1. Introduction
In the last few years, low CO2 generation of electrical energy has garnered much public attention. Electricity generation in nuclear power plants is an alternative to carbon-based electricity generation by combustion. The concept of a gas-cooled high-temperature reactor (HTR) is being actively discussed and further developed with an emphasis on achieving the highest possible level of safety compared with classic light-water reactor systems. The HTR is a future nuclear technology that can realistically replace classical reactor systems in the coming decades. The reason for this is its outstanding features such as scalability in the form of modular design and passive safety through the selection of suitable mechanisms. Due to the high coolant output temperatures, process heat up to 950 °C can also be provided for industrial applications.
In the event of an accident, the maximum fuel-element temperature is below the designed temperature of the overall system because of the suitable choice of reactor geometry [
1]. This prevents the reactor core from melting.
Because of the potential of using process heat for, e.g., large-scale hydrogen production and synfuel production or downstream ammonia synthesis, strong efforts are currently being made in several countries to introduce such a system.
Two experimental reactors are currently in operation: the HTR-10 in China, which has spherical fuel elements, and the HTTR in Japan, which has prismatic fuel elements. Both are used to investigate the operating behavior of the entire plant and to test new materials. The results obtained here will be used to design high-performance plants. The near completion of a prototype, the HTR-PM in China, which has two blocks of 250 MW
th each, suggests that the introduction of the technology will be intensified in other countries. Plans are currently underway to build HTRs in Poland [
2], Saudi Arabia, and Jordan. In Indonesia, first a 50 MW
th experimental reactor [
3] and later a reactor with 200 MW
th [
4] will be built.
Because of the use of graphite as a cladding material for the fuel elements and for the lining and reflector structures in the reactor pressure vessel, larger quantities of graphite dust can be generated by mechanical or chemical processes and can be distributed in the primary circuit. Dust has been found in considerable quantities during the decommissioning of previous plants [
5] and can negatively affect the reactor’s operation, with dust acting as a contaminant in the coolant [
6,
7]. One reason for the formation of graphite dust is the use of helium as a coolant, which significantly reduces the abrasion resistance of the graphite [
8]. Decken et al. [
5] note that graphite has a significantly higher coefficient of friction under reactor conditions than in an air atmosphere. In addition to mechanical dust formation, chemical abrasion also occurs on the surfaces of graphite components such as the reflector and fuel elements. This is caused by unavoidable impurities in the helium atmosphere. Radiation-induced structural changes in graphite also increase the formation rate of graphite particles. Quantitative and generalizable statements on the generated dust quantities have only been able to be obtained, to a limited extent, from the previous operating experiences of the various HTR plants, since each plant has a very specific operating history. The dust quantities estimated by elaborate investigations into the Arbeitsgemeinschaft Versuchs Reaktor (AVR) Power Plant must also be evaluated in this way [
5].
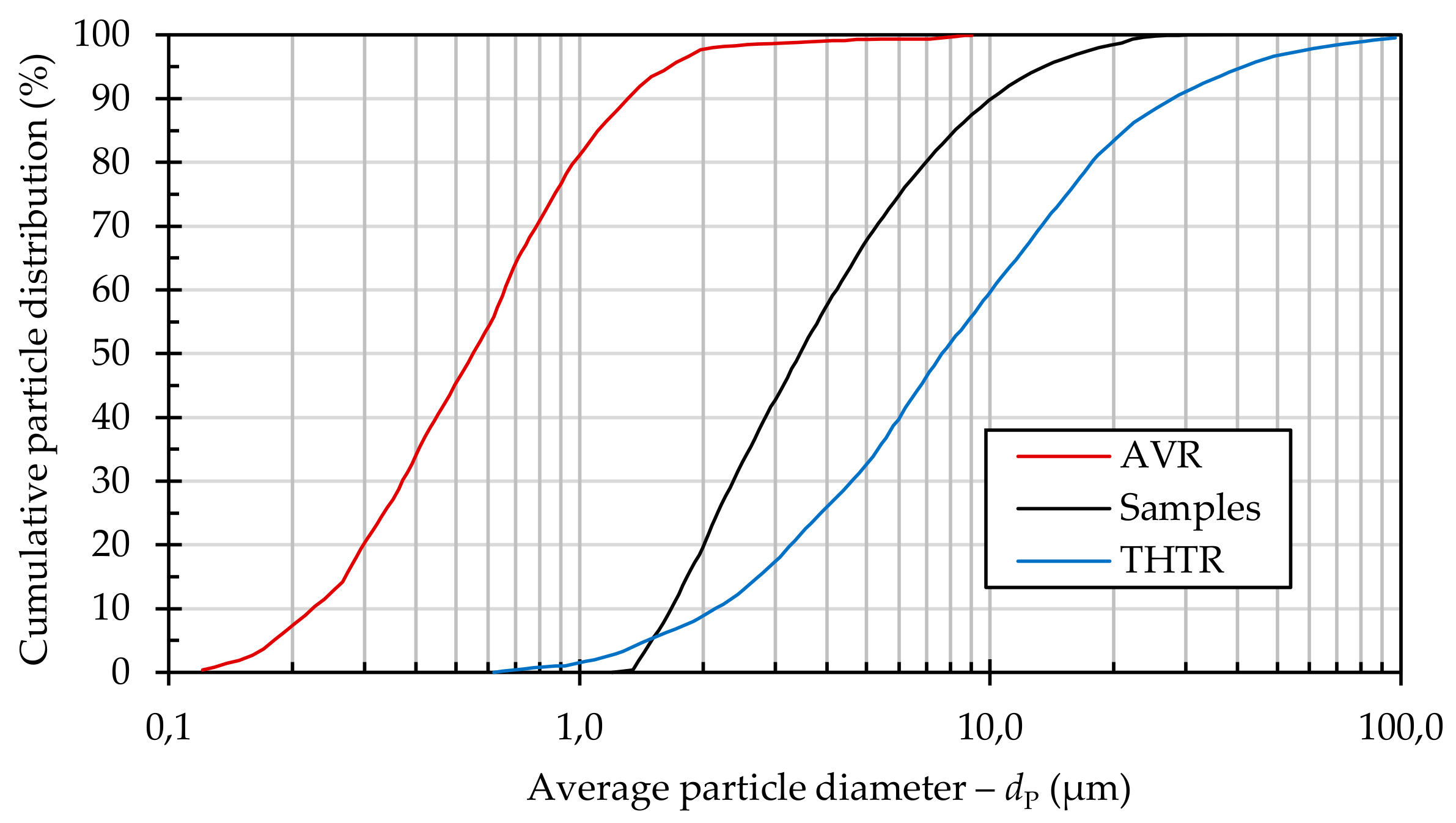
Different sources thus indicate different quantities for the entire operating period: 46 kg [
9], 60 kg [
5], and 100–200 kg [
10]. In the interpretation of these quantities it must also be considered that the measurements were carried out locally in different pipe segments by means of a wipe test and then scaled up to the overall plant. Furthermore, the applicability of these quantities to large industrial plants is questionable, since the dust production in these cases was influenced by contamination from oil, water, and air intrusion, as well as by the use of different test fuel elements with different composition and abrasion behavior.
In modern plants, such as the Chinese HTR-10, dust production rates of ~3 kg/a [
11] have been measured during operation. Here, it must be noted that these were local extractions, and that upscaling to the entire primary circuit occurred.
However, sufficiently detailed models are missing for an exact description of dust transport and deposition behavior under different thermo-fluid dynamic conditions [
12]. This requires the use and further development of experimentally confirmed models. However, there is a lack of sufficiently validated experimental data for this purpose [
13]. A large number of the theoretical models used to describe the processes, so far, are considered to be faulty [
14]. For an exact description of the mobilization behavior, it is therefore necessary to underlie the time-dependent change of the adhesive force as well as the chemical bond between graphite particles and the surfaces with experimental data.
Previous calculations have shown that 86% of the graphite dust produced in the primary circuit is deposited on the graphite surfaces. Two-thirds of the remaining quantity is deposited on the various steam generator tubes in the low-temperature intermediate heat exchanger, or on the high-temperature heat exchanger, as well as on the connecting pipes of the primary circuit [
15]. Firstly, experimental investigations under ambient air have shown that particles in the flow area primarily collect in flow dead spaces and lead to increased deposits there [
16]. This dust can grow into multi-layered systems over time. It cannot be ruled out that the dust will be remobilized again because of its brittle structure and because of the flowing cooling gas. So far, insufficient data are available on the long-term and corrosion behavior of these layers under HTR conditions [
9].
Until now, only metallic alloys have been exposed to the hot cooling gas. Taking into account the strong helium flow, chemical interactions, such as decarburization or the change in creep strength, have been investigated [
17].
Further investigations have shown that the mobile dust present in the primary circuit binds a considerable amount of gaseous fission products [
1]. This sorption process of radioactive fission products takes place continuously during normal operation [
15]. The most important fission products are cesium (Cs-134 and Cs-137), iodine (I-131), and silver (Ag-110 m) [
18]. The graphitic dust acts as a transport medium for non-gaseous radionuclides. In the AVR and HTR-10, values of 106–108 Bq/g Cs-137 [
5] and 100–150 Bq/g Cs-137 [
11], respectively, have been measured.
In addition to considering the loading of the graphite particles with radionuclides, the structure of the mobile particles present in the primary circuit is altered in the event of a release accident. This is due to the strong physical and chemical processes that take place when the released materials leave the primary system and the containment [
19]. The particles, which are reduced in size, among other things can have a far-reaching negative influence on the environment of a reactor plant, because of their improved ability to float. The fact that smaller particles are respirable should not be neglected.
