No Effects of New Zealand Blackcurrant Extract on Physiological and Performance Responses in Trained Male Cyclists Undertaking Repeated Testing across a Week Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Physical Activity and Dietary Standardization
2.4. Incremental Cycling Test
2.5. Maximal Rate of Oxygen Uptake
2.6. Submaximal Cycling Intensity
2.7. Time Trial (16.1 km)
2.8. Calculations and Statistical Analysis
3. Results
3.1. Blood Lactate Levels during the Incremental Cycling Protocol
3.2. Substrate Oxidation Rate and Physiological Data at Submaximal Cycling Intensity
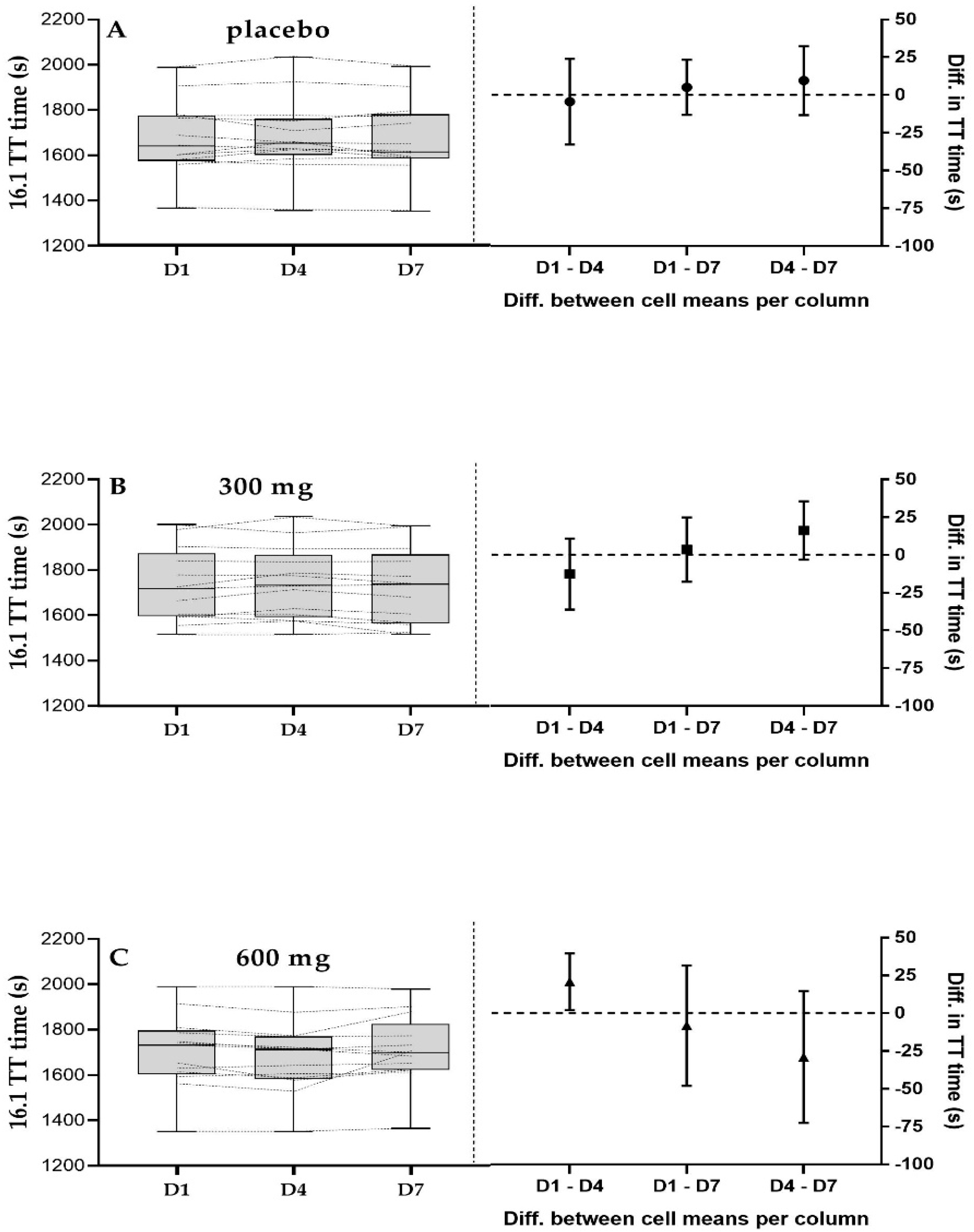
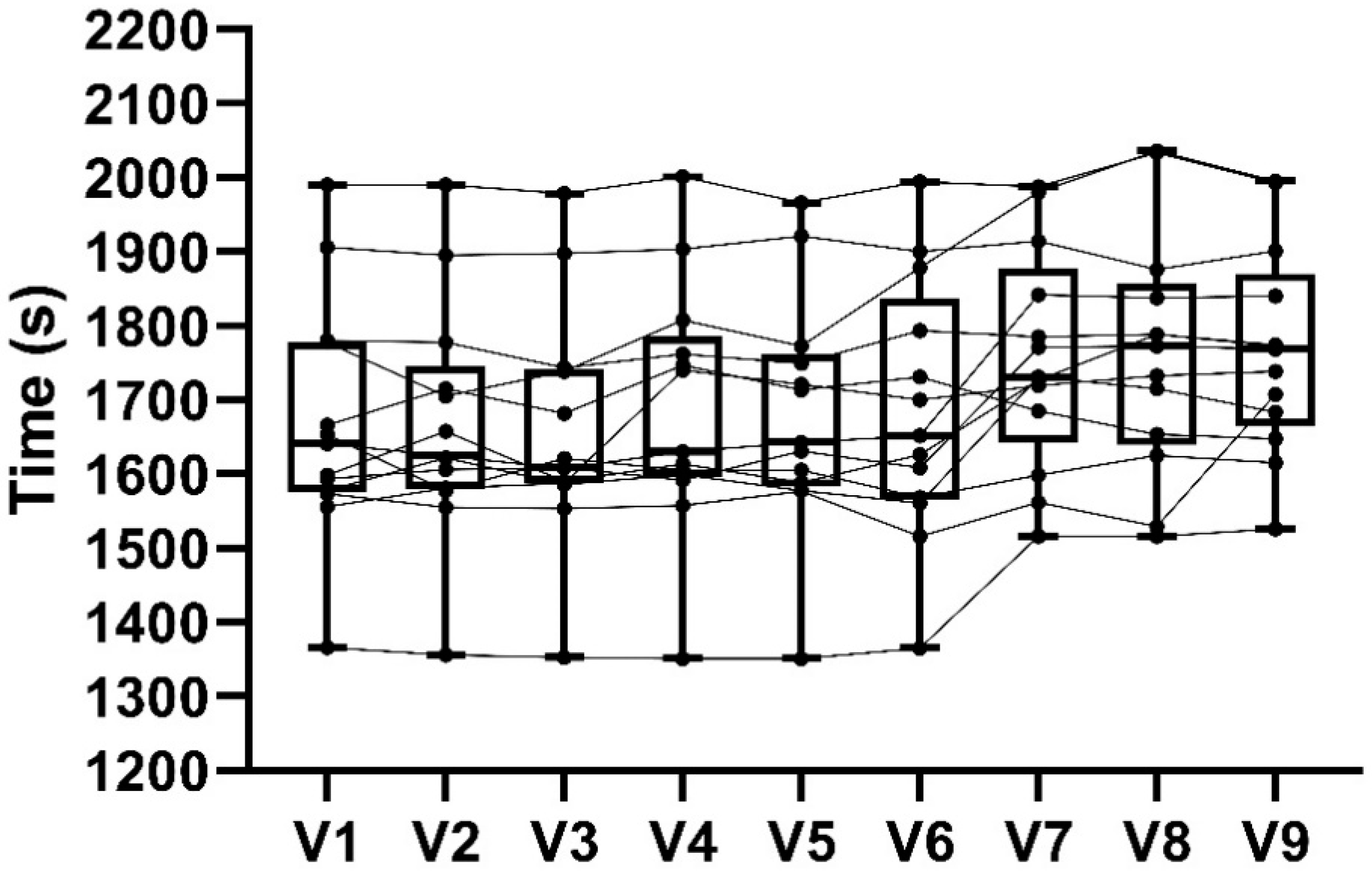
3.3. 16.1 km Time Trial Performance
4. Discussion
4.1. Metabolic and Physiological Responses
4.1.1. Lactate Curve
4.1.2. Substrate Oxidation
4.2. 16.1 km TT
5. Conclusions and Practical Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harborne, J.B.; Grayer, R.J. The Anthocyanins. The Flavonoids; Harborne, J.B., Ed.; Springer: Boston, MA, USA, 1988; pp. 1–20. [Google Scholar]
- Connolly, D.A.J.; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The effects of montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Deut. Lebensm. Rundsch. 2007, 103, 369–378. [Google Scholar]
- García-Alonso, M.; Rimbach, G.; Rivas-Gonzalo, J.C.; De Pascual-Teresa, S. Antioxidant and cellular activities of anthocyanins and their corresponding Vitisins A—Studies in platelets, monocytes, and human endothelial cells. J. Agric. Food Chem. 2004, 52, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Perkins, I.C.; Vine, S.A.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves high-intensity intermittent running. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 487–493. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Cousins, L.; Williams, D.; Blacker, S.D. Beneficial effects of New Zealand blackcurrant extract on maximal sprint speed during the Loughborough Intermittent Shuttle Test. Sports 2016, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Godwin, C.; Cook, M.D.; Willems, M.E.T. Effect of New Zealand blackcurrant extract on performance during the running based anaerobic sprint test in trained youth and recreationally active male football players. Sports 2017, 5, 69. [Google Scholar] [CrossRef]
