Effects of Heavy Isotopes (2H1 and 18O16) Depleted Water Con-Sumption on Physical Recovery and Metabolic and Immunological Parameters of Healthy Volunteers under Regular Fitness Load
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Drinking Water under Investigation
2.3. Assessment of Anthropometric Changes
2.4. Assessment of Heart/Vascular Physiology Parameters
2.5. Reagents and Assay Kits
2.6. Assessment of Heavy Water Isotopes (2H and 18O) in Different Water Samples and in Human Plasma
2.7. Redox and Oxidation Marker Assays
2.8. ATP Assay
2.9. Biochemical Analyses
2.10. Immunological Assays
2.11. Statistical Analysis
3. Results
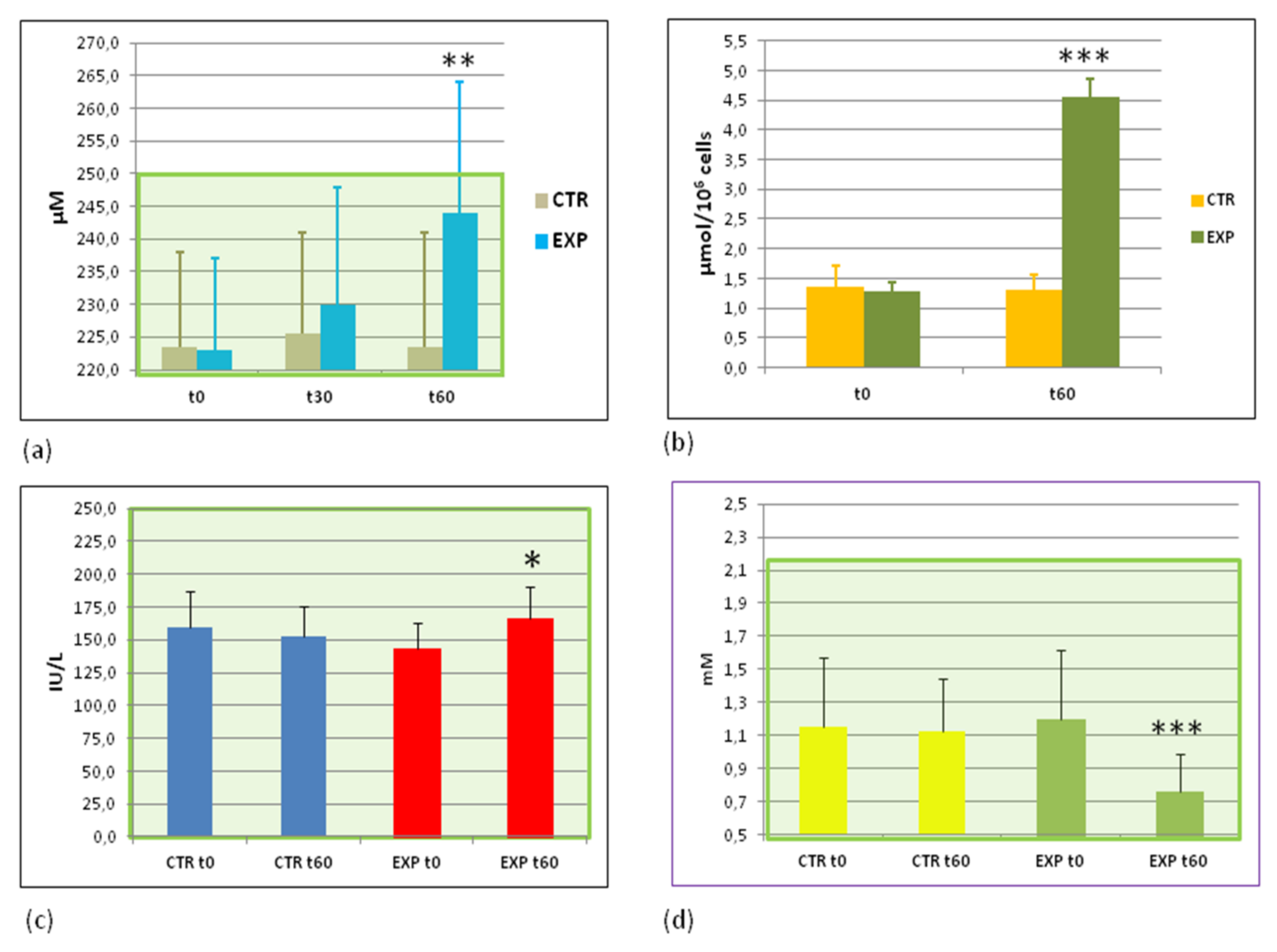
3.1. Effects of HIDW Consumption on Plasma Content of 2H and 18O Isotopes
3.2. Effects of HIDW Consumption on Anthropometric Parameters
3.3. Effects of HIDW Consumption on Aerobic Endurance, Fitness Levels, and Recovery Rate
3.4. Parameters of Energy Metabolism before and after the Trial
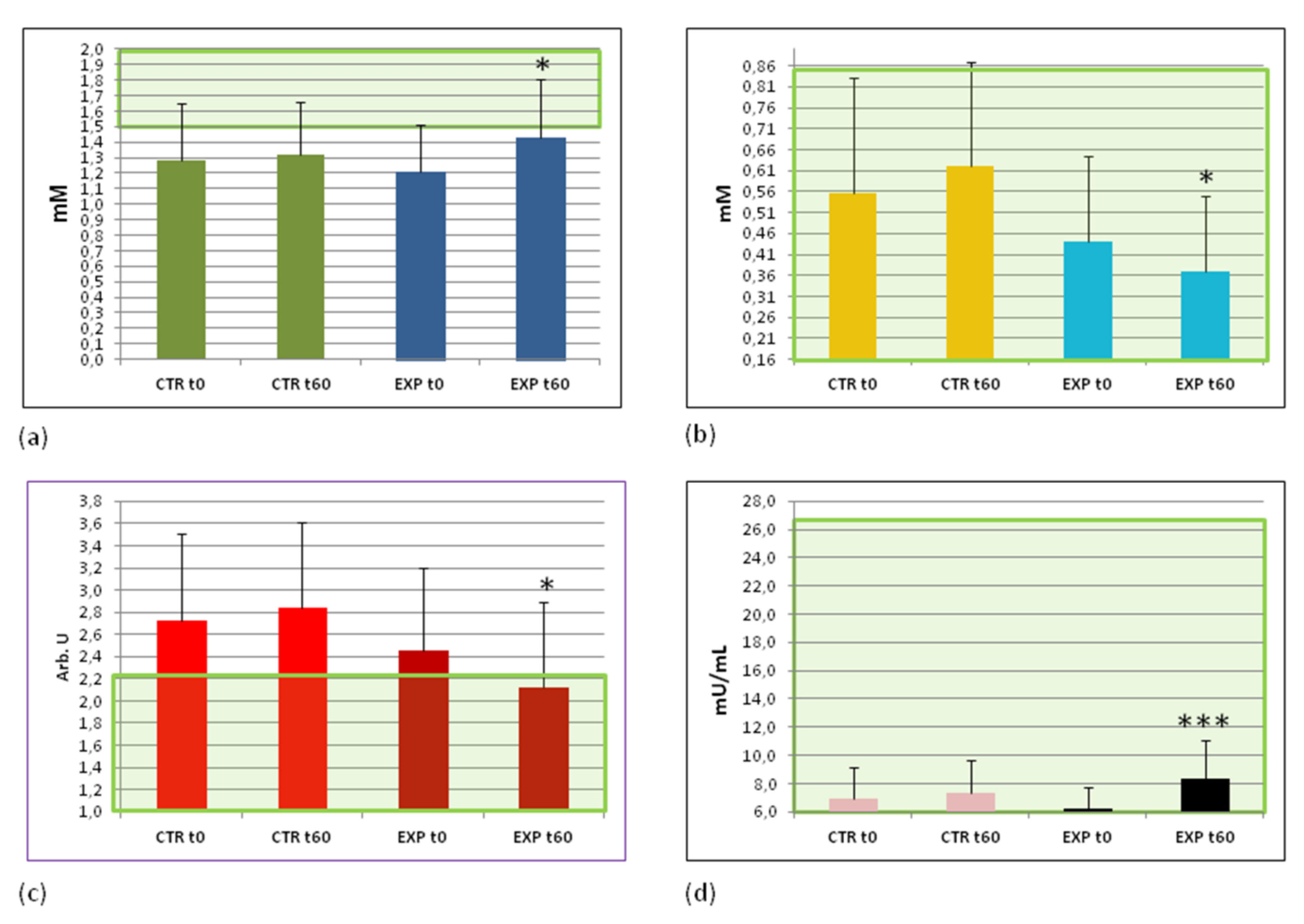
3.5. Effects of Heavy Isotopes Depleted Water Consumption on Lipid and Glucose Metabolism Markers
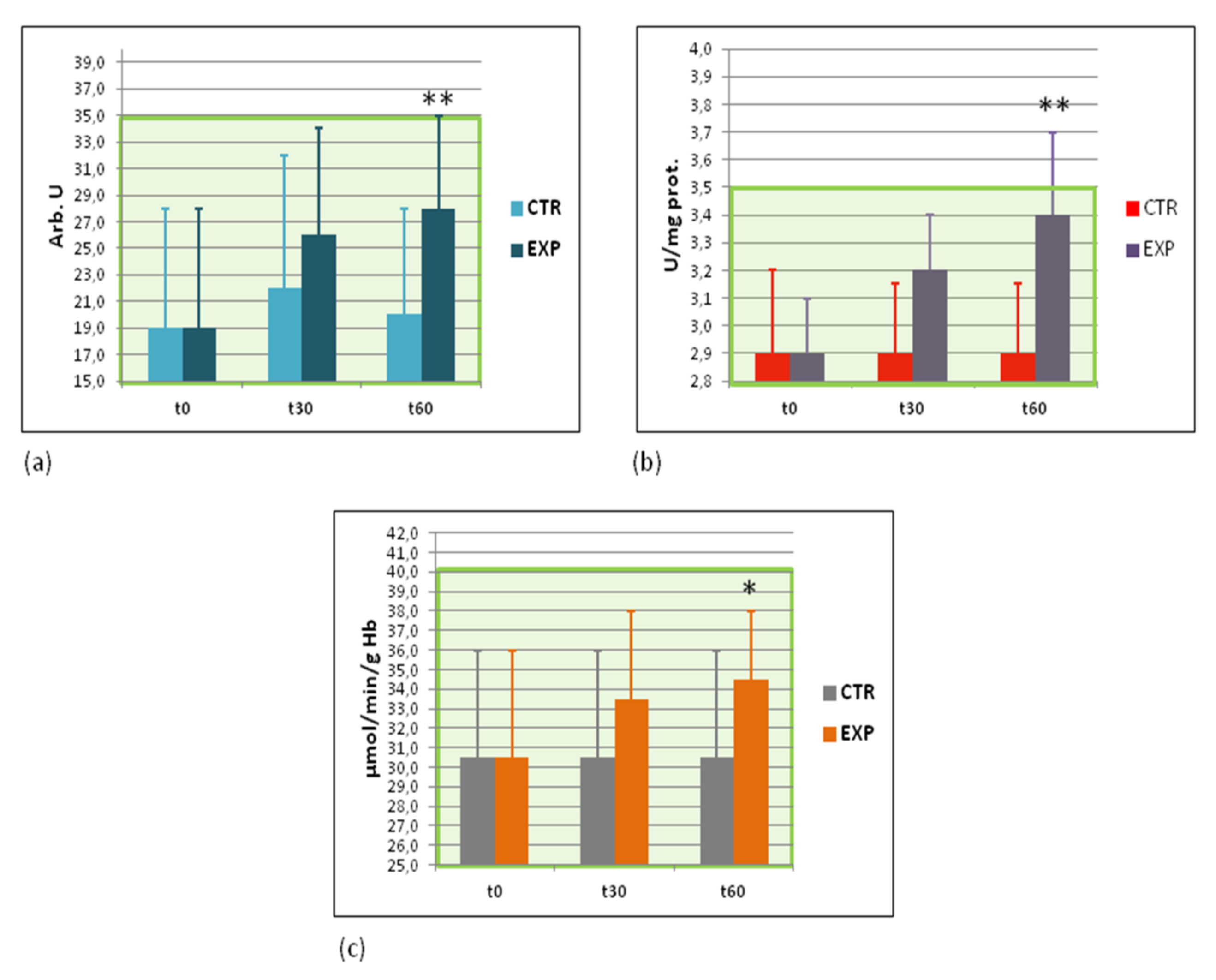
3.6. Effects of Heavy Isotopes Depleted Water Consumption on Free Radical Production and Antioxidant Enzymes
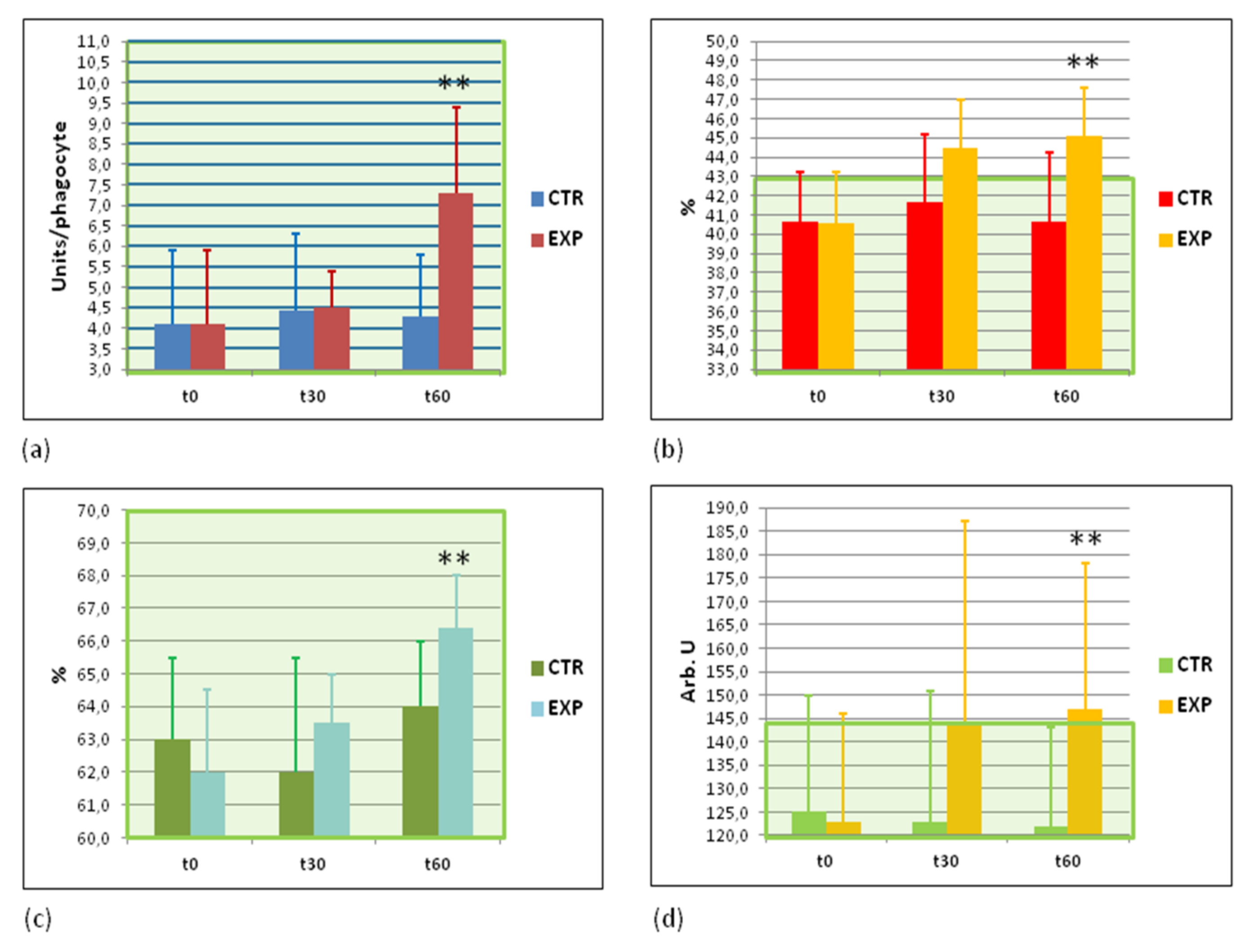
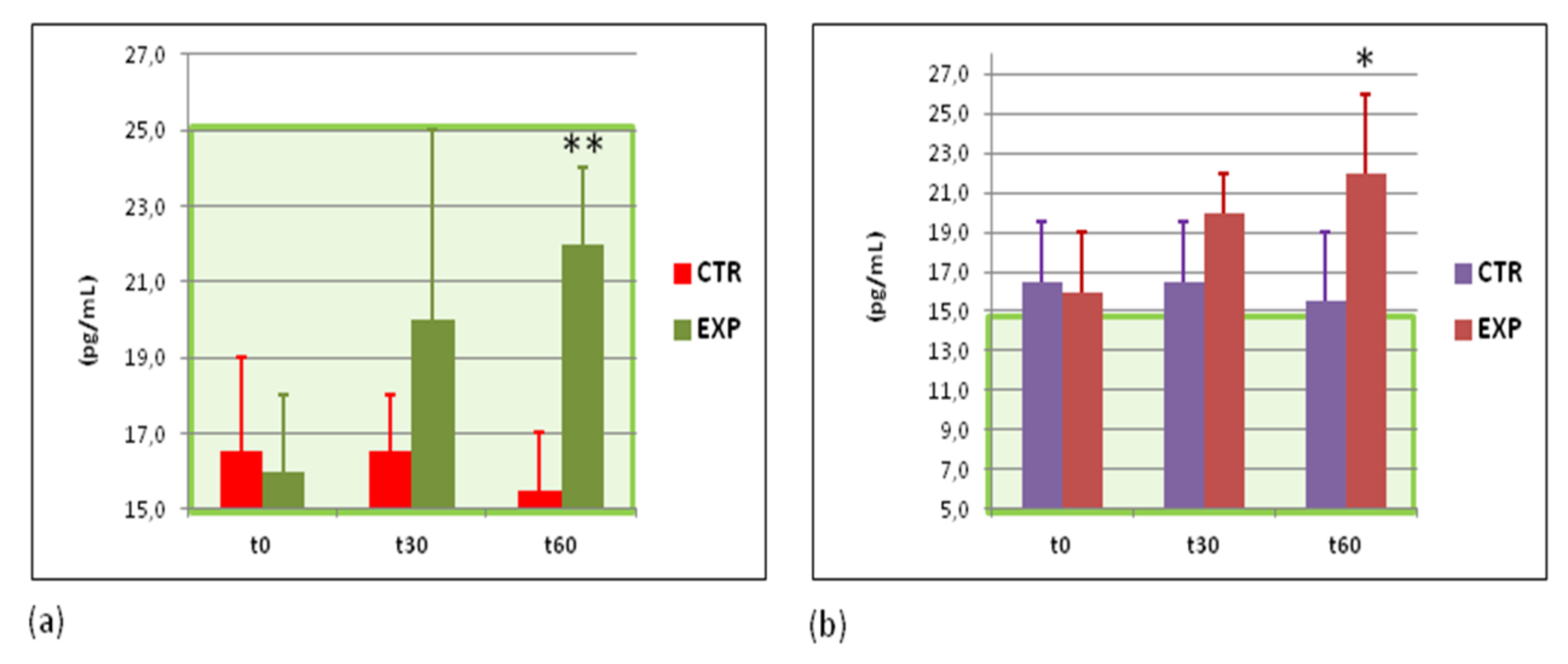
3.7. Effects of Heavy Isotopes Depleted Water Consumption on Immune Defence of Circulating White Blood Cells
