Acute Low-Dose Hyperoxia during a Single Bout of High-Intensity Interval Exercise Does Not Affect Red Blood Cell Deformability and Muscle Oxygenation in Trained Men—A Randomized Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Ethical Aspects
2.2. Study Design
2.3. Aerobic Performance
2.4. Exercise Loading
2.5. Primary Outcome
Red blood cell deformability
2.6. Secondary Outcomes of Interest
2.6.1. Muscle Oxygenation
2.6.2. BLa, HR and RPE
2.6.3. Basic Blood Count
2.7. Statistical Analysis
3. Results
3.1. RBC-D
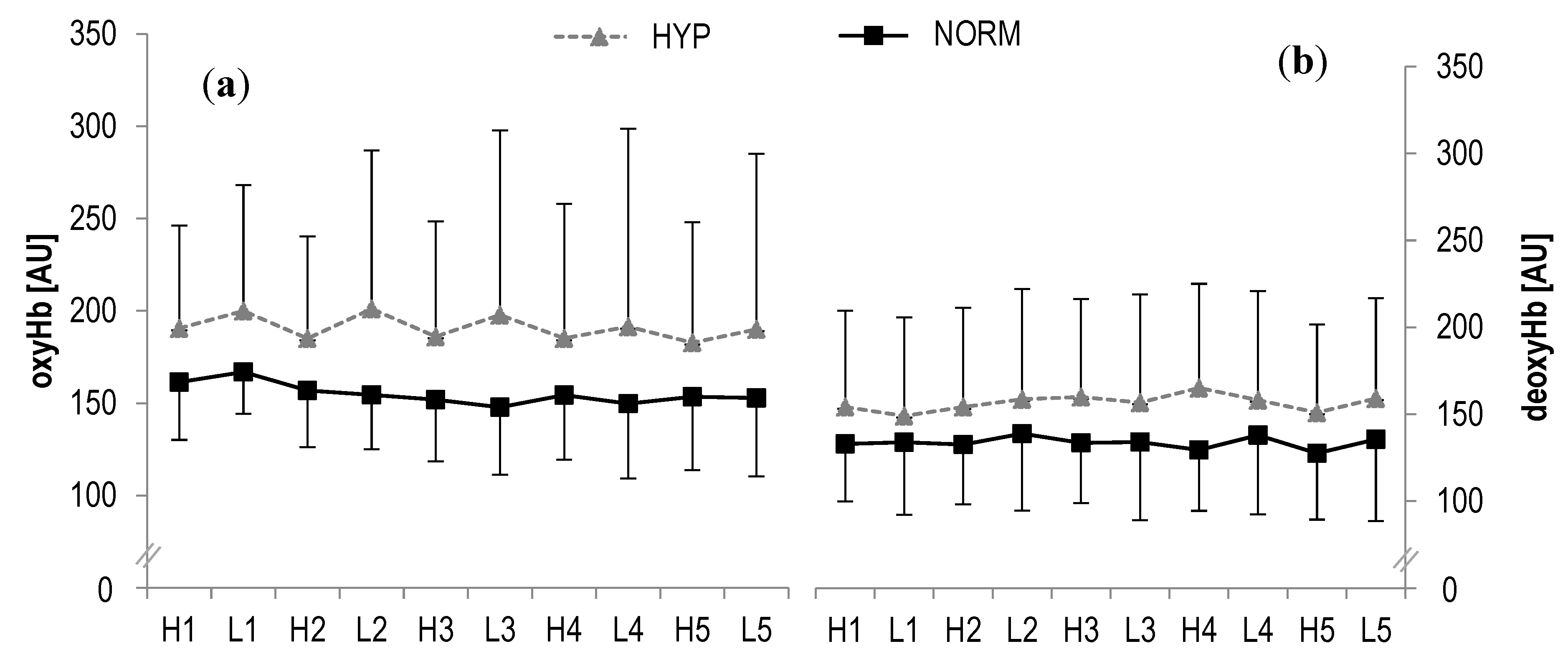
3.2. Muscle Oxygenation
3.3. BLa, HR and RPE
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bassett, D.R.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.A.; Ekblom, B. Hyperoxia for performance and training. J. Sports Sci. 2018, 36, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Agency WA-D. Manipulation of Blood and Blood Components|World Anti-Doping Agency. Available online: https://www.wada-ama.org/en/content/what-is-prohibited/prohibited-at-all-times/manipulation-of-blood-and-blood-components (accessed on 25 November 2019).
