Abstract
This article reviews tendon and ligament injury incidence and severity within elite rugby union and rugby league. Furthermore, it discusses the biological makeup of tendons and ligaments and how genetic variation may influence this and predisposition to injury. Elite rugby has one of the highest reported injury incidences of any professional sport. This is likely due to a combination of well-established injury surveillance systems and the characteristics of the game, whereby high-impact body contact frequently occurs, in addition to the high intensity, multispeed and multidirectional nature of play. Some of the most severe of all these injuries are tendon and ligament/joint (non-bone), and therefore, potentially the most debilitating to a player and playing squad across a season or World Cup competition. The aetiology of these injuries is highly multi-factorial, with a growing body of evidence suggesting that some of the inter-individual variability in injury susceptibility may be due to genetic variation. However, little effort has been devoted to the study of genetic injury traits within rugby athletes. Due to a growing understanding of the molecular characteristics underpinning the aetiology of injury, investigating genetic variation within elite rugby is a viable and worthy proposition. Therefore, we propose several single nucleotide polymorphisms within candidate genes of interest; COL1A1, COL3A1, COL5A1, MIR608, MMP3, TIMP2, VEGFA, NID1 and COLGALT1 warrant further study within elite rugby and other invasion sports.
1. Introduction
Due to the characteristics of the game of rugby, whereby high-impact body contact frequently occurs through multiple physical collisions and tackles, musculoskeletal injuries are extremely common [1,2]. Rugby Union (RU) has one of the highest reported incidences of match injuries within professional sports, regardless of the injury definition used [3]. This is likely in part due to the well-established and frequently applied injury surveillance research compared to other collision sports. Rugby League (RL) does not currently have a comparable level of injury surveillance research which limits our understanding somewhat. The majority of injuries in both RU and RL occur during tackles [1,4,5,6,7,8]. However, numerous other causes have been documented, including but not limited to rucks, mauls, scrums [9] and via tripping, twisting, slipping, falling, overexertion, and overuse [10]. A meta-analysis by Williams et al. [11] reported the total incidence of injury (injuries per 1000 player h) as 81/1000 in matches (~3 injuries per match) and 3/1000 in training in men’s professional RU.
The regular occurrence of injury in RU limits competitive success. For example, Williams et al.’s [12] recent seven-year prospective study assessing playing time loss from injury and team success in elite RU found clear negative associations between injury measures (injury burden and injury days per match) and team success (league points tally and Euro Rugby club ranking). Thus, reductions in injury incidence and severity could enhance team success.
Due to the high incidence of injury in RU, numerous injury surveillance studies have been conducted during international competitions, particularly during the five Rugby World Cups from 1995 to 2015 [7,8,13,14], as well as single and multiple seasons for professional [6,15,16] and community level rugby [17,18]. Although numerous injury surveillance studies have been carried out in RU, only studies from 2007 were consistent with the international consensus statement for epidemiological studies in rugby [19]. Therefore, comparisons with earlier studies are problematic. This consistency has not existed to the same degree for RL, although recent steps have been taken towards a consensus-driven approach [20].
Injury data collection is an essential part of trying to understand the risk (incidence and severity) of participation in sports and how that risk changes over time. van Mechelen et al. [21] designed a four-step model for injury prevention within sport. It involves (i) identifying the extent of the sports injury problem, (ii) identifying the characteristics and mechanisms that contribute to the development of injury, (iii) introducing measures to reduce future risk and/or severity of injury, (iv) an evaluation of those measures by repeating the first step. A similar risk management model was proposed by Fuller and Drawer [22], which aimed to identify risk factors and estimates that could be evaluated and then communicated to the sports community. Having a deeper understanding of these areas enables coaches, doctors and strength and conditioning staff to assess current practices in injury prevention, treatment, and rehabilitation, and make adjustments accordingly. It also allows governing bodies to identify areas of high risk and to introduce strategies to mitigate such a risk. Finally, longitudinal injury data allows researchers to monitor the impact and effectiveness of any interventions. What is apparent from the research undertaken thus far is that injuries vary considerably in location, diagnosis and profile.
