Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review
Abstract
1. Introduction
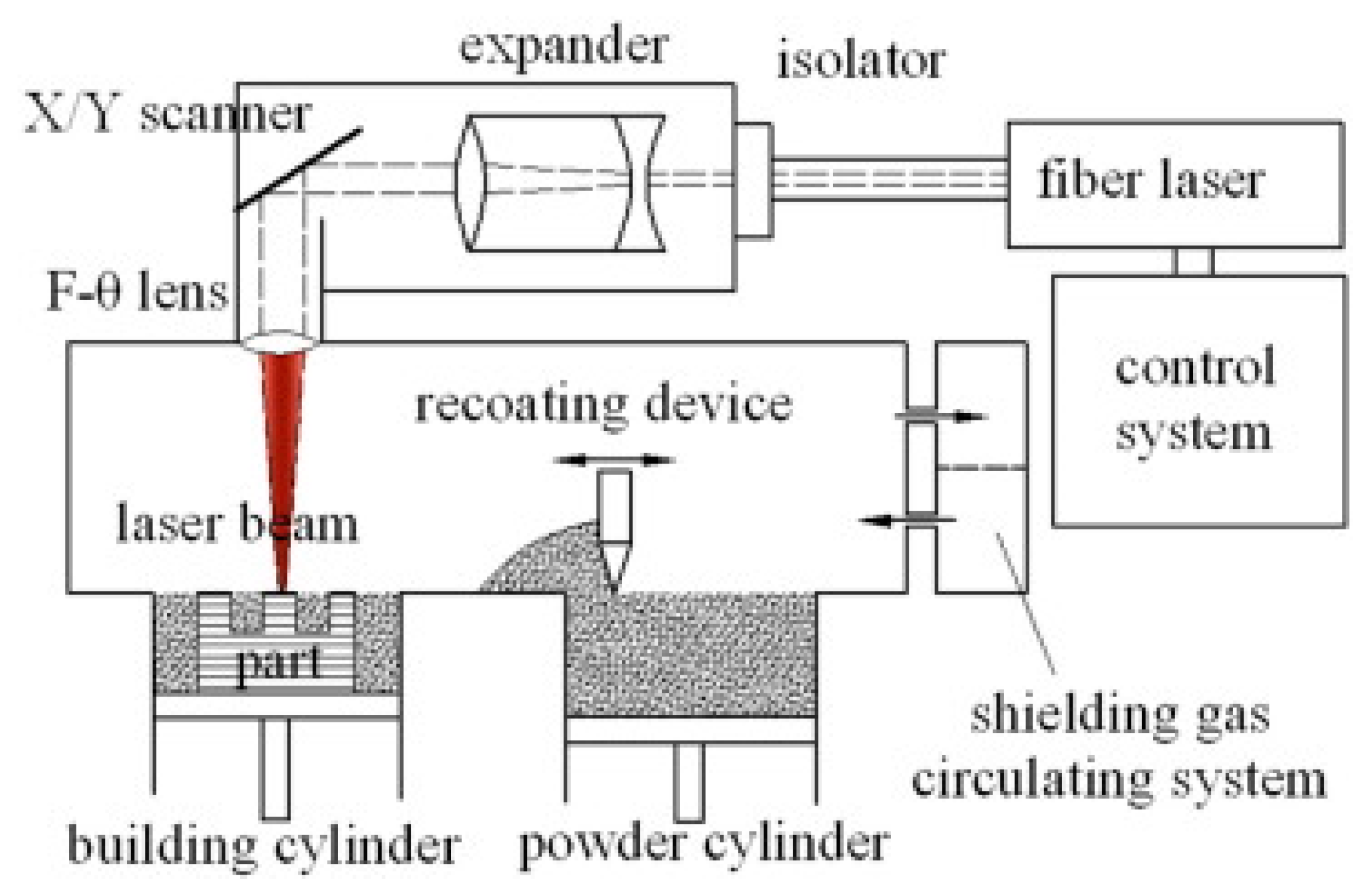
2. Microstructure of Al Alloys after SLM
2.1. In as-Built State
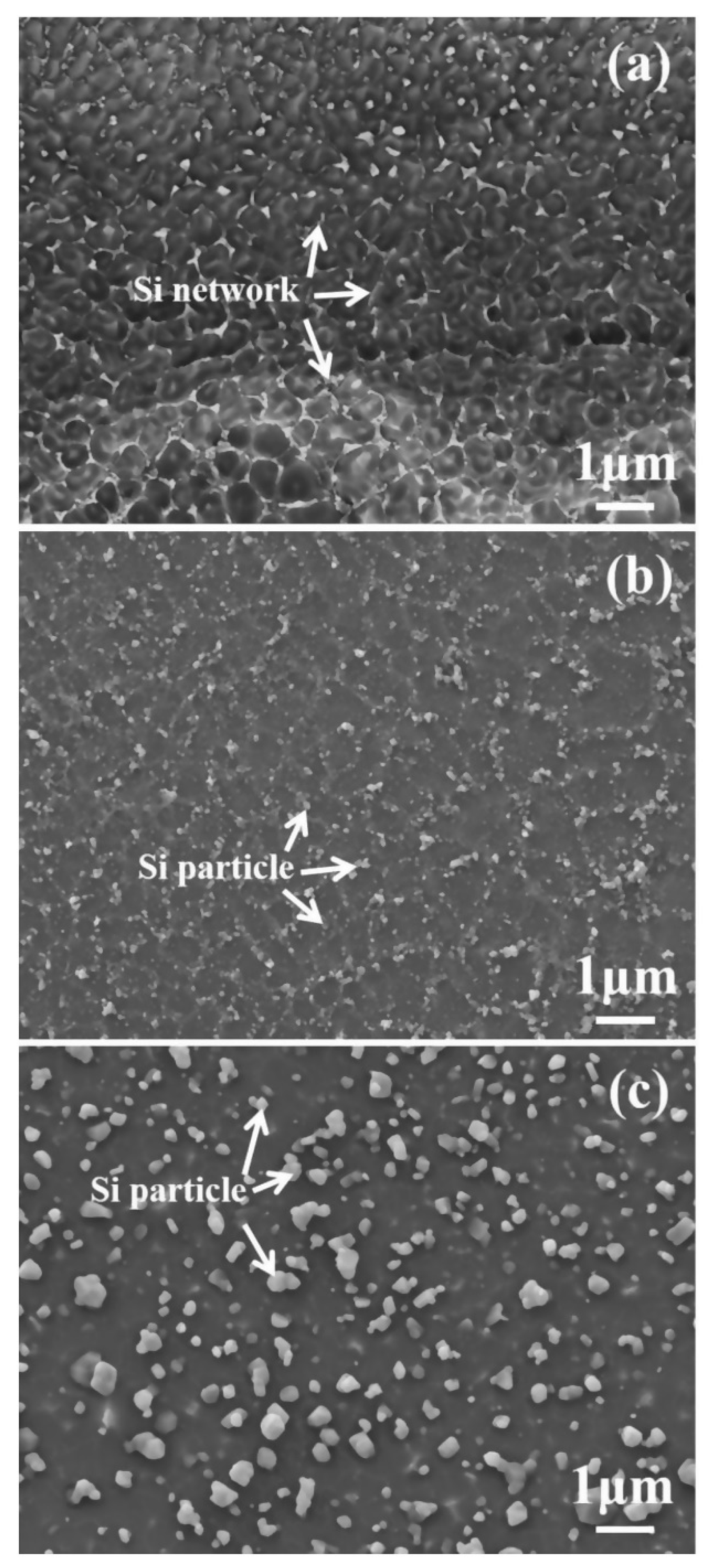
2.2. In Heat Treated State
3. Corrosion Behavior in as-SLMed State
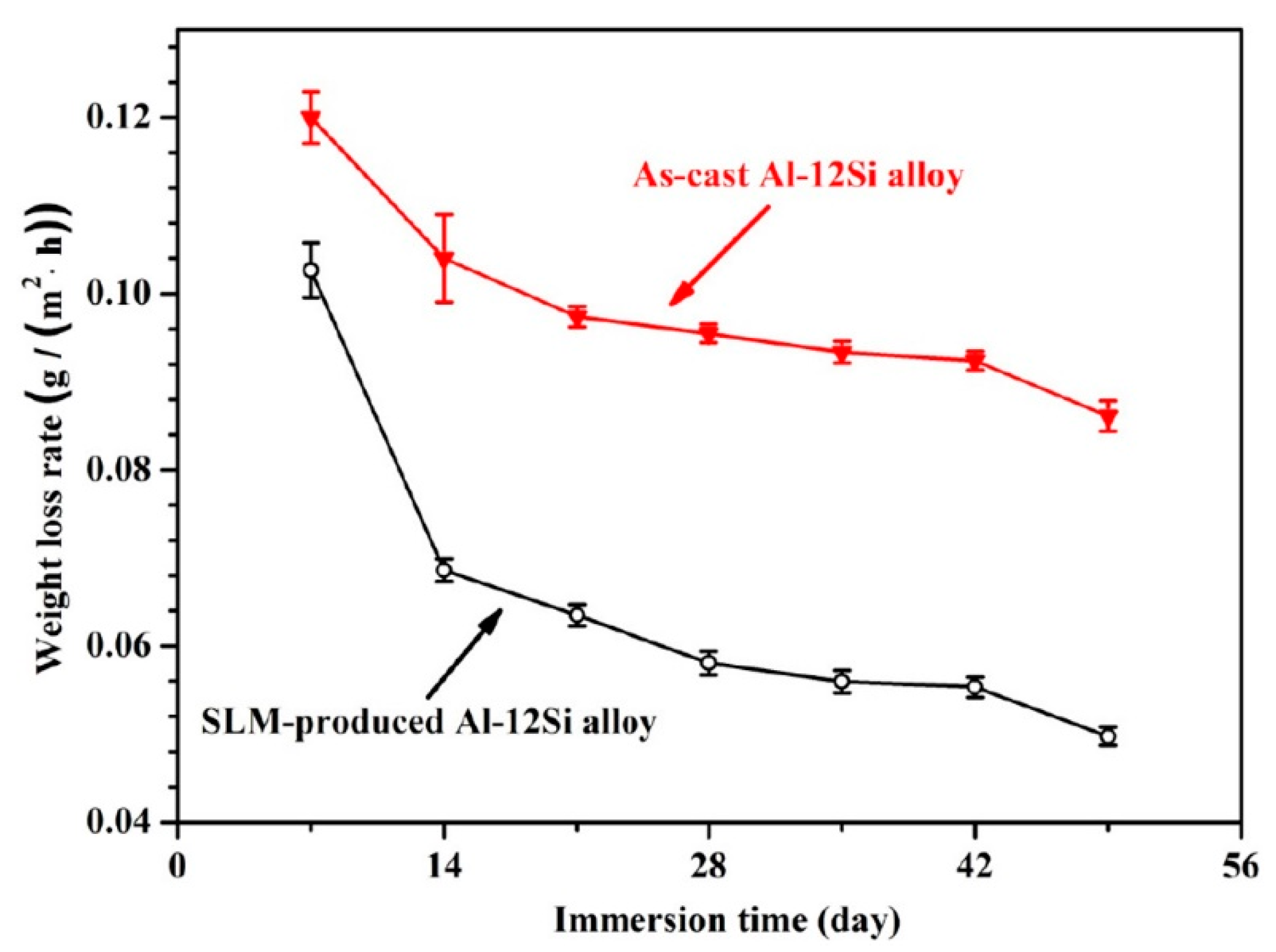
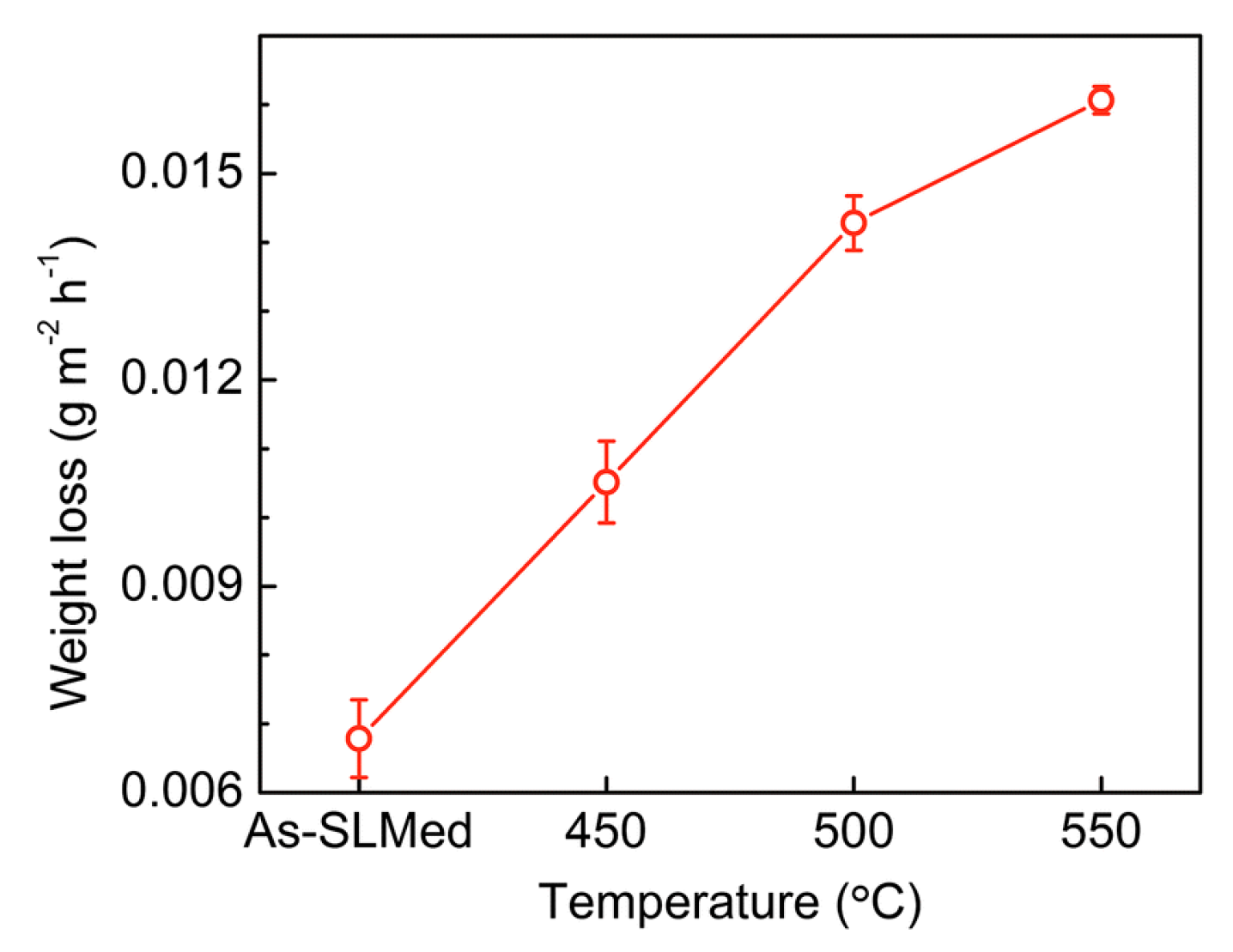
3.1. Corrosion Rate (Weight Loss Tests)
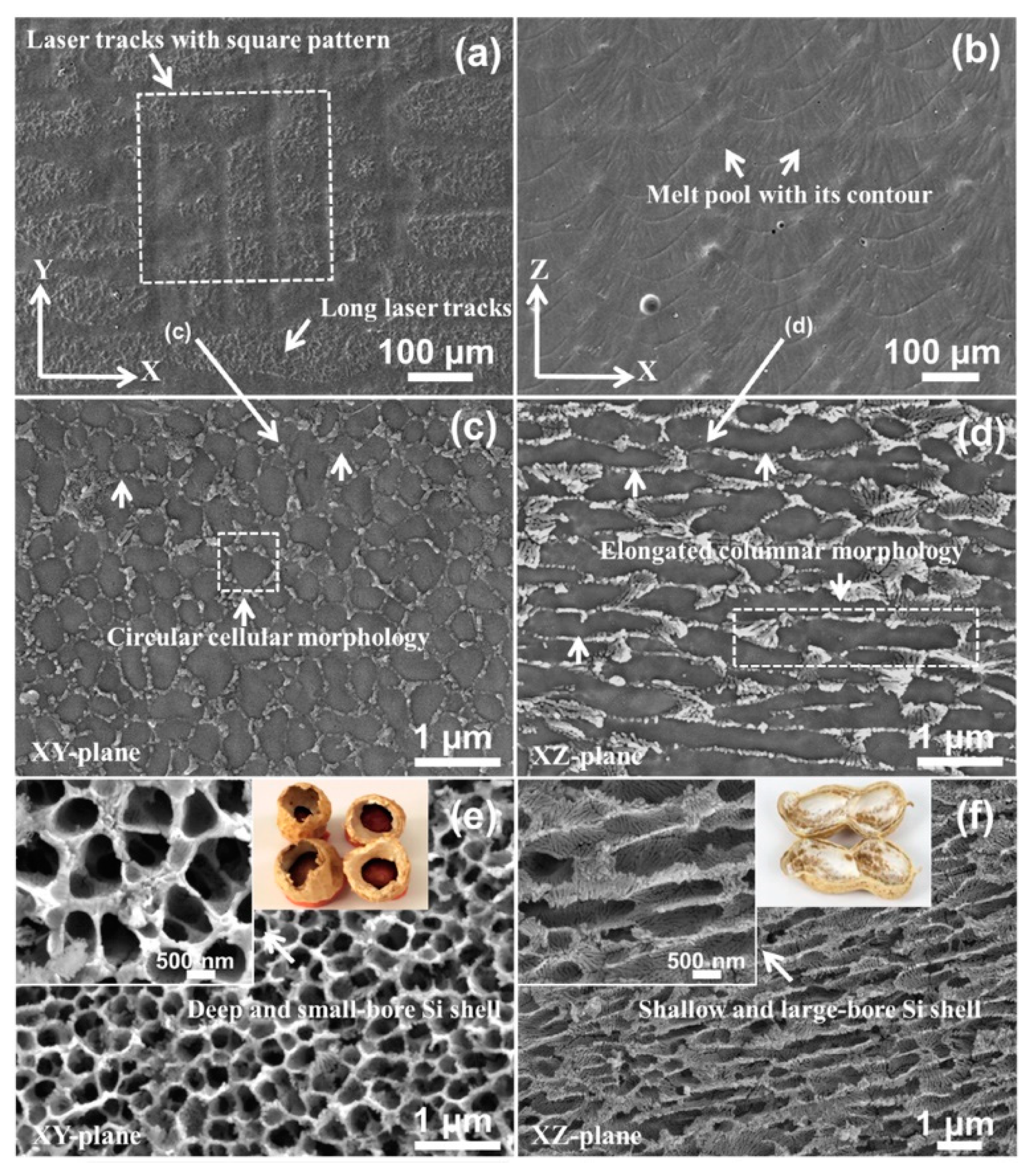
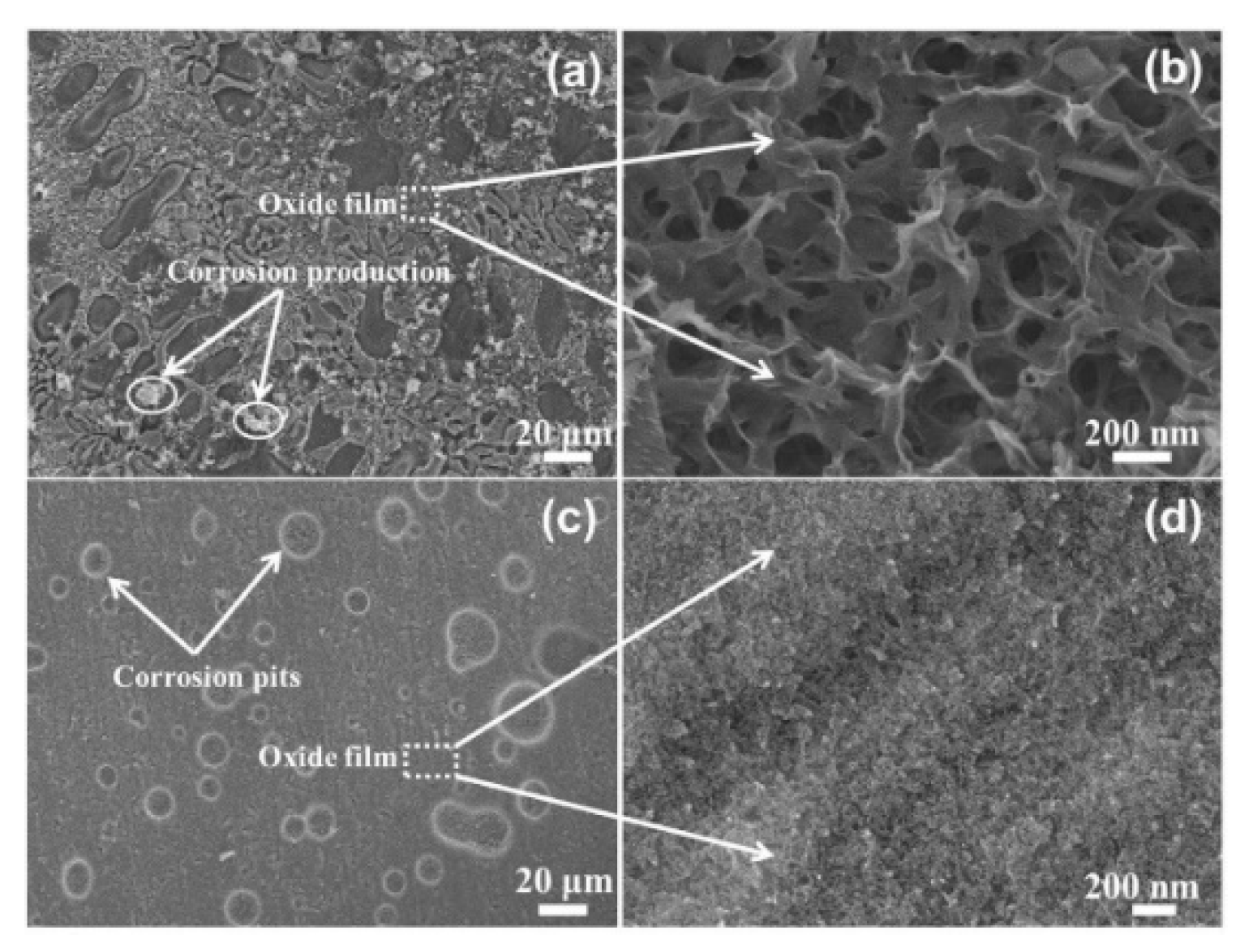
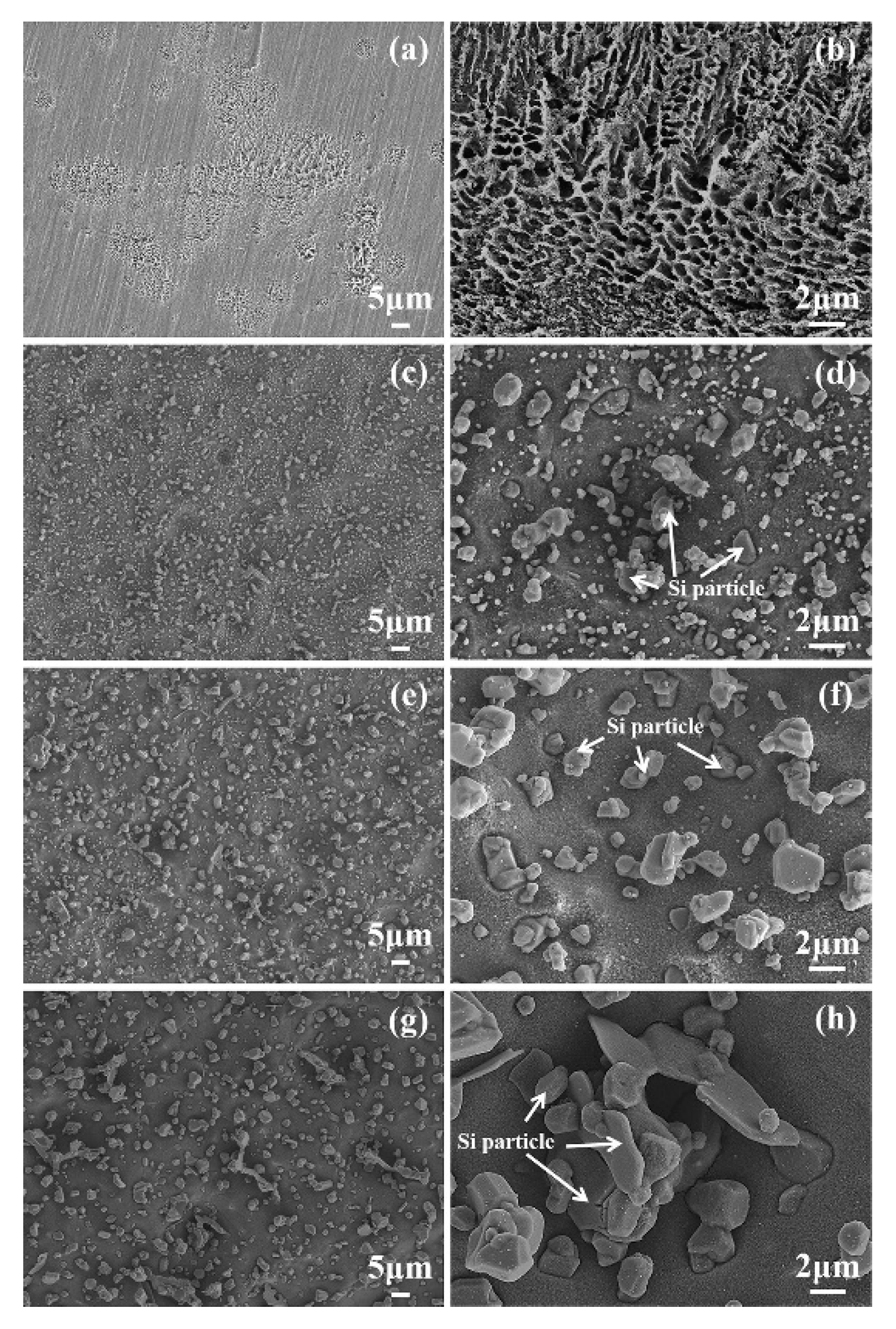
