Hot Deformation Characteristics of 18Cr-5Ni-4Cu-N Stainless Steel Using Constitutive Equation and Processing Map
Abstract
1. Introduction
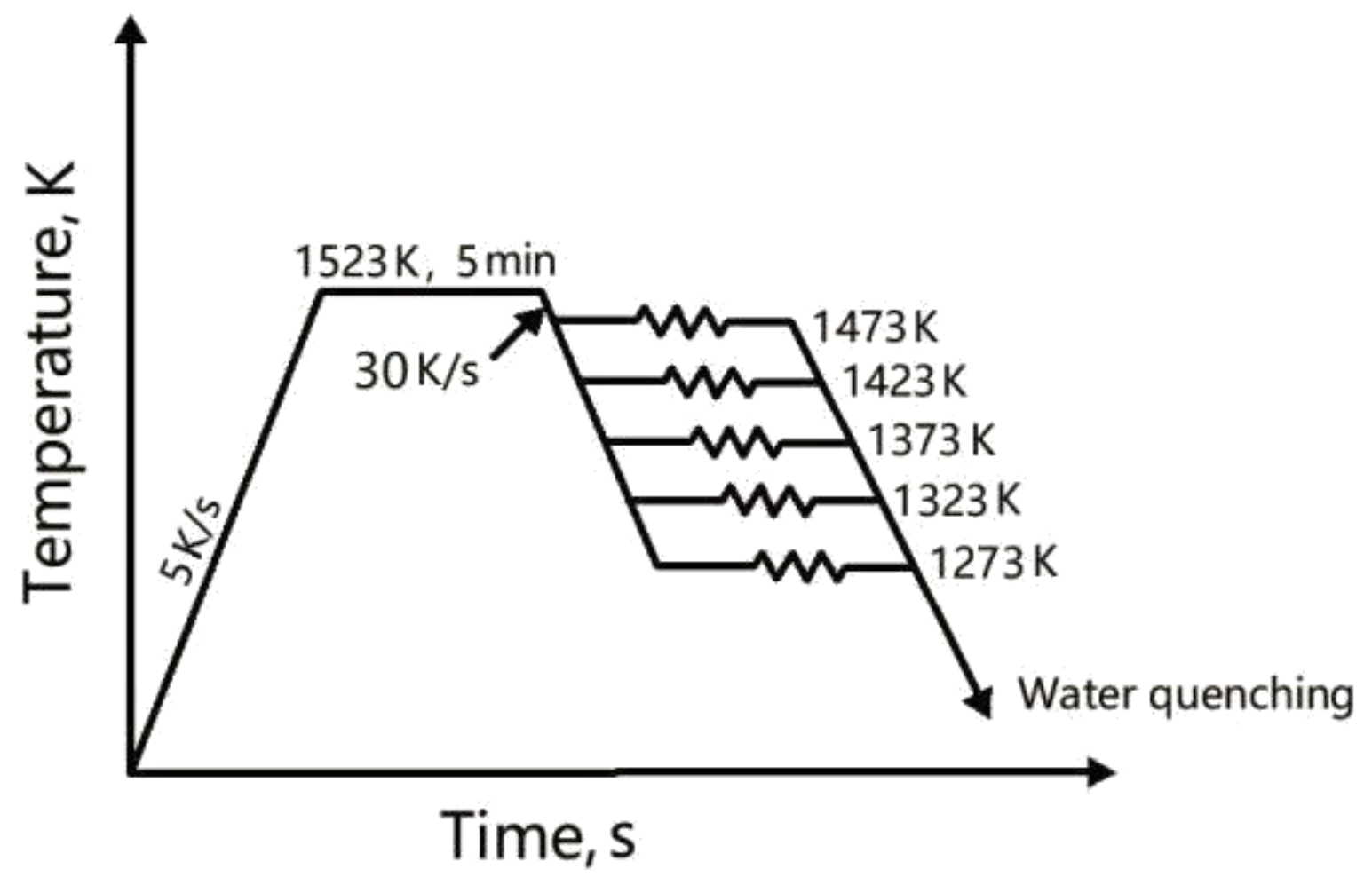
2. Experimental Procedures
Hot Deformation
3. Experimental Results and Analysis
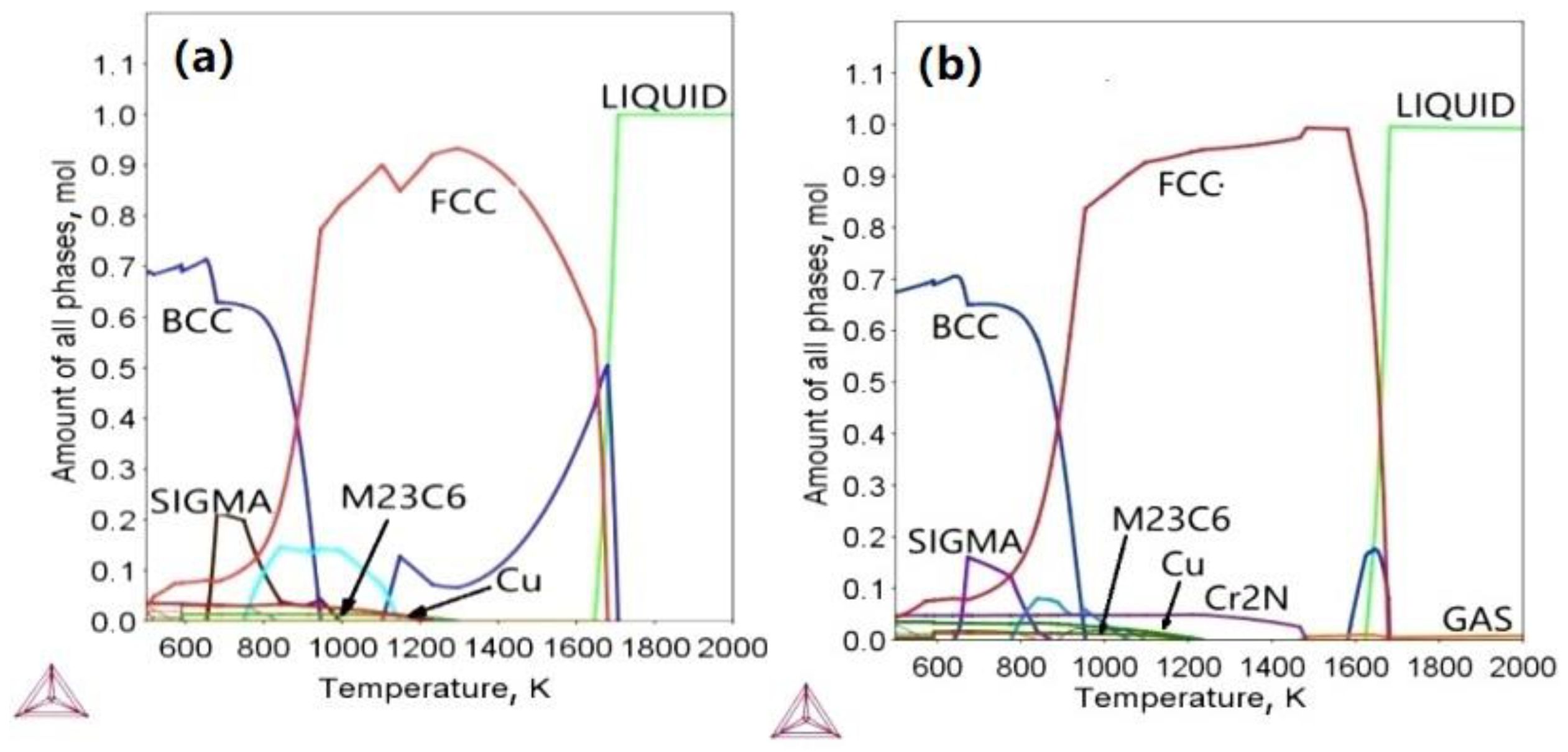
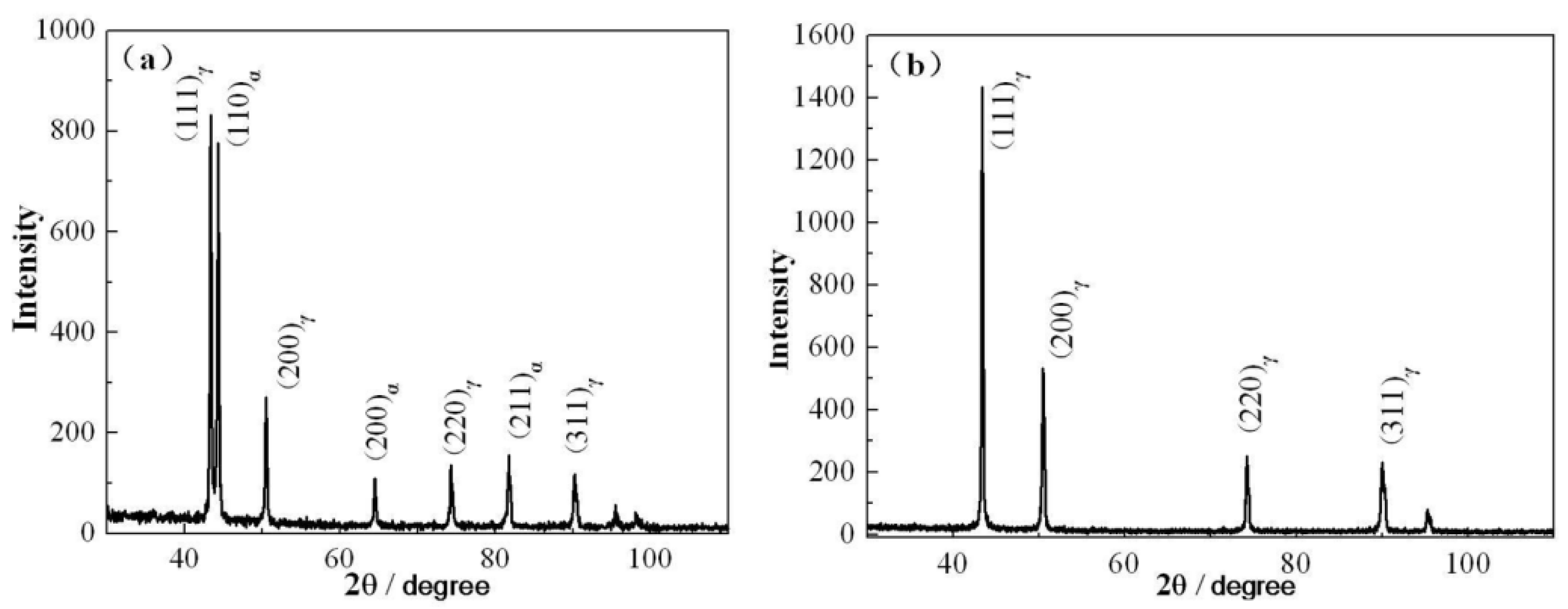
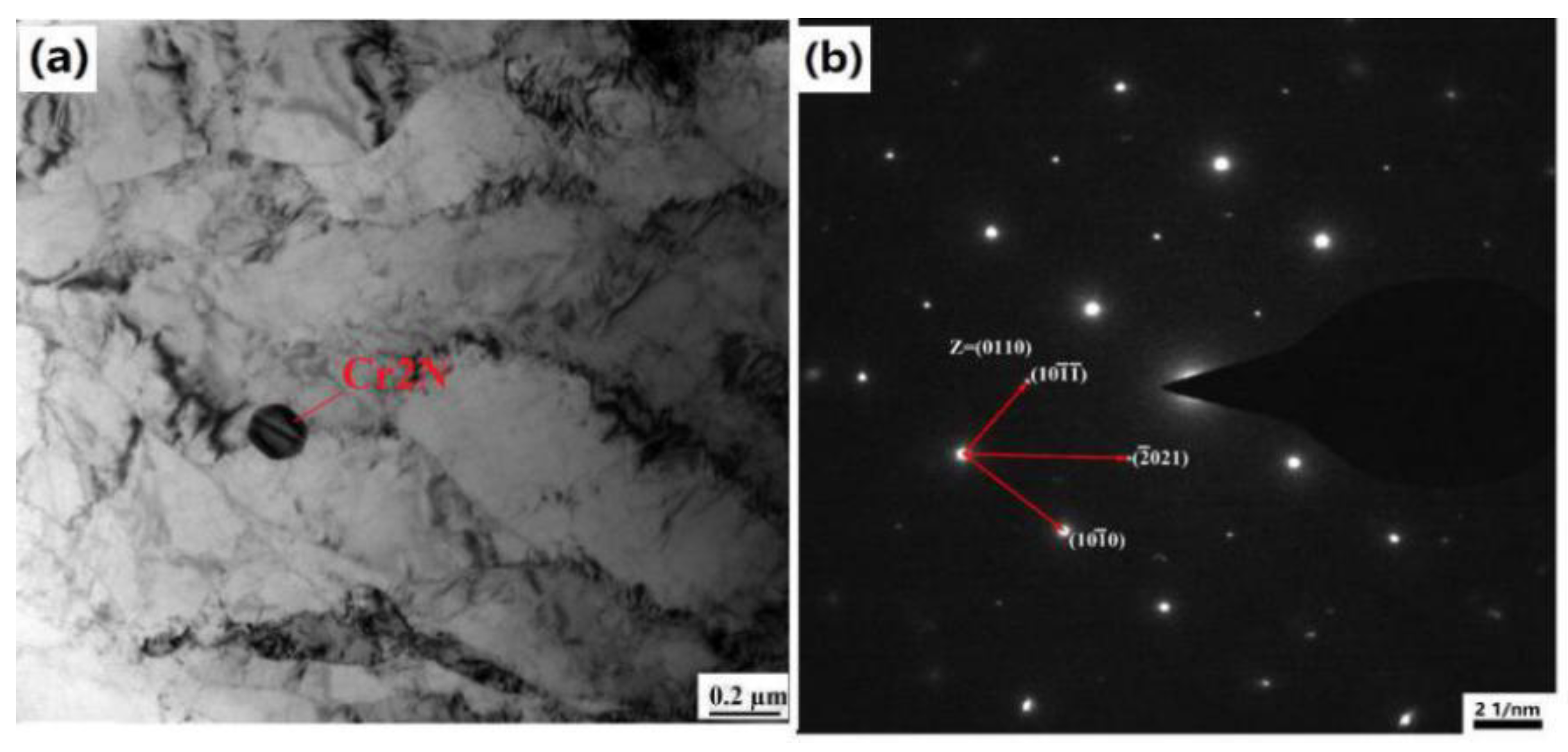
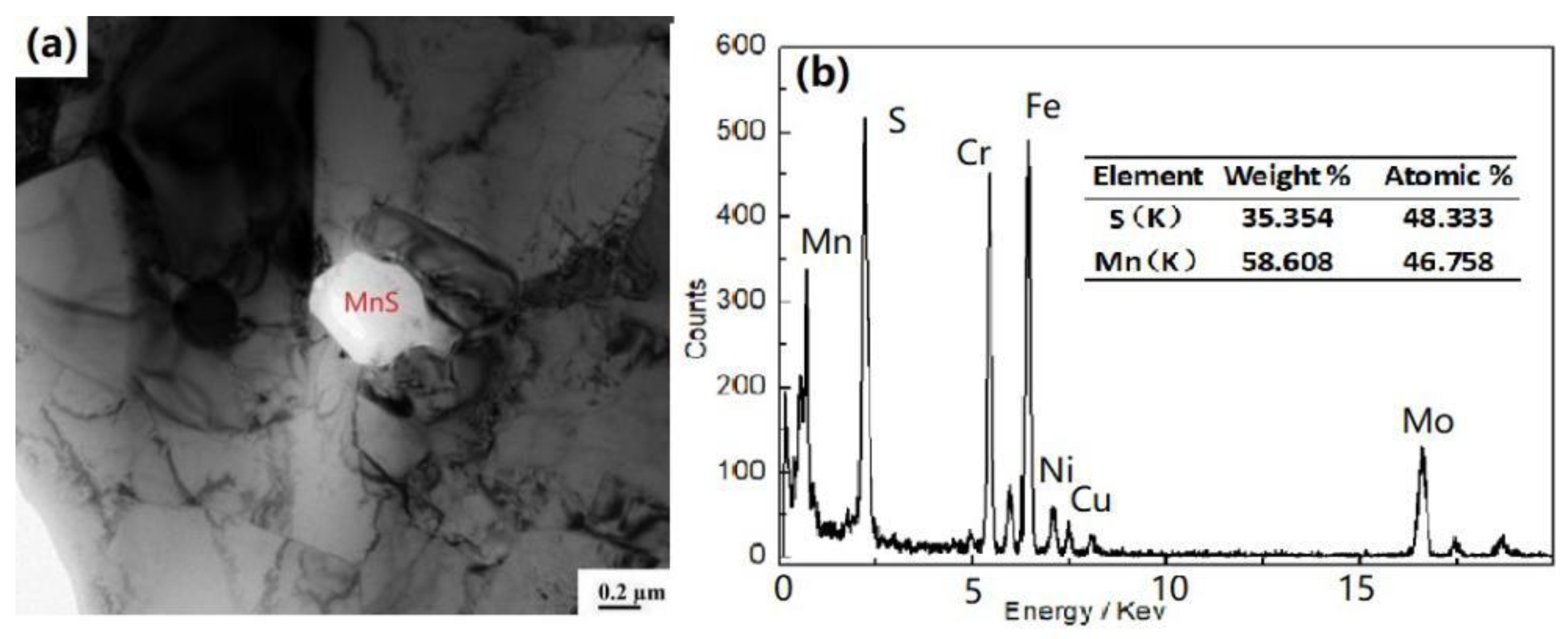
3.1. Thermodynamic Calculation of the Tested Steel and XRD Phase Results
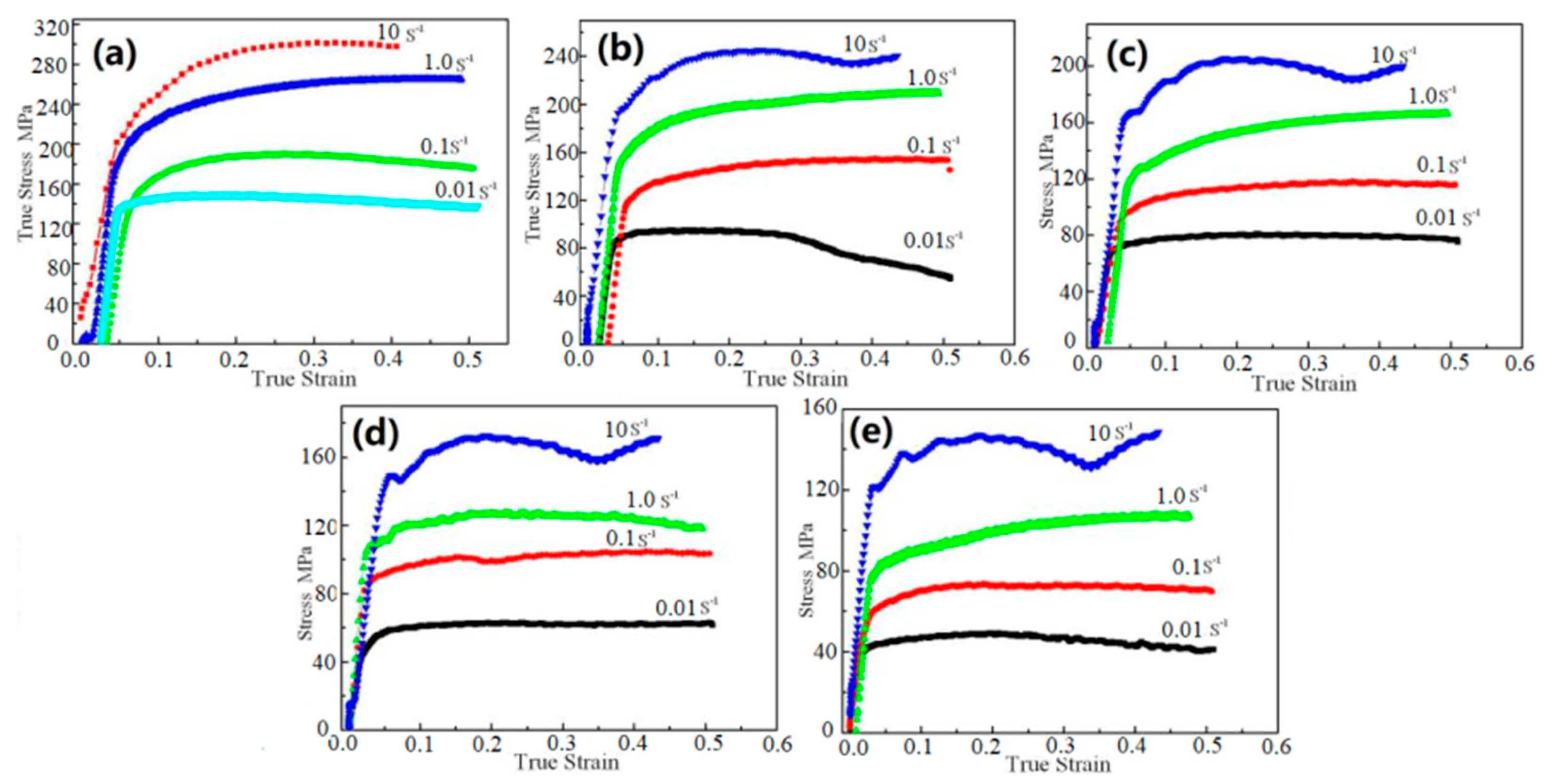
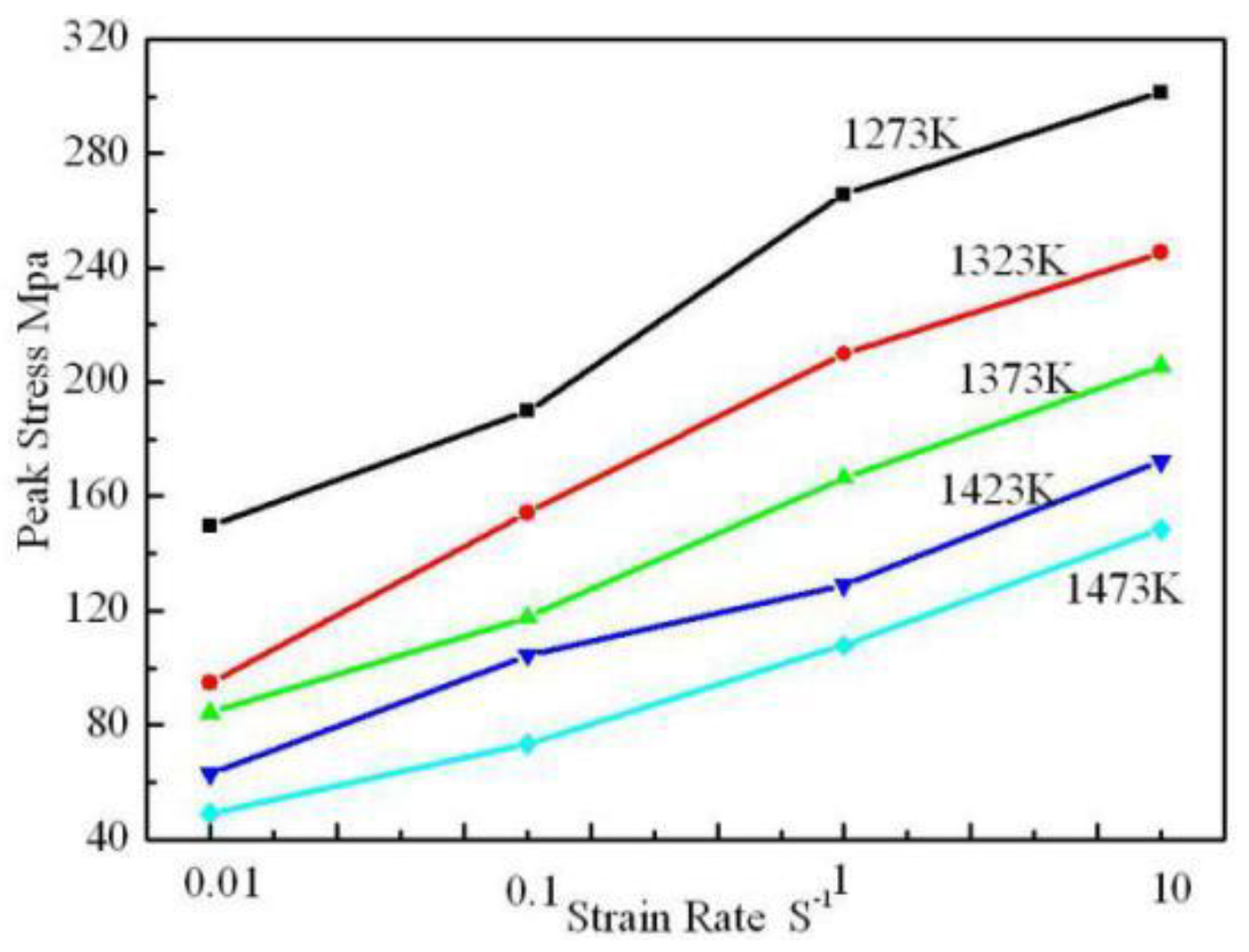
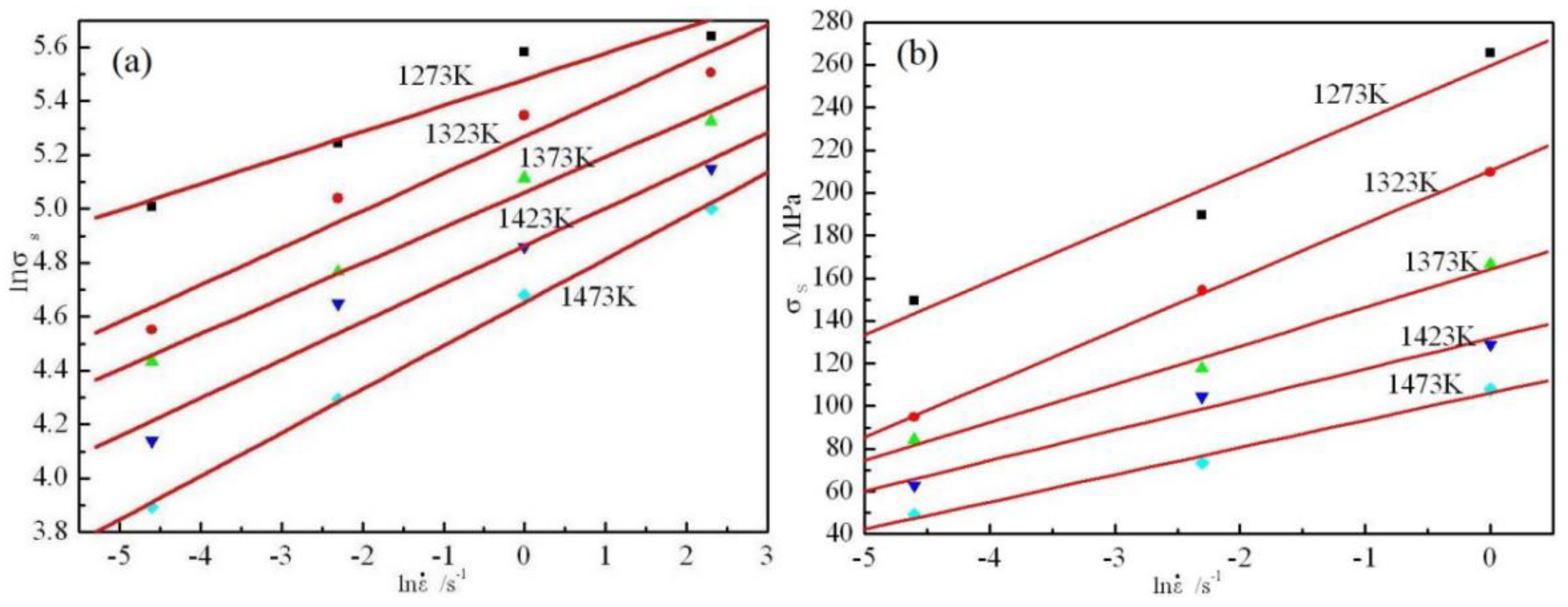
3.2. Flow Stress Model
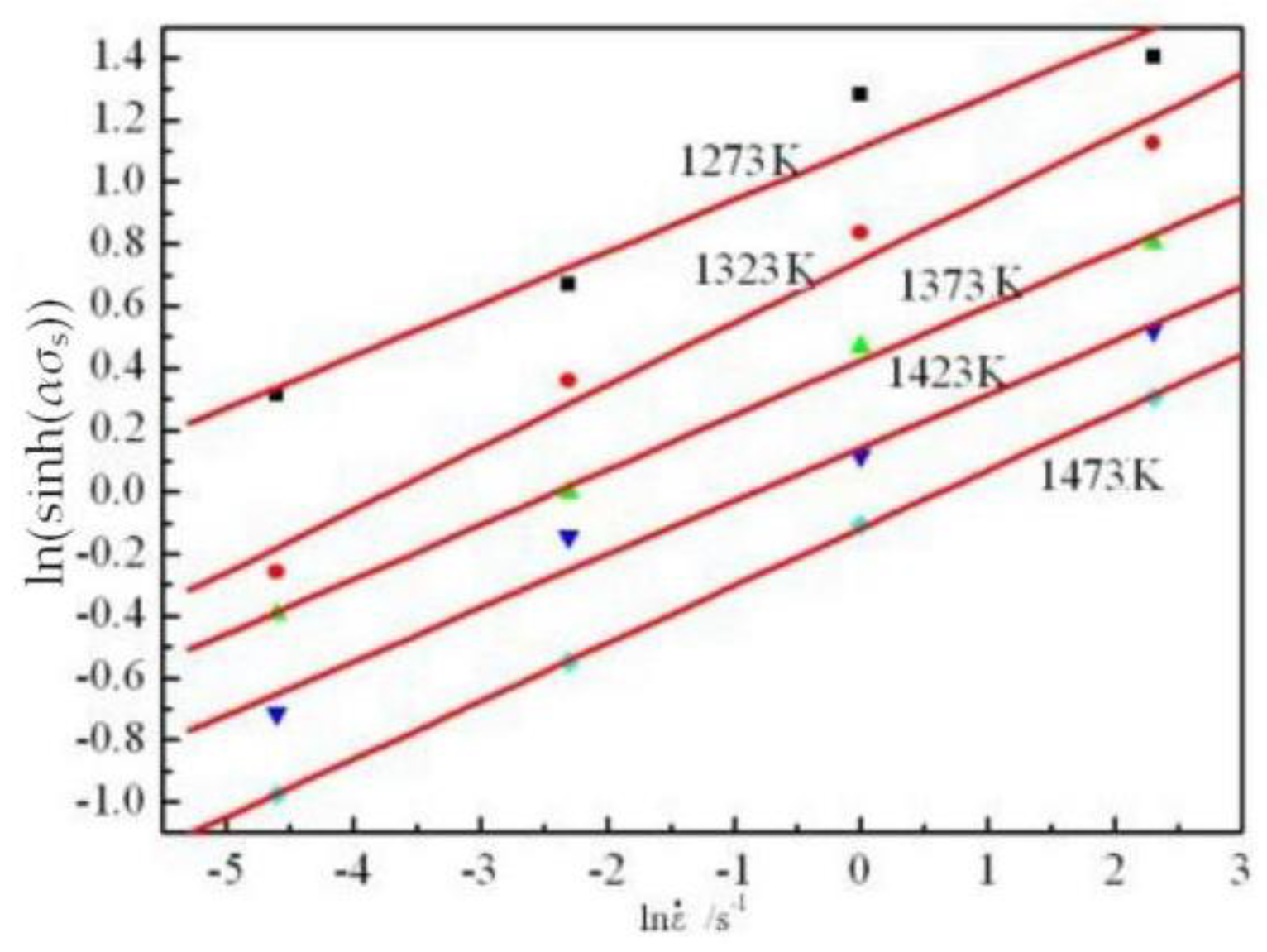
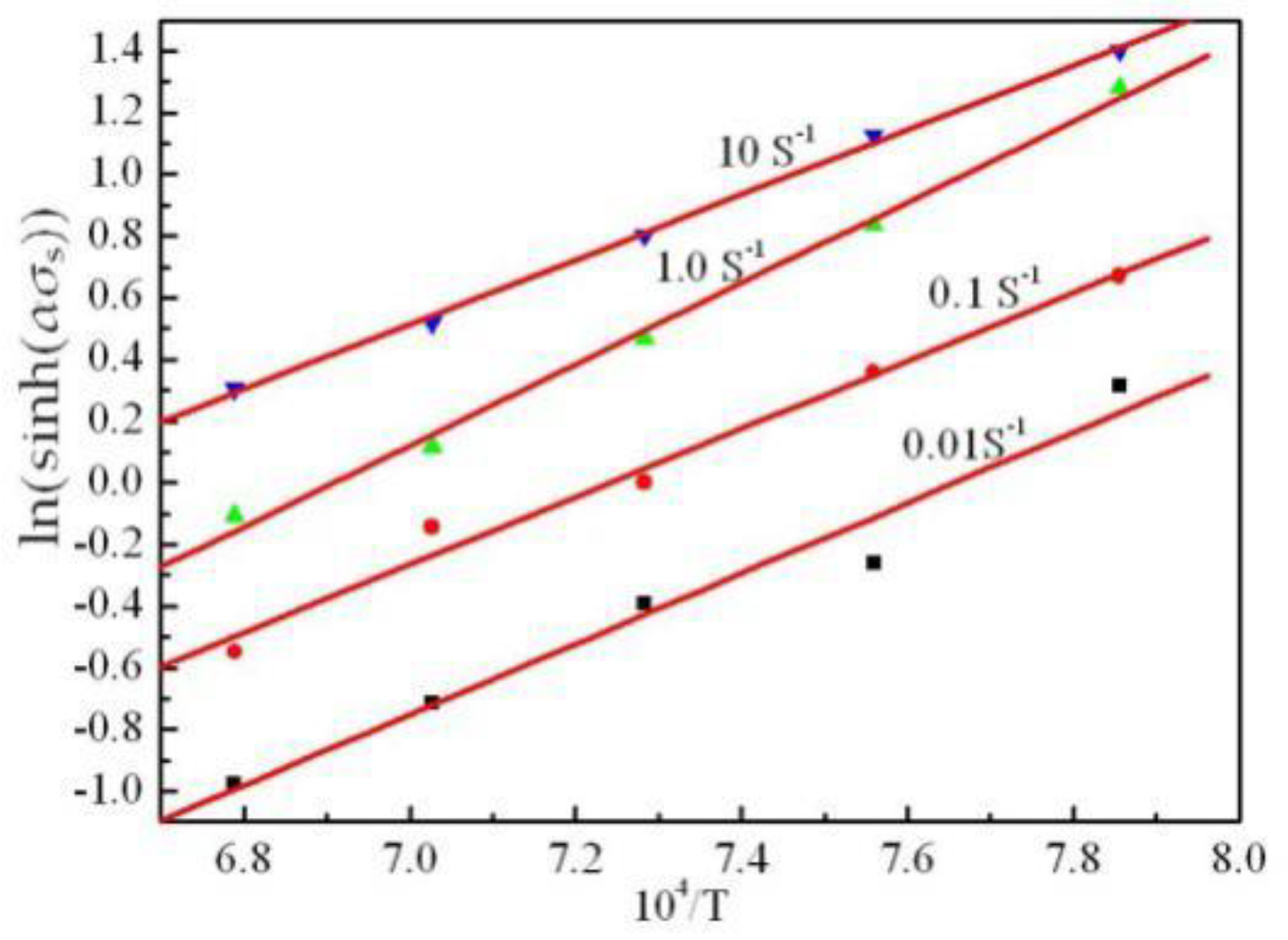
3.3. Flow Stress Model/Kinetic Analysis
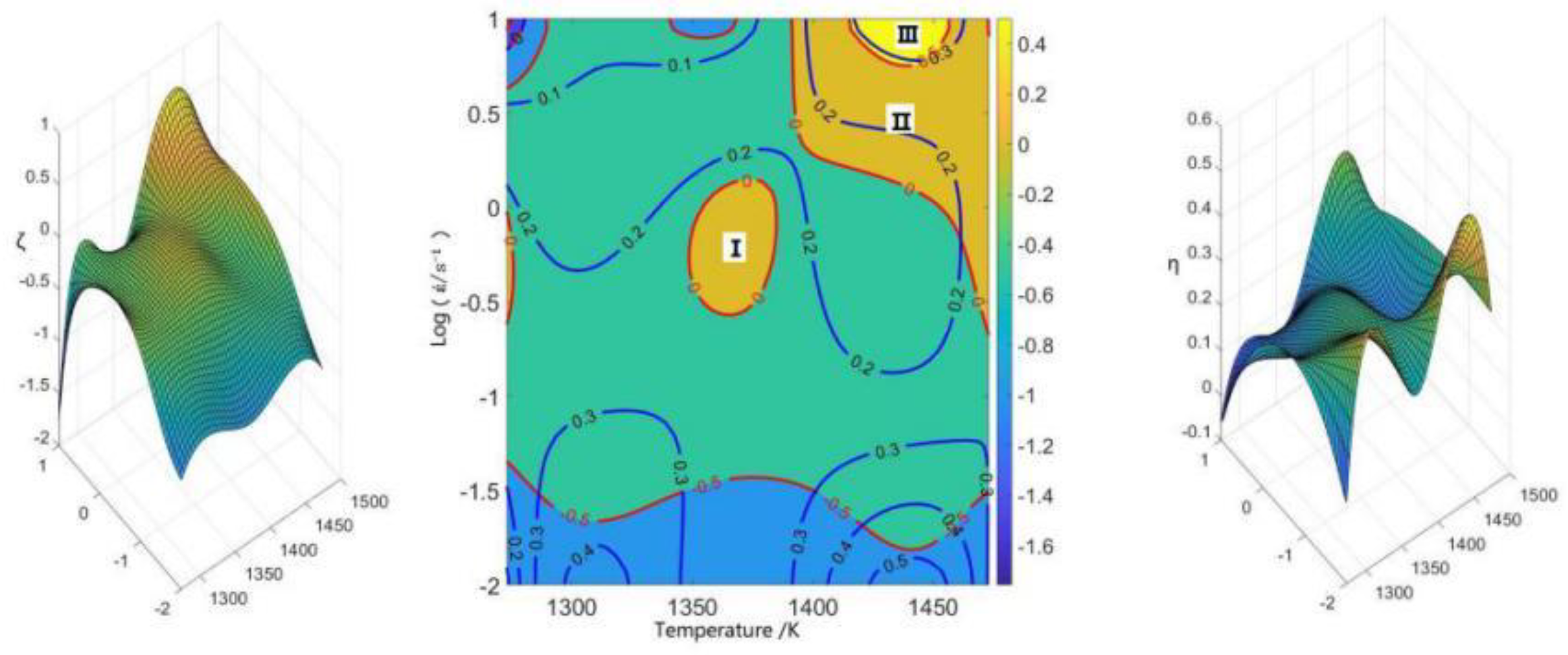
3.4. Energy Dissipation Diagram
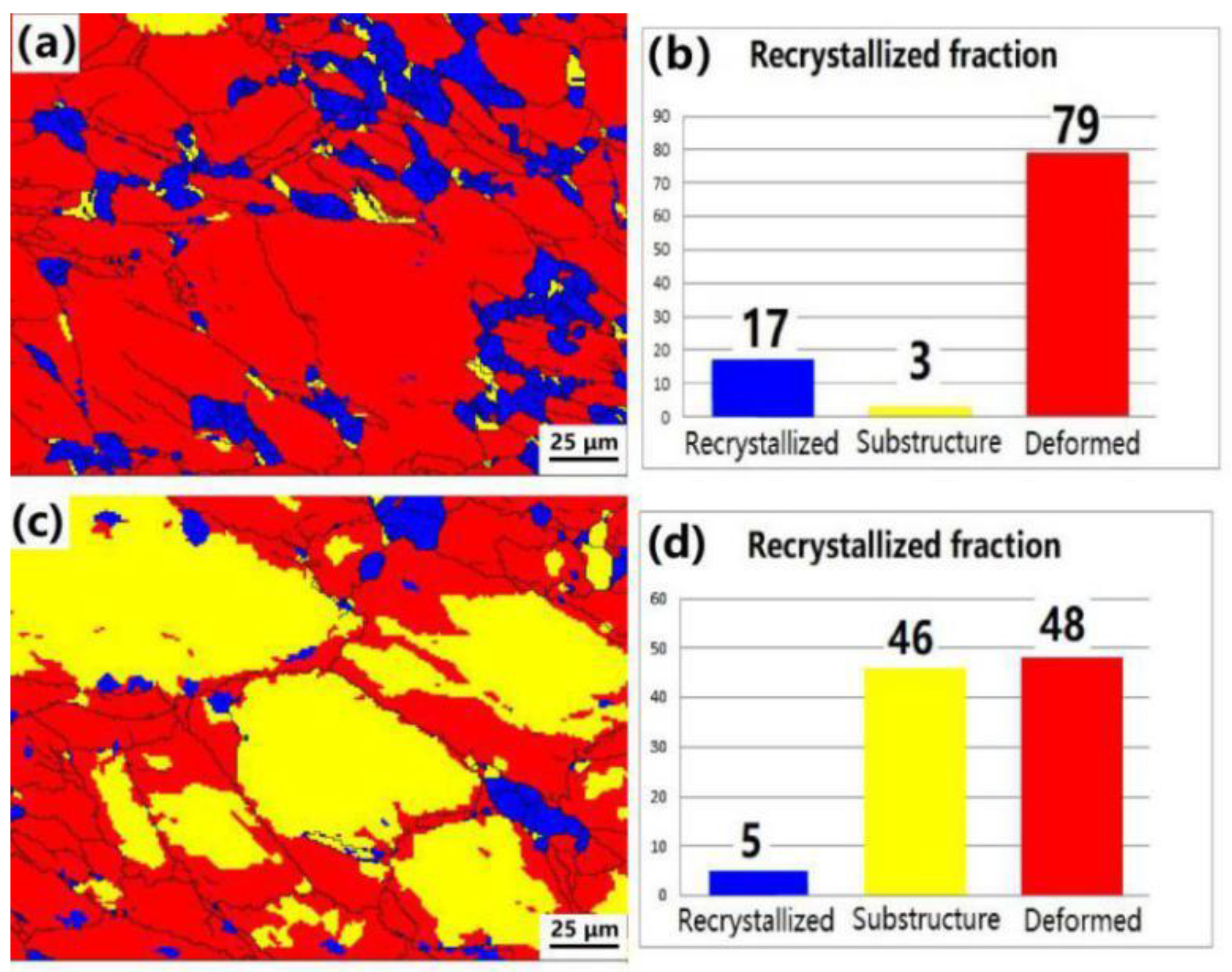
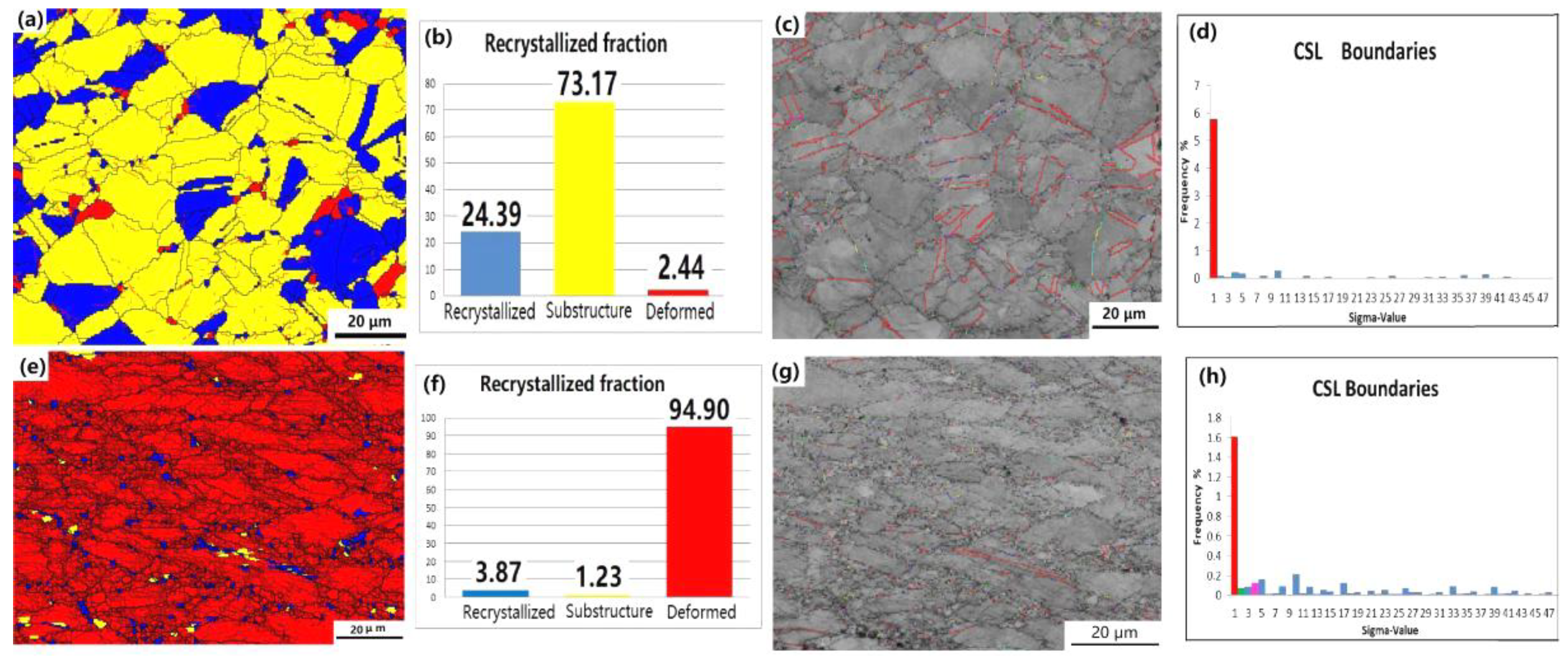