3.8. Effects of Heavy Isotopes Depleted Water Consumption on Antibacterial and Antiviral Immunity of Circulating White Blood Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
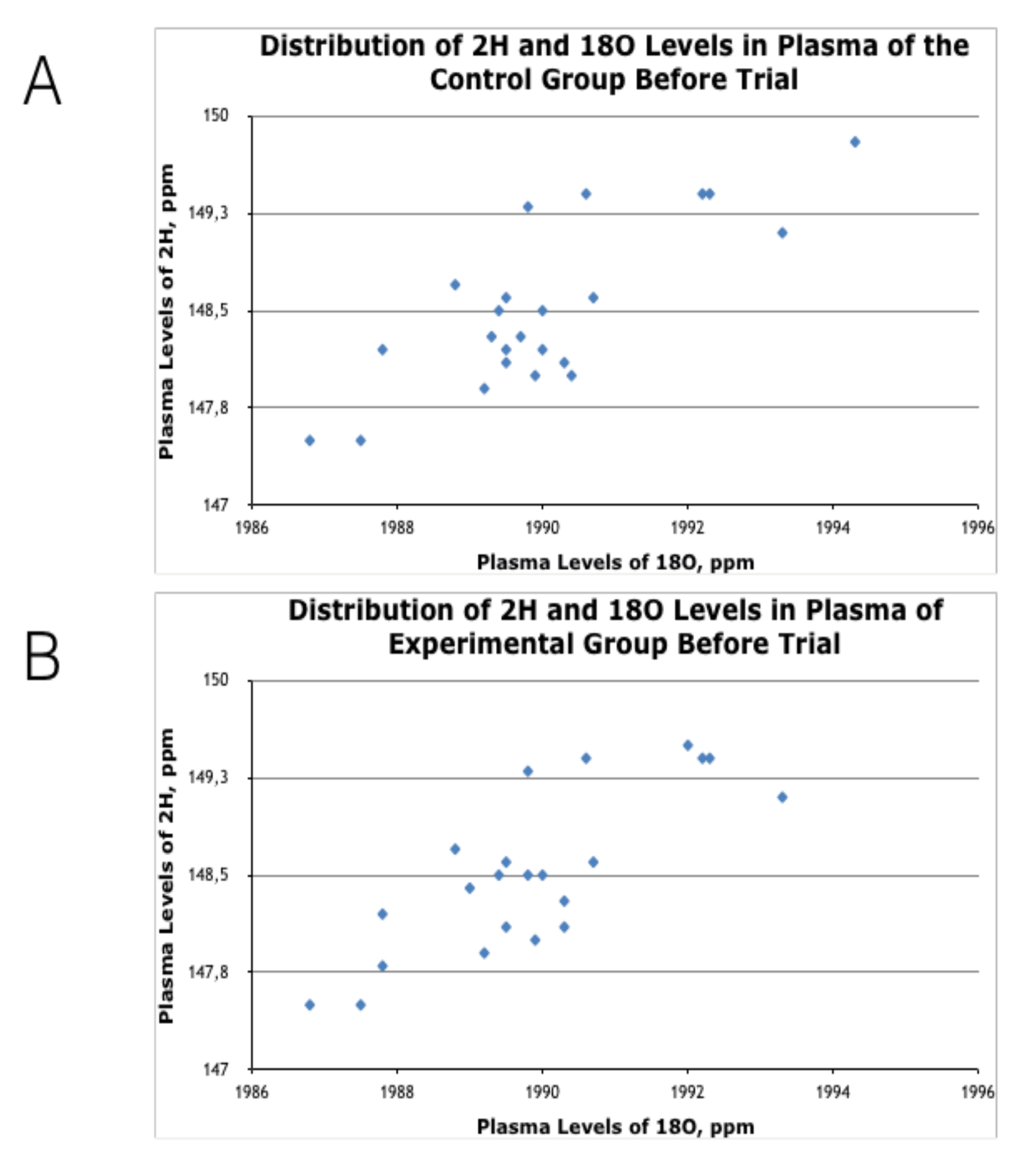
Appendix B
| CTR | EXP | ||||
|---|---|---|---|---|---|
| t0 | t30 | t60 | t0 | t30 | t60 |
| 23.8 ± 3.7 | 23.8 ± 2.2 | 23.8 ± 1.9 | 23.7 ± 4.8 | 23.4 ± 4.6 | 23.4 ± 4.7 |
| Parameter, Units | Control Group | Experimental Group | Normal Range | ||||
|---|---|---|---|---|---|---|---|
| t0 | t30 | t60 | t0 | t30 | t60 | ||
| Red blood cell SOD, IU/mg prot | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.3 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.2 | 0.7–1.8 |
| Plasma NO2−/NO3−, μM | 9.5 ± 5.5 | 9.4 ± 5.6 | 10.0 ± 5.4 | 10.0 ± 6.0 | 10.0 ± 5.0 | 10.0 ± 5.5 | 5.0–15.2 |
| Red blood cell GSH, μM | 650 ± 128 | 650 ± 130 | 640 ± 135 | 640 ± 130 | 680 ± 68 | 675 ± 100 | 500–870 |
| Plasma IL-1β, pg/mL | 51 ± 6 | 52 ± 4 | 52 ± 4 | 50 ± 4 | 50 ± 4 | 52 ± 6 | 45–66 |
| Plasma TNFα, pg/mL | 21 ± 5 | 22 ± 6 | 21 ± 6 | 19 ±6 | 19 ± 5 | 24 ± 5 | 15–26 |
| Glucose, mM | 4.8 ± 0.2 | ND | 4.9 ± 0.1 | 4.8 ± 0.2 | ND | 4.7 ± 0.1 | 4.1–5.9 |
| Triglycerides, mM | 1.3 ± 0.4 | ND | 1.3 ± 0.4 | 1.0 ± 0.3 | ND | 0.9 ± 0.5 | >1.8 |
| Total cholesterol, mM | 5.2 ± 0.4 | ND | 5.2 ±0.3 | 5.0 ± 0.3 | ND | 4.4 ± 0.4 | >5.2 |
| Cholesterol/LDL, mM | 3.0 ± 1.0 | ND | 3.7 ± 0.8 | 2.7 ± 0.8 | ND | 2.4 ± 0.8 | >3.8 |
