Study of the Structure of FeOx-CaO-SiO2-MgO and FeOx-CaO-SiO2-MgO-Cu2O-PdO Slags Relevant to Urban Ores Processing through Cu Smelting
Abstract
1. Introduction
2. Previous Studies on Structures of Copper-Making Slags
3. Methodology of Experiments
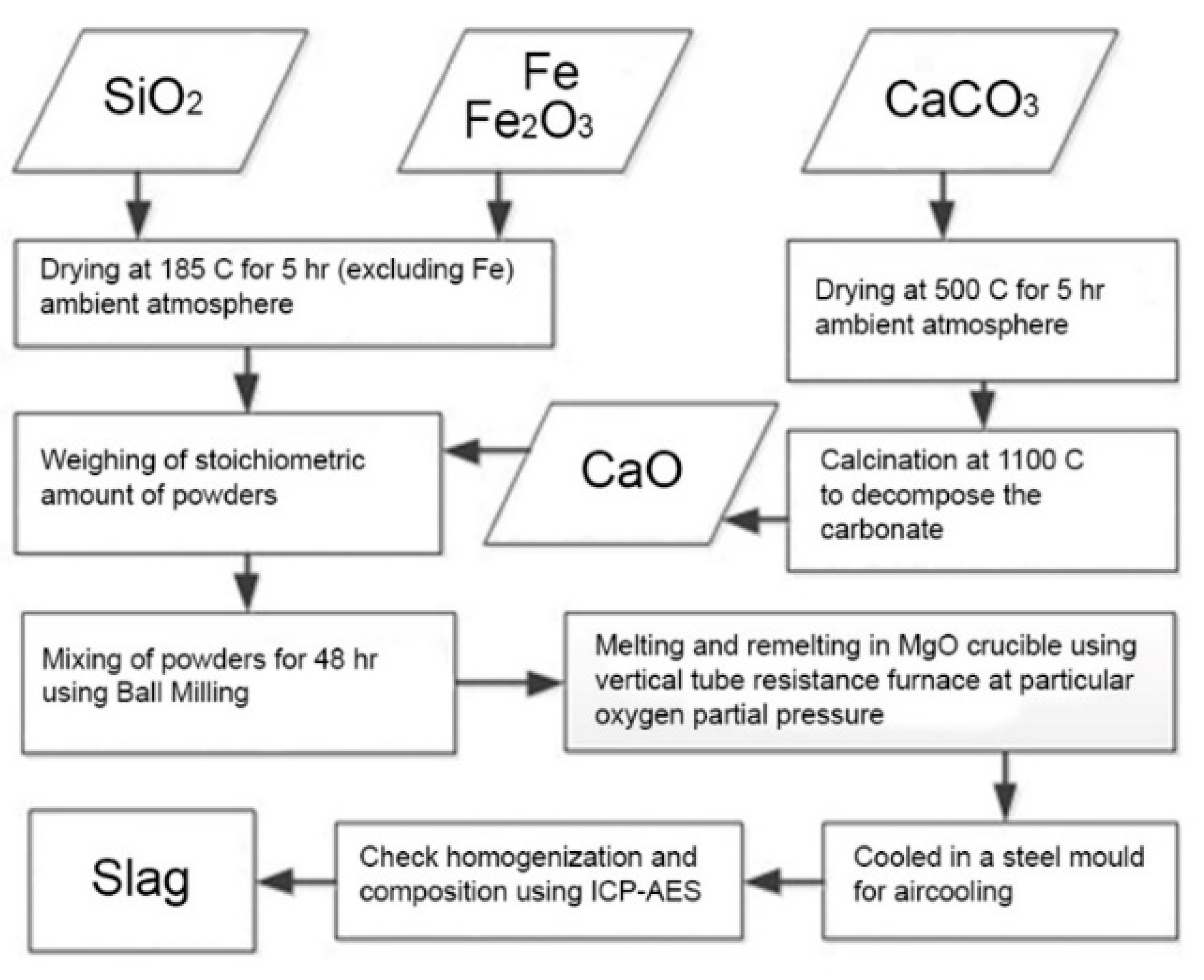