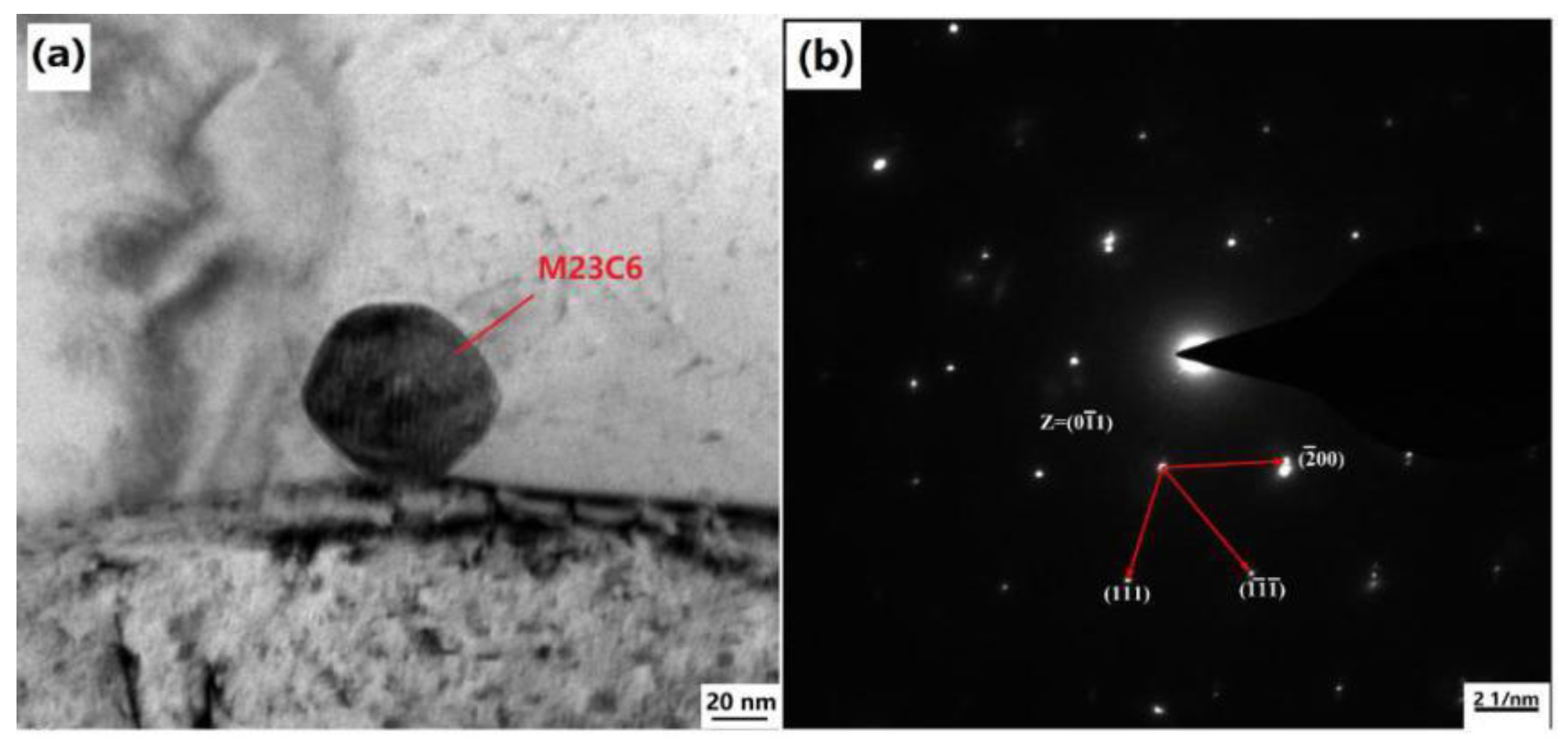
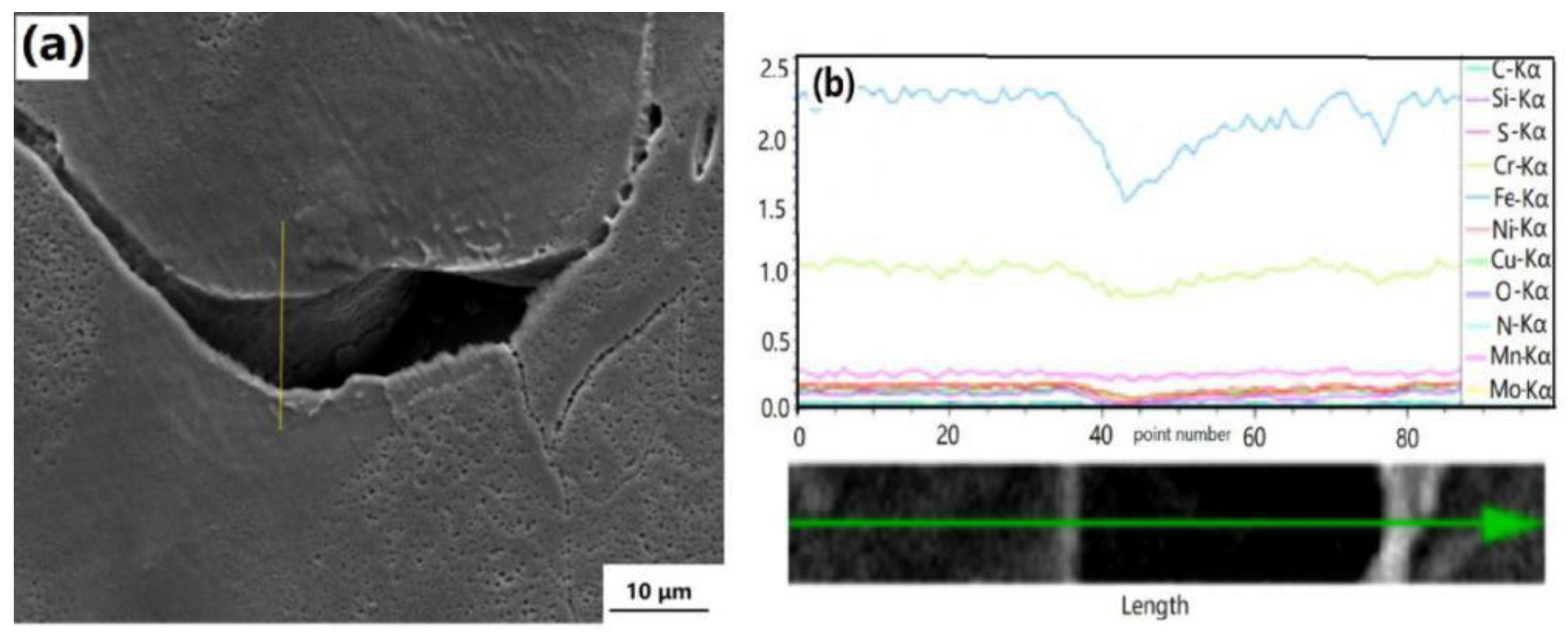
3.5. Dynamic Recrystallization Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, S.-P.; Wang, Z.-H.; Cheng, S.-C.; Liu, Z.-D.; Han, J.-C.; Fu, W.-T. Effect of Cu Content on Aging Precipitation Behaviors of Cu-Rich Phase in Fe-Cr-Ni Alloy. J. Iron Steel Res. Int. 2010, 17, 63–68. [Google Scholar] [CrossRef]
- Xi, T.; Shahzad, M.B.; Xu, D.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Copper precipitation behavior and mechanical properties of Cu-bearing 316L austenitic stainless steel: A comprehensive cross-correlation study. Mater. Sci. Eng. A 2016, 675, 243–252. [Google Scholar] [CrossRef]
- San, X.Y.; Zhang, B.; Wu, B.; Wei, X.X.; Oguzie, E.E.; Ma, X.L. Investigating the effect of Cu-rich phase on the corrosion behavior of Super304H austenitic stainless steel by TEM. Corros. Sci. 2018, 130, 143–152. [Google Scholar] [CrossRef]