- Ekblom, B.; Huot, R.; Stein, E.M.; Thorstensson, A.T. Effect of changes in arterial oxygen content on circulation and physical performance. J. Appl. Physiol. 1975, 39, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Mallette, M.M.; Stewart, D.G.; Cheung, S.S. The Effects of Hyperoxia on Sea-Level Exercise Performance, Training, and Recovery: A Meta-Analysis. Sports Med. 2018, 49, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Sperlich, B.; Zinner, C.; Hauser, A.; Holmberg, H.C.; Wegrzyk, J. The Impact of Hyperoxia on Human Performance and Recovery. Sports Med. 2017, 47, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Prieur, F.; Benoit, H.; Busso, T.; Castells, J.; Geyssant, A.; Denis, C. Effects of moderate hyperoxia on oxygen consumption during submaximal and maximal exercise. Eur. J. Appl. Physiol. 2002, 88, 235–242. [Google Scholar] [CrossRef]
- Linossier, M.T.; Dormois, D.; Arsac, L.; Denis, C.; Gay, J.P.; Geyssant, A.; Lacour, J.R. Effect of hyperoxia on aerobic and anaerobic performances and muscle metabolism during maximal cycling exercise. Acta Physiol. Scand. 2000, 168, 403–411. [Google Scholar] [CrossRef]
- Peltonen, J.E.; Rantamäki, J.; Niittymäki, S.P.T.; Sweins, K.; Viitasalo, J.T.; Rusko, H.K. Effects of oxygen fraction in inspired air on rowing performance. Med. Sci. Sports Exerc. 1995, 27, 573–579. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Boushel, R.; Madsen, P.; Secher, N.H. Cerebral desaturation during exercise reversed by O2 supplementation. Am. J. Physiol. 1999, 277 Pt 2, 1045–1052. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10484427 (accessed on 29 March 2018). [CrossRef]
- Karpovich, P.V. The effect of oxygen inhalation on swimming performance. Res. Q. Am. Phys. Educ. Assoc. 1934, 5, 24–30. [Google Scholar] [CrossRef]
- Eves, N.D.; Petersen, S.R.; Jones, R.L. Hyperoxia improves maximal exercise with the self-contained breathing apparatus (SCBA). Ergonomics 2002, 45, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Oussaidene, K.; Prieur, F.; Bougault, V.; Borel, B.; Matran, R.; Mucci, P. Cerebral oxygenation during hyperoxia-induced increase in exercise tolerance for untrained men. Eur. J. Appl. Physiol. 2013, 113, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.; Kayser, B.; Rae, E.; Rauch, L.; Bosch, A.; Noakes, T. Hyperoxia improves 20 km cycling time trial performance by increasing muscle activation levels while perceived exertion stays the same. Eur. J. Appl. Physiol. 2007, 101, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Ploutz-Snyder, L.L.; Simoneau, J.A.; Gilders, R.M.; Staron, R.S.; Hagerman, F.C. Cardiorespiratory and metabolic adaptations to hyperoxic training. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 38–48. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8861667 (accessed on 28 March 2018). [CrossRef] [PubMed]
- Haseler, L.J.; Richardson, R.S.; Videen, J.S.; Hogan, M.C. Phosphocreatine hydrolysis during submaximal exercise: The effect of FI(O2). J. Appl. Physiol. 1998, 85, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Richardson, R.S.; Haseler, L.J. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: A31P-MRS study. J. Appl. Physiol. 1999, 86, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Glazier, L.; Watt, M.J.; LeBlanc, P.J.; Heigenhauser, G.J.F.; Spriet, L.L. Effects of hyperoxia on skeletal muscle carbohydrate metabolism during transient and steady-state exercise. J. Appl. Physiol. 2005, 98, 250–256. [Google Scholar] [CrossRef]
- Hogan, M.C.; Cox, R.H.; Welch, H.G. Lactate accumulation during incremental exercise with varied inspired oxygen fractions. J. Appl. Physiol. 1983, 55, 1134–1140. [Google Scholar] [CrossRef]
- Goodrich, J.A.; Ryan, B.J.; Byrnes, W.C. The Influence of Oxygen Saturation on the Relationship Between Hemoglobin Mass and VO2max. Sports Med. Int. Open 2018, 2, E98–E104. [Google Scholar] [CrossRef]
- Harms, C.A.; McClaran, S.R.; Nickele, G.A.; Pegelow, D.F.; Nelson, W.B.; Dempsey, J.A. Effect of exercise-induced arterial O2 desaturation on VO2max in women. Med. Sci. Sports Exerc. 2000, 32, 1101–1108. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10862536 (accessed on 22 November 2018). [CrossRef]
- Williams, J.H.; Powers, S.K.; Stuart, M.K. Hemoglobin desaturation in highly trained athletes during heavy exercise. Med. Sci. Sports Exerc. 1986, 18, 168–173. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3702644 (accessed on 26 September 2019). [CrossRef] [PubMed]
- Connes, P.; Bouix, D.; Durand, F.; Kippelen, P.; Mercier, J.; Prefaut, C.; Brun, J.F.; Caillaud, C. Is hemoglobin desaturation related to blood viscosity in athletes during exercise? Int. J. Sports Med. 2004, 25, 569–574. [Google Scholar] [CrossRef]
- Mairbäurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Jenkins, D. Factors Affecting the Rate of Phosphocreatine Resynthesis Following Intense Exercise. Sports Med. 2002, 32, 761–784. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, K.; Harris, R.C.; Hultman, E. Resynthesis of creatine phosphate in human muscle after exercise in relation to intramuscular pH and availability of oxygen. Scand. J. Clin. Lab. Investig. 1979, 39, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Bouix, D.; Py, G.; Prefaut, C.; Mercier, J.; Brun, J.F.; Caillaud, C. Opposite effects of in vitro lactate on erythrocyte deformability in athletes and untrained subjects. Clin. Hemorheol. Microcirc. 2004, 31, 311–318. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15567902 (accessed on 8 June 2018).