3.1. Materials, Master Alloys and Master Slags
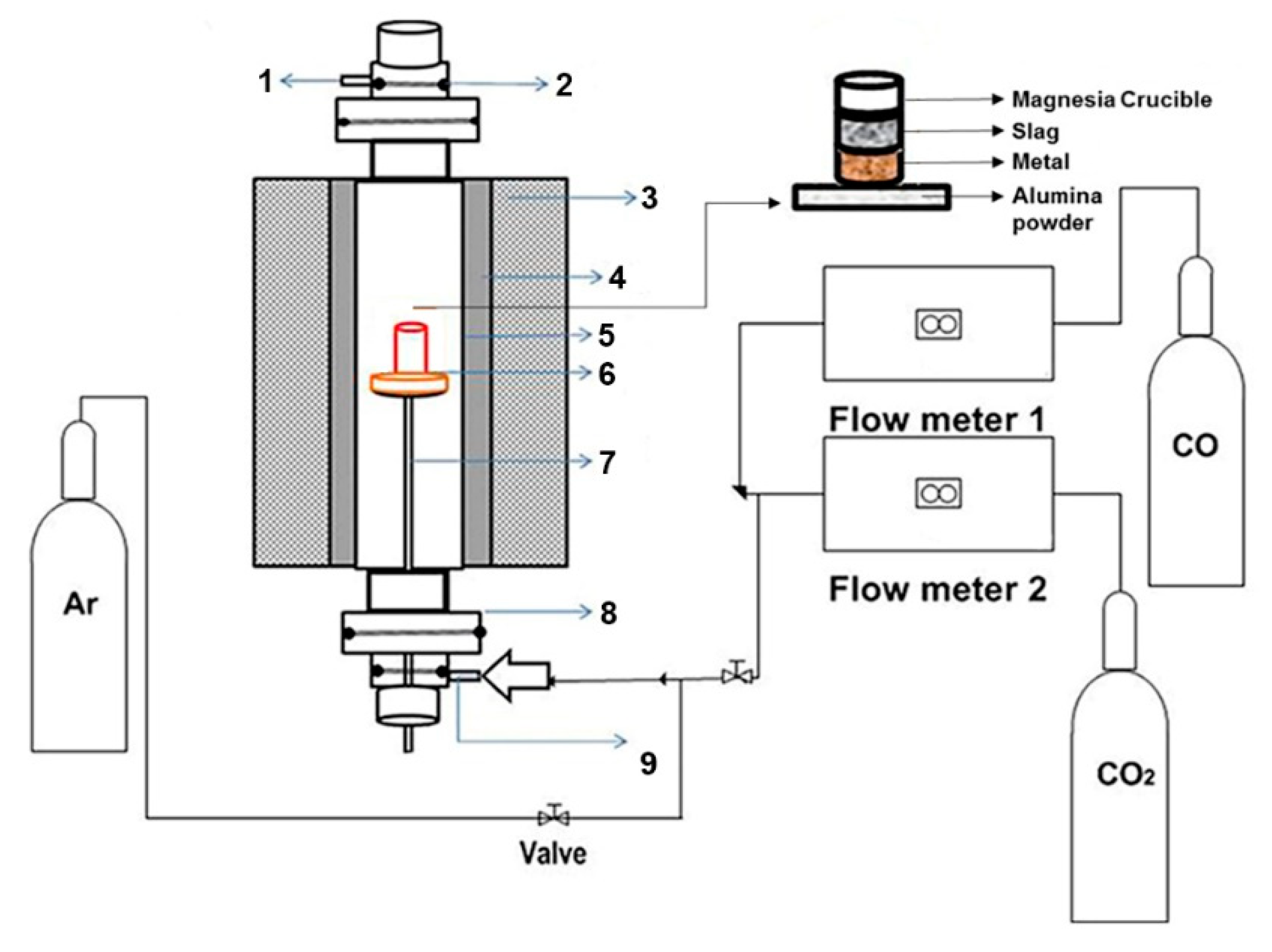
3.2. Experimental Apparatus and Plan
3.3. Slag and Metal Samples Characterization
4. Results and Discussion
4.1. Structure Analysis of the FeOx-CaO-SiO2-MgO (FCSM) Slags
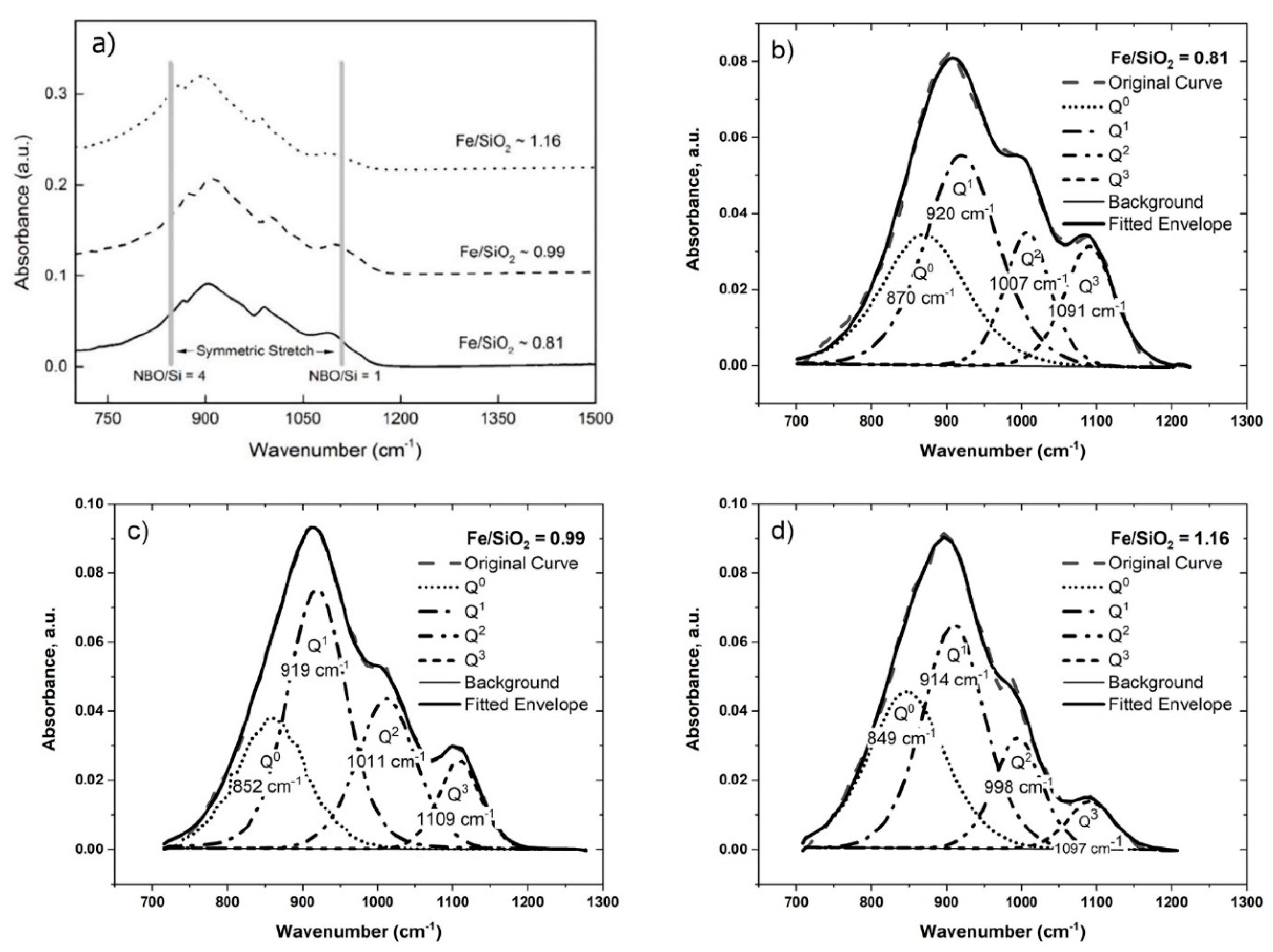
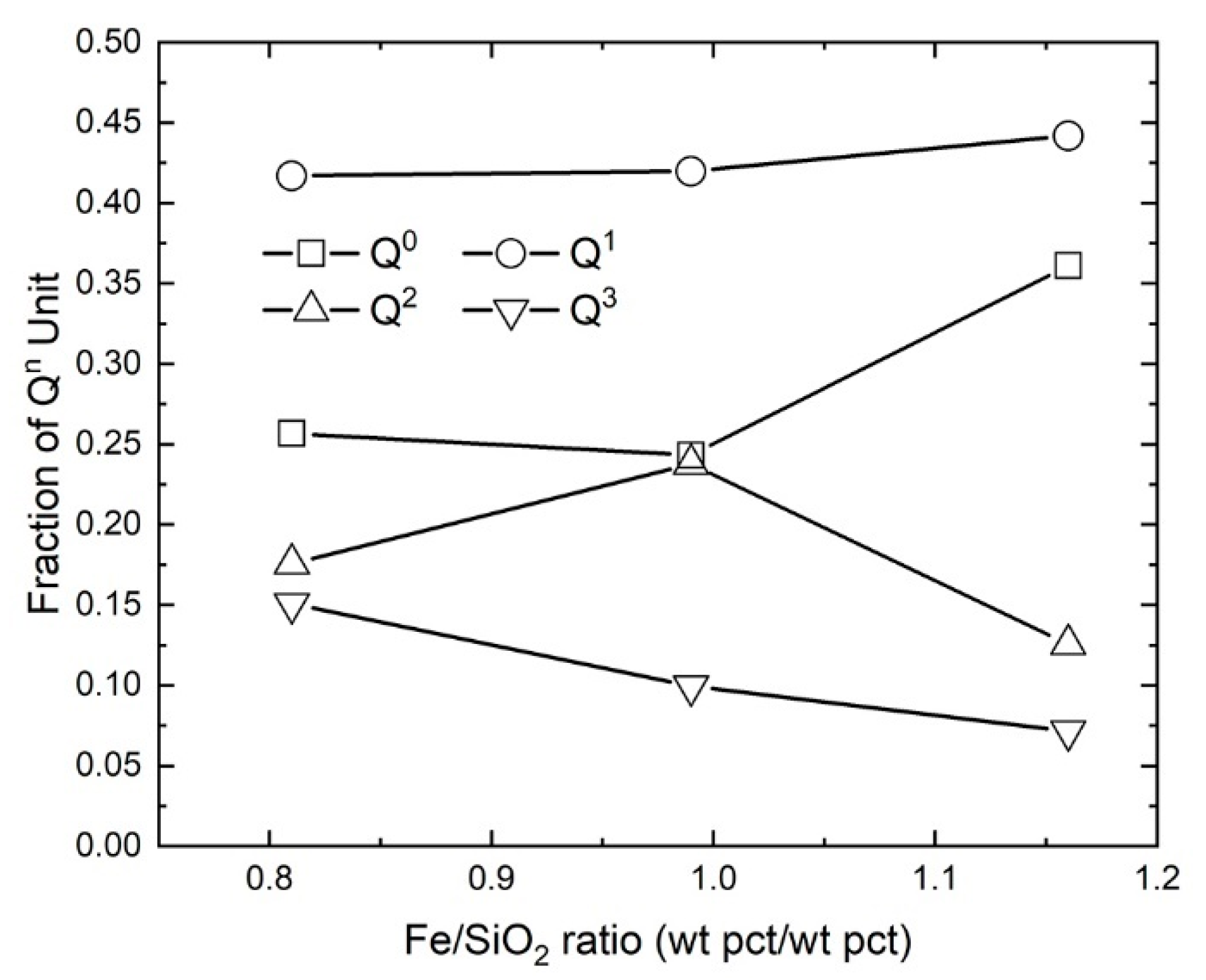