2. Previews
In a typical HTR hot gas atmosphere, thermochemically-induced interactions can have an influence on the composition, binding, or adhesive force of individual or different particles; this can have a significant effect on the remobilization and release of separated particles in the event of an accident.
To describe the potential chemical processes, the small amounts of impurities inevitably contained in the cooling gas, helium (quality helium 4.6), in an HTR during typical reactor operation must be considered. The most important impurities are H
2, H
2O, CH
4, CO, CO
2, N
2, and O
2 [
20].
These impurities can be introduced into the cooling gas from the cooling gas filling, which maintains the operating pressure, in the form of contamination by maintenance or by other chemical processes [
9]. The following are some possible reactions that can occur in the reactor core at high temperatures (with a maximum fuel temperature in the core of 900 °C) [
21].
Taking into account the temperature and pressure conditions present in the HTR, the following altered equilibrium states apply to the evaluation of the reactions taking place with priority [
1].
Previous investigations by Meng et al. [
14] have shown that in addition to mechanical processes, the chemical production of graphite particles takes place; hydrogen has a notable influence on the production of graphite particles. The existing carbon dioxide reacts with hydrogen to form graphite and water vapor (i.e., chemical vapor deposition occurs). For example, graphite has been found to form and to be deposited on Inconel 617 as large particle agglomerates and as individual particles with a diameter of 0.2–1 µm [
14].
Further investigations have been carried out on the formation of reaction products from the fission products that diffuse from the fuel elements. The influence of the O
2 impurity is the main reason for the phase transformation from cesium to Cs
2O
2, which is present in solid form up to a temperature of 610 °C and also as a gas. Strontium first forms SrO
2 and SrO with increasing temperature. Silver in the form of AgO remains stable at all temperature ranges. With a further increase in temperature, a turning point is reached where cesium and iodine bind [
22].
With regard to the evaluation of the structural material, investigations into the influence of thermal annealing on the internal complex transformation processes have been carried out. A negative influence on the structural integrity has been confirmed by time- and temperature-dependent phase changes [
23].
Taking these aspects into account, chemical and thermally-induced reactions of the base material have an indirect influence on the adhesive force,
Fadh, of particles deposited on surfaces. The main influencing factor is the alteration of the material-dependent Hamaker constant,
Cadh, which is necessary for the description of the force between two particles specifically in which Van der Waals forces act. It is influenced over time by the change in or interaction between particles (particle size
dP), the medium between the particles and the substrate, and the substrate itself (surface condition and chemical composition). Because of chemical influence (e.g., the formation of an oxide layer), the boundary layer thickness,
δ, between the particle and surface changes, i.e.,
Extensive investigations on material resistance carried out over past decades have shown that oxidation phenomena occur on the corrosion-resistant alloys used in the HTR. These phenomena are achieved by the formation of layers (passivation) that are only a few micrometers thick [
9].
In addition, extensive investigations have been carried out on the resistance to the reactions caused by impurities in the cooling gas helium. By varying the oxygen concentration and simulating water contamination in the cooling gas, the formation of corrosion layers on chromium-nickel-based alloys has been confirmed [
20].
To date, the presence of graphite particles has been insufficiently accounted for such research. Hence, the work presented herein aims to clarify the influence of graphite particles on structural design in order to consider countermeasures within plant design, if necessary. Thus, the determination of possible reaction products and their reaction speed is essential. This forms the basis for determining the residual amounts of radionuclides that could be released in the event of mobilization of particles.
3. Methods
3.2. Test Plant DRESDEN-TANK
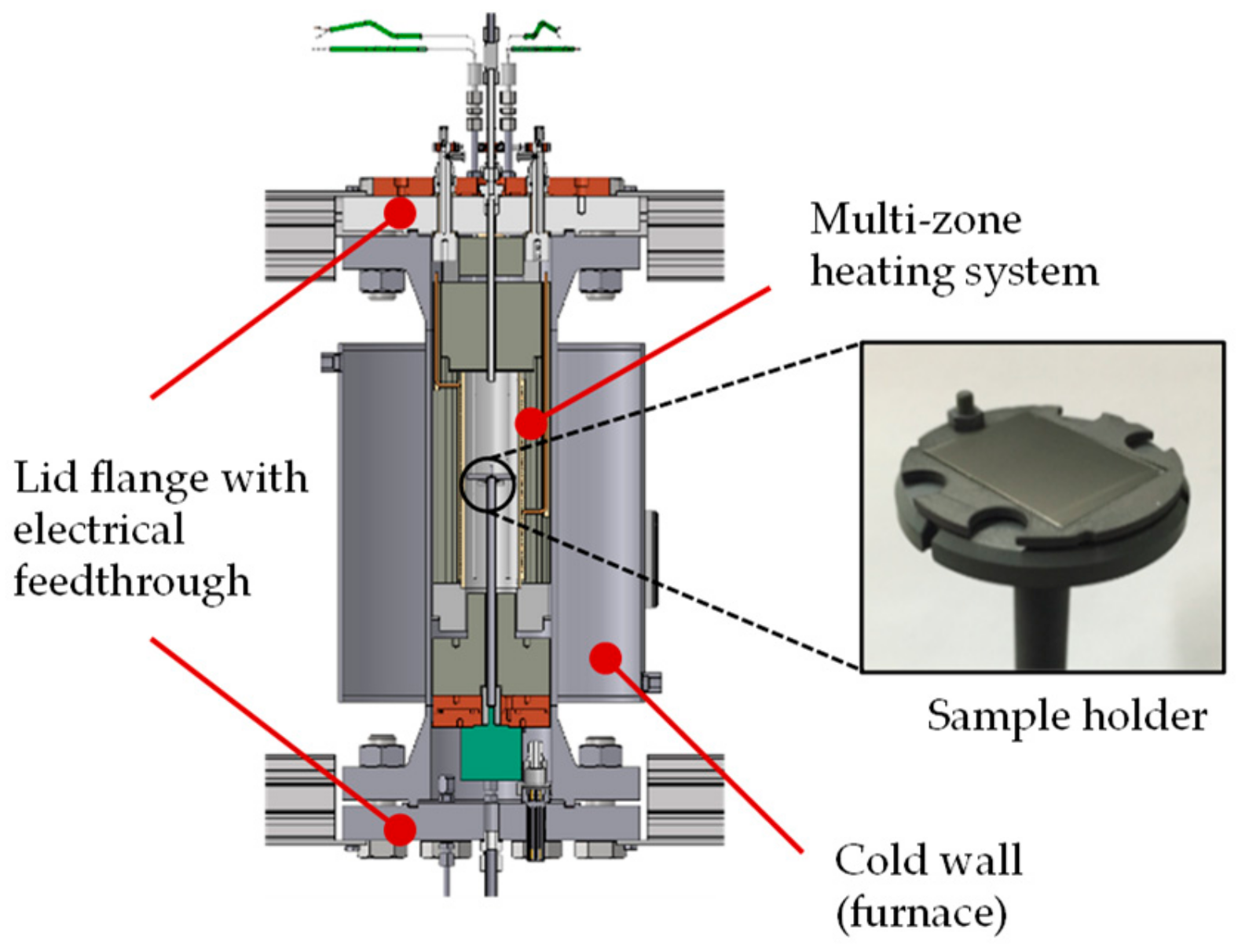
The dust resuspension and deposition in tank geometry (DRESDEN-TANK) test plant was set up at the Technische Universität Dresden in order to investigate these processes and deepen an understanding of graphite dust behavior under HTR conditions (
Figure 3).
The experiments in the test plant have focused on the investigation of the safety-relevant, long-term interactions of graphite particles on the various surfaces of the HTR (primarily the circuit components).
The DRESDEN-TANK test plant enables long-term tests to be carried out under representative HTR temperatures and pressures. It was designed for test durations longer than 1000 h at up to 1000 °C and 6 MPa. The overall structure and the inner specimen chamber, as well as one of the specimen grips, are shown schematically in
Figure 4.
The main component of the test plant is the high-pressure temperature autoclave, which was actualized as a cold-wall furnace that can be filled with gaseous fluids. In the pressure-tight sealable sample chamber (Ø = 55 mm and height = 200 mm), substrates loaded in various states (graphitic or metallic) can be introduced and thermally annealed in various atmospheres, and can include helium, argon, nitrogen, air, or mixtures of these. The temperature can be varied within a range of 25 to 1000 °C. Controllable multi-zone heating (3 × 1 kW) ensures a stable and homogeneous temperature distribution in the area of the particle-laden sample during stationary operation. The axial temperature field is monitored by 20 thermocouples that are mounted radially between the sample holder and the inner wall over the entire height. At the same time, up to eight samples can be positioned in the center of the chamber with the help of a corresponding sample holder. In addition, axial rotation of the samples is possible in order to allow centrifugal forces to act on the particles.
The rest of the internal structure is filled with dense insulation material on the outside, which leads to savings in gas consumption during the long-term tests thanks to the reduced space requirement. The cover flanges on the top and bottom are equipped with electrical feedthroughs for heating and measuring technology.
In order to minimize any external influences during thermal treatment, accurate and contamination-free sample handling is essential. The samples are stored separately in antistatic containers and allowed to dry between each step.