| Insulin resistance, arb.units | 0.7 ± 1.0 | ND | 0.7 ± 1.2 | 0.7 ± 0.7 | ND | 0.7 ± 0.8 | >1.1 |
| C-reactive peptide, ng/mL | 2.2 ± 1.2 | ND | 2.2 ± 1.1 | 1.6 ± 0.3 | ND | 1.7 ± 0.3 | 0.8–4.0 |
| Adiponectin, μg/mL | 6.8 ± 1.1 | ND | 6.8 ± 1.0 | 7.0 ± 1.1 | ND | 7.1 ± 1.0 | 5.0–10.0 |
| HOMA, arb.units | 1.0 ± 0.7 | ND | 1.2 ± 1.0 | 1.8 ± 0.2 | ND | 1.8 ± 0.4 | >3.0 |
Appendix C
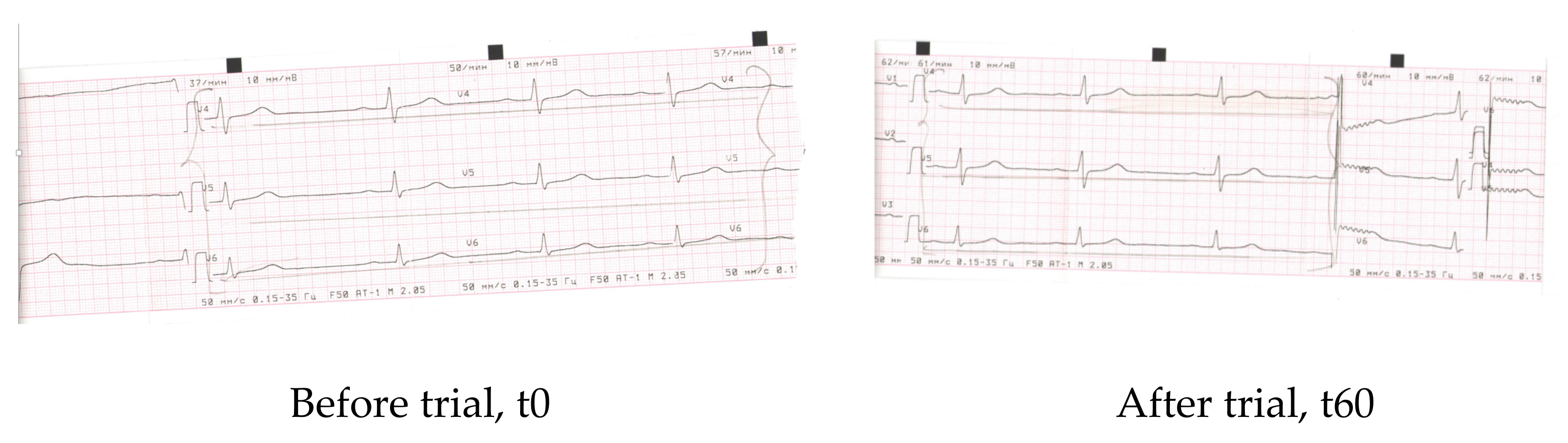
| Parameter | Before | After 60 days |
| Rhyme | Synus | Synus |
| Heart rate | 52 | 69 |
| PQ | 0.22″ | 0.15″ |
| QRS | 0.1″ | 0.1″ |
| QT | 0.41″ | 0.40″ |
| QRS axis | Vertical +88 | Vertical +88 |
| Conclusions | Bradicardia, dysmetabolic alterations of myocardium (frontal and lateral walls of the left ventricle) (V4–V6) | Normal rhyme, improvement of re-polarisation (V4–V6) |
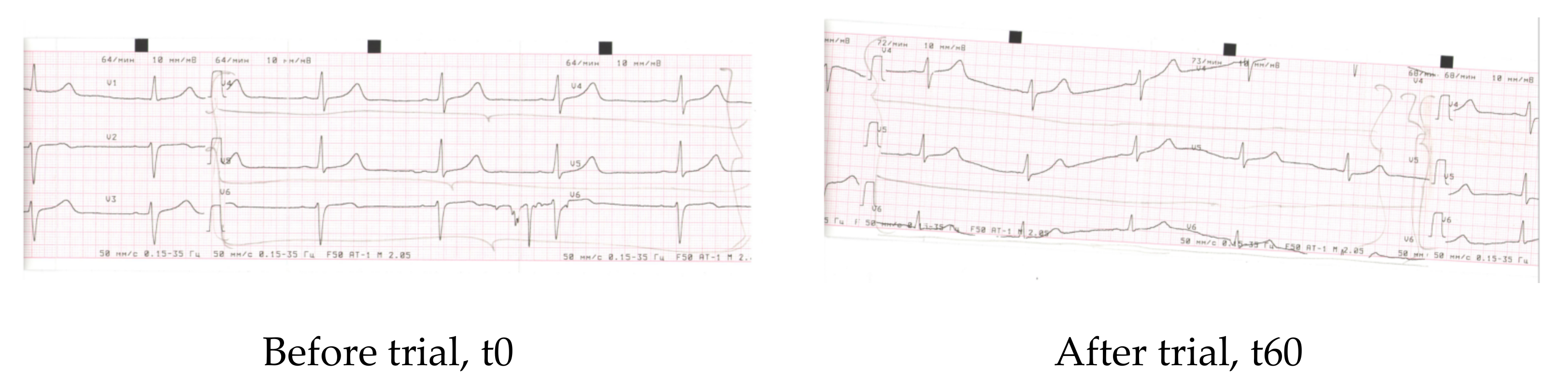
| Parameter | Before | After 60 days |
| Rhyme | Synus | Synus |
| Heart rate | 68 | 70 |
| PQ | 0.12″ | 0.14″ |
| QRS | 0.08″ | 0.08″ |
| QT | 0.38″ | 0.38″ |
| QRS axis | Vertical +77 | Vertical +77 |
| Conclusions | Dysmetabolic alterations of myocardium (frontal and lateral walls of the left ventricle) (V4–V6) | Improvement of re-polarisation along frontal and lateral walls of the left ventricle (V4–V6) |
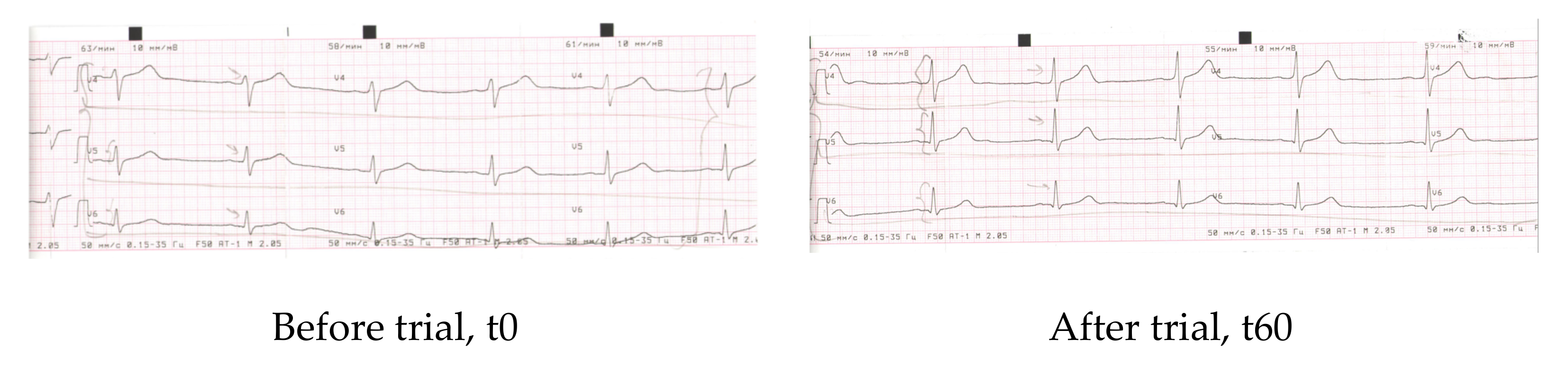
| Parameter | Before | After 60 days |
| Rhyme | Sinus | Sinus |
| Heart rate | 74 | 67 |
| PQ | 0.18″ | 0.16″ |
| QRS | 0.08″ | 0.08″ |