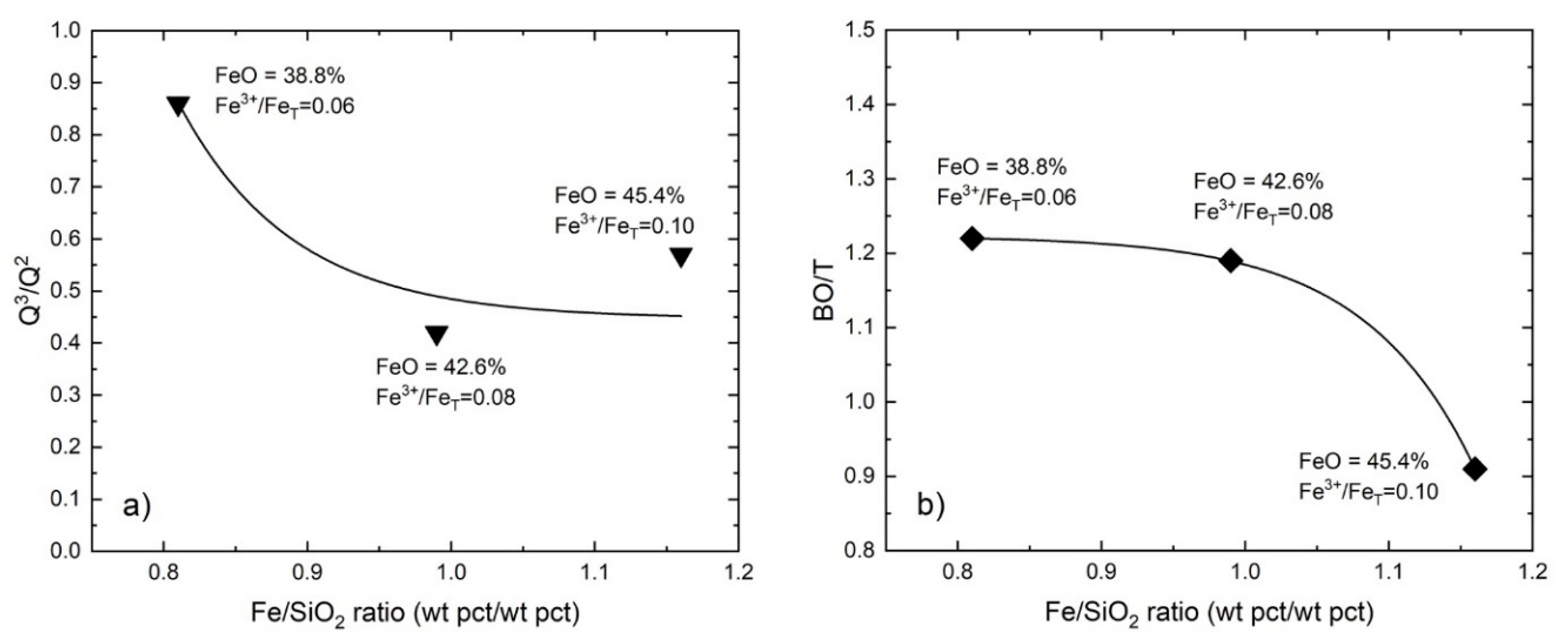
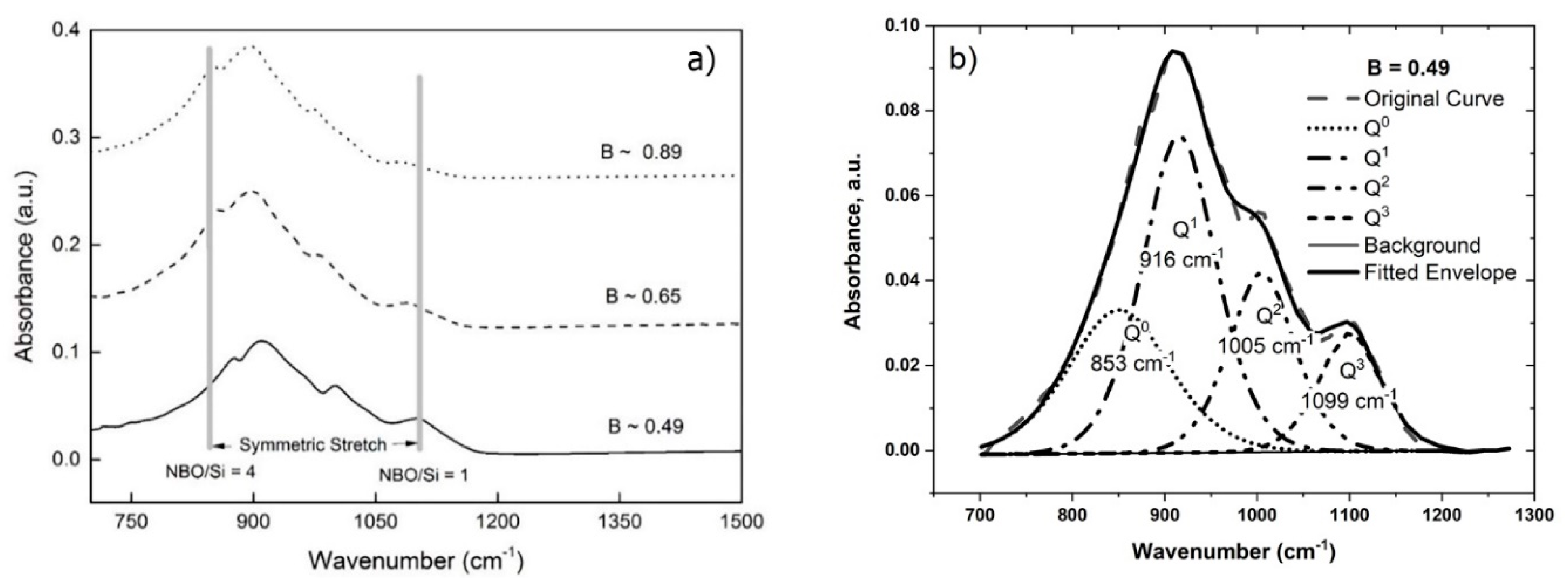
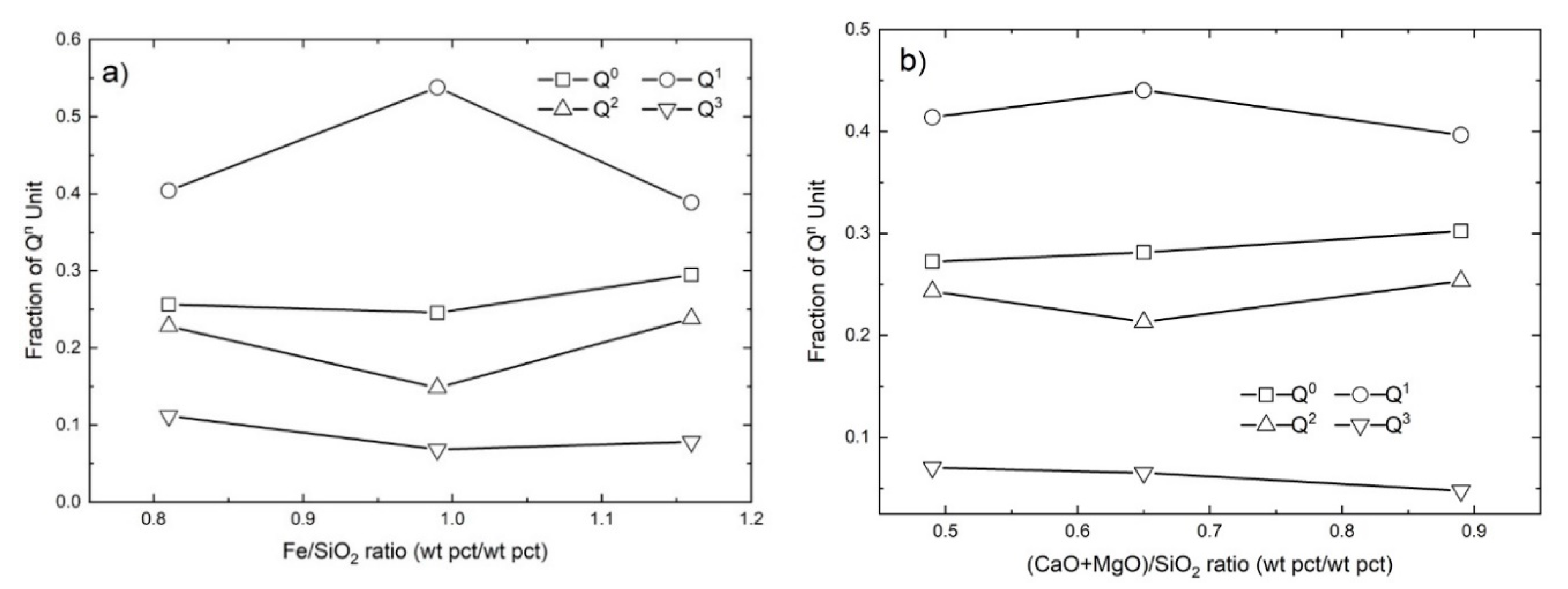
4.1.1. The Effect of Fe/SiO2
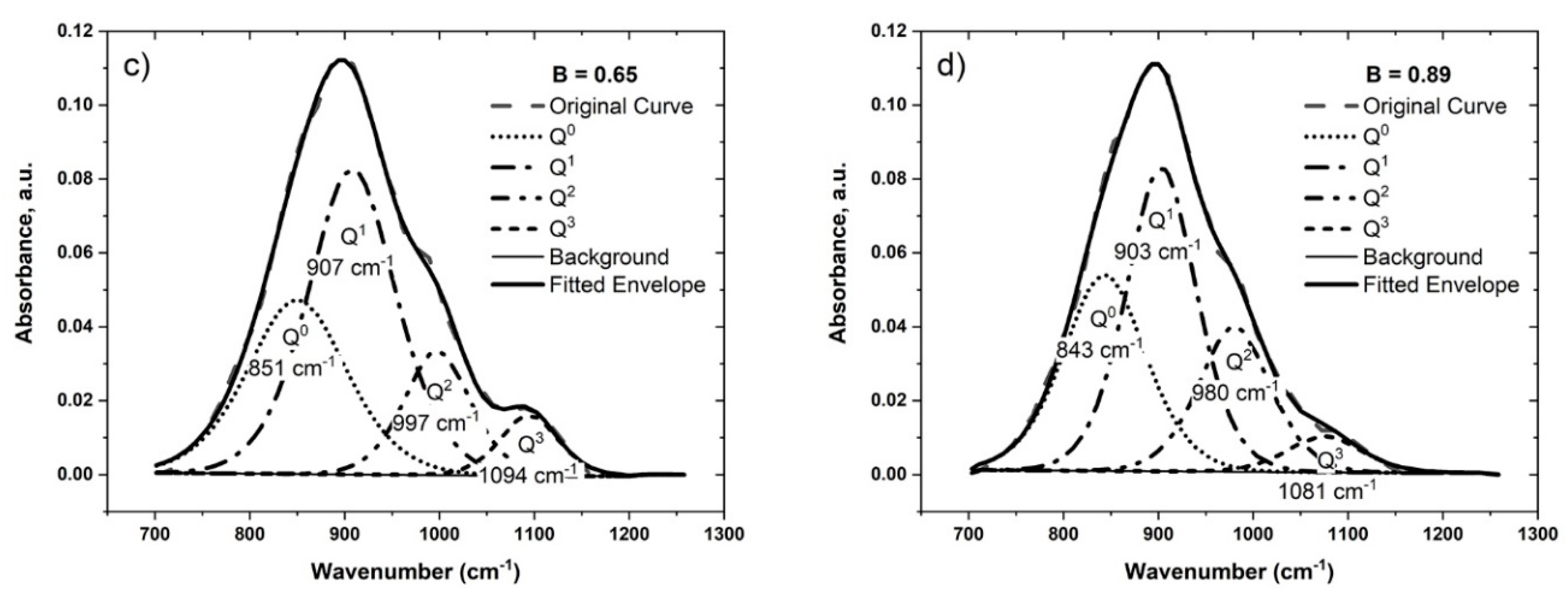
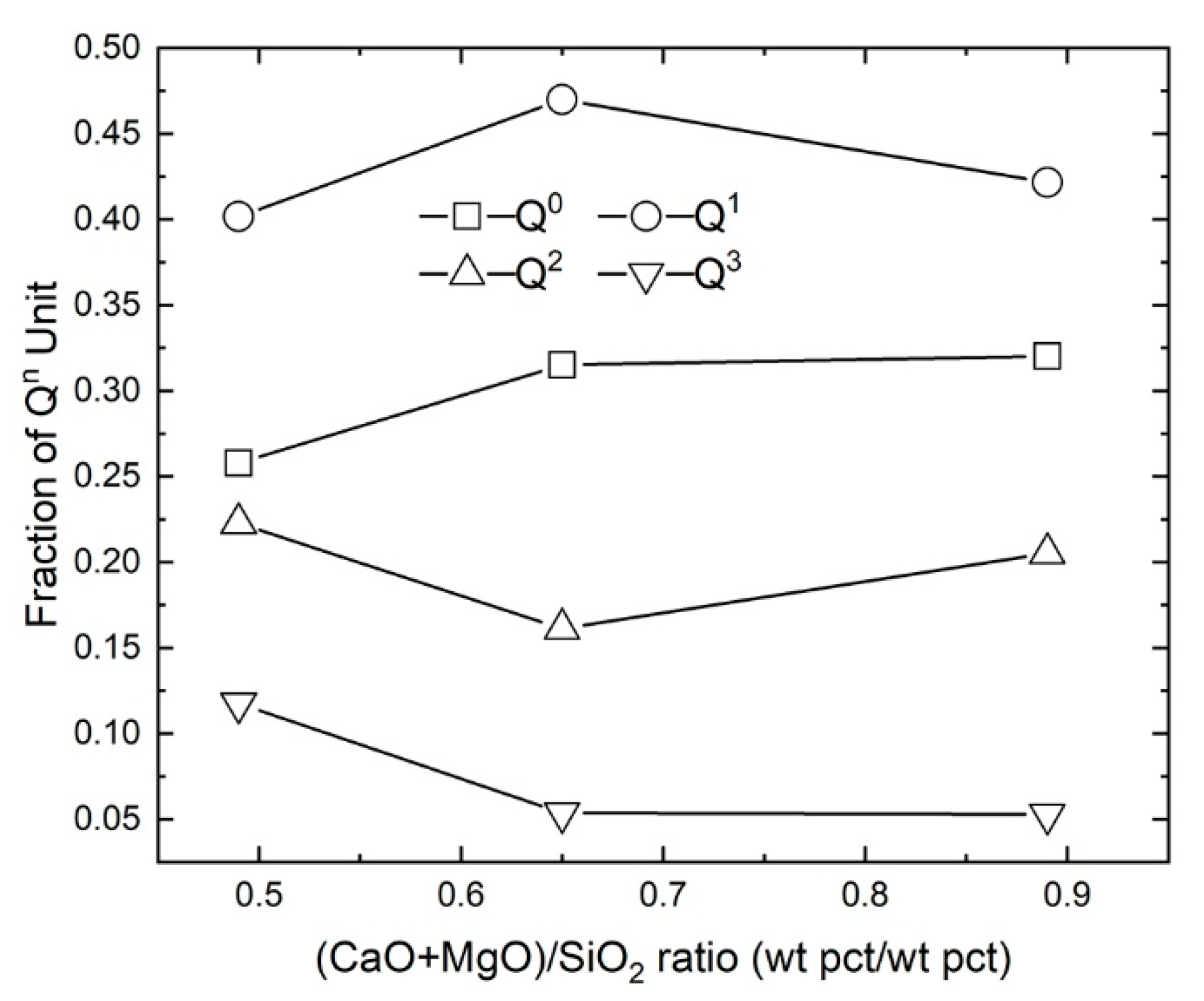
4.1.2. The Effect of Basicity