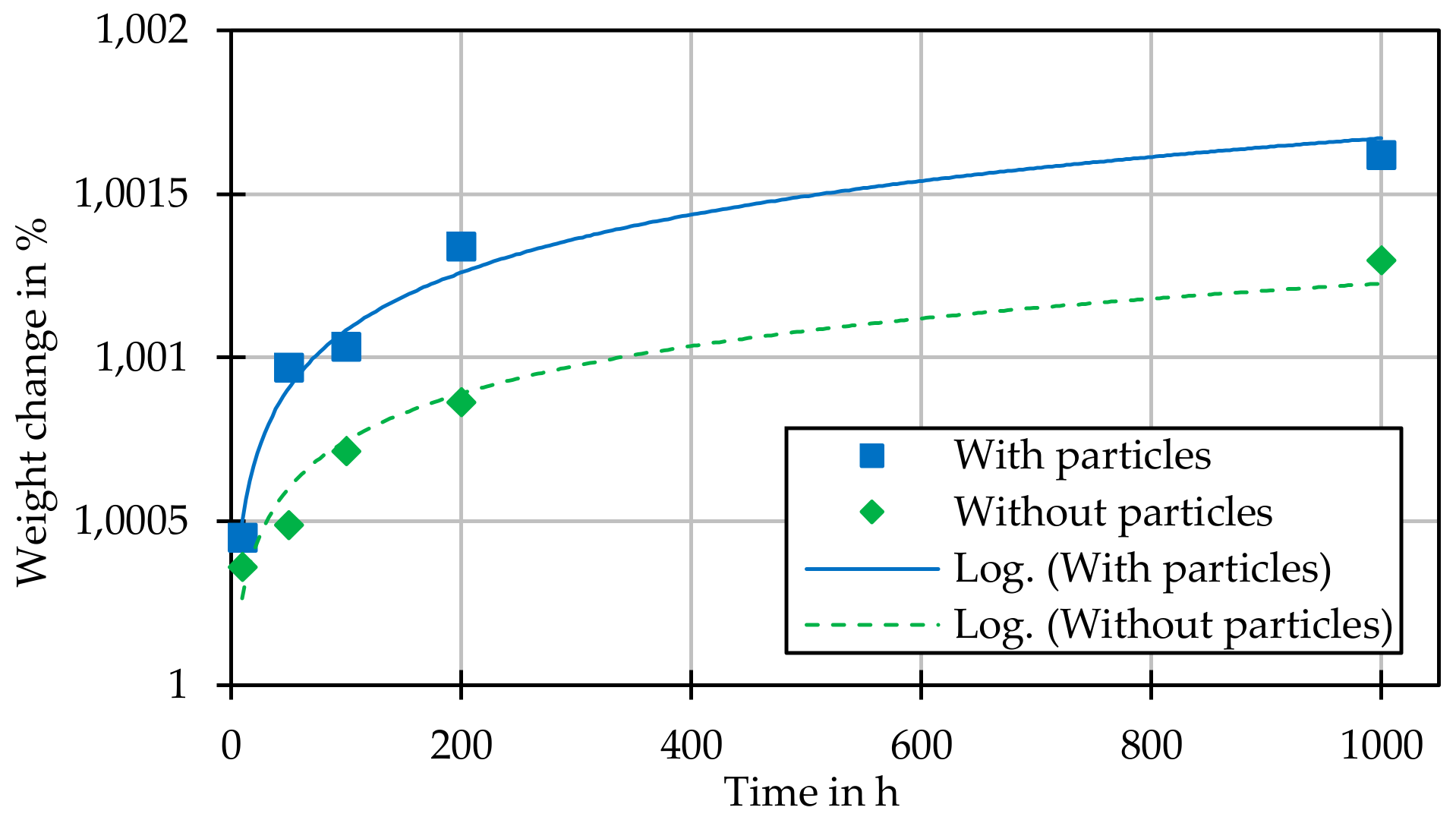
In each individual test, the change in weight of the sample and the change in the geometry of the individual particles (diameter and height) are determined.
After loading and insertion of the sample holder, the inner volume is evacuated three times to 19 Pa and rinsed with helium 5.0. At the beginning of the heating phase, the internal pressure is increased to 1 MPa. The particle-laden substrates are heated to the target temperatures at a rate of 8 k/min. After reaching the target temperature, the internal pressure is finally increased to 6 MPa. This prevents any pressure increases during heating because of gas expansions above the filling pressure limit of the system.
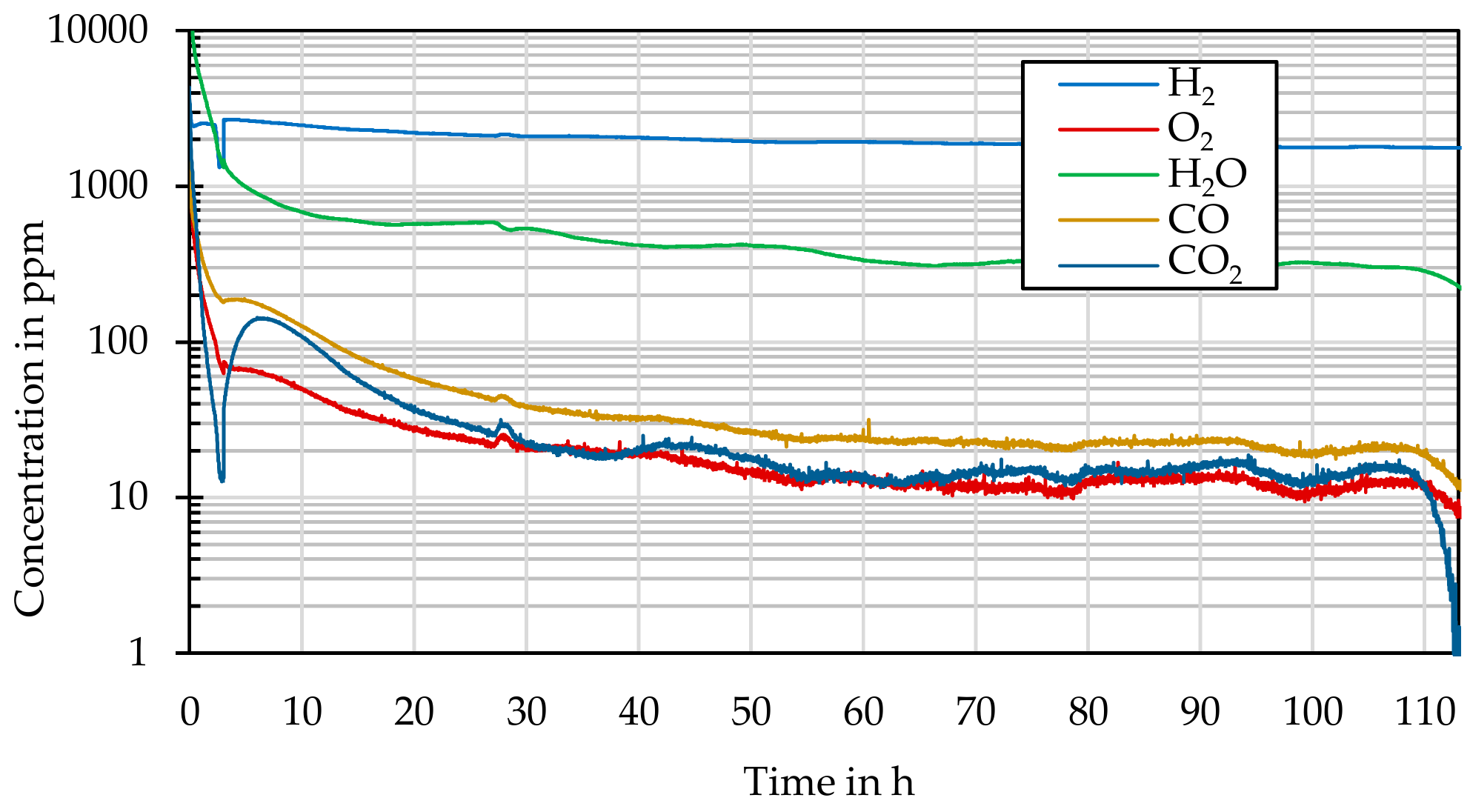
The experiments presented were carried out in a stable helium atmosphere (750 °C, 6 MPa) over periods of 10, 20, 50, 100, 200, 500, and 1000 h. A three-fold test repetition per test time and sample material was chosen in order to statistically confirm the statements.
With the help of the experimental data obtained here, thermally driven annealing processes such as surface carbonization or nitriding, oxidation, and changes in adhesive force can be demonstrated. Thus, the influence of a given process on the change in single particles, up to agglomerated particle accumulations, can be described in detail. In addition to the determination of the induced reaction mechanisms and speeds, the generated data have been made available for the development of scalable HTR-CFD codes and their validation.