- Brugniaux, J.V.; Coombs, G.B.; Barak, O.F.; Dujic, Z.; Sekhon, M.S.; Ainslie, P.N. Highs and lows of hyperoxia: Physiological, performance, and clinical aspects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1–R27. [Google Scholar] [CrossRef]
- Ulker, P.; Ozen, N.; BasralA, F.; Cengiz, M. Acute and Short Term Hyperoxemia: How about Hemorheology and Tissue Perfusion? J. Intensive Crit. Care 2017, 3, 18. [Google Scholar] [CrossRef]
- Lindholm, P.; Larsson, Å.; Frånberg, O.; Gullstrand, L. A Portable Device for Intermittent Oxygen Supplementation during High-Intensity Exercise. J. Biomed. Sci. Eng. 2017, 10, 304–316. [Google Scholar] [CrossRef]
- Chatila, W.; Nugent, T.; Vance, G.; Gaughan, J.; Criner, G.J. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest 2004, 126, 1108–1115. [Google Scholar] [CrossRef]
- Burgos, C.; Henríquez-Olguín, C.; Andrade, D.C.; Ramírez-Campillo, R.; Araneda, O.F.; White, A.; Cerda-Kohler, H. Effects of exercise training under hyperbaric oxygen on oxidative stress markers and endurance performance in young soccer players: A pilot study. J. Nutr. Metab. 2016, 2016, 5647407. [Google Scholar] [CrossRef] [PubMed]
- Lock, S.H.; Blower, G.; Prynne, M.; Wedzicha, J.A. Comparison of liquid and gaseous oxygen for domiciliary portable use. Thorax 1992, 47, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Nasilowski, J.; Przybylowski, T.; Zielinski, J.; Chazan, R. Comparing supplementary oxygen benefits from a portable oxygen concentrator and a liquid oxygen portable device during a walk test in COPD patients on long-term oxygen therapy. Respir. Med. 2008, 102, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Burns, B.; Goadsby, P.J. High-flow oxygen for treatment of cluster headache: A randomized trial. JAMA J. Am. Med. Assoc. 2009, 302, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Lawler, J.; Dempsey, J.A.; Dodd, S.; Landry, G. Effects of incomplete pulmonary gas exchange on VO2 max. J. Appl. Physiol. 1989, 66, 2491–2495. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Hollmann, W.; Venrath, H. Höchst- und Dauerleistungsfähigkeit des Sportlers: Spiroergometrische Beurteilung und Untersuchungsergebnisse von Männlichen Und Weiblichen Personen des 1. bis 8. Lebensjahrzehnts; Barth: Munich, Germany, 1963. [Google Scholar]
- Kuipers, H.; Verstappen, F.T.J.; Keizer, H.A.; Geurten, P.; van Kranenburg, G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int. J. Sports Med. 1985, 6, 197–201. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Wisloff, U.; Stoylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior Cardiovascular Effect of Aerobic Interval Training versus Moderate Continuous Training in Heart Failure Patients: A Randomized Study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef]
- Tschakert, G.; Hofmann, P. High-intensity intermittent exercise: Methodological and physiological aspects. Int. J. Sports Physiol. Perform. 2013, 8, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Schumann, M.; Schulz, H.; Hackney, A.C.; Bloch, W. Feasibility of high-intensity interval training with hyperoxia vs. intermittent hyperoxia and hypoxia in cancer patients undergoing chemotherapy—Study protocol of a randomized controlled trial. Contemp. Clin. Trials Commun. 2017, 8, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.J.; Baskurt, O.K.; Meiselman, H.J.; Marshall-Gradisnik, S.M. A comparison of capillary and venous blood sampling methods for the use in haemorheology studies. Clin. Hemorheol. Microcirc. 2011, 47, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Data reduction methods for ektacytometry in clinical hemorheology. Clin. Hemorheol. Microcirc. 2013, 54, 99–107. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.J.; Collison, J.; Cooper, C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018, 23, 15007–15014. [Google Scholar] [CrossRef]