4.2. Structure Analysis of the FeOx-CaO-SiO2-MgO-Cu2O-PdO (FCSM-Cu2O-PdO) Slags
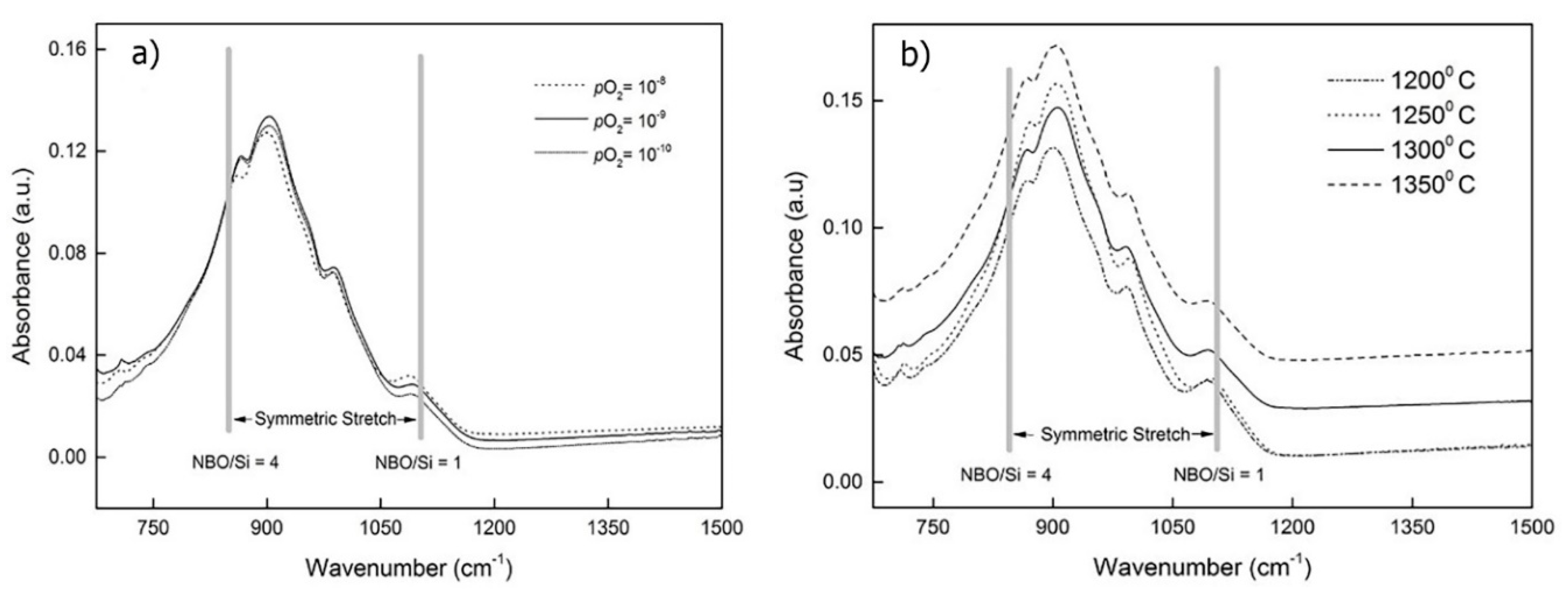
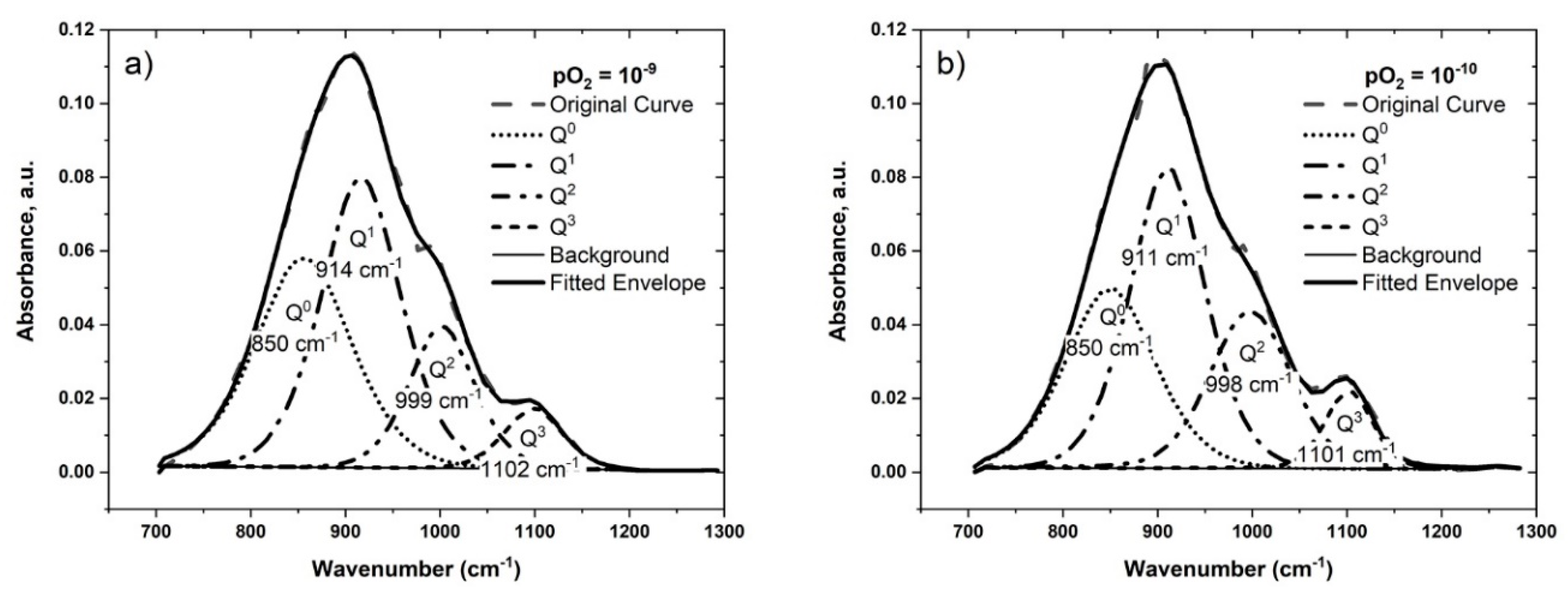
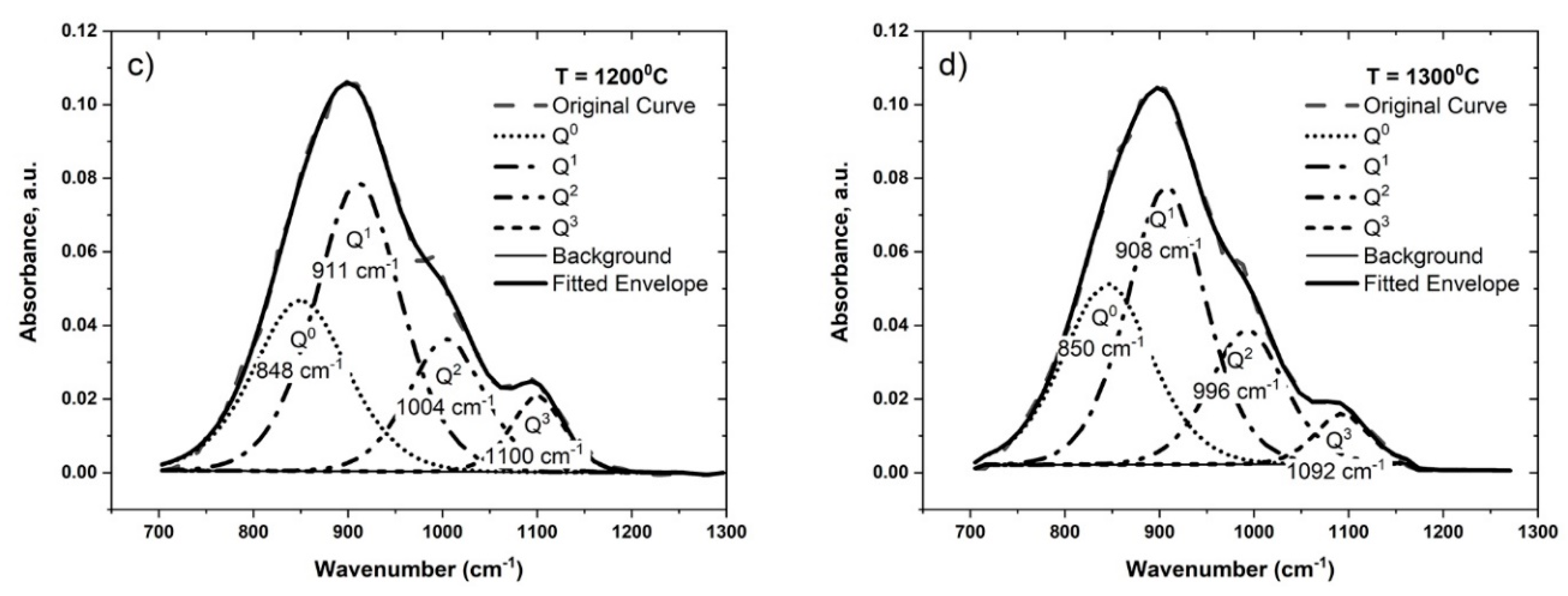
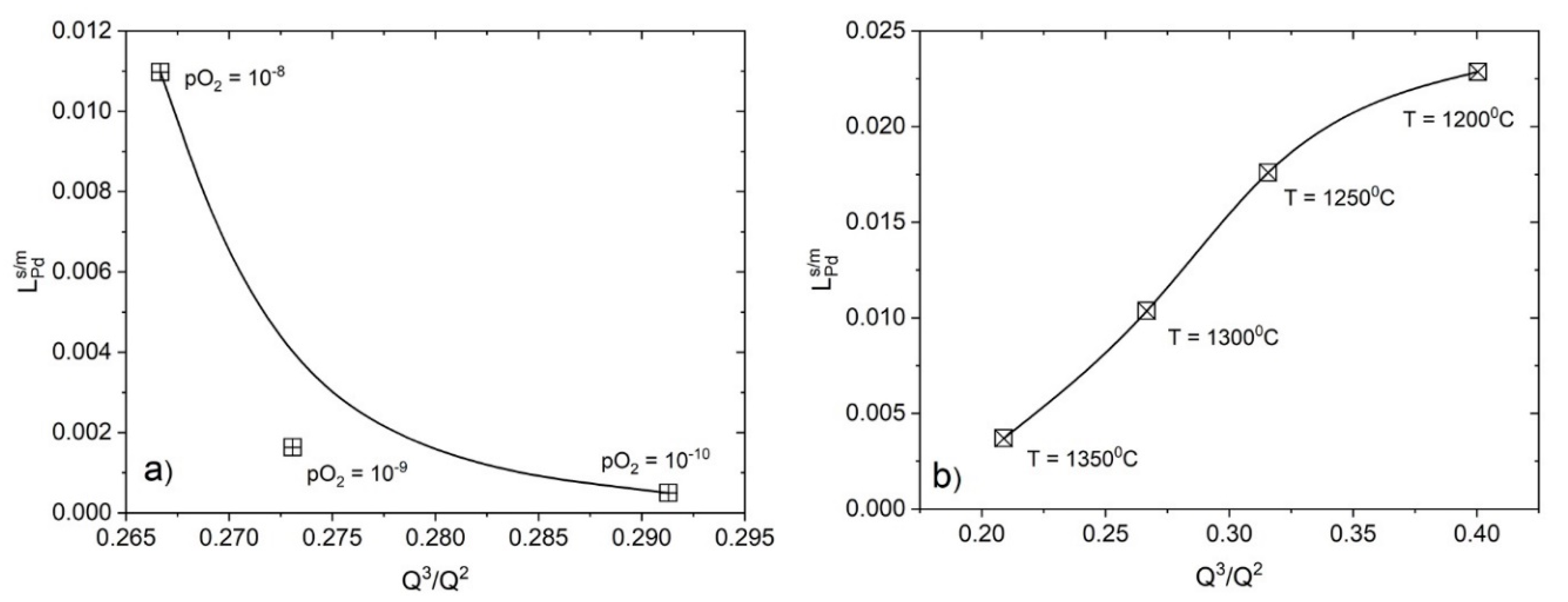
4.2.1. The Effect of Oxygen Potential and Temperature
4.2.2. The Effect of Slag Composition