| QT | 0.40″ | 0.38″ |
| QRS axis | Horizontal +38 | Horizontal +38 |
| Conclusions | Dysmetabolic alterations of myocardium (frontal and lateral walls of the left ventricle) (V4-V5) | Improvement of re-polarisation of (V4–V5), increment of peaks V4–V5 |
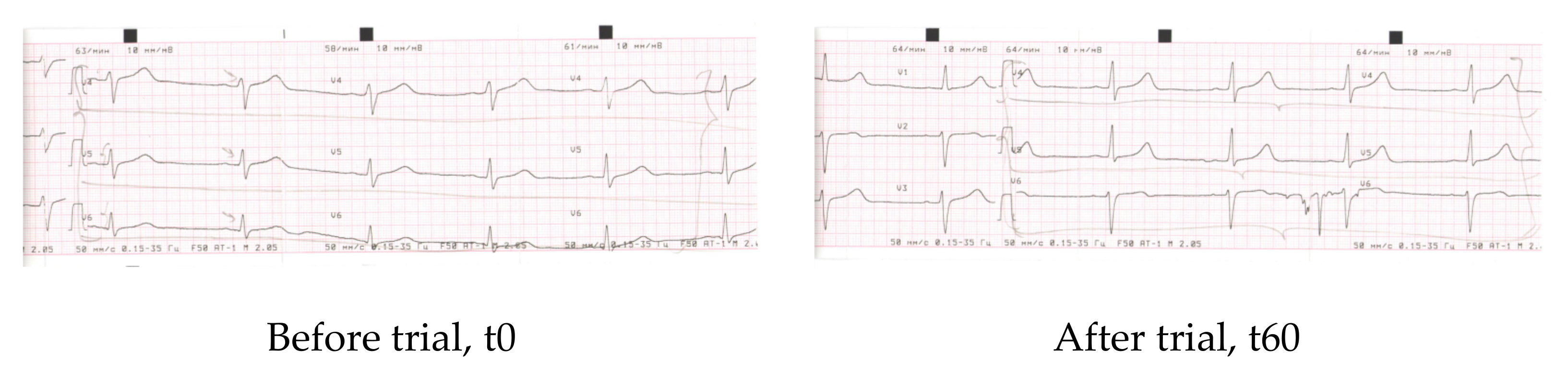
| Parameter | Before | After 60 days |
| Rhyme | Synus | Synus |
| Heart rate | 68 | 70 |
| PQ | 0.12″ | 0.14″ |
| QRS | 0.08″ | 0.08″ |
| QT | 0.38″ | 0.38″ |
| QRS axis | Vertical +77 | Vertical +77 |
| Conclusions | Dysmetabolic alterations of myocardium (frontal and lateral walls of the left ventricle) (V4–V6) | Dysmetabolic alterations of myocardium (frontal and lateral walls of the left ventricle) (V4–V6) |
References
- Mitchell, H.H.; Hamilton, T.S.; Steggerda, F.R.; Bean, H.W. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J. Biol. Chem. 1945, 158, 625–637. [Google Scholar] [CrossRef]
- Katz, J.J.; Crespi, H.L.; Miller, A.; Maunsbach, A.B. Deuterated Organisms: Cultivation and Uses. Science 1966, 151, 1187–1194. [Google Scholar] [CrossRef]
- Steckel, F.; Szapiro, S. Physical and chemical properties of heavy water. Part 1.—Density and thermal expansion. Trans. Faraday Soc. 1963, 59, 331–343. [Google Scholar] [CrossRef]
- Kudish, A.I.; Wolf, D.; Steckel, F. Physical properties of heavy-oxygen water. Absolute viscosity of H218O between 15 and 35 °C. J. Chem. Soc. Faraday Trans. 1972, 68, 2041–2046. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; A Kavitskaya, A.; Romanyukina, I.Y.; A Loboda, O. Revealing water’s secrets: Deuterium depleted water. Chem. Central J. 2013, 7, 103. [Google Scholar] [CrossRef][Green Version]