3.2. Corrosion Morphology
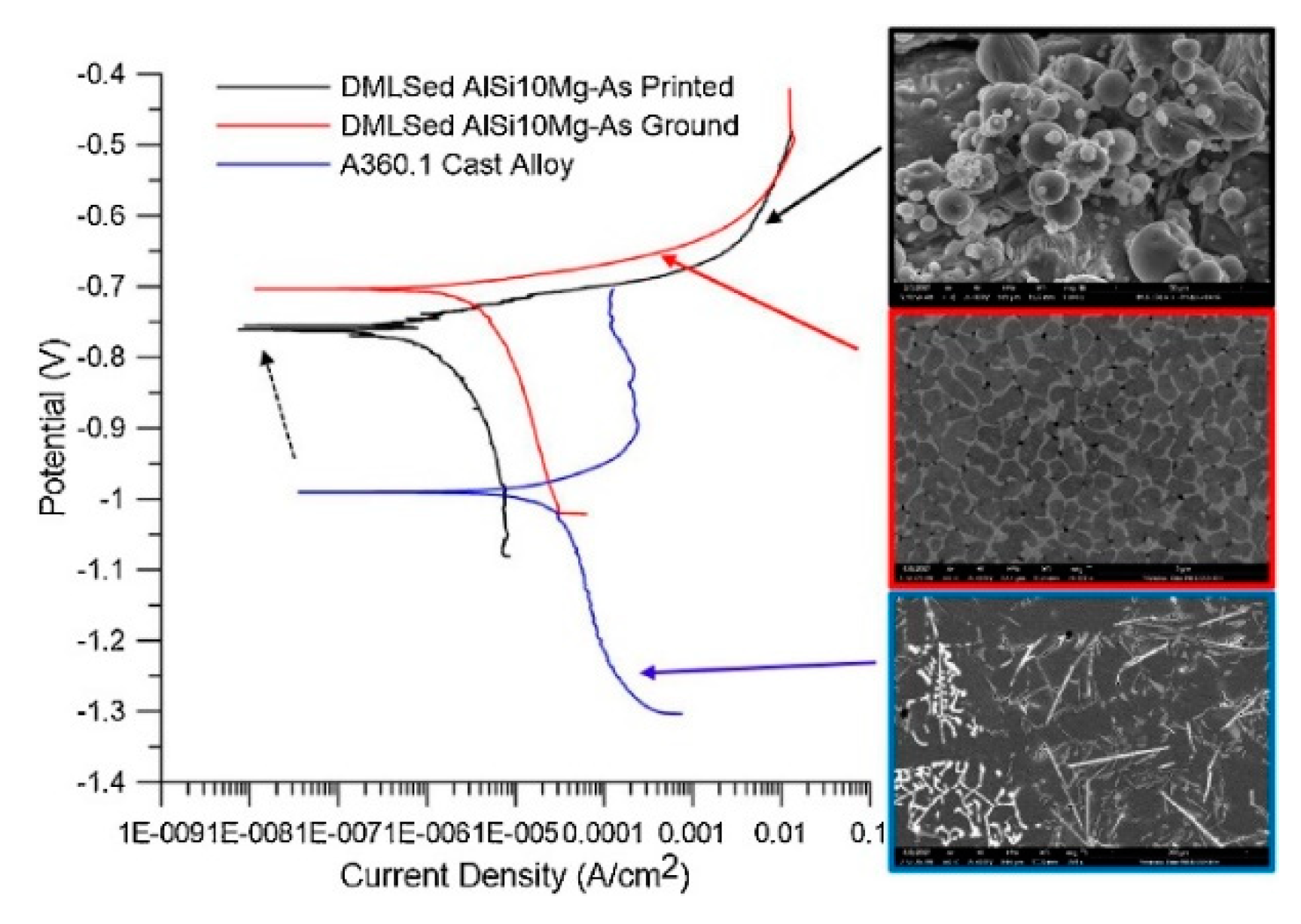
3.3. Electrochemical Properties
3.3.1. Polarization Curves
3.3.2. Electrochemical Impedance Spectrum
4. Corrosion Behavior after Post Heat Treatments
4.1. Corrosion Rate
4.2. Corrosion Morphology
4.3. Electrochemical Properties
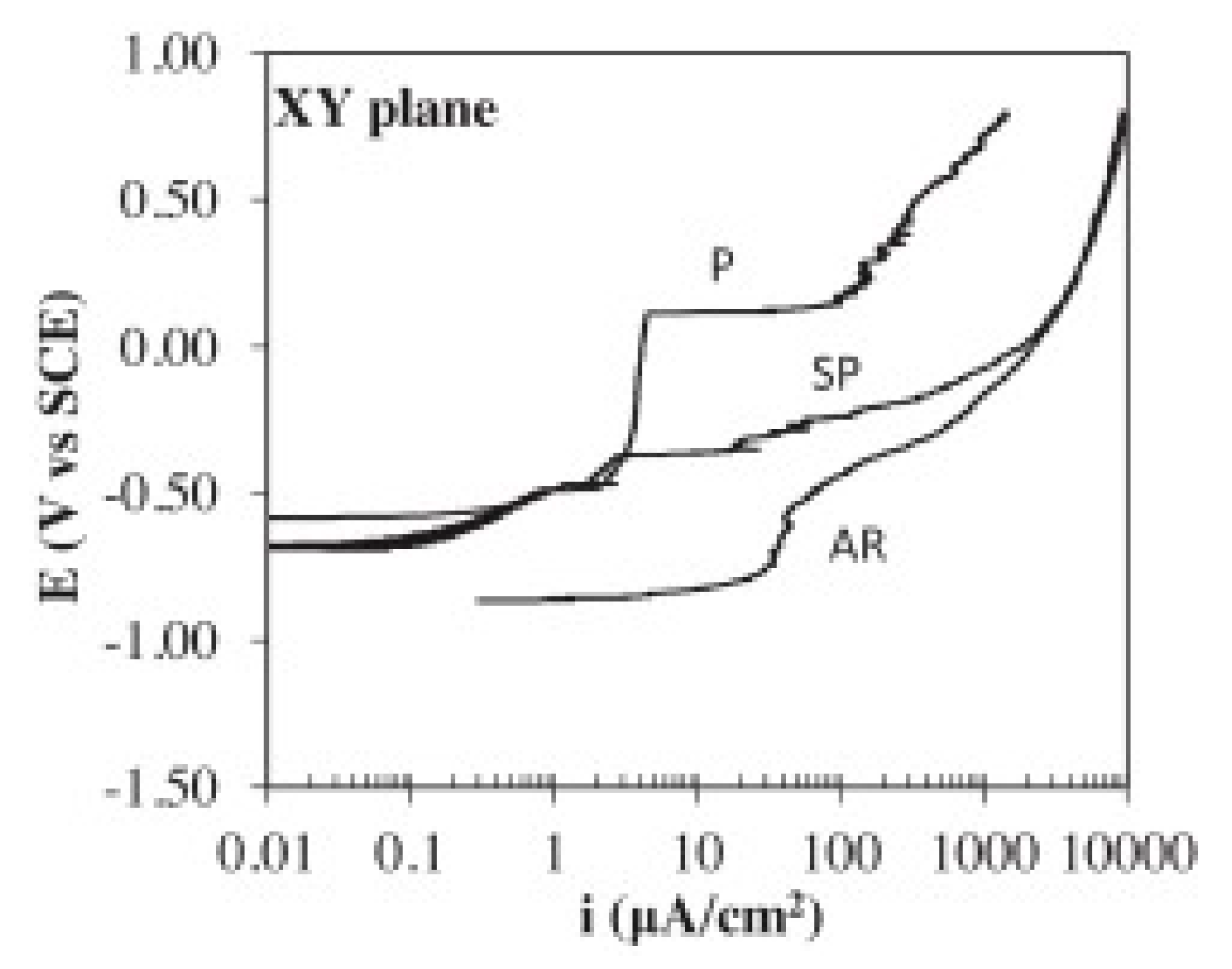
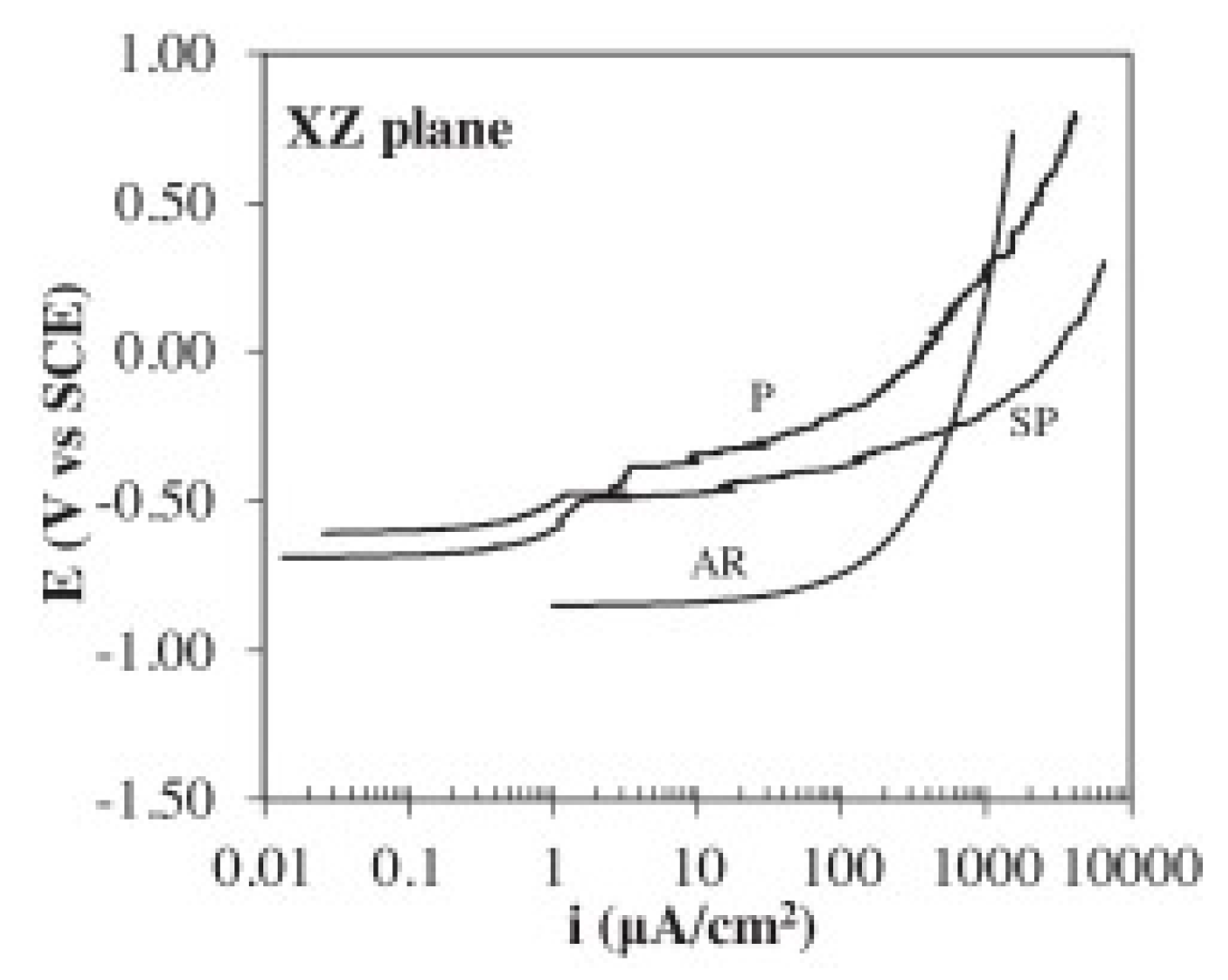
4.3.1. Polarization Curves
4.3.2. Electrochemical Impedance Spectrum
5. Summary and Outlook
- (1)
- (2)
- (3)
- (4)