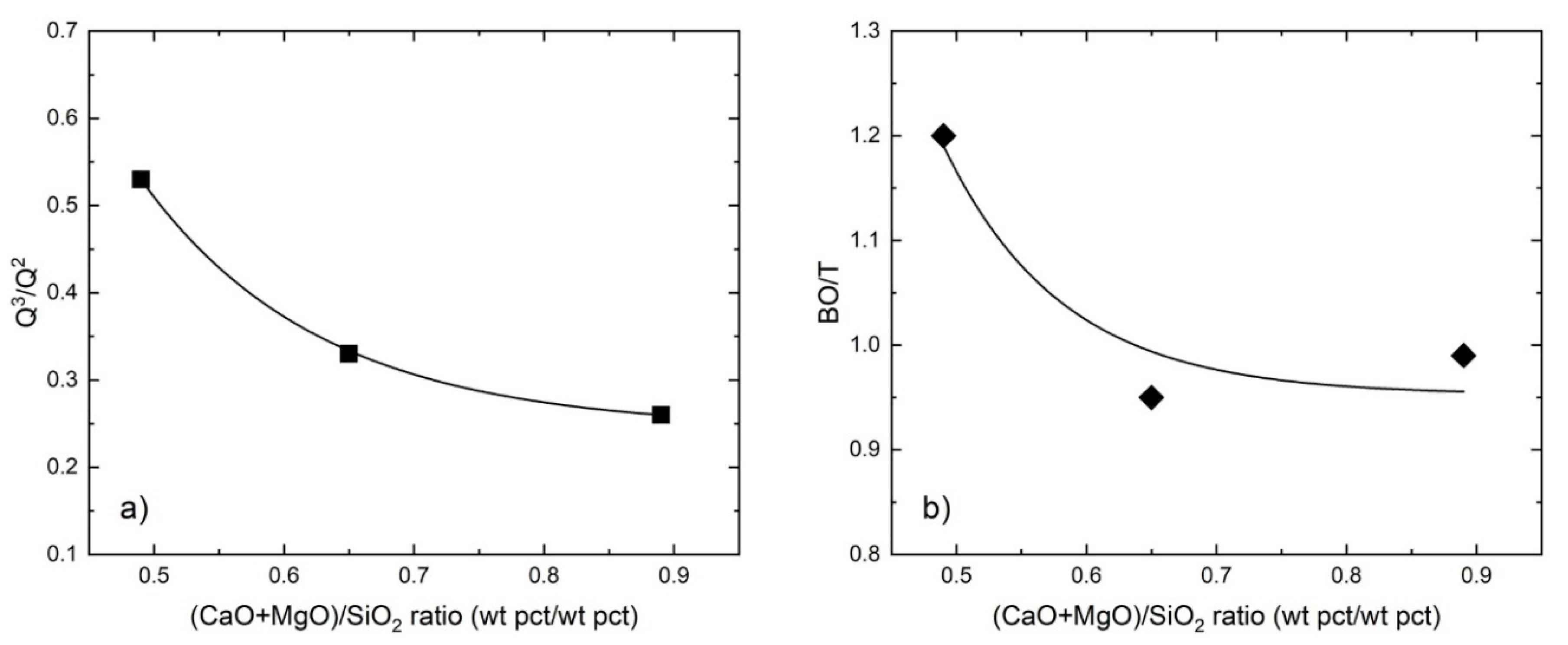
4.3. Correlations of the Slag Structure, with Composition and Experimental Parameters
5. Summary
- The relative intensity of high-frequency band Q3 to the low-frequency band Q0 in each slag was affected by Fe/SiO2 ratio, basicity, oxygen partial pressure and temperature.