- Powers, S.K.; Radák, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Takenami, E.; Iwasaki-Kurashige, K.; Osada, T.; Katsumura, T.; Hamaoka, T. Effects of blackcurrant anthocyanin intake on peripheral muscle circulation during typing work in humans. Eur. J. Appl. Physiol. 2004, 94, 36–45. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Willems, M.E.T. Blackcurrant alters physiological responses and femoral artery diameter during sustained isometric contraction. Nutrients 2017, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.T.; Myers, S.D.; Gault, M.L.; Cook, M.D. Beneficial physiological effects with blackcurrant intake in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Žiberna, L.; Lunder, M.; Tramer, F.; Drevenšek, G.; Passamonti, S. The endothelial plasma membrane transporter bilitranslocase mediates rat aortic vasodilation induced by anthocyanins. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.S.D.O.; De Aguiar, R.A.; Turnes, T.; Dos Santos, R.P.; De Oliveira, M.F.M.; Caputo, F. Intracellular shuttle: The lactate aerobic metabolism. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.C.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.M.; Wagenmakers, A.J.M. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Dose effects of New Zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur. J. Appl. Physiol. 2017, 117, 1207–1216. [Google Scholar] [CrossRef]
- Strauss, J.A.; Willems, M.E.T.; Shepherd, S.O. New Zealand blackcurrant extract enhances fat oxidation during prolonged cycling in endurance-trained females. Eur. J. Appl. Physiol. 2018, 118, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Arkinstall, M.J.; Bruce, C.R.; Clark, S.A.; Rickards, C.A.; Burke, L.M.; Hawley, J.A. Regulation of fuel metabolism by preexercise muscle glycogen content and exercise intensity. J. Appl. Physiol. 2004, 97, 2275–2283. [Google Scholar] [CrossRef] [Green Version]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ferrars, R.M.; Cassidy, A.; Curtis, P.J.; Kay, C.D. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol. Nutr. Food Res. 2013, 58, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of montmorency tart cherry (L. Prunus Cerasus) consumption on nitric oxide biomarkers and exercise performance. Scand. J. Med. Sci. Sport. 