- Post heat treatment parameters (temperature and time) affect the microstructure (grain size, Si distribution, etc.) of SLMed Al alloys, consequently affect the corrosion behaviors. Larger Al grains and broken Si shells results in the acceleration of the corrosion reaction, and the isolated Si particles hinder the compact oxide film formation on aluminum surfaces [63,65,66].
- (5)
- Due to the distinctly different microstructures in the two directions, the XZ-plane (parallel to the building direction) exhibits better corrosion resistance than the XY-plane [74]. Samples with as-printed surface show the worse corrosion resistance and the lower corrosion fatigue cycles, and this is owing to the rougher surface obtained from the direct print compared to the polished surface [64].
- (6)
- It is reported that proper post heat treatments could eliminate the residual stress existed in the SLM aluminum alloys, but the corrosion resistance decreases with increasing temperature of post heat treatment. Therefore, further studies on post heat treatments of SLMed Al alloys and other stress-relief techniques are needed to reduce the residual stress and meanwhile keep the corrosion resistance in a high level [67].
- (7)
- To further study the corrosion behaviors of SLMed alloy in corrosive atmosphere, it is inevitable to conduct the salt spray test in the future.
- (8)
- Other corrosion behavior aspects, which have been studied for conventional aluminum alloys, such as, intergranular corrosion and exfoliation corrosion, also need to be studied for SLMed aluminum alloys to gain more insights into the corrosion mechanism of SLMed Al alloys.