- The degree of polymerization, represented by Q3/Q2, and the average number of bridging oxygens (BO/T) were found to decrease with increasing both Fe/SiO2 ratio and basicity.
- There are interrelationships between the partition ratio , the Q3/Q2 and the slag chemistry, which are affected by the experimental conditions; e.g., temperature and pO2.
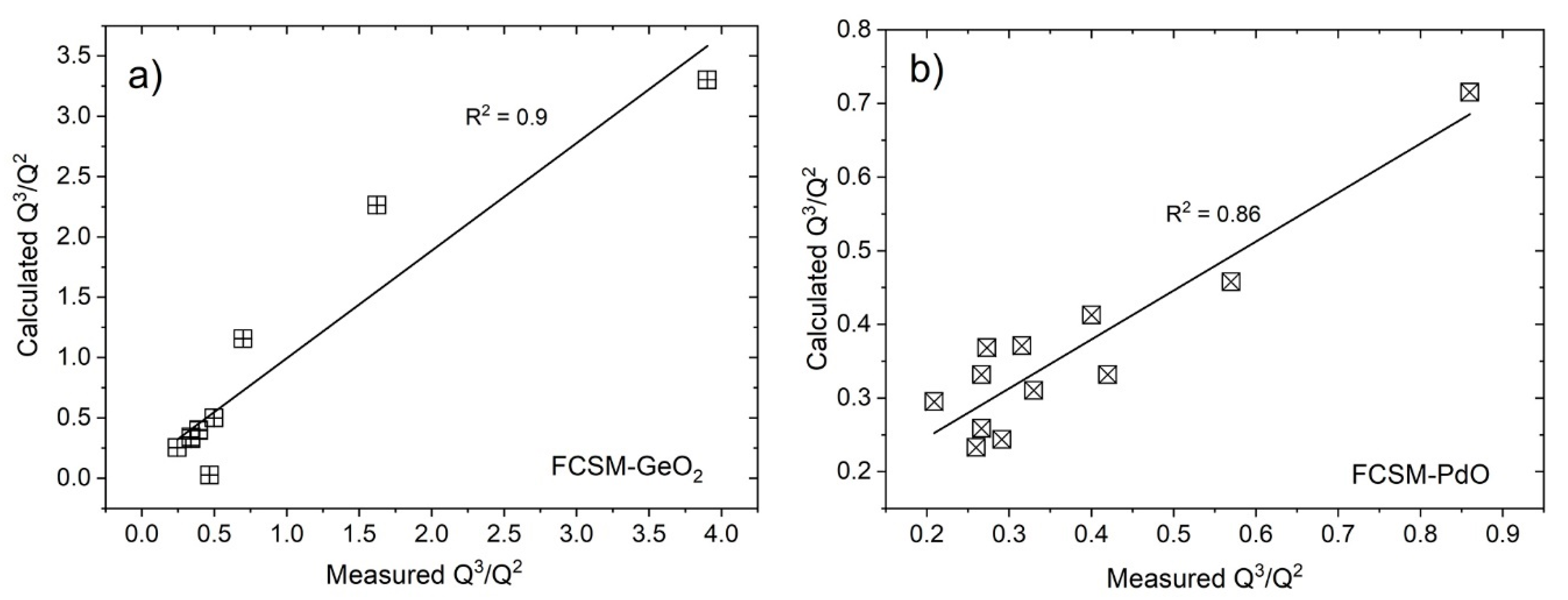
- Semi-empirical equations have been developed using multiple regression analysis to correlate the Q3/Q2, temperature, pO2 and slag compositions. These correlations can be used to predict the Q3/Q2 of the FCSM slag, within the range of compositions investigated.
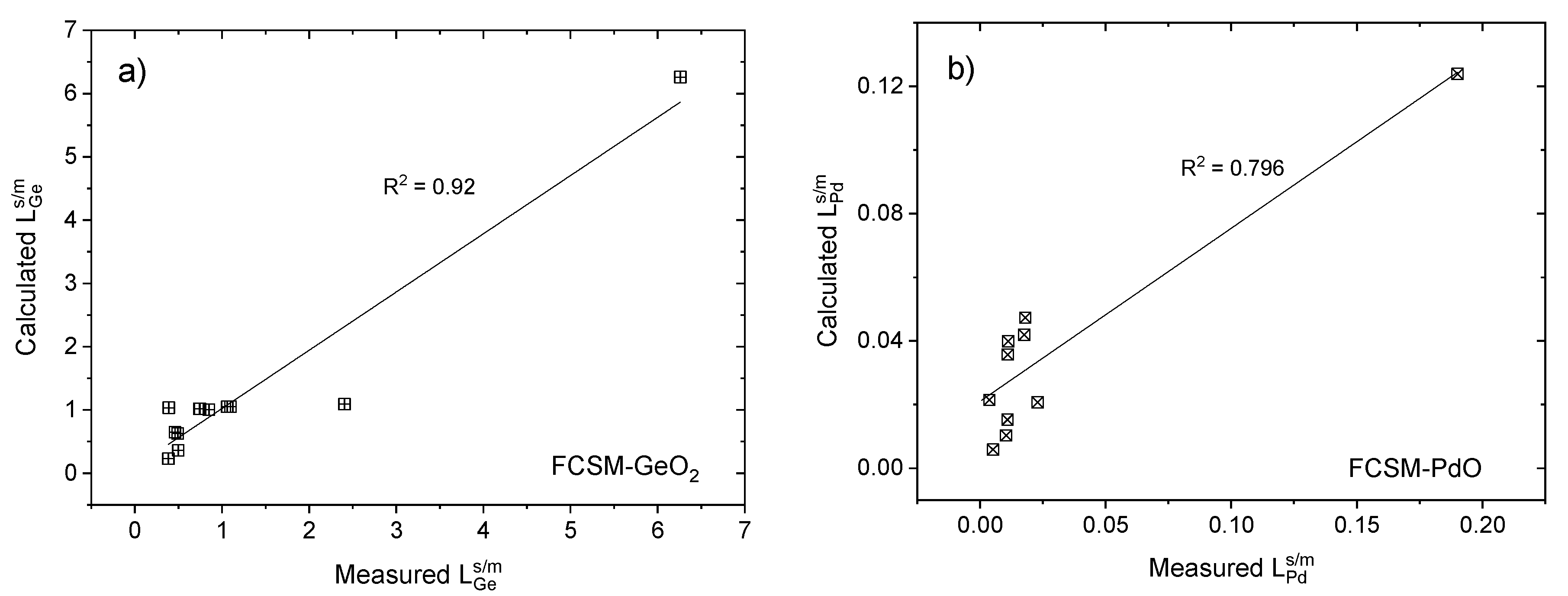
- The partition ratios of Pd and Ge can be reasonably correlated with the pO2, temperature and Q3/Q2. Similar equations for the various valuable elements (Au, Ag, Pt, In, Ru, etc.) should also be developed. These sets of equations will be useful for evaluating and designing slags to be used for secondary black copper process for an optimized process with maximum recovery of all valuable metals.