3.3. Measurement Techniques
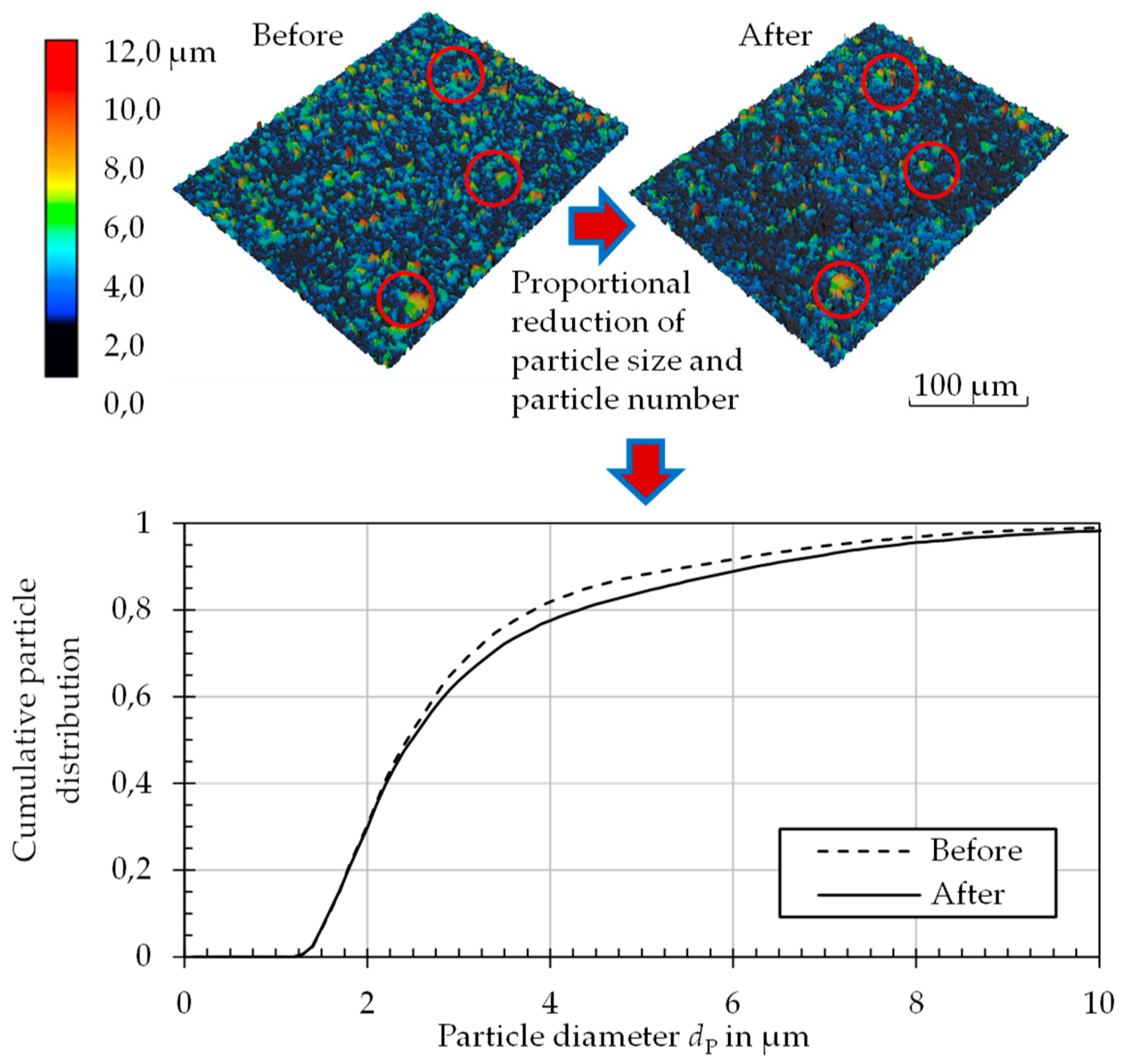
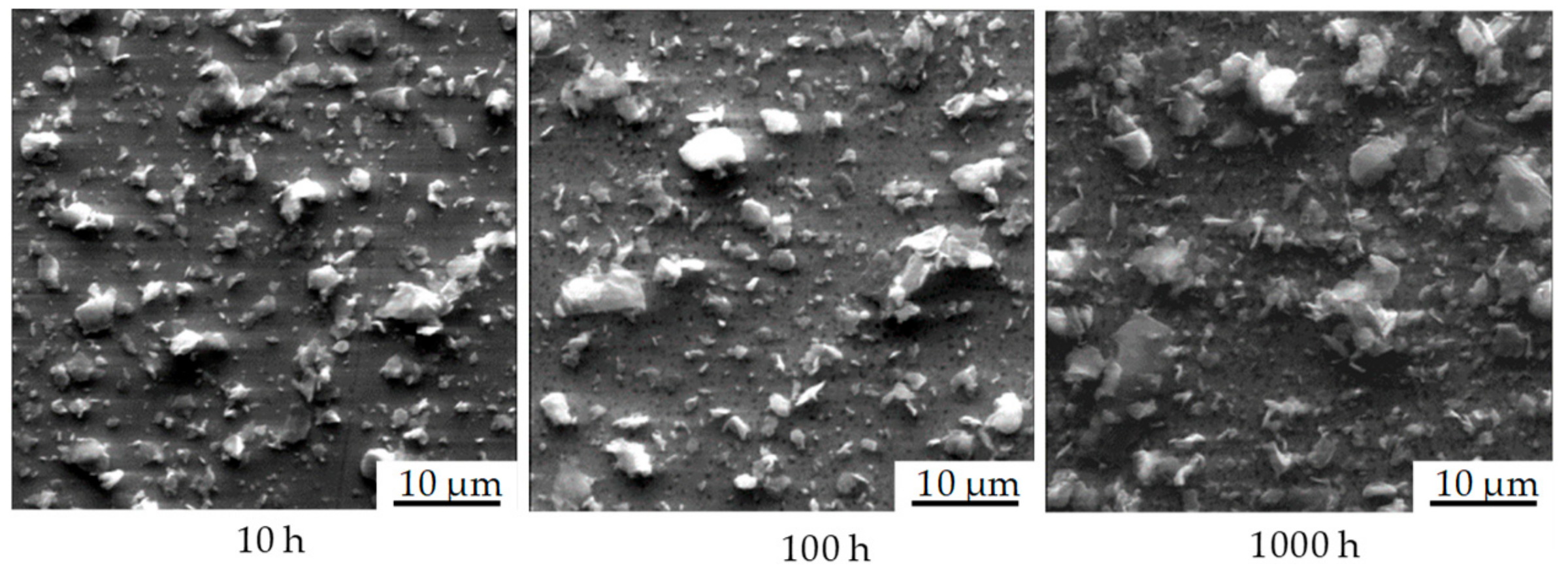
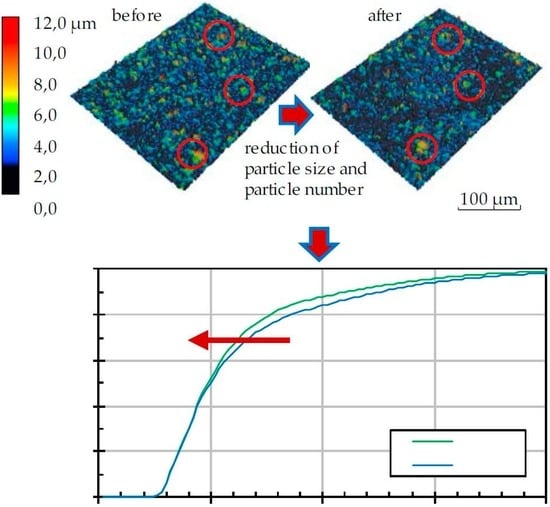
The geometric change in the particle morphology was determined by laser scanning microscopy (Keyence VK9300, Osaka, Japan). For this purpose, a height profile of each particle-laden surface was created before and after thermal annealing, and both were compared with one another; exact sample positioning makes it possible to find and measure the same particle before and after thermal annealing.
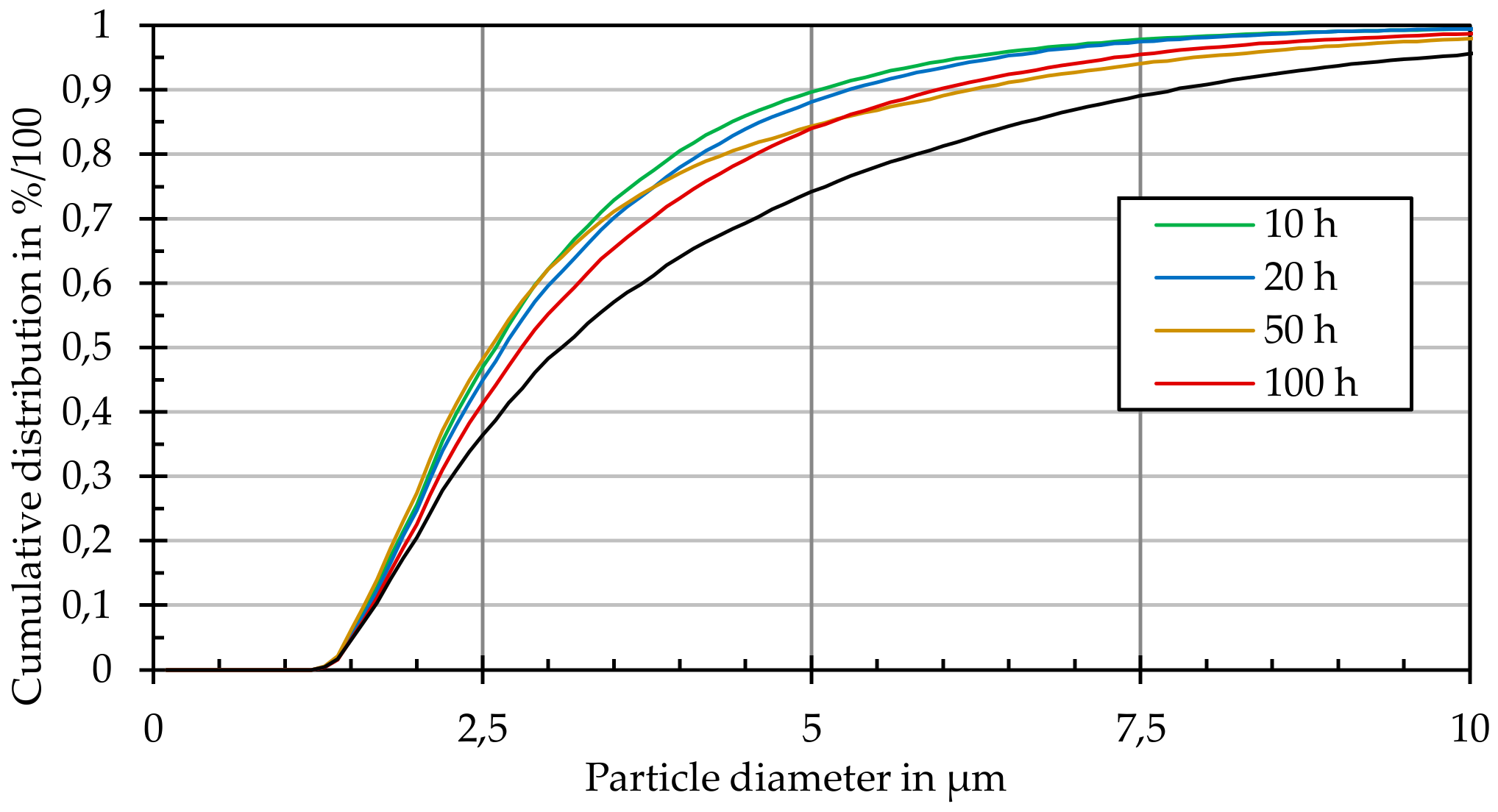
Figure 5 shows this process. For each sample, 400 individual profiles with dimensions of 283 × 213 µm
2 were recorded. By extrapolating these results over the entire sample area, statements could be made about the entire particle distribution within a given sample. With a maximum of 400 detected particles per profile, the geometric data of up to 100,000 particles were available for the generation of the cumulative frequency distribution of particle size. Thus, very detailed conclusions on particle size changes could be made.