- (9)
- The corrosion behaviors of more types of Al alloys, including conventional Al alloys (1xxx-8xxx) and new Al alloys specially developed for SLM (for example, Scalmalloy), also need to be investigated for their potential future industrial applications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhang, S.S.; Zhu, H.H.; Wang, G.Q.; Zeng, X.Y. Investigation on the angular accuracy of selective laser melting. Int. J. Adv. Manuf. Techcol. 2019, 104, 3147–3153. [Google Scholar] [CrossRef]
- Rakesh, C.S.; Raja, A.; Nadig, P.; Jayaganthan, R.; Vasa, N.J. Influence of working environment and built orientation on the tensile properties of selective laser melted AlSi10Mg alloy. Mater. Sci. Eng. A 2019, 750, 141–151. [Google Scholar] [CrossRef]
- Mueller, M.; Riede, M.; Eberle, S.; Reutlinger, A.; Brandao, A.D.; Pambaguian, L.; Seidel, A.; Lopez, E.; Brueckner, F.; Beyer, E.; et al. Microstructural, mechanical, and thermo-physical characterization of hypereutectic AlSi40 fabricated by selective laser melting. J. Laser Appl. 2019, 31, 022321. [Google Scholar] [CrossRef]
- Li, Z.; Kuai, Z.; Bai, P.; Nie, Y.; Fu, G.; Liu, W.; Yang, S. Microstructure and Tensile Properties of AlSi10Mg Alloy Manufactured by Multi-Laser Beam Selective Laser Melting (SLM). Metals 2019, 9, 1337. [Google Scholar] [CrossRef]
- Zaharin, A.H.; Abdul Rani, M.A.; Azam, I.F.; Ginta, L.T.; Sallih, N.; Ahmad, A.; Yunus, A.N.; Zulkifli, Z.T. Effect of Unit Cell Type and Pore Size on Porosity and Mechanical Behavior of Additively Manufactured Ti6Al4V Scaffolds. Materials 2018, 11, 2402. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydłowski, J.K.; Święszkowski, W. Laser and Electron Beam Additive Manufacturing Methods of Fabricating Titanium Bone Implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Strakosova, A.; Kubásek, J.; Michalcová, A.; Průša, F.; Vojtěch, D.; Dvorský, D. High Strength X3NiCoMoTi 18-9-5 Maraging Steel Prepared by Selective Laser Melting from Atomized Powder. Materials 2019, 12, 4174. [Google Scholar] [CrossRef]
- Mugwagwa, L.; Yadroitsev, I.; Matope, S. Effect of Process Parameters on Residual Stresses, Distortions, and Porosity in Selective Laser Melting of Maraging Steel 300. Metals 2019, 9, 1042. [Google Scholar] [CrossRef]
- Narvan, M.; Al-Rubaie, S.K.; Elbestawi, M. Process-Structure-Property Relationships of AISI H13 Tool Steel Processed with Selective Laser Melting. Materials 2019, 12, 2284. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, C.; Wang, D.; Liu, W.; Fang, X.; Wellmann, D.; Zhao, Y.; Tian, Y. A Review on Laser Powder Bed Fusion of Inconel 625 Nickel-Based Alloy. Appl. Sci. 2019, 10, 81. [Google Scholar] [CrossRef]
- Calandri, M.; Yin, S.; Aldwell, B.; Calignano, F.; Lupoi, R.; Ugues, D. Texture and Microstructural Features at Different Length Scales in Inconel 718 Produced by Selective Laser Melting. Materials 2019, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Dehghanghadikolaei, A.; Fotovvati, B.; Vosooghnia, A.; Emamian, S.S.; Gisario, A. The Potential of Additive Manufacturing in the Smart Factory Industrial 4.0: A Review. Appl. Sci. 2019, 9, 3865. [Google Scholar] [CrossRef]
- Manca, D.R.; Churyumov, A.Y.; Pozdniakov, A.V.; Prosviryakov, A.S.; Ryabov, D.K.; Krokhin, A.Y.; Korolev, V.A.; Daubarayte, D.K. Microstructure and Properties of Novel Heat Resistant Al-Ce-Cu Alloy for Additive Manufacturing. Met. Mater. Int. 2019, 25, 633–640. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T.; Ozaki, T.; Sugita, K.; Mizuno, M.; Araki, H. Microstructural formation and characterization mechanisms of selective laser melted Al-Si-Mg alloys with increasing magnesium content. Mater. Sci. Eng. A 2019, 754, 786–798. [Google Scholar] [CrossRef]
- Karolus, M.; Maszybrocka, J.; Stwora, A.; Skrabalak, G. Residual stresses of alsi10mg fabricated by selective laser melting (SLM). Arch. Metall. Mater. 2019, 64, 1011–1016. [Google Scholar] [CrossRef]
- Rahmani, R.; Rosenberg, M.; Ivask, A.; Kollo, L. Comparison of Mechanical and Antibacterial Properties of TiO2/Ag Ceramics and Ti6Al4V-TiO2/Ag Composite Materials Using Combined SLM-SPS Techniques. Metals 2019, 9, 874. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhang, H.; Zhu, H.H.; Xiao, Z.X.; Nie, X.J.; Zeng, X.Y. Microstructure, mechanical properties and strengthening mechanisms of AlCu5MnCdVA aluminum alloy fabricated by selective laser melting. Mater. Sci. Eng. A 2019, 759, 154–166. [Google Scholar] [CrossRef]
- Gu, H.Y.; Li, S.; Pavier, M.; Attallah, M.M.; Paraskevoulakos, C.; Shterenlikht, A. Fracture of three-dimensional lattices manufactured by selective laser melting. Int. J. Solids Struct. 2019, 180, 147–159. [Google Scholar] [CrossRef]
- Delahaye, J.; Tchuindjang, J.T.; Lecomte-Beckers, J.; Rigo, O.; Habraken, A.M.; Mertens, A. Influence of Si precipitates on fracture mechanisms of AlSi10Mg parts processed by Selective Laser Melting. Acta Mater. 2019, 175, 160–170. [Google Scholar] [CrossRef]
- Zhou, Y.; Duan, L.C.; Wen, S.F.; Wei, Q.S.; Shi, Y.S. Enhanced micro-hardness and wear resistance of Al-15Si/TiC fabricated by selective laser melting. Compos. Commun. 2018, 10, 64–67. [Google Scholar] [CrossRef]
- Zhao, J.W.; Easton, M.; Qian, M.; Leary, M.; Brandt, M. Effect of building direction on porosity and fatigue life of selective laser melted AlSi12Mg alloy. Mater. Sci. Eng. A 2018, 729, 76–85. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, D.D.; Yang, J.K.; Dai, D.H.; Zhao, T.; Hong, C.; Gasser, A.; Poprawe, R. Selective laser melting of rare earth element Sc modified aluminum alloy: Thermodynamics of precipitation behavior and its influence on mechanical properties. Addit. Manuf. 2018, 23, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.C.; Shi, J.; Lu, S.Q.; Xiao, W.H. Investigation of Porosity and Mechanical Properties of Graphene Nanoplatelets-Reinforced AlSi10 Mg by Selective Laser Melting. J. Micro Nano-Manuf. 2018, 6, 010902. [Google Scholar] [CrossRef]
- Uzan, N.E.; Shneck, R.; Yeheskel, O.; Frage, N. High-temperature mechanical properties of AlSi10Mg specimens fabricated by additive manufacturing using selective laser melting technologies (AM-SLM). Addit. Manuf. 2018, 24, 257–263. [Google Scholar] [CrossRef]
- Takata, N.; Kodaira, H.; Suzuki, A.; Kobashi, M. Size dependence of microstructure of AlSi10Mg alloy fabricated by selective laser melting. Mater. Charact. 2018, 143, 18–26. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Mai, S.; Wang, D.; Song, C. Investigation into spatter behavior during selective laser melting of AISI 316L stainless steel powder. Mater. Des. 2015, 87, 797–806. [Google Scholar] [CrossRef]
- Spierings, A.B.; Dawson, K.; Dumitraschkewitz, P.; Pogatscher, S.; Wegener, K. Microstructure characterization of SLM-processed Al-Mg-Sc-Zr alloy in the heat treated and HIPed condition. Addit. Manuf. 2018, 20, 173–181. [Google Scholar] [CrossRef]
- Liu, M.L.; Takata, N.; Suzuki, A.; Kobashi, M. Microstructural characterization of cellular AlSi10Mg alloy fabricated by selective laser melting. Mater. Des. 2018, 157, 478–491. [Google Scholar] [CrossRef]
- Liu, J.; Gu, D.D.; Chen, H.Y.; Dai, D.H.; Zhang, H. Influence of substrate surface morphology on wetting behavior of tracks during selective laser melting of aluminum-based alloys. J. Zhejiang Univ. Sci. A 2018, 19, 111–121. [Google Scholar] [CrossRef]
- Fousova, M.; Dvorsky, D.; Vronka, M.; Vojtech, D.; Lejcek, P. The Use of Selective Laser Melting to Increase the Performance of AlSi9Cu3Fe Alloy. Materials 2018, 11, 1918. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhu, Y.; Pan, M.; Xie, T.; Chen, X.; Yang, H. A study on cavitation erosion behavior of AlSi10Mg fabricated by selective laser melting (SLM). Wear 2017, 376, 496–506. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zhu, H.H.; Hu, Z.H.; Zeng, X.Y. Study on the Selective Laser Melting of AlSi10Mg. Acta Metall. Sin. 2017, 53, 918–926. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.T.; Liao, W.H.; MacDonald, E.; Wei, H.L.; Chen, X.Y.; Jiang, L.Y. The influence of process parameters on vertical surface roughness of the AlSi10Mg parts fabricated by selective laser melting. J. Mater. Process. Technol. 2019, 266, 26–36. [Google Scholar] [CrossRef]