Author Contributions
Funding
Conflicts of Interest
References
- Sommerville, I.; Sosinsky, D. Solubility, Solubility, Capacity and Stability of Species in Metallurgical Slags and Glasses. In Pyrometallurgy for Complex Materials and Wastes; TMS publication: Pittsburgh, PA, USA, 1994; pp. 73–91. [Google Scholar]
- Wang, L.; Wang, Y.; Wang, Q.; Chou, K. Raman Structure Investigations of CaO-MgO-Al2O3-SiO2-CrOx and its Correlation with Sulfide Capacity. Metall. Mater. Trans. B 2016, 47, 10–15. [Google Scholar] [CrossRef]
- Halter, W.E.; Mysen, B.O. Melt speciation in the system Na2O–SiO2. Chem. Geol. 2004, 213, 115–123. [Google Scholar] [CrossRef]
- Park, J.H. Effect of silicate structure on thermodynamic properties of calcium silicate melts: Quantitative analysis of Raman spectra. Met. Mater. Int. 2013, 19, 577–584. [Google Scholar] [CrossRef]
- Mills, K.C. The influence of structure on the physico-chemical properties of slags. ISIJ Int. 1993, 33, 148–155. [Google Scholar] [CrossRef]
- Toop, G.W.; Somis, C.S. Some new ionic concepts of silicate slags. Can. Metall. Q. 1962, 1, 129–152. [Google Scholar] [CrossRef]
- Virgo, D.B.; Mysen, B.; Kushiro, I. Anionic constitution of 1-atmosphere silicate melts: Implications for the structure of igneous melts. Science 1980, 208, 1371–1373. [Google Scholar] [CrossRef]
- Takanori, H.; Ryuichi, A.; Youichi, M.; Minoru, N.; Yasuhiro, T.; Takao, A. Techniques to separate metal from waste printed circuit boards from discarded personal computers. J. Mater. Cycles Waste Manag. 2009, 11, 42–54. [Google Scholar]
- Palladium Spot Price History & Current Prices, in Money Metal Exchange. Available online: https://www.moneymetals.com/precious-metals-charts/palladium-price (accessed on 18 December 2019).
- Brooks, G.A.; Hasan, M.M.; Rhamdhani, M.A. Slag Basicity: What Does It Mean? In 10th International Symposium on High-Temperature Metallurgical Processing; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2019; pp. 297–308. [Google Scholar]
- Kongoli, F.; McBow, I.; Yazawa, A. Liquidus Surface of “Lime Ferrite” Slags at Intermediate Oxygen Potentials. Mater. Trans. 2003, 44, 2136–2140. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Cardona, N.; Coursol, P.; Mackey, P.J.; Parra, R. Physical chemistry of copper smelting slags and copper losses at the Paipote smelterPart 1—Thermodynamic modelling. Can. Metall. Q. 2011, 50, 318–329. [Google Scholar] [CrossRef]
- Kaur, R. FCS Slag for Continuous Copper Converting. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2007. [Google Scholar]
- Kaur, R.; Swinbourne, D.; Wadsley, M.; Nexhip, C. Comparison of ferrous calcium silicate slag and calcium ferrite slag interactions with magnesia-chrome refractories. Metall. Mater. Trans. B 2011, 42, 451–459. [Google Scholar] [CrossRef]
- Paulina, L.; Swinbourne, D.; Kho, T. Distribution of bismuth between copper and FeOx-CaO-SiO2 slags under copper converting conditions. Min. Proc. Extr. Metall. 2013, 122, 79–86. [Google Scholar] [CrossRef]
- Anindya, A.A. Minor Elements Distribution During the Smelting of WEEE with Copper Scrap. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2012. [Google Scholar]
- Anindya, A.; Swinbourne, D.R.; Reuter, M.A.; Matusewicz, R.W. Distribution of elements between copper and FeOx-CaO-SiO2 slags during pyrometallurgical processing of WEEE: Part 1—Tin. Min. Proc. Extr. Metall. 2013, 122, 165–173. [Google Scholar] [CrossRef]
- Anindya, A.; Swinbourne, D.R.; Reuter, M.A.; Matusewicz, R.W. Distribution of elements between copper and FeOx-CaO-SiO2 slags during pyroprocessing of WEEE: Part 2—Indium. Min. Proc. Extr. Metall. 2014, 123, 43–52. [Google Scholar] [CrossRef]
- Avarmaa, K.; Yliaho, S.; Taskinen, P. Recoveries of rare elements Ga, Ge, In and Sn from waste electric and electronic equipment through secondary copper smelting. Waste Manag. 2018, 71, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Shuva, M.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.H.; Reuter, M.A. Thermodynamics Behavior of Germanium During Equilibrium Reactions between FeOx-CaO-SiO2-MgO Slag and Molten Copper. Metall. Mater. Trans. B 2016, 47, 2889–2903. [Google Scholar] [CrossRef]
- Shuva, M.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.H.; Reuter, M.A. Thermodynamics of palladium (Pd) and tantalum (Ta) relevant to secondary copper smelting. Metall. Mater. Trans. B 2017, 48, 317–327. [Google Scholar] [CrossRef]
- Shuva, M.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.H.; Reuter, M.A. Thermodynamics data of valuable elements relevant to e-waste processing through primary and secondary copper production: A review. J. Clean. Prod. 2016, 131, 795–809. [Google Scholar] [CrossRef]
- Jiao, Q.; Themelis, N. Correlations of electrical conductivity to slag composition and temperature. Metall. Trans. B 1988, 19, 133–140. [Google Scholar] [CrossRef]
- Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of basicity and FeO content on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1283–1290. [Google Scholar] [CrossRef]
- Kim, H.S.; Min, D.J.; Park, J.H. Foaming behavior of CaO-SiO2-FeO-MgOsatd-X (X= Al2O3, MnO, P2O5, and CaF2) slags at high temperatures. ISIJ Int. 2001, 41, 317–324. [Google Scholar] [CrossRef]
- McMillan, P. Raman spectroscopic study of glasses in the system CaO-MgO-SiO2. Am. Miner. 1984, 69, 645–659. [Google Scholar]
- Brandriss, M.E.; Stebbins, J.F. Effects of temperature on the structures of silicate liquids: 29Si NMR results. Geochim. Cosmochim. Acta 1988, 52, 2659–2669. [Google Scholar] [CrossRef]
- Cooney, T.F.; Sharma, S.K. Structure of glasses in the systems Mg2SiO4-Fe2SiO4, Mn2SiO4-Fe2SiO4, Mg2SiO4-CaMgSiO4, and Mn2SiO4-CaMnSiO4. J. Non-Cryst. Solids 1990, 122, 10–32. [Google Scholar] [CrossRef]
- Sohn, I.; Min, D.J. A Review of the Relationship between Viscosity and the Structure of Calcium-Silicate-Based Slags in Ironmaking. Steel Res. Int. 2012, 83, 611–630. [Google Scholar] [CrossRef]
- Gao, Y.M.; Wang, S.B.; Hong, C.; Ma, X.J.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9wt% Al2O3 slag system. Int. J. Min. Met. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.J.; Chang, W.S.; Sohn, I. Effect of FeOt Content and CaO/SiO2 Ratio on Hydrogen Dissolution in CaF2–CaO–SiO2-Based Welding Fluxes. J. Am. Ceram. Soc. 2012, 95, 1756–1763. [Google Scholar] [CrossRef]
- Shuva, M.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.H.; Reuter, M.A. Structural Analysis of Germanium (Ge)-Containing Ferrous Calcium Silicate Magnesia Slag for Applications of Black Copper Smelting. In 9th International Symposium on High-Temperature Metallurgical Processing, Phoenix, AZ, USA, 11–15 March 2018; Springer: Cham, Switzerland, 2018; pp. 295–304. [Google Scholar]
- Yamaguchi, K. Distribution Ratios of Pt and Pd between Iron Oxide Slags and Molten Copper. In Proceedings of the European Metallurgical Conference, EMC 2011, Aachen, Germany, 26–29 June 2011. [Google Scholar]