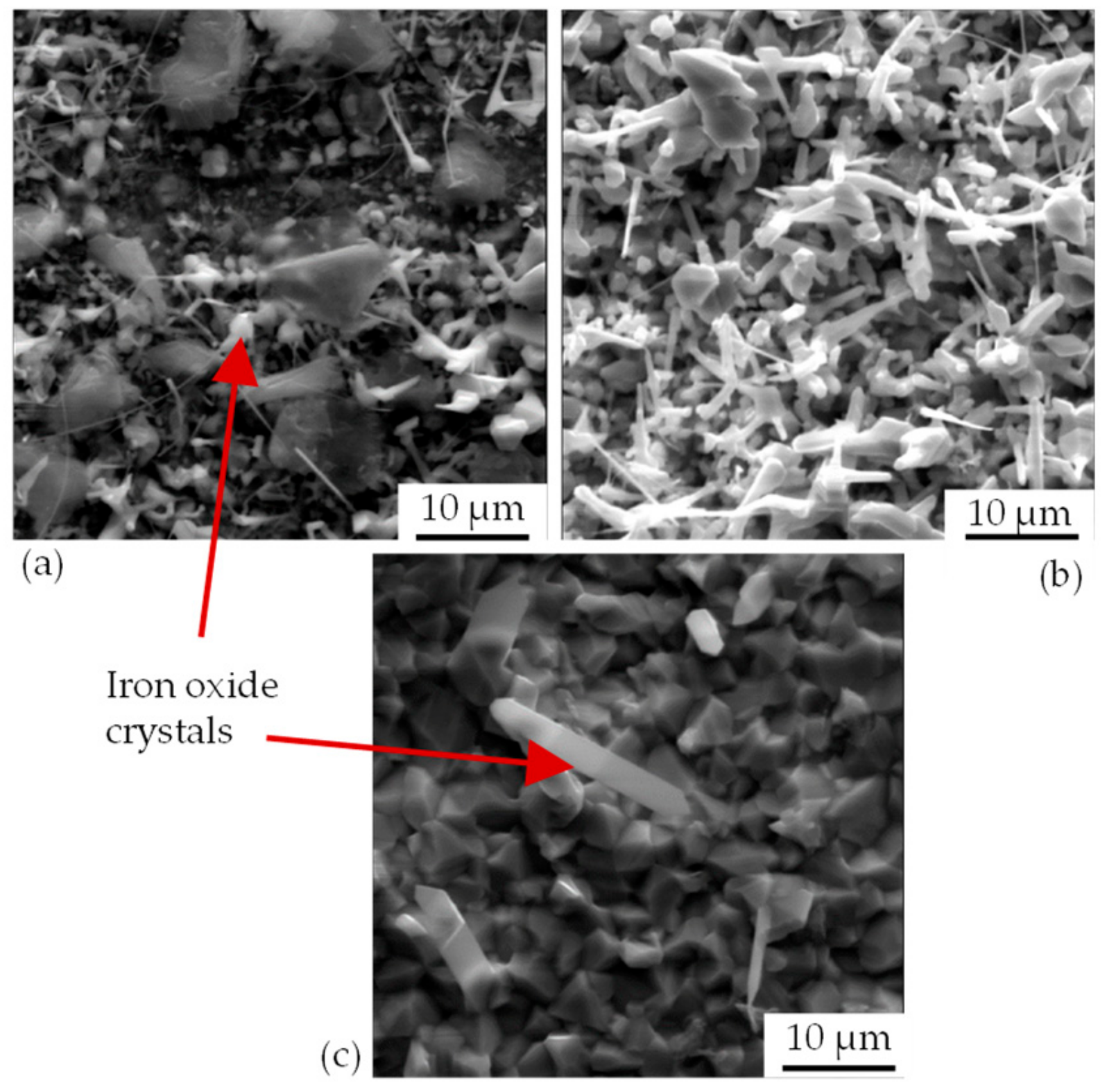
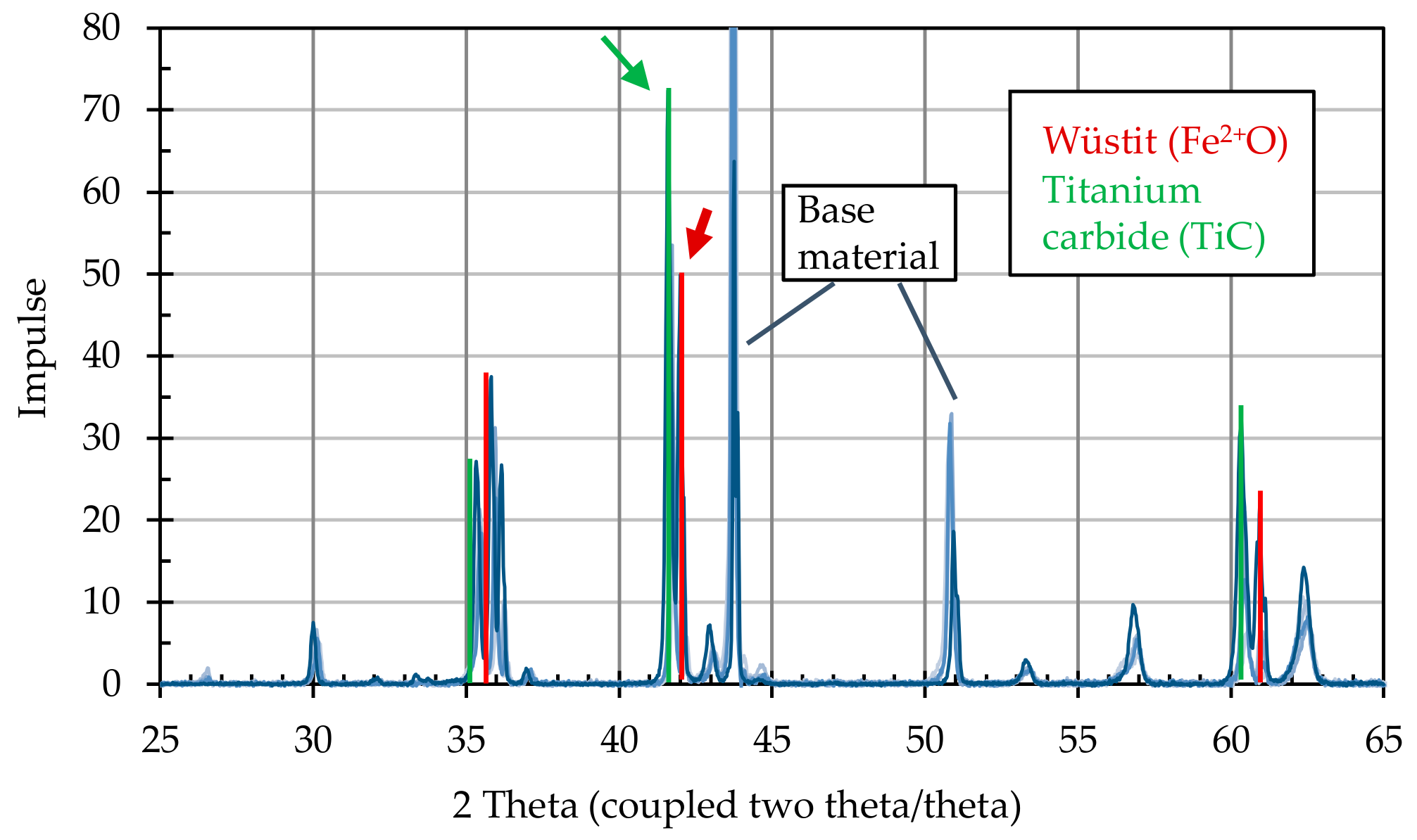
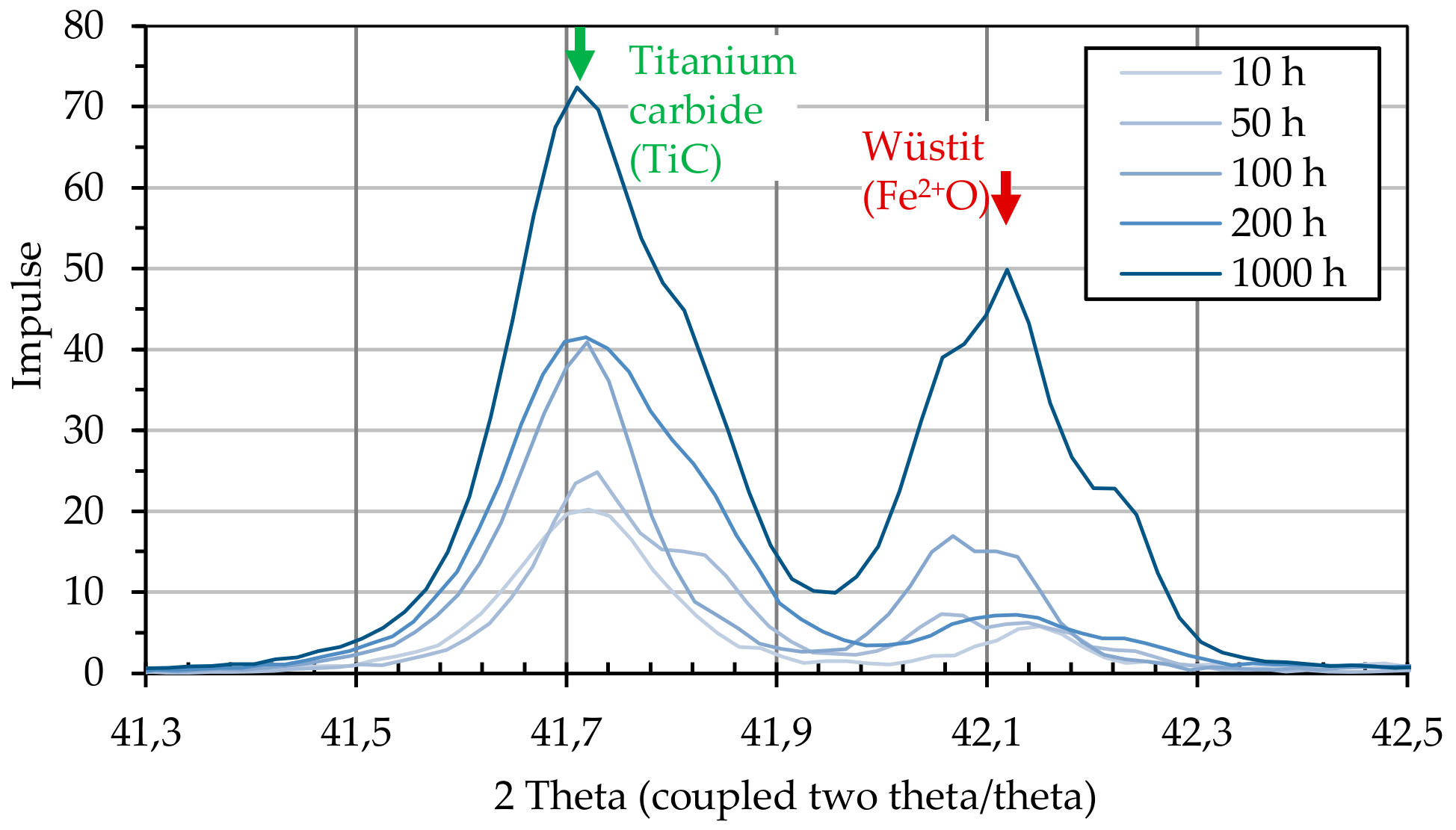
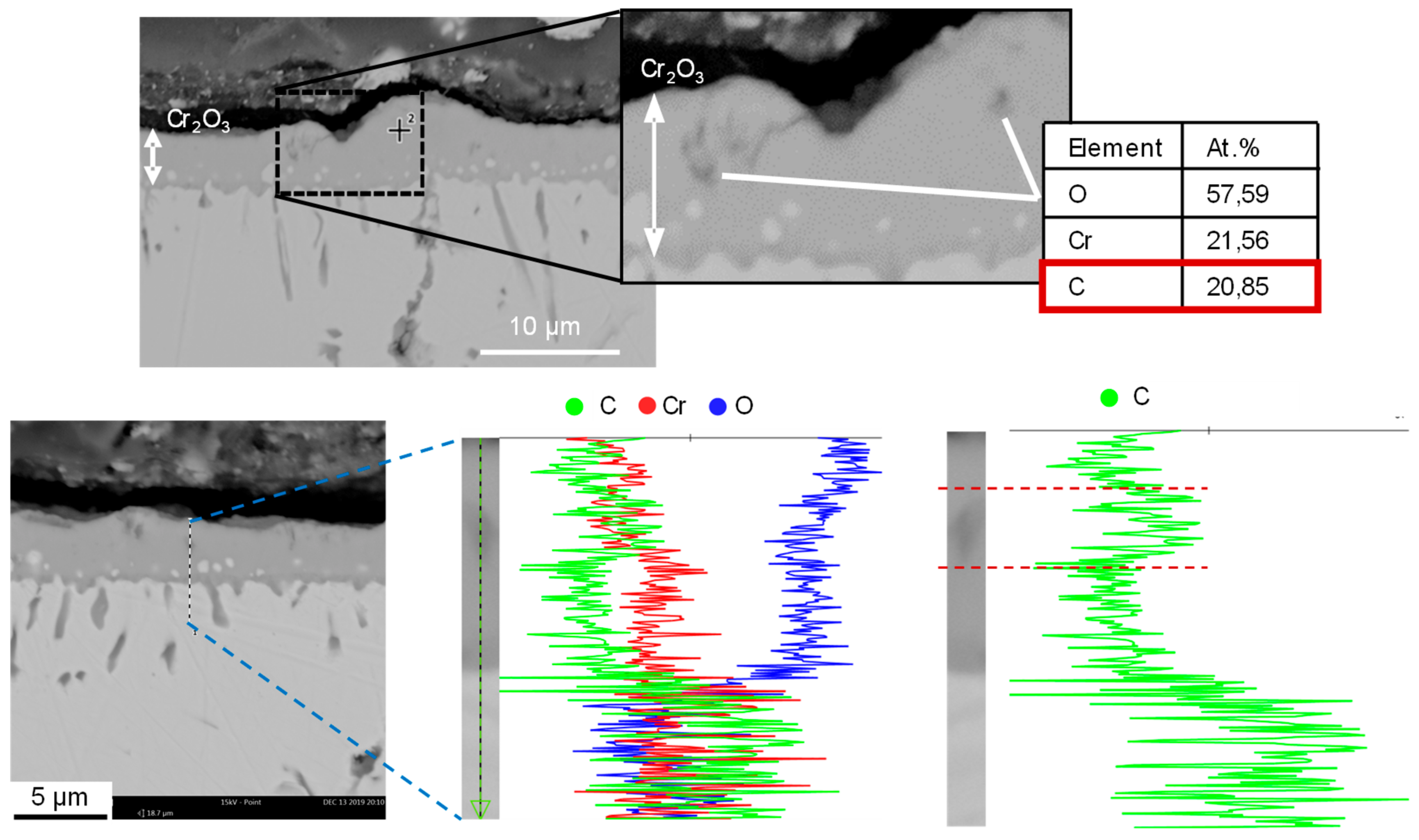
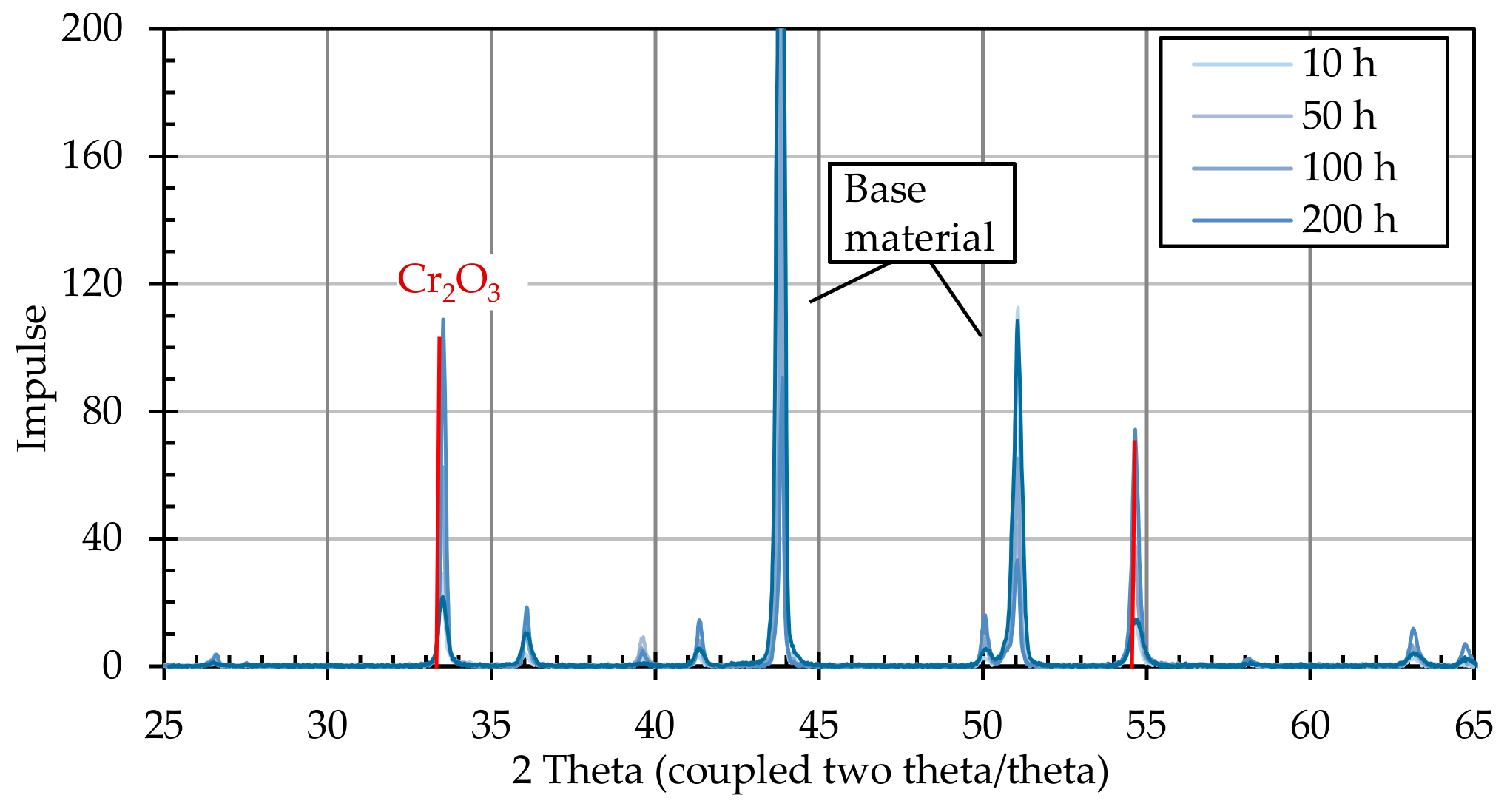
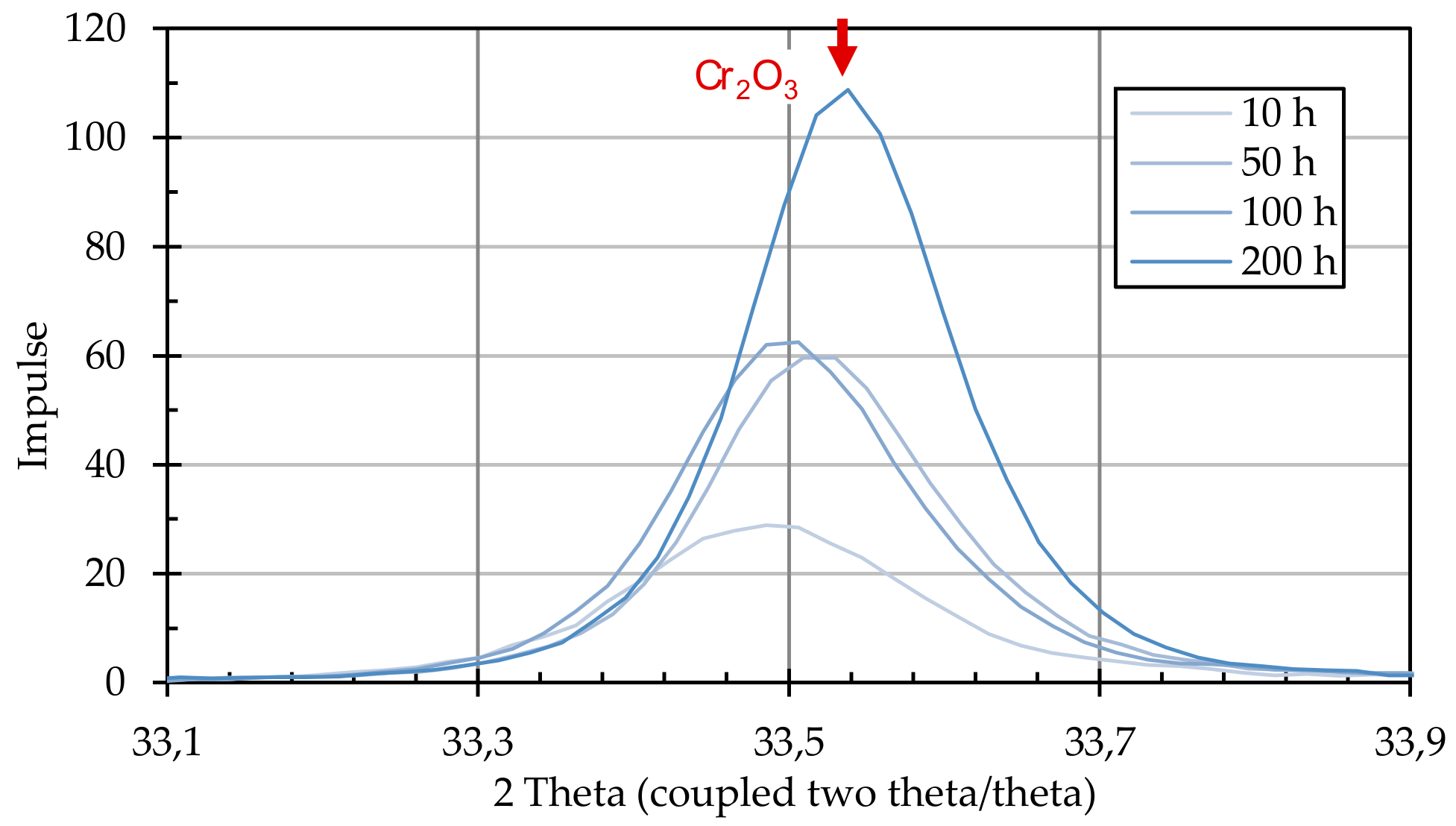
After the annealing process, the particle-laden substrates were examined for the formation of crystalline structures using X-ray diffractometric phase analysis (XRD, D8 Advance A25 Eco System, Bruker Corporation, Billerica, MA, USA). The changes induced by the presence of graphite particles were detected by comparing particle-loaded and non-loaded substrates.
In order to describe the chemical processes on the substrate surface during thermal treatment, substrates were analyzed by means of scanning electron microscopy (SEM) and energy-dispersive element analysis (EDX). The SEM-EDX system used was Phenom Pro (LOT-QuantumDesign, Les Ulis, France). In addition, segments of substrates were embedded in epoxy resin to produce the specimens. Subsequent SEM and EDX analyses were used to investigate the surface-related material changes in cross section.