2018, 28, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P.E. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.M.; Peeling, P. Methodologies for investigating performance changes with supplement use. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef]
- Neveu, V.; Pérez-Jiménez, J.; Vos, F.; Crespy, V.; Du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26, S28–S37. [Google Scholar] [CrossRef]
- Newell, J.; Higgins, D.; Madden, N.; Cruickshank, J.; Einbeck, J.; McMillan, K.; McDonald, R. Software for calculating blood lactate endurance markers. J. Sports Sci. 2007, 25, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F.; Mora-Rodriguez, R.; Byerley, L.O.; Coyle, E.F. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am. J. Physiol. 1997, 273, E768–E775. [Google Scholar] [CrossRef]
- Montain, S.J.; Hopper, M.K.; Coggan, A.R.; Coyle, E.F. Exercise metabolism at different time intervals after a meal. J. Appl. Physiol. 1991, 70, 882–888. [Google Scholar] [CrossRef]
- Desai, T.; Bottoms, L.; Roberts, M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur. J. Appl. Physiol. 2018, 118, 2523–2539. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Serafini, M.; Crozier, A. Bioavailability of Pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J. Agric. Food Chem. 2008, 56, 713–719. [Google Scholar] [CrossRef]
- Klein, S.; Coyle, E.F.; Wolfe, R.R. Fat metabolism during low-intensity exercise in endurance-trained and untrained men. Am. J. Physiol. 1994, 267, E934–E940. [Google Scholar] [CrossRef]
- Watt, M.J.; Howlett, K.F.; Febbraio, M.A.; Spriet, L.L.; Hargreaves, M. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J. Physiol. 2001, 534, 269–278. [Google Scholar] [CrossRef]
- Lyall, K.A.; Hurst, S.M.; Cooney, J.M.; Jensen, D.; Lo, K.; Hurst, R.D.; Stevenson, L.M. Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R70–R81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, P.T.; Barton, M.J.; Bowtell, J.L. Montmorency cherry supplementation improves 15-km cycling time-trial performance. Eur. J. Appl. Physiol. 2019, 119, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, C.D.; Hopkins, W.G. Variation in performance of elite cyclists from race to race. Eur. J. Sport Sci. 2006, 6, 25–31. [Google Scholar] [CrossRef]
