scholar] [CrossRef]
- Phuraya, N.; Phung-On, I.; Terasaki, H.; Komizo, Y. Direct observation of liquation in Ni-base superalloy by using confocal laser scanning microscopy. Key Eng. Mater. 2015, 658, 36–41. [Google Scholar] [CrossRef]
- Xu, H.; Liu, W.; Lu, F.; Wang, P.; Ding, Y. Evolution of carbides and its characterization in HAZ during NG-TIG welding of Alloy 617B. Mater. Charact. 2017, 130, 270–277. [Google Scholar] [CrossRef]
- Fink, C.; Zinke, M. Welding of nickel-based alloy 617 using modified dip arc processes. Weld. World 2013, 57, 323–333. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 55–56. [Google Scholar]
- Tytko, D.; Choi, P.-P.; Klöwer, J.; Kostka, A.; Inden, G.; Raabe, D. Microstructural evolution of a Ni-based superalloy (617B) at 700 C studied by electron microscopy and atom probe tomography. Acta Mater. 2012, 60, 1731–1740. [Google Scholar] [CrossRef]
- Jo, T.S.; Lim, J.H.; Do Kim, Y. Dissociation of Cr-rich M23C6 carbide in alloy 617 by severe plastic deformation. J. Nucl. Mater. 2010, 406, 360–364. [Google Scholar] [CrossRef]
- Inoue, A.; Masumoto, T. Carbide reactions (M3C→M7C3→M23C6→M6C) during tempering of rapidly solidified high carbon Cr-W and Cr-Mo steels. Metall. Trans. A 1980, 11, 739–747. [Google Scholar] [CrossRef]
- Kuo, K.; Jia, C. Crystallography of M23C6 and M6C precipitated in a low alloy steel. Acta Metall. 1985, 33, 991–996. [Google Scholar] [CrossRef]
- Chen, W.; Chaturvedi, M.C.; Richards, N.L. Effect of boron segregation at grain boundaries on heat-affected zone cracking in wrought INCONEL 718. Metall. Mater. Trans. A 2001, 32, 931–939. [Google Scholar] [CrossRef]
- Huang, X.; Chaturvedi, M.C.; Richards, N.L.; Jackman, J. The effect of grain boundary segregation of boron in cast alloy 718 on HAZ microfissuring—A SIMS analysis. Acta Mater. 1997, 45, 3095–3107. [Google Scholar] [CrossRef]
- Guo, H.; Chaturvedi, M.C.; Richards, N.L. Effect of boron concentration and grain size on weld heat affected zone microfissuring in Inconel 718 base superalloys. Sci. Technol. Weld. Join. 2013, 4, 257–264. [Google Scholar] [CrossRef]
- Ojo, O.A. Intergranular liquation cracking in heat affected zone of a welded nickel based superalloy in as cast condition. Mater. Sci. Technol. 2007, 23, 1149–1155. [Google Scholar] [CrossRef]
- Böllinghaus, T.; Herold, H. Hot Cracking Phenomena in Welds; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Miller, W.A.; Chadwick, G.A. On the magnitude of the solid/liquid interfacial energy of pure metals and its relation to grain boundary melting. Acta Metall. 1967, 15, 607–614. [Google Scholar] [CrossRef]
| Elements | C | Co | Cr | Fe | Mo | Ti | Al | Nb | B | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| BM | 0.052 | 12.05 | 22.38 | 0.33 | 9.02 | 0.44 | 1.03 | 0.03 | 0.0045 | Bal. |
| Filler metal | 0.07 | 11.20 | 22.50 | - | 8.90 | 0.39 | 1.27 | - | - | Bal. |
| Current (A) | Voltage (V) | Welding Speed (cm/min) | Heat Input (kJ/cm) |
|---|---|---|---|
| 180 | 12 | 8–9 | 16–19 |
| Elements | C | Al | Ti | Mo | Cr | Co | Ni |
|---|---|---|---|---|---|---|---|
| Cr-rich phase (A) | 2.26 | 0.41 | - | 24.31 | 50.03 | 5.94 | 17.04 |
| Mo-rich phase (B) | 1.51 | 0.51 | 1.67 | 46.48 | 21.26 | 7.92 | 20.70 |
| Elements | C | Al | Ti | Mo | Cr | Co | Ni |
|---|---|---|---|---|---|---|---|
| M23C6 in network-shaped structure | 4.2 | 0.3 | - | 21.7 | 38.4 | 6.3 | 21.1 |
| M6C in network-shaped structure | 3.6 | 0.53 | 0.2 | 34.6 | 28.3 | 5.8 | 20.3 |
| M23C6 in fine precipitates | 3.5 | 0.48 | - | 24.8 | 29.5 | 8.7 | 30.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, K.; Hu, M.; Wu, Y.; Cai, Z.; Pan, J. The Mechanism for HAZ Liquation of Nickel-Based Alloy 617B During Gas Tungsten Arc Welding. Metals 2020, 10, 94. https://doi.org/10.3390/met10010094
Li S, Li K, Hu M, Wu Y, Cai Z, Pan J. The Mechanism for HAZ Liquation of Nickel-Based Alloy 617B During Gas Tungsten Arc Welding. Metals. 2020; 10(1):94. https://doi.org/10.3390/met10010094
Chicago/Turabian StyleLi, Shanlin, Kejian Li, Mengjia Hu, Yao Wu, Zhipeng Cai, and Jiluan Pan. 2020. "The Mechanism for HAZ Liquation of Nickel-Based Alloy 617B During Gas Tungsten Arc Welding" Metals 10, no. 1: 94. https://doi.org/10.3390/met10010094
APA StyleLi, S., Li, K., Hu, M., Wu, Y., Cai, Z., & Pan, J. (2020). The Mechanism for HAZ Liquation of Nickel-Based Alloy 617B During Gas Tungsten Arc Welding. Metals, 10(1), 94. https://doi.org/10.3390/met10010094