- Boros, L.G.; D’Agostino, D.P.; Katz, H.E.; Roth, J.P.; Meuillet, E.; Somlyai, G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med. Hypotheses 2016, 87, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sessions, A.; Burgoyne, T.W.; Schimmelmann, A.; Hayes, J.M. Fractionation of hydrogen isotopes in lipid biosynthesis. Org. Geochem. 1999, 30, 1193–1200. [Google Scholar] [CrossRef]
- Schmidt, H.-L.; Werner, R.A.; Eisenreich, W. Systematics of 2H patterns in natural compounds and its importance for the elucidation of biosynthetic pathways. Phytochem. Rev. 2003, 2, 61–85. [Google Scholar] [CrossRef]
- Gyöngyi, Z.; Budán, F.; Szabó, I.; Ember, I.; Kiss, I.; Krempels, K.; Somlyai, I.; Somlyai, G. Deuterium Depleted Water Effects on Survival of Lung Cancer Patients and Expression of Kras, Bcl2, and Myc Genes in Mouse Lung. Nutr. Cancer 2013, 65, 240–246. [Google Scholar] [CrossRef]
- Strekalova, T.; Evans, M.; Chernopiatko, A.; Couch, Y.; Costa-Nunes, J.P.; Cespuglio, R.; Chesson, L.; Vignisse, J.; Steinbusch, H.W.; Anthony, D.; et al. Deuterium content of water increases depression susceptibility: The potential role of a serotonin-related mechanism. Behav. Brain Res. 2015, 277, 237–244. [Google Scholar] [CrossRef]