- Henao, H.; Yamaguchi, H.M.; Ueda, K. Distribution precious metals between copper metal and iron silicate slag at 1573K. In Proceedings of the TMS Fall Extraction & Processing: Sohn International Symposium 2006, San Diego, CA, USA, 27–31 August 2006; pp. 723–729. [Google Scholar]
- Avarmaa, K.; O’Brien, H.; Johto, H.; Taskinen, P. Equilibrium Distribution of Precious Metals between Slag and Copper Matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rhamdhani, M.A.; Shuva, M.; Brooks, G.A. Structure-Thermodynamics Interrelation for the GeO2 and PdO Containing MgO-Saturated Ferrous Calcium Silicate (FCS) Slag Relevant to E-Waste Processing. In 11th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 23–27 February 2020; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Yazawa, A.; Takeda, Y. Equilibrium relations between liquid copper and calcium ferrite slag. Trans. Jpn. Inst. Met. 1982, 23, 328–333. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D. Solubility mechanisms of carbon dioxide in silicate melts; a Raman spectroscopic study. Am. Miner. 1980, 65, 885–899. [Google Scholar]
- Mysen, B.O.; Richet, P. Silicate Glasses and Melts; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Park, J.H. Composition–structure–property relationships of CaO–MO–SiO2 (M= Mg2+, Mn2+) systems derived from micro-Raman spectroscopy. J. Non-Cryst. Solids 2012, 358, 3096–3102. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Mysen, B.O.; Dubinsky, E.V. Melt structural control on olivine/melt element partitioning of Ca and Mn. Geochim. Cosmochim. Acta 2004, 68, 1617–1633. [Google Scholar] [CrossRef]
- Shuva, M.A.H.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.; Reuter, M.A.; Firdaus, M. Analysis for optimum conditions for recovery of valuable metals from e-waste through black copper smelting. In 8th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 26 February–2 March 2017; Springer: Cham, Switzerland, 2017; pp. 419–427. [Google Scholar]
- Mysen, B.O.; Frantz, J.D. Raman spectroscopy of silicate melts at magmatic temperatures: Na2O-SiO2, K2O-SiO2 and Li2O-SiO2 binary compositions in the temperature range 25–1475 °C. Chem. Geol. 1992, 96, 321–332. [Google Scholar] [CrossRef]


| Temperature °C (K) | pO2 (atm) | Slag Composition (wt.%) | FeT/SiO2 | Basicity | Samples Group No. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FeT | SiO2 | CaO | MgO | Cu2O | PdO (ppm) | |||||
| FCSM slags | ||||||||||
| 1300 (1573) | 10−8 | 27.4 | 33.9 | 9.7 | 6.8 | - | - | 0.81 | 0.49 | #1 |
| 32.8 | 33.1 | 9.9 | 6.5 | - | - | 0.99 | 0.49 | #2 | ||
| 37.9 | 32.8 | 9.8 | 6.5 | - | - | 1.16 | 0.49 | #3 | ||
| 33.4 | 33.6 | 14.5 | 7.5 | - | - | 0.99 | 0.65 | #4 | ||
| 30.7 | 31.3 | 19.4 | 8.8 | - | - | 0.99 | 0.89 | #5 | ||
| FCSM-Cu2O-PdO slags | ||||||||||
| 1300 (1573) | 10−8 | 31.1 | 32.6 | 14.5 | 7.8 | 1.06 | 592 | 0.95 | 0.68 | #4-a |
| 10−9 | 1.80 | 99 | #4-b | |||||||
| 10−10 | 2.56 | 29 | #4-c | |||||||
| 1200 (1473) | 10−8 | 32.8 | 33.1 | 9.9 | 6.5 | 2.82 | 1397 | 0.99 | 0.49 | #2-a |
| 1250 (1523) | 1.84 | 1131 | #2-b | |||||||
| 1350 (1623) | 1.13 | 282 | #2-d | |||||||
| 1300 (1573) | 10−8 | 37.3 | 35.3 | 5.6 | 6.3 | 1.61 | 1061 | 1.06 | 0.34 | #6 |
| 33.4 | 33.6 | 14.5 | 7.5 | 1.65 | 353 | 0.99 | 0.65 | #4-d | ||
| 27.4 | 33.9 | 9.7 | 6.8 | 1.55 | 11,219 | 0.81 | 0.49 | #1-a | ||
| 32.8 | 33.1 | 9.9 | 6.5 | 1.61 | 661 | 0.99 | 0.49 | #2-c | ||
| 37.9 | 32.8 | 9.8 | 6.5 | 1.52 | 50 | 1.16 | 0.49 | #3-a | ||
| NBO/T (Qn) | Unit | Frequency (cm−1) | Mode | Ref. |
|---|---|---|---|---|
| 0 (Q4) | SiO2 | 1200, 1190 | Asymmetric stretch | [7] |
| 1 (Q3) | [Si2O5]2− | 1100–1050 | Symmetric stretch | [7] |
| 2 (Q2) | [SiO3]2− | 980–950 | Symmetric stretch | [7] |
| 3(Q1) | [Si2O7]6− | 920–900 | Symmetric stretch | [7] |
| 4 (Q0) | [SiO4]4− | 880–850 | Symmetric stretch | [7] |
| - | 800–780 | Asymmetric bending | [27] | |
| - | 650–500 | Symmetric bending | [27] | |
| - | 480–440 | Rocking | [27] |
| Sample | Fe/SiO2 | Peak Position (cm−1) | Fraction of Different Qn Units | Q3/Q2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | Q1 | Q2 | Q3 | Q0 | Q1 | Q2 | Q3 | |||
| #1 | 0.81 | 870 | 920 | 1007 | 1091 | 0.26 | 0.42 | 0.18 | 0.15 | 0.86 |
| #2 | 0.99 | 852 | 919 | 1011 | 1109 | 0.24 | 0.42 | 0.24 | 0.10 | 0.42 |
| #3 | 1.16 | 849 | 914 | 998 | 1097 | 0.36 | 0.44 | 0.13 | 0.07 | 0.57 |
| Sample | Peak Position (cm−1) | Fraction of Different Qn Units | Q3/Q2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Basicity | Q0 | Q1 | Q2 | Q3 | Q0 | Q1 | Q2 | Q3 | ||
| #3 | 0.49 | 853 | 916 | 1005 | 1099 | 0.26 | 0.40 | 0.22 | 0.12 | 0.53 |
| #4 | 0.65 | 851 | 907 | 997 | 1094 | 0.32 | 0.47 | 0.16 | 0.05 | 0.33 |
| #5 | 0.89 | 843 | 903 | 980 | 1081 | 0.32 | 0.42 | 0.21 | 0.05 | 0.26 |
| Sample | T (°C) | log pO2 | Peak Position (cm−1) | Fraction of Different Qn Units | Q3/Q2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | Q1 | Q2 | Q3 | Q0 | Q1 | Q2 | Q3 | ||||
| #4-b | 1300 | −9 | 850 | 914 | 999 | 1102 | 0.29 | 0.45 | 0.20 | 0.06 | 0.27 |
| #4-c | 1300 | −10 | 850 | 911 | 998 | 1102 | 0.29 | 0.41 | 0.23 | 0.07 | 0.29 |
| #2-a | 1200 | −8 | 850 | 911 | 1004 | 1100 | 0.29 | 0.45 | 0.18 | 0.07 | 0.40 |
| #2-c | 1300 | −8 | 845 | 907 | 992 | 1092 | 0.32 | 0.43 | 0.20 | 0.05 | 0.27 |
| Sample | Fe/SiO2 | Basicity, B | Peak Position (cm−1) | Fraction of Different Qn Units | Q3/Q2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | Q1 | Q2 | Q3 | Q0 | Q1 | Q2 | Q3 | ||||
| #1-a | 0.81 | 0.49 | 845 | 904 | 995 | 1090 | 0.26 | 0.40 | 0.23 | 0.11 | 0.49 |
| #2-c | 0.99 | 0.49 | 840 | 908 | 997 | 1091 | 0.25 | 0.54 | 0.15 | 0.07 | 0.46 |
| #3-a | 1.16 | 0.49 | 845 | 911 | 991 | 1093 | 0.29 | 0.39 | 0.24 | 0.08 | 0.33 |
| #6 | 1.06 | 0.34 | 847 | 909 | 995 | 1091 | 0.27 | 0.41 | 0.24 | 0.07 | 0.29 |
| #2-c | 0.99 | 0.55 | 840 | 905 | 990 | 1095 | 0.28 | 0.44 | 0.21 | 0.07 | 0.31 |
| #4-d | 0.99 | 0.65 | 842 | 905 | 988 | 1099 | 0.30 | 0.40 | 0.25 | 0.05 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Rhamdhani, M.A.; Shuva, M.A.H.; Brooks, G.A. Study of the Structure of FeOx-CaO-SiO2-MgO and FeOx-CaO-SiO2-MgO-Cu2O-PdO Slags Relevant to Urban Ores Processing through Cu Smelting. Metals 2020, 10, 78. https://doi.org/10.3390/met10010078
Hasan MM, Rhamdhani MA, Shuva MAH, Brooks GA. Study of the Structure of FeOx-CaO-SiO2-MgO and FeOx-CaO-SiO2-MgO-Cu2O-PdO Slags Relevant to Urban Ores Processing through Cu Smelting. Metals. 2020; 10(1):78. https://doi.org/10.3390/met10010078
Chicago/Turabian StyleHasan, Mohammad Mehedi, M. Akbar Rhamdhani, M. Al Hossaini Shuva, and Geoffrey A. Brooks. 2020. "Study of the Structure of FeOx-CaO-SiO2-MgO and FeOx-CaO-SiO2-MgO-Cu2O-PdO Slags Relevant to Urban Ores Processing through Cu Smelting" Metals 10, no. 1: 78. https://doi.org/10.3390/met10010078
APA StyleHasan, M. M., Rhamdhani, M. A., Shuva, M. A. H., & Brooks, G. A. (2020). Study of the Structure of FeOx-CaO-SiO2-MgO and FeOx-CaO-SiO2-MgO-Cu2O-PdO Slags Relevant to Urban Ores Processing through Cu Smelting. Metals, 10(1), 78. https://doi.org/10.3390/met10010078