- Prabha, B.; Sundaramoorthy, P.; Suresh, S.; Manimozhi, S.; Ravishankar, B. Studies on Stress Corrosion Cracking of Super 304H Austenitic Stainless Steel. J. Mater. Eng. Perform. 2009, 18, 1294–1299. [Google Scholar] [CrossRef]
- Hattestrand, M.; Nilsson, J.-O.; Stiller, K.; Liu, P.; Andersson, M. Precipitation hardening in a 12%Cr–9%Ni–4%Mo–2%Cu stainless steel. Acta Mater. 2004, 52, 1023–1037. [Google Scholar] [CrossRef]
- Sen, I.; Kumar, E.A.S.; Fleury, E.; Oh-ishi, K.; Hono, K.; Ramamurty, U. Microstructure and mechanical properties of annealed SUS 304H austeniticstainless steel with copper. Mater. Sci. Eng. A 2011, 528, 4491–4499. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, S.C.; Gang, Y. Effect of Cu Addition on the Creep Rupture Properties of Super304H Steel. Mater. Mech. Eng. 2002, 26, 23–25. (In Chinese) [Google Scholar]
- Raj, R. Development of a processing map for use in warm forming and hot forming process. Metall. Trans. A 1981, 12, 1089–1097. [Google Scholar] [CrossRef]
- Saadatkia, S.; Mirzadeh, H.; Cabrera, J.-M. Hot deformation behavior, dynamic recrystallization, andphysically-based constitutive modeling of plain carbon steels. Mater. Sci. Eng. A 2015, 636, 196–202. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, X.-M. A critical review of experimental results and constitutive descriptions for metals and alloys in hot working. Mater. Des. 2011, 32, 1733–1759. [Google Scholar] [CrossRef]
- Tan, S.; Wang, Z.; Cheng, S.; Liu, Z.; Han, J.; Fu, W. Processing maps and hot workability of Super304H austenitic heat-resistantstainless steel. Mater. Sci. Eng. A 2009, 517, 312–315. [Google Scholar] [CrossRef]
- Taylor, A.S.; Hodgson, P.D. Dynamic behaviour of 304 stainless steel during high Z deformation. Mater. Sci. Eng. A 2011, 528, 3310–3320. [Google Scholar] [CrossRef]