- Ávila, D.S.; Somlyai, G.; Somlyai, I.; Aschner, M. Anti-aging effects of deuterium depletion on Mn-induced toxicity in a C. elegans model. Toxicol. Lett. 2012, 211, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.-S.; Zhang, Y.-R.; Sheng, H.-C.; Ao, Z.-H.; Zhang, S.-Y.; Wang, J.-Y. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Exp. Ther. Med. 2010, 1, 277–283. [Google Scholar] [CrossRef]
- Somlyai, G.; Jancsó, G.; Jákli, G.; Vass, K.; Barna, B.; Lakics, V.; Gaál, T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993, 317, 1–4. [Google Scholar] [CrossRef]
- Pomytkin, I.A.; Kolesova, O.E. Relationship between natural concentration of heavy water isotopologos and rate of H2O2 generation by mitochondria. Bull. Exp. Biol. Med. 2006, 142, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Pomytkin, I.A.; Kolesova, O.E. Effect of insulin on the rate of hydrogen peroxide generation in mitochondria. Bull. Exp. Biol. Med. 2003, 135, 541–542. [Google Scholar] [CrossRef]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and Negative Regulation of Insulin Signaling by Reactive Oxygen and Nitrogen Species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef]
- Physical Status: The Use and Inter-Pretation of Anthropometry; Report of a WHO expert committee; World Health Organization: Geneva, Switzerland, 1995; Available online: https://apps.who.int/iris/bitstream/handle/10665/37003/W?sequence=1 (accessed on 10 July 2021).