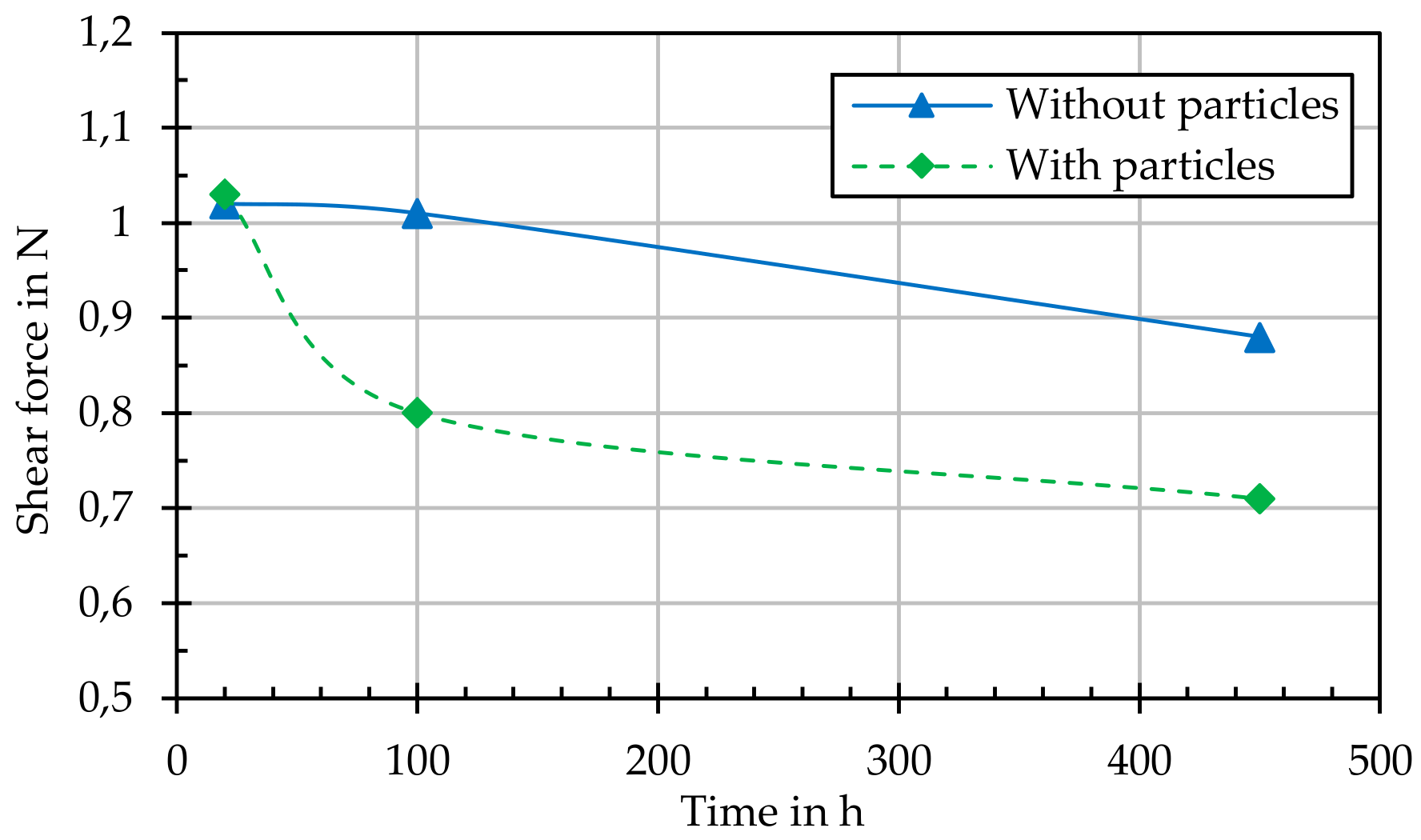
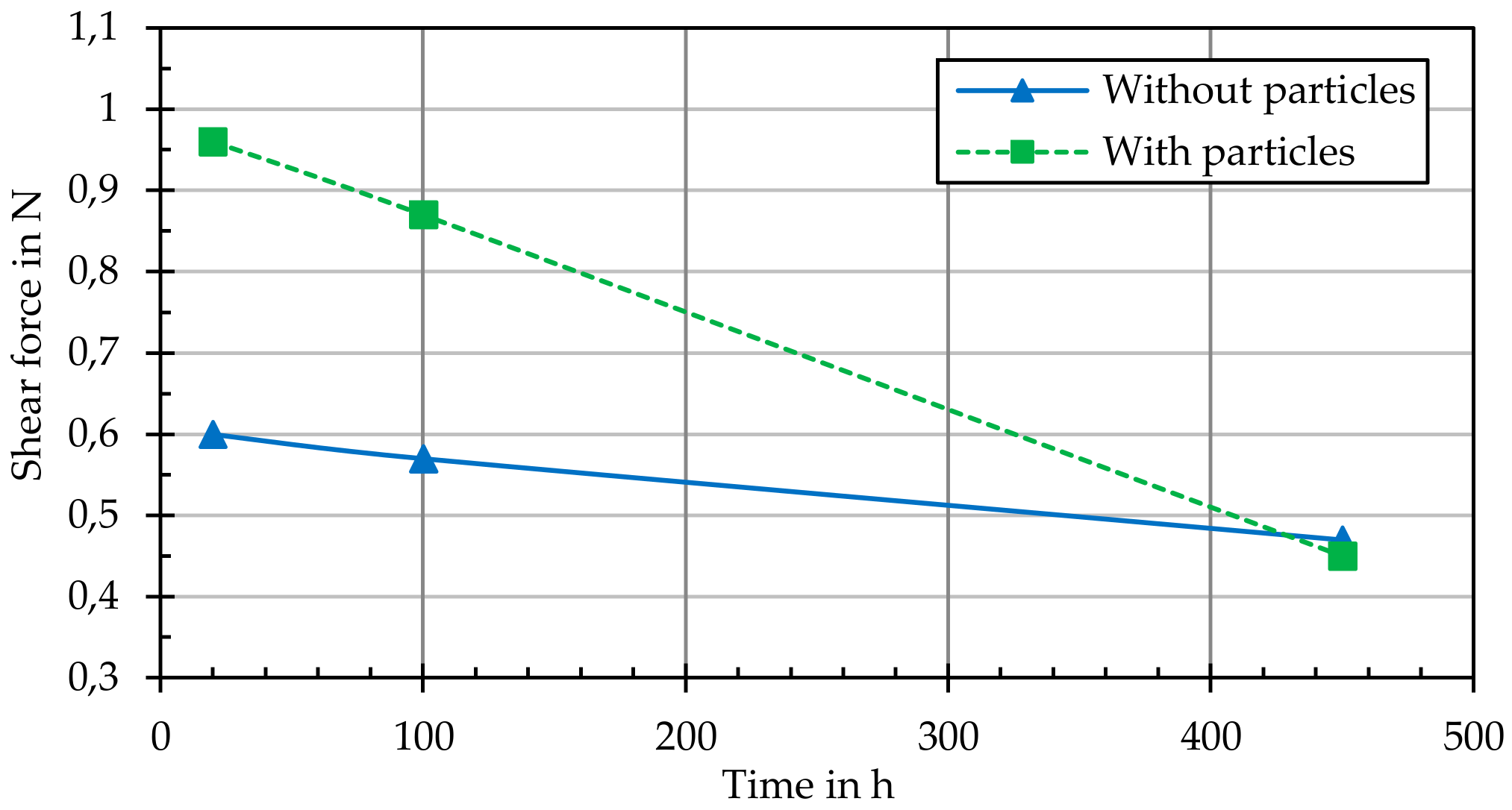
In addition, the adhesive strength of the grown layers or structures on the thermally annealed samples (with and without particle loading) was determined by means of a scratch test (performed according to DIN EN ISO 1071-3). With one measuring or scribing tip (tip radius 200 µm), five scribe marks with an increasing load were introduced to each sample and the critical force at which the grown layer detached from the substrate was determined.
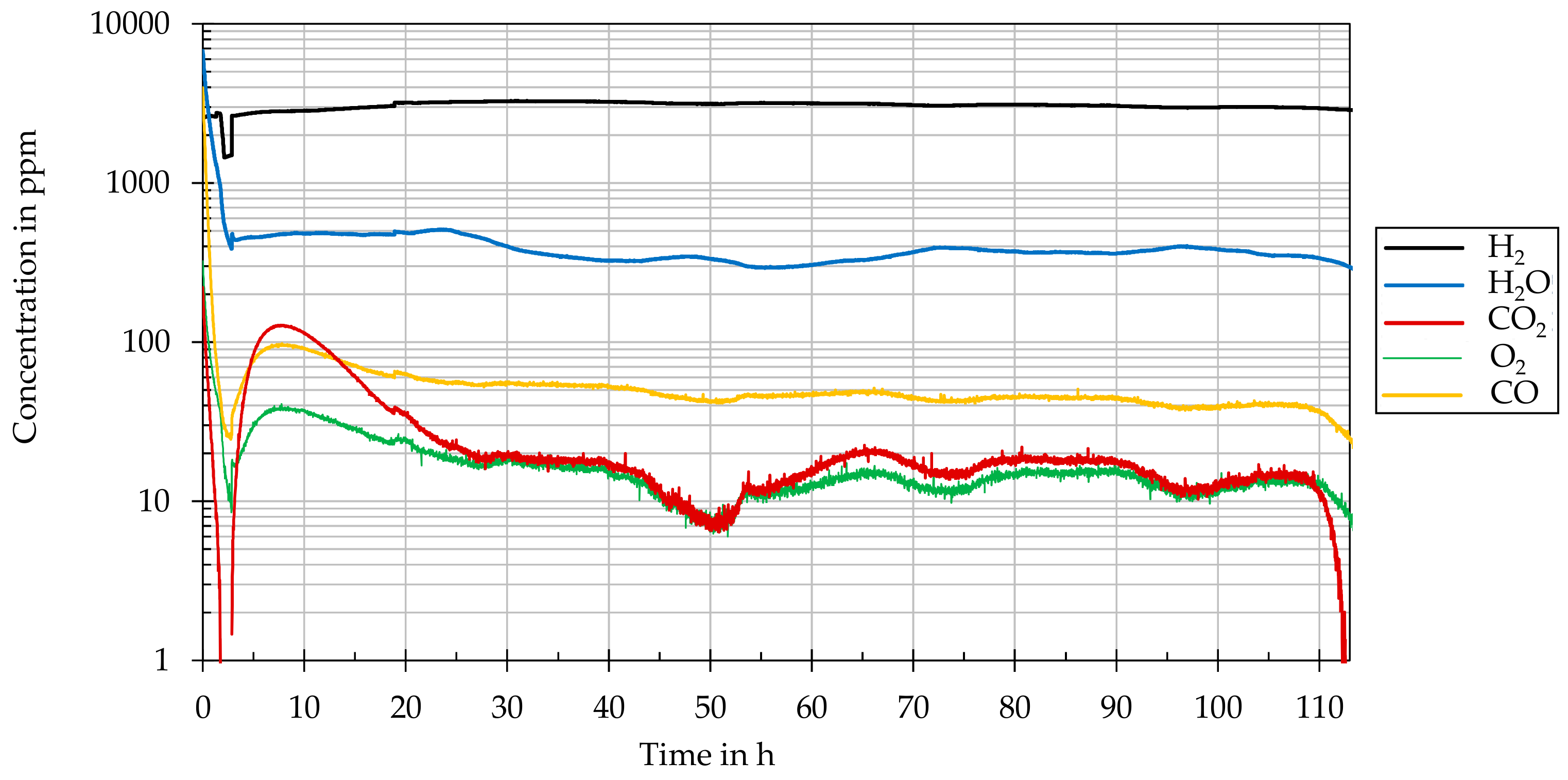
A mass spectrometer (GSD 301 O2 C, Pfeiffer Vacuum, Asslar, Germany) was used to characterize the gaseous reaction products or compounds that formed during thermal annealing as a function of time, temperature, and concentration. The concentrations of H, H2O, CO, CO2, and O2 were determined with a resolution in the single-digit part per million (ppm) range.