- Narayana, S.V.S.; Murty, B.; Rao, N.; Kashyap, B.P. Identification of flow instabilities in the p rocessingmaps of AISI 304 stainless steel. J. Mater. Process. Technol. 2005, 166, 268–278. [Google Scholar] [CrossRef]
- Zener, C.; Hollomon, J.H. Effect of Strain Rate Upon Plastic Flow of Steel. J. Appl. Phys. 1944, 15, 22–32. [Google Scholar] [CrossRef]
- CSellars, M.; Tegart, W.J.M.G. Relationship between strength and structure in deformation at elevated temperatures. Mem. Sci. Rev. Met. 1966, 63, 731–746. [Google Scholar]
- Mwembela, A.; Konopleva, E.B.; McQueen, H.J. Microstructural development in Mg alloy AZ31 during hot working. Scr. Mater. 1997, 37, 1789–1795. [Google Scholar] [CrossRef]
- Jonas, J.J.; Sellars, C.M.; Tegart, W.J.M. Strength and structure under hot-working conditions. Metall. Rev. 1969, 14, 1–24. [Google Scholar]
- McQueen, H.J.; Wong, W.A.; Jonas, J.J. Deformation of aluminum at high temperatures and strain rates. Can. J. Phys. 1967, 45, 1225–1234. [Google Scholar] [CrossRef]
- Arun, K.; Babu, S.; Mandal, C.N.; Athreya, B.; Shakthipriya, V. Subramanya Sarma.Hot deformation characteristics and processing map of a phosphorous modified super austenitic stainless steel. Mater. Des. 2017, 115, 262–275. [Google Scholar]
- Changjun, W.; Han, F.; Wenjie, Z.; Zhigang, S.; Qilong, Y. Dynamic Recrystallization Behavior and Microstructure Evolution of AISI304N Stainless Steel. J. Iron Steel Res. Int. 2013, 20, 107–112. [Google Scholar]
- Belyakov, A.; Miura, H.; Sakai, T. Dynamic Recrystallization Under Warm Deformation of a 304 Type Austenitic Stainless Steel. Mater. Sci. Eng. 1998, 255A, 139–147. [Google Scholar] [CrossRef]
- Prasad, Y.V.R.K.; Gegel, H.L.; Doraivelu, S.M. Modeling of dynamic material behavior in hot deformation: Forging of Ti-6242. Metall. Trans. A 1984, 15, 1883–1892. [Google Scholar] [CrossRef]