- Thorniley, M.S.; Sinclair, J.S.; Barnett, N.J.; Shurey, C.B.; Green, C.J. The use of near-infrared spectroscopy for assessing flap viability during reconstructive surgery. Br. J. Plast Surg. 1998, 51, 218–226. [Google Scholar] [CrossRef]
- Blake, O.M.; Champoux, Y.; Wakeling, J.M. Muscle coordination patterns for efficient cycling. Med. Sci. Sports Exerc. 2012, 44, 926–938. [Google Scholar] [CrossRef]
- da Silva, J.C.L.; Tarassova, O.; Ekblom, M.M.; Andersson, E.; Ronquist, G.; Arndt, A. Quadriceps and hamstring muscle activity during cycling as measured with intramuscular electromyography. Eur. J. Appl. Physiol. 2016, 116, 1807–1817. [Google Scholar] [CrossRef]
- Chin, L.M.K.; Kowalchuk, J.M.; Barstow, T.J.; Kondo, N.; Amano, T.; Shiojiri, T.; Koga, S. The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J. Appl. Physiol. 2011, 111, 1259–1265. [Google Scholar] [CrossRef]
- Bakker, A.; Smith, B.; Ainslie, P.; Smith, K. Near-Infrared Spectroscopy. In Applied Aspects of Ultrasonography in Humans; Ainslie, P., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Shin, S. Advances in the measurement of red blood cell deformability: A brief review. J. Cell. Biotechnol. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Suhr, F.; Brenig, J.; Müller, R.; Behrens, H.; Bloch, W.; Grau, M. Moderate Exercise Promotes Human RBC-NOS Activity, NO Production and Deformability through Akt Kinase Pathway. PLoS ONE. 2012, 7, e45982. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Bloch, W.; Mester, J.; Born, D.P.; Sperlich, B. Effects of different levels of compression during sub-maximal and high-intensity exercise on erythrocyte deformability. Eur. J. Appl. Physiol. 2012, 112, 2163–2169. [Google Scholar] [CrossRef]
- Connes, P.; Simmonds, M.J.; Brun, J.F.; Baskurt, O.K. Exercise hemorheology: Classical data, recent findings and unresolved issues. Clin. Hemorheol. Microcirc. 2013, 53, 187–199. [Google Scholar] [CrossRef]
- Uyuklu, M.; Meiselman, H.J.; Baskurt, O.K. Effect of hemoglobin oxygenation level on red blood cell deformability and aggregation parameters. Clin. Hemorheol. Microcirc. 2009, 41, 179–188. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Temiz, A.; Meiselman, H.J. Effect of superoxide anions on red blood cell rheologic properties. Free Radic. Biol. Med. 1998, 24, 102–110. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9436619 (accessed on 13 June 2018). [CrossRef]
- Kwak, D.J.; Kwak, S.D.; Gauda, E.B. The Effect of Hyperoxia on Reactive Oxygen Species (ROS) in Rat Petrosal Ganglion Neurons During Development Using Organotypic Slices. Pediatr. Res. 2006, 60, 371–376. [Google Scholar] [CrossRef]
- Brueckl, C.; Kaestle, S.; Kerem, A.; Habazettl, H.; Krombach, F.; Kuppe, H.; Kuebler, W.M. Hyperoxia-Induced Reactive Oxygen Species Formation in Pulmonary Capillary Endothelial Cells In Situ. Am. J. Respir. Cell Mol. Biol. 2006, 34, 453–463. [Google Scholar] [CrossRef]