- Jousselin, E. Le test de Ruffier, improperment appele test de Ruffier-Dickson. Med. Sport 2007, 83, 33–34. [Google Scholar]
- Dzhimak, S.S.; Baryshev, M.G.; A Basov, A.; A Timakov, A. Influence of deuterium depleted water on freeze-dried tissue isotopic composition and morphofunctional body performance in rats of different generations. Biofizika 2014, 59, 749–756. [Google Scholar] [CrossRef]
- Giovannoni, G.; Land, J.M.; Keir, G.; Thompson, E.J.; Heales, S.J.R. Adaptation of the Nitrate Reductase and Griess Reaction Methods for the Measurement of Serum Nitrate plus Nitrite Levels. Ann. Clin. Biochem. 1997, 34, 193–198. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- De Luca, C.; Mikhal’Chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Sandström, J.; Nilsson, P.; Karlsson, K.; Marklund, S. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J. Biol. Chem. 1994, 269, 19163–19166. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- E Paglia, D.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Kharaeva, Z.; Gostova, E.; De Luca, C.; Raskovic, D.; Korkina, L. Clinical and biochemical effects of coenzyme Q10, vitamin E, and selenium supplementation to psoriasis patients. Nutrition 2009, 25, 295–302. [Google Scholar] [CrossRef]
- Lubrano, C.; Valacchi, G.; Specchia, P.; Gnessi, L.; Rubanenko, E.P.; Shuginina, E.A.; Trukhanov, A.I.; Korkina, L.G.; De Luca, C. Integrated Haematological Profiles of RedoxStatus, Lipid, and Inflammatory Protein Biomarkers in Benign Obesity and Unhealthy Obesity with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dzhimak, S.S.; Basov, A.A.; Kopytov, G.F.; Kashaev, D.V.; Sokolov, M.E.; Artsybasheva, O.M.; Sharapov, K.S.; Baryshev, M.G. Application of NMR spectroscopy to the determination of low concentrations of nonradioactive isotopes in liquid media. Rus. Phys. J. 2015, 58, 923–929. [Google Scholar] [CrossRef]
- Basov, A.; Fedulova, L.; Baryshev, M.; Dzhimak, S. Deuterium-Depleted Water Influence on the Isotope 2H/1H Regulation in Body and Individual Adaptation. Nutrition 2019, 11, 1903. [Google Scholar] [CrossRef]
- Halenova, T.; Zlatskiy, I.; Syroeshkin, A.; Maximova, T.; Pleteneva, T. Deuterium-Depleted Water as Adjuvant Therapeutic Agent for Treatment of Diet-Induced Obesity in Rats. Molecules 2019, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Sartor, F.; Bonato, M.; Papini, G.; Bosio, A.; Mohammed, R.A.; Bonomi, A.G.; Moore, J.P.; Merati, G.; La Torre, A.; Kubis, H.-P. A 45-Second Self-Test for Cardiorespiratory Fitness: Heart Rate-Based Estimation in Healthy Individuals. PLoS ONE 2016, 11, e0168154. [Google Scholar] [CrossRef] [PubMed]
- Rehakova, R.; Klimentova, J.; Cebova, M.; Barta, A.; Matuskova, Z.; Labas, P.; Pechanova, O. Effect of Deuterium-Depleted Water on Selected Cardiometabolic Parameters in Fructose-Treated Rats. Physiol. Res. 2016, 65, S401–S407. [Google Scholar] [CrossRef]
- Tesch, P.; Sjödin, B.; Thorstensson, A.; Karlsson, J. Muscle fatigue and its relation to lactate accumulation and LDH activity in man. Acta Physiol. Scand. 1978, 103, 413–420. [Google Scholar] [CrossRef]
- Juel, C.; Klarskov, C.; Nielsen, J.J.; Krustrup, P.; Mohr, M.; Bangsbo, J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am. J. Physiol. Metab. 2004, 286, E245–E251. [Google Scholar] [CrossRef]
- Hoehn, K.L.; Salmon, A.B.; Hohnen-Behrens, C.; Turner, N.; Hoy, A.; Maghzal, G.J.; Stocker, R.; Van Remmen, H.; Kraegen, E.W.; Cooney, G.J.; et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 17787–17792. [Google Scholar] [CrossRef]
- Stump, C.S.; Short, K.; Bigelow, M.L.; Schimke, J.M.; Nair, K.S. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. USA 2003, 100, 7996–8001. [Google Scholar] [CrossRef]
- Boirie, Y. Insulin regulation of mitochondria proteins and oxidative phosphorylation in human muscle. Trends Endocrinol. Metab. 2003, 14, 393–394. [Google Scholar] [CrossRef]
- Pomytkin, I.A. H2O2 Signalling Pathway: A Possible Bridge between Insulin Receptor and Mitochondria. Curr. Neuropharmacol. 2012, 10, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Bejma, J.; Ramires, P.; Ji, L.L. Free radical generation and oxidative stress with ageing and exercise: Differential effects in the myocardium and liver. Acta Physiol. Scand. 2000, 169, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-induced oxidative stress and dietary antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef]
- Knez, W.L.; Coombes, J.S.; Jenkins, D.G. Ultra-endurance exercise and oxidative damage: Implications for cardiovascular health. Sports Med. 2006, 36, 429–441. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Taylor, A.W.; Ohno, H.; Goto, S. Adaptation to exercise-induced oxidative stress: From muscle to brain. Exerc. Immunol. Rev. 2001, 7, 90–107. [Google Scholar] [PubMed]
- Powers, S.K.; Talbert, E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.-C.; Martínez, A.; Santangelo, G.; Pallardó, F.V.; Sastre, J.; Viña, J. Oxidative stress in marathon runners: Interest of antioxidant supplementation. Br. J. Nutr. 2006, 96, S31–S33. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Pastore, S. The role of redox regulation in the normal physiology and inflammatory diseases of skin. Front. Biosci. 2009, 1, 123–141. [Google Scholar] [CrossRef]
- Vasilaki, A.; McArdle, F.; Iwanejko, L. Adaptive responses of mouse skeletal muscle to contractile activity: The effect of age. Mech. Ageing Dev. 2006, 127, 830–839. [Google Scholar] [CrossRef]