- Zavorsky, G.S.; Murias, J.M.; Gow, J.; Kim, D.J.; Poulin-Harnois, C.; Kubow, S.; Lands, L.C. Laboratory 20-km cycle time trial reproducibility. Int. J. Sports Med. 2007, 28, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Cugini, P.; Di Palma, L.; Di Simone, S.; Lucia, P.; Battisti, P.; Coppola, A.; Leone, G. Circadian rhythm of cardiac output, peripheral vascular resistance, and related variables by a beat-to-beat monitoring. Chronobiol. Int. 1993, 10, 73–78. [Google Scholar] [CrossRef]
| N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | N10 | N11 | N12 | N13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomized allocation | 1/2/3 | 2/1/3 | 3/2/1 | 3/1/2 | 3/2/1 | 2/1/3 | 3/2/1 | 3/2/1 | 2/1/3 | 3/2/1 | 1/3/2 | 3/2/1 | 2/1/3 |
| Time of testing | 6 p.m. | 6 p.m. | 3 p.m. | 6 p.m. | 9 a.m. | 6 p.m. | 6 p.m. | 6 p.m. | 8 a.m. | 6 p.m. | 9 a.m. | 1 p.m. | 4 p.m. |
| Time to complete the study (months) | 8.5 | 8 | 8.5 | 8.5 | 7 | 7 | 6.5 | 11 | 3 | 5 | 3 | 7 | 3 |
| Condition | Day 1 | Day 4 | Day 7 |
|---|---|---|---|
| Lactate (mmol∙L−1) | |||
| 30% | |||
| PLA | 0.75 ± 0.35 | 0.76 ± 0.30 | 0.76 ± 0.37 |
| 300 mg | 0.73 ± 0.20 | 0.79 ± 0.28 | 0.71 ± 0.20 |
| 600 mg | 0.67 ± 0.25 | 0.71 ± 0.18 | 0.76 ± 0.28 |
| 40% | |||
| PLA | 0.87 ± 0.45 | 0.83 ± 0.38 | 0.86 ± 0.48 |
| 300 mg | 0.79 ± 0.27 | 0.86 ± 0.40 | 0.80 ± 0.31 |
| 600 mg | 0.77 ± 0.32 | 0.81 ± 0.20 | 0.81 ± 0.26 |
| 50% | |||
| PLA | 1.21 ± 0.60 | 1.21 ± 0.49 | 1.18 ± 0.64 |
| 300 mg | 1.09 ± 0.37 | 1.15 ± 0.56 | 1.10 ± 0.51 |
| 600 mg | 1.07 ± 0.39 | 1.18 ± 0.42 | 1.16 ± 0.44 |
| 60% | |||
| PLA | 1.82 ± 0.84 | 1.89 ± 0.77 | 1.78 ± 0.85 |
| 300 mg | 1.70 ± 0.59 | 1.73 ± 0.81 | 1.85 ± 0.86 |
| 600 mg | 1.60 ± 0.53 | 1.86 ± 0.85 | 1.92 ± 0.93 |
| Condition | Day 1 | Day 4 | Day 7 |
|---|---|---|---|
| O2 (L·min−1) | |||
| PLA | 2.63 ± 0.35 | 2.62 ± 0.29 | 2.62 ± 0.29 |
| 300 mg | 2.62 ± 0.32 | 2.58 ± 0.39 | 2.64 ± 0.31 |
| 600 mg | 2.61 ± 0.33 | 2.62 ± 0.30 | 2.61 ± 0.30 |
| CO2 (L·min−1) | |||
| PLA | 2.39 ± 0.32 | 2.35 ± 0.27 | 2.37 ± 0.38 |