- Majeed, A.; Lv, J.X.; Zhang, Y.F.; Muzamil, M.; Waqas, A.; Shamim, K.; Qureshi, M.E.; Zafar, F. An investigation into the influence of processing parameters on the surface quality of AlSi10Mg parts by SLM process. In Proceedings of the 2019 16th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 8–12 January 2019; pp. 143–147. [Google Scholar]
- Maamoun, A.H.; Xue, Y.F.; Elbestawi, M.A.; Veldhuis, S.C. The Effect of Selective Laser Melting Process Parameters on the Microstructure and Mechanical Properties of A16061 and AlSi10Mg Alloys. Materials 2019, 12, 12. [Google Scholar] [CrossRef]
- Montero-Sistiaga, M.L.; Mertens, R.; Vrancken, B.; Wang, X.; Van Hooreweder, B.; Kruth, J.-P.; Van Humbeeck, J. Changing the alloy composition of Al7075 for better processability by selective laser melting. J. Mater. Process. Technol. 2016, 238, 437–445. [Google Scholar] [CrossRef]
- Kairy, S.K.; Gharbi, O.; Nicklaus, J.; Jiang, D.; Hutchinson, C.R.; Birbilis, N. On the Characterization of a Hitherto Unreported Icosahedral Quasicrystal Phase in Additively Manufactured Aluminum Alloy AA7075. Metall. Mater. Trans. A 2018, 50, 529–533. [Google Scholar] [CrossRef]
- Bagherifard, S.; Beretta, N.; Monti, S.; Riccio, M.; Bandini, M.; Guagliano, M. On the fatigue strength enhancement of additive manufactured AlSi10Mg parts by mechanical and thermal post-processing. Mater. Des. 2018, 145, 28–41. [Google Scholar] [CrossRef]
- Strößner, J.; Terock, M.; Glatzel, U. Mechanical and Microstructural Investigation of Nickel-Based Superalloy IN718 Manufactured by Selective Laser Melting (SLM). Adv. Eng. Mater. 2015, 17, 1099–1105. [Google Scholar] [CrossRef]
- Wang, L.F.; Sun, J.; Zhu, X.G.; Cheng, L.Y.; Shi, Y.; Guo, L.; Yan, B. Effects of T2 Heat Treatment on Microstructure and Properties of the Selective Laser Melted Aluminum Alloy Samples. Materials 2018, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Kodaira, H.; Sekizawa, K.; Suzuki, A.; Kobashi, M. Change in microstructure of selectively laser melted AlSi10Mg alloy with heat treatments. Mater. Sci. Eng. A 2017, 704, 218–228. [Google Scholar] [CrossRef]
- Casati, R.; Lemke, J.; Alarcon, A.; Vedani, M. Aging Behavior of High-Strength Al Alloy 2618 Produced by Selective Laser Melting. Metall. Mater. Trans. A 2017, 48, 575–579. [Google Scholar] [CrossRef]
- Tradowsky, U.; White, J.; Ward, R.M.; Read, N.; Reimers, W.; Attallah, M.M. Selective laser melting of AlSi10Mg: Influence of post-processing on the microstructural and tensile properties development. Mater. Des. 2016, 105, 212–222. [Google Scholar] [CrossRef]
- Chen, B.; Moon, S.K.; Yao, X.; Bi, G.; Shen, J.; Umeda, J.; Kondoh, K. Strength and strain hardening of a selective laser melted AlSi10Mg alloy. Scr. Mater. 2017, 141, 45–49. [Google Scholar] [CrossRef]
- Aversa, A.; Lorusso, M.; Cattano, G.; Manfredi, D.; Calignano, F.; Ambrosio, E.P.; Biamino, S.; Fino, P.; Lombardi, M.; Pavese, M. A study of the microstructure and the mechanical properties of an Al-Si-Ni alloy produced via selective laser melting. J. Alloys Compd. 2017, 695, 1470–1478. [Google Scholar] [CrossRef]
- Ma, P.; Jia, Y.D.; Prashanth, K.G.; Scudino, S.; Yu, Z.S.; Eckert, J. Microstructure and phase formation in Al-20Si-5Fe-3Cu-1Mg synthesized by selective laser melting. J. Alloys Compd. 2016, 657, 430–435. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Everitt, N.M.; Maskery, I.; Ashcroft, I.; Tuck, C. Selective laser melting of aluminum alloys. MRS Bull. 2017, 42, 311–319. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T. Microstructures and mechanical properties of A356 (AlSi7Mg0.3) aluminum alloy fabricated by selective laser melting. Mater. Des. 2016, 89, 1294–1301. [Google Scholar] [CrossRef]
- Biffi, C.A.; Fiocchi, J.; Tuissi, A. Selective laser melting of AlSi10 Mg: Influence of process parameters on Mg2Si precipitation and Si spheroidization. J. Alloys Compd. 2018, 755, 100–107. [Google Scholar] [CrossRef]
- Fiocchi, J.; Tuissi, A.; Bassani, P.; Biffi, C.A. Low temperature annealing dedicated to AlSi10Mg selective laser melting products. J. Alloys Compd. 2017, 695, 3402–3409. [Google Scholar] [CrossRef]
- Roberts, C.E.; Bourell, D.; Watt, T.; Cohen, J. A novel processing approach for additive manufacturing of commercial aluminum alloys. Phys. Procedia 2016, 83, 909–917. [Google Scholar] [CrossRef]
- Wang, L.F.; Sun, J.; Yu, X.L.; Shi, Y.; Zhu, X.G.; Cheng, L.Y.; Liang, H.H.; Yan, B.; Guo, L.J. Enhancement in mechanical properties of selectively laser-melted AlSi10Mg aluminum alloys by T6-like heat treatment. Mater. Sci. Eng. A 2018, 734, 299–310. [Google Scholar] [CrossRef]
- Fousova, M.; Dvorsky, D.; Michalcova, A.; Vojtech, D. Changes in the microstructure and mechanical properties of additively manufactured AlSi10Mg alloy after exposure to elevated temperatures. Mater. Charact. 2018, 137, 119–126. [Google Scholar] [CrossRef]
- Chen, T.; Wang, L.Z.; Tan, S. Effects of vacuum annealing treatment on microstructures and residual stress of AlSi10Mg parts produced by selective laser melting process. Mod. Phys. Lett. B 2016, 30, 1650255. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Klauss, H.J.; Surreddi, K.B.; Lober, L.; Wang, Z.; Chaubey, A.K.; Kuhn, U.; Eckert, J. Microstructure and mechanical properties of Al-12Si produced by selective laser melting: Effect of heat treatment. Mater. Sci. Eng. A 2014, 590, 153–160. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, L.C.; Fang, M.H.; Sercombe, T.B. The effect of atmosphere on the structure and properties of a selective laser melted Al-12Si alloy. Mater. Sci. Eng. A 2014, 597, 370–375. [Google Scholar] [CrossRef]
- Shuai, C.J.; He, C.X.; Xu, L.; Li, Q.; Chen, T.; Yang, Y.W.; Peng, S.P. Wrapping effect of secondary phases on the grains: Increased corrosion resistance of Mg-Al alloys. Virtual Phys. Prototyp. 2018, 13, 292–300. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Testa, C.; Manfredi, D.; Ambrosio, E.P.; Calignano, F.; Lorusso, M.; Fino, P. Analisys of corrosion resistance of the aismomg alloy obtained by additive manufacturing in chloride solution. Metall. Ital. 2016, 108, 137–146. [Google Scholar]
- Cottam, R.; Brandt, M. Laser Surface Treatment to Improve the Surface Corrosion Properties of Nickel-Aluminum Bronze. In Laser Surface Engineering; Woodhead Publishing: Cambridge, UK, 2015; pp. 469–481. [Google Scholar] [CrossRef]
- Fefelov, A.S.; Merkushev, A.G.; Chikova, O.A.; Finkel’shtein, A.B. Microstructure and Mechanical Properties of Objects Prepared by Selective Laser Melting of AKD12 Powder. Metallurgist 2018, 62, 470–475. [Google Scholar] [CrossRef]
- Rafieazad, M.; Mohammadi, M.; Nasiri, A.M. On microstructure and early stage corrosion performance of heat treated direct metal laser sintered AlSi10Mg. Addit. Manuf. 2019, 28, 107–119. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, J.X.; Gu, X.H.; Qin, P.; Dai, N.W.; Li, X.P.; Kruth, J.P.; Zhang, L.C. Improved corrosion behavior of ultrafine-grained eutectic Al-12Si alloy produced by selective laser melting. Mater. Des. 2018, 146, 239–248. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Fan, X.; Dai, N.; Xiao, Y.; Zhang, L.-C. Abnormal corrosion behavior of selective laser melted AlSi10Mg alloy induced by heat treatment at 300 °C. J. Alloys Compd. 2019, 803, 314–324. [Google Scholar] [CrossRef]
- Rubben, T.; Revilla, R.I.; De Graeve, I. Influence of heat treatments on the corrosion mechanism of additive manufactured AlSi10Mg. Corros. Sci. 2019, 147, 406–415. [Google Scholar] [CrossRef]