- Hollander, J.; Fiebig, R.; Gore, M.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflug. Arch. 2001, 442, 426–434. [Google Scholar] [CrossRef]
- Rauma, A.-L.; Mykkänen, H. Antioxidant status in vegetarians versus omnivores. Nutrition 2000, 16, 111–119. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Saso, L.; De Luca, C.; Korkina, L. Redox Control of Multidrug Resistance and Its Possible Modulation by Antioxidants. Oxidative Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Asp. Med. 2004, 25, 365–451. [Google Scholar] [CrossRef]
- Holmström, K.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.; Abramov, A. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef]
- Sharma, K. Mitochondria hormesis and diabetic complications. Diabetes 2015, 64, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Barbato, D.L.; Tatulli, G.; Aquilano, K.; Ciriolo, M.R. The role of nNOS and PGC-1α in skeletal muscle cells. J. Cell Sci. 2014, 127, 4813–4820. [Google Scholar] [CrossRef]
- Cornelius, C.; Perrotta, R.; Graziano, A.; Calabrese, E.J.; Calabrese, V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: Mitochondria as a “chi”. Immun. Ageing 2013, 10, 1–13. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, D.; Yang, H.; Wang, W.; Xiao, J.; Zhou, L.; Fu, H. Neuroprotective Effects of Deuterium-Depleted Water (DDW) Against H2O2-Induced Oxidative Stress in Differentiated PC12 Cells Through the PI3K/Akt Signaling Pathway. Neurochem. Res. 2020, 45, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral Actions of Interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef]
- Huang, S.; Hendriks, W.; Althage, A.; Hemmi, S.; Bluethmann, H.; Kamijo, R.; Vilcek, J.; Zinkernagel, R.M.; Aguet, M. Immune response in mice that lack the interferon-gamma receptor. Science 1993, 259, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Inducible nitric oxide synthase: What difference does it make? J. Clin. Investig. 1997, 100, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, Y.; Iwabuchi, K.; Onoe, K. Cooperative action of interleukin-10 and interferon-γ to regulate dendritic cell functions. Immunology 2008, 127, 345–353. [Google Scholar] [CrossRef]
| Water Samples | 2H Content | 18O Content | ||
|---|---|---|---|---|
| ppm | δ2Hv-SMOW *, ‰ | ppm | δ18Ov-SMOW *, ‰ | |
| HIDW | 58.5 ± 0.3 | −624.4 ± 1.9 | 1780.7 ± 0.1 | −111.9 ± 0.4 |
| Natural local tap water | 146.8 ± 0.3 | −57.5 ± 1.7 | 1981.3 ± 0.2 | −11.8 ± 0.7 |
| Natural local spring water | 148.1 ± 0.3 | −49.2 ± 1.9 | 1984.5 ± 0.2 | −10.2 ± 0.8 |
| Mineral/Anion/Physical Parameter | Value, Units |
|---|---|
| Potassium | 4.4 mg/L |
| Sodium | 10.0 mg/L |
| Calcium | 50.1 mg/L |
| Magnesium | 10.4 mg/L |
| Chloride | 70.0 mg/L |
| Fluoride | 0.5 mg/L |
| Sulphate | 10.0 mg/L |
| Hydrocarbonate | 70.8 mg/L |
| Total mineralisation | 226.0 mg/L |
| Electroconductivity | 320 μS/cm |
| pH | 7.2 |
| Isotope/Group | Plasma 2H Content, ppm | Plasma 18O Content, ppm | ||
|---|---|---|---|---|
| Before (t0) | After (t60) | Before (t0) | After (t60) | |
| EXP HIDW (n = 25) | 148.5 ± 0.1 | 129.9 ± 1.0 *** | 1986 ± 1.0 | 1961 ± 10.0 * |
| CTR Local water (n = 25) | 148.5 ± 0.1 | 148.5 ± 0.1 | 1990 ± 3.0 | 1990 ± 1.0 |
| Change of the Parameter (t60–t0), Units | Group | Significance of the Change | |
|---|---|---|---|
| Control | Experimental | ||
| Body weight, kg | 0.04 ± 0.03 | −0.59 ± 0.32 ** | p < 0.01 |
| Chest circumference, cm | 0.35 ± 0.10 | −0.52 ± 0.28 *** | p < 0.001 |
| Waist circumference, cm | 0.04 ± 0.03 | −0.70 ± 0.19 ** | p < 0.01 |
| Hip circumference, cm | 0.09 ± 0.06 | −0.63 ± 0.21 ** | p < 0.01 |
| Thigh circumference, cm | −0.04 ± 0.01 | −0.50 ± 0.12 ** | p < 0.01 |
| Forearm circumference, cm | −0.04 ± 0.01 | −0.17 ± 0.05 ** | p < 0.01 |
| CTR | EXP | ||||
|---|---|---|---|---|---|
| t0 | t30 | t60 | t0 | t30 | t60 |
| 9.1 ± 1.3 | 9.0 ± 1.8 | 8.9 ± 1.9 | 10.2 ± 1.5 | 9.2 ± 1.9 | 8.0 ± 1.1 * |
| CTR | EXP | ||||
|---|---|---|---|---|---|
| t0 | t30 | t60 | t0 | t30 | t60 |
| 8.6 ± 0.7 | 8.2 ± 1.2 | 7.7 ± 1.9 | 9.3 ± 1.3 | 8.1 ± 0.6 * | 6.6 ± 0.8 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharaeva, Z.; Hokonova, T.; Elmurzaeva, J.; Dzamihova, I.; Mayer, W.; De Luca, C.; Trakhtman, I.; Korkina, L. Effects of Heavy Isotopes (2H1 and 18O16) Depleted Water Con-Sumption on Physical Recovery and Metabolic and Immunological Parameters of Healthy Volunteers under Regular Fitness Load. Sports 2021, 9, 110. https://doi.org/10.3390/sports9080110
Kharaeva Z, Hokonova T, Elmurzaeva J, Dzamihova I, Mayer W, De Luca C, Trakhtman I, Korkina L. Effects of Heavy Isotopes (2H1 and 18O16) Depleted Water Con-Sumption on Physical Recovery and Metabolic and Immunological Parameters of Healthy Volunteers under Regular Fitness Load. Sports. 2021; 9(8):110. https://doi.org/10.3390/sports9080110
Chicago/Turabian StyleKharaeva, Zaira, Tamara Hokonova, Jannet Elmurzaeva, Irlana Dzamihova, Wolfgang Mayer, Chiara De Luca, Ilya Trakhtman, and Liudmila Korkina. 2021. "Effects of Heavy Isotopes (2H1 and 18O16) Depleted Water Con-Sumption on Physical Recovery and Metabolic and Immunological Parameters of Healthy Volunteers under Regular Fitness Load" Sports 9, no. 8: 110. https://doi.org/10.3390/sports9080110
APA StyleKharaeva, Z., Hokonova, T., Elmurzaeva, J., Dzamihova, I., Mayer, W., De Luca, C., Trakhtman, I., & Korkina, L. (2021). Effects of Heavy Isotopes (2H1 and 18O16) Depleted Water Con-Sumption on Physical Recovery and Metabolic and Immunological Parameters of Healthy Volunteers under Regular Fitness Load. Sports, 9(8), 110. https://doi.org/10.3390/sports9080110