Fundamental understanding of injury mechanisms and differences in inter-individual risk begins with the genome and the biological composition of tissues that depend on coordinated expression of selected genes at the protein level. Being able to utilize these genetic data alongside the traditional injury prevention practices may enable a more personalized approach to injury risk management at the elite level of rugby. The aim of this narrative review, therefore, is firstly to highlight the incidence and severity rates of tendon and ligament injury within elite rugby. Secondly, to discuss the biological composition of tendons and ligaments and how genomics may influence this and subsequent predisposition to injury. The steps necessary to better understand the genomic aspects of injury within elite rugby will then be considered. A structured literature search was performed for empirical research studies and review articles. The search terms included “elite rugby injury”, “injury mechanisms in rugby”, “tendon and ligament epidemiology”, “pathophysiology of tendon and ligament injury”, “molecular characteristics of tendon and ligament injury”, “genetics of tendon and ligament injury”. The reference lists of all articles were also examined for eligible studies to minimize the possibility of relevant articles being omitted.
2. Tendon and Ligament Injury Incidence Rates and Severity in Rugby
2.1. Tendon and Ligament Injury Incidence Rates in Rugby
Numerous injury surveillance studies have been carried out within professional RL, with muscle/tendon and ligament/joint (non-bone) injuries consistently the two most frequent types of injury [4,23,24,25,26,27]. However, the majority of professional RL studies are dated, have limited application to present day RL, and inconsistent methodological approaches and definitions were used. Cross et al. [28] demonstrated the importance of utilizing consistent definitions for injury by showing that incidence of injury with a >24-hour time-loss definition was approximately double that when using a >7 day definition. For example, Gissane et al.’s [23] injury definition was “the onset of pain or a disability resulting from either training for or playing rugby league,” while Seward et al.’s [24] definition was “that which caused a player to be unavailable for selection in a match, or participation in a training session or any other injury which required medical treatment, other than routine conservative measures.” These differences provide substantially different portrayals of injury risk. When the injury definition is more exclusive and includes only more severe injuries, joint/ligament injuries are most frequent. However, when the definition is more inclusive, muscular, head and neck injuries are most frequent [2]. This has led to much debate on definitions of injury within RL [29,30,31]. A very recent attempt was made at a consensus-driven approach to standardize epidemiological studies in RL [20], and these data are probably more valid than those previously reported. Three different ligament injuries were in the top five for incidence: medial collateral ligament (MCL) 3.9/1000 h, syndesmosis 2.7/1000 h, ankle lateral ligament 2.6/1000 h [20].
In RU, injury incidence rates are easier to identify than RL due to the consensus statement on injury definitions and data collection procedures for studies in RU [19]. However, much like RL, muscle/tendon and ligament/joint (non-bone) injuries are consistently the top two most frequently occurring injury groups in elite RU [6,7,8,11,14,32] with more muscle/tendon injuries in backs than forwards at English Premiership and International level. For ligament/joint (non-bone) injuries, forwards appear to have more frequent occurrence at international level, while backs have more at English Premiership level [6,7,14]. It should be noted, however, that these apparent differences between forwards and backs are based on data provided in the literature but not statistical testing. Table 1 summarizes the match injury incidence of muscle/tendon and ligament/joint (non-bone) injuries from post-2007 studies where methodologies align with the consensus statement on injury definitions and data collection procedures [19]. It is worth noting that, at World Cup competitions, although muscle/tendon injuries have a high incidence, this is mainly due to the presence of muscle rather than tendon injuries [7,8,14]. It is likely that this also occurs in the English Premiership and Super 14 competitions, but the data are not clear.

Table 1.
Muscle/tendon and ligament/joint (non-bone) injury incidence rates in elite rugby union.
In the English Premiership RU competition across the seven most recently reported seasons from 2011–2018, ligament injuries were consistently amongst the top five most common injuries [16,37,38], with MCL in the top five every season apart from 2015–2016. The Professional Rugby Injury Surveillance Project (PRISP) reports individual injuries such as MCL, hamstring or ankle lateral ligament, rather than grouping all muscle/tendon or ligament/joint (non-bone) injuries together. Outside of the top five injuries, there are no available data on further muscle/tendon and ligament/joint (non-bone) injuries, making more detailed or