- Gu, X.-H.; Zhang, J.-X.; Fan, X.-L.; Zhang, L.-C. Corrosion Behavior of Selective Laser Melted AlSi10Mg Alloy in NaCl Solution and Its Dependence on Heat Treatment. Acta Metall. Sin. (Engl. Lett.) 2019. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Gu, X.; Dai, N.; Qin, P.; Zhang, L.-C. Distinction of corrosion resistance of selective laser melted Al-12Si alloy on different planes. J. Alloys Compd. 2018, 747, 648–658. [Google Scholar] [CrossRef]
- Bian, P.Y.; Shao, X.D.; Du, J.L. Wear and Corrosion Resistance of AlSi10Mg by Selective Laser Melting and Micro-arc Oxidation. China Surf. Eng. 2018, 31, 88–95. [Google Scholar] [CrossRef]
- Spierings, A.B.; Dawson, K.; Kern, K.; Palm, F.; Wegener, K. SLM-processed Sc- and Zr- modified Al-Mg alloy: Mechanical properties and microstructural effects of heat treatment. Mater. Sci. Eng. A 2017, 701, 264–273. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.R.; Singer, L.; Thomas, S.; Kelly, R.G.; Scully, J.R.; Birbilis, N. Corrosion of Additively Manufactured Alloys: A Review. Corrosion 2018, 74, 1318–1350. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Li, X. Corrosion of metallic materials fabricated by selective laser melting. npj Mater. Degrad. 2019, 3, 24. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Maskery, I.; Tuck, C.; Ashcroft, I.; Everitt, N.M. The microstructure and mechanical properties of selectively laser melted AlSi10Mg: The effect of a conventional T6-like heat treatment. Mater. Sci. Eng. A 2016, 667, 139–146. [Google Scholar] [CrossRef]
- Manfredi, D.; Calignano, F.; Krishnan, M.; Canali, R.; Ambrosio, E.P.; Atzeni, E. From Powders to Dense Metal Parts: Characterization of a Commercial AlSiMg Alloy Processed through Direct Metal Laser Sintering. Materials 2013, 6, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Giet, S.; Yang, K.; Wu, X.; Davies, C.H.J. The influence of processing parameters on aluminium alloy A357 manufactured by Selective Laser Melting. Mater. Des. 2016, 109, 334–346. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, D. A study on the residual stress during selective laser melting (SLM) of metallic powder. Int. J. Adv. Manuf. Technol. 2016, 87, 647–656. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Masood, S.H.; Ruan, D.; Palanisamy, S.; Rashid, R. High strain rate dynamic behaviour of AlSi12 alloy processed by selective laser melting. Int. J. Adv. Manuf. Technol. 2018, 97, 1023–1035. [Google Scholar] [CrossRef]
- Uzan, N.; Rosenthal, I.; Stern, A. Macro- and Microstructural Characterization of Cup-Shaped AlSi10Mg Components Fabricated by Selective Laser Melting (SLM). Metallogr. Microstruct. Anal. 2016, 5, 512–519. [Google Scholar] [CrossRef]
- Gharbi, O.; Jiang, D.; Feenstra, D.R.; Kairy, S.K.; Wu, Y.; Hutchinson, C.R.; Birbilis, N. On the corrosion of additively manufactured aluminium alloy AA2024 prepared by selective laser melting. Corros. Sci. 2018, 143, 93–106. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Debalina, B.; Wang, Z.; Gostin, P.F.; Gebert, A.; Calin, M.; Kuhn, U.; Kamaraj, M.; Scudino, S.; Eckert, J. Tribological and corrosion properties of Al-12Si produced by selective laser melting. J. Mater. Res. 2014, 29, 2044–2054. [Google Scholar] [CrossRef]
- Ma, P.; Prashanth, K.G.; Scudino, S.; Jia, Y.D.; Wang, H.W.; Zou, C.M.; Wei, Z.J.; Eckert, J. Influence of Annealing on Mechanical Properties of Al-20Si Processed by Selective Laser Melting. Metals 2014, 4, 28–36. [Google Scholar] [CrossRef]
- Zakay, A.; Aghion, E. Effect of Post-heat Treatment on the Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing. JOM 2019, 71, 1150–1157. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.H.; Nie, X.J.; Qi, T.; Hu, Z.H.; Zeng, X.Y. Fabrication and Heat Treatment of High Strength Al-Cu-Mg alloy Processed Using Selective Laser Melting. Laser 3D Manufacturing III; International Society for Optics and Photonics: San Francisco, CA, USA, 2016. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Ambrosio, E.P.; Calignano, F.; Manfredi, D.; Pavese, M.; Fino, P. Effect of heat treatment on corrosion resistance of DMLS AlSi10Mg alloy. Electrochim. Acta 2016, 206, 346–355. [Google Scholar] [CrossRef]
- Leon, A.; Aghion, E. Effect of surface roughness on corrosion fatigue performance of AlSi10Mg alloy produced by Selective Laser Melting (SLM). Mater. Charact. 2017, 131, 188–194. [Google Scholar] [CrossRef]
- Leon, A.; Shirizly, A.; Aghion, E. Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals 2016, 6, 148. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Pavese, M.; Fino, P.; Ambrosio, E.P.; Calignano, F.; Manfredi, D. Corrosion resistance of direct metal laser sintering AlSiMg alloy. Surf. Interface Anal. 2016, 48, 818–826. [Google Scholar] [CrossRef]
- Revilla, R.I.; De Graeve, I. Influence of Si Content on the Microstructure and Corrosion Behavior of Additive Manufactured Al-Si Alloys. J. Electrochem. Soc. 2018, 165, C926–C932. [Google Scholar] [CrossRef]
- Fathi, P.; Mohammadi, M.; Duan, X.; Nasiri, A.M. A comparative study on corrosion and microstructure of direct metal laser sintered AlSi10Mg_200C and die cast A360.1 aluminum. J. Mater. Process. Technol. 2018, 259, 1–14. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Manfredi, D.; Fino, P.; Biamino, S.; Badini, C. Evaluation of corrosion resistance of Al–10Si–Mg alloy obtained by means of Direct Metal Laser Sintering. J. Mater. Process. Technol. 2016, 231, 326–335. [Google Scholar] [CrossRef]


| Alloys | Electrolyte Solution | Corrosion Measurement | Influence Factors | Reference |
|---|---|---|---|---|
| AlSi10Mg | 3.5 wt. % NaCl | Polarization curve, EIS, corrosion morphology, weight loss | Temperature of post heat treatment | [65] |
| AlSi10Mg | 3.5 wt. % NaCl | OCP, Polarization curve, EIS, corrosion morphology | Temperature of post heat treatment | [63] |
| AlSi10Mg | Harrison’s solution | OCP, Polarization curve, EIS, corrosion morphology | Temperature of post heat treatment, surface roughness | [85] |
| AlSi10Mg | 0.1 M NaCl solution | OCP, Polarization curve, corrosion morphology, SKPFM | Temperature of post heat treatment | [66] |
| AlSi10Mg | 3.5 wt. % NaCl | Polarization curve, EIS, corrosion morphology, weight loss | Temperature of post heat treatment | [67] |
| AlSi10Mg | 3.5 wt. % NaCl | Polarization curve, EIS, corrosion morphology, weight loss, low cycle fatigue | Surface roughness | [86] |
| AlSi10Mg | 3.5 wt. % NaCl | Polarization curve, weight loss, corrosion morphology, low cycle corrosion fatigue | SLM and cast | [87] |
| AlSi10Mg | Harrison’s solution | Polarization curve, corrosion morphology | Different surface treatment | [88] |
| Al-12Si | 0.01 M, 0.1 M, 1 M HNO3 solution | weight loss, corrosion morphology | Temperature of post heat treatment | [81] |
| Al-12Si | 3.5 wt. % NaCl | Polarization curve, EIS, corrosion morphology, weight loss | SLM and cast | [64] |
| Al-12Si | 3.5 wt. % NaCl | OCP, Polarization curve, EIS, corrosion morphology | Printing plane | [68] |
| AA2024 | 0.01 M, 0.1 M, 0.6 M NaCl | Polarization curve, elemental dissolution profiles | SLM and cast | [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhang, C.; Jia, D.; Wellmann, D.; Liu, W. Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review. Metals 2020, 10, 102. https://doi.org/10.3390/met10010102
Chen H, Zhang C, Jia D, Wellmann D, Liu W. Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review. Metals. 2020; 10(1):102. https://doi.org/10.3390/met10010102
Chicago/Turabian StyleChen, Hongwei, Chaoqun Zhang, Dan Jia, Daniel Wellmann, and Wen Liu. 2020. "Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review" Metals 10, no. 1: 102. https://doi.org/10.3390/met10010102
APA StyleChen, H., Zhang, C., Jia, D., Wellmann, D., & Liu, W. (2020). Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review. Metals, 10(1), 102. https://doi.org/10.3390/met10010102