| 300 mg | 2.39 ± 0.34 | 2.36 ± 0.38 | 2.42 ± 0.34 |
| 600 mg | 2.37 ± 0.31 | 2.39 ± 0.30 | 2.37 ± 0.30 |
| E (L·min−1) | |||
| PLA | 56.23 ± 7.77 | 54.87 ± 6.16 | 55.77 ± 5.97 |
| 300 mg | 55.70 ± 7.45 | 54.95 ± 8.57 | 56.99 ± 7.50 |
| 600 mg | 55.89 ± 7.81 | 56.15 ± 8.17 | 56.48 ± 6.92 |
| HR (bpm) | |||
| PLA | 140 ± 13 | 140 ± 13 | 141 ± 13 |
| 300 mg | 144 ± 11 | 139 ± 12 | 142 ± 13 |
| 600 mg | 140 ± 14 | 140 ± 13 | 140 ± 12 |
| RER | |||
| PLA | 0.90 ± 0.03 | 0.89 ± 0.04 | 0.91 ± 0.03 |
| 300 mg | 0.92 ± 0.04 | 0.91 ± 0.04 | 0.91 ± 0.04 |
| 600 mg | 0.91 ± 0.04 | 0.91 ± 0.04 | 0.91 ± 0.04 |
| FATox (g·min−1) | |||
| PLA | 0.41 ± 0.18 | 0.44 ± 0.17 | 0.38 ± 0.17 |
| 300 mg | 0.34 ± 0.15 | 0.36 ± 0.18 | 0.37 ± 0.18 |
| 600 mg | 0.40 ± 0.20 | 0.37 ± 0.19 | 0.39 ± 0.16 |
| CHO (g·min−1) | |||
| PLA | 2.21 ± 0.41 | 2.13 ± 0.45 | 2.27 ± 0.53 |
| 300 mg | 2.38 ± 0.56 | 2.28 ± 0.58 | 2.34 ± 0.64 |
| 600 mg | 2.22 ± 0.54 | 2.18 ± 0.71 | 2.26 ± 0.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montanari, S.; Şahin, M.A.; Lee, B.J.; Blacker, S.D.; Willems, M.E.T. No Effects of New Zealand Blackcurrant Extract on Physiological and Performance Responses in Trained Male Cyclists Undertaking Repeated Testing across a Week Period. Sports 2020, 8, 114. https://doi.org/10.3390/sports8080114
Montanari S, Şahin MA, Lee BJ, Blacker SD, Willems MET. No Effects of New Zealand Blackcurrant Extract on Physiological and Performance Responses in Trained Male Cyclists Undertaking Repeated Testing across a Week Period. Sports. 2020; 8(8):114. https://doi.org/10.3390/sports8080114
Chicago/Turabian StyleMontanari, Stefano, Mehmet A. Şahin, Ben J. Lee, Sam D. Blacker, and Mark E.T. Willems. 2020. "No Effects of New Zealand Blackcurrant Extract on Physiological and Performance Responses in Trained Male Cyclists Undertaking Repeated Testing across a Week Period" Sports 8, no. 8: 114. https://doi.org/10.3390/sports8080114
APA StyleMontanari, S., Şahin, M. A., Lee, B. J., Blacker, S. D., & Willems, M. E. T. (2020). No Effects of New Zealand Blackcurrant Extract on Physiological and Performance Responses in Trained Male Cyclists Undertaking Repeated Testing across a Week Period. Sports, 8(8), 114. https://doi.org/10.3390/sports8080114