- Wilson, C.; González-Billault, C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: Implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S.; Ferrari, M. Muscle Oximetry in Sports Science: A Systematic Review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Hamaoka, T. Near-Infrared Spectroscopy: What Can It Tell Us about Oxygen Saturation in Skeletal Muscle? Exerc. Sport Sci. Rev. 2000, 28, 123–127. [Google Scholar] [PubMed]
- Plet, J.; Pedersen, P.K.; Jensen, F.B.; Hansen, J.K. Increased working capacity with hyperoxia in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 171–177. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1396641 (accessed on 28 March 2018). [CrossRef] [PubMed]




| Characteristics | Values |
|---|---|
| N | 13 |
| Age [y] | 23.6 ± 2.5 |
| Height [cm] | 184.8 ± 5.3 |
| Weight [kg] | 78.5 ± 9.0 |
| Fat mass [kg] | 11.2 ± 2.4 |
| Fat mass [%] | 14.1 ± 1.9 |
| Lean mass [kg] | 67.4 ± 7.0 |
| Lean mass [%] | 85.9 ± 1.9 |
| VO2peak [mL min−1∙kg−1] | 53.3 ± 5.2 |
| Wmax [W] | 310 ± 34 |
| PO [W∙kg−1] | 4.0 ± 0.2 |
| HYP | NORM | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Pre | Post | Δ | 95%CI | Pre | Post | Δ | 95%CI |
| RBC [106 μL] | 4.9 ± 0.3 | 5.2 ± 0.3 * | 0.26 | 0.15; 0.37 | 5.0 ± 0.4 | 5.2 ± 0.5 * | 0.23 | 0.14; 0.32 |
| HGB [g dL−1] | 15.4 ± 1.2 | 16.2 ± 1.1 * | 0.80 | 0.44; 1.16 | 15.5 ± 1.3 | 16.2 ± 1.3 * | 0.72 | 0.41; 1.03 |
| HCT [%] | 45.0 ± 2.9 | 47.3 ± 2.6 * | 2.37 | 1.35; 3.39 | 45.4 ± 3.3 | 47.7 ± 3.6 * | 2.27 | 1.38; 3.16 |
| Ratio SS1/2 to EImax | 2.38 ± 0.3 | 2.29 ± 0.3 | −0.10 | −0.22; 0.02 | 2.34 ± 0.3 | 2.19 ± 0.3 * | −0.16 | −0.23; −0.08 |
| BLa [mmol∙L−1] | 1.2 ± 0.4 | 8.6 ± 3.0 * | 7.4 | 5.42; 9.42 | 1.2 ± 0.3 | 10.0 ± 2.5 * | 8.8 | 7.15; 10.45 |
| HR [beats∙min−1 ] | 85.9 ± 8.0 | 179.5 ± 11.2 * | 93.6 | 84.4; 102.8 | 81.6 ± 10.6 | 174.3 ± 11.4 * | 92.8 | 83.0; 102.6 |
| RPE [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] | 6.2 ± 0.4 | 19.1 ± 0.8 * | 12.9 | 12.1; 13.7 | 6.1 ± 0.3 | 18.1 ± 1.5 * | 12.0 | 10.8; 13.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitag, N.; Böttrich, T.; Weber, P.D.; Manferdelli, G.; Bizjak, D.A.; Grau, M.; Sanders, T.C.; Bloch, W.; Schumann, M. Acute Low-Dose Hyperoxia during a Single Bout of High-Intensity Interval Exercise Does Not Affect Red Blood Cell Deformability and Muscle Oxygenation in Trained Men—A Randomized Crossover Study. Sports 2020, 8, 4. https://doi.org/10.3390/sports8010004
Freitag N, Böttrich T, Weber PD, Manferdelli G, Bizjak DA, Grau M, Sanders TC, Bloch W, Schumann M. Acute Low-Dose Hyperoxia during a Single Bout of High-Intensity Interval Exercise Does Not Affect Red Blood Cell Deformability and Muscle Oxygenation in Trained Men—A Randomized Crossover Study. Sports. 2020; 8(1):4. https://doi.org/10.3390/sports8010004
Chicago/Turabian StyleFreitag, Nils, Tim Böttrich, Pia D. Weber, Giorgio Manferdelli, Daniel A. Bizjak, Marijke Grau, Tanja C. Sanders, Wilhelm Bloch, and Moritz Schumann. 2020. "Acute Low-Dose Hyperoxia during a Single Bout of High-Intensity Interval Exercise Does Not Affect Red Blood Cell Deformability and Muscle Oxygenation in Trained Men—A Randomized Crossover Study" Sports 8, no. 1: 4. https://doi.org/10.3390/sports8010004
APA StyleFreitag, N., Böttrich, T., Weber, P. D., Manferdelli, G., Bizjak, D. A., Grau, M., Sanders, T. C., Bloch, W., & Schumann, M. (2020). Acute Low-Dose Hyperoxia during a Single Bout of High-Intensity Interval Exercise Does Not Affect Red Blood Cell Deformability and Muscle Oxygenation in Trained Men—A Randomized Crossover Study. Sports, 8(1), 4. https://doi.org/10.3390/sports8010004