- HaÈnninen, H.; Romu, J.; Ilola, R.; Tervo, J.; Laitinen, A. Effects of processing and manufacturing of high nitrogen-containing stainless steels on their mechanical, corrosion and wear properties. J. Mater. Process. Technol. 2001, 117, 424–430. [Google Scholar] [CrossRef]
- Simmons, J.W. Influence of nitride (Cr2N) precipitation on the plastic flow behavior of high-nitrogen austenitic stainless steel. Scr. Metall. Et Mater. 1995, 32, 265–270. [Google Scholar] [CrossRef]
- Maurizo, V.; David, D.; Aldo, M. Characterization of Grain-boundary Precipitates after Hot-ductility Tests of Microalloyed Steels. ISIJ Int. 2009, 49, 446–452. [Google Scholar]
- Ohadi, D.; Parsan, M.H.; Mirzadeh, H. Development of dynamic recrystallization maps based on the initial grain size. Mater. Sci. Eng. A 2013, 565, 90–95. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.-y.; Bai, P.-c.; Yang, J.-c. Hot Deformation Characteristics of 18Cr-5Ni-4Cu-N Stainless Steel Using Constitutive Equation and Processing Map. Metals 2020, 10, 82. https://doi.org/10.3390/met10010082
Fu X-y, Bai P-c, Yang J-c. Hot Deformation Characteristics of 18Cr-5Ni-4Cu-N Stainless Steel Using Constitutive Equation and Processing Map. Metals. 2020; 10(1):82. https://doi.org/10.3390/met10010082
Chicago/Turabian StyleFu, Xiao-yang, Pu-cun Bai, and Ji-chun Yang. 2020. "Hot Deformation Characteristics of 18Cr-5Ni-4Cu-N Stainless Steel Using Constitutive Equation and Processing Map" Metals 10, no. 1: 82. https://doi.org/10.3390/met10010082
APA StyleFu, X.-y., Bai, P.-c., & Yang, J.-c. (2020). Hot Deformation Characteristics of 18Cr-5Ni-4Cu-N Stainless Steel Using Constitutive Equation and Processing Map. Metals, 10(1), 82. https://doi.org/10.3390/met10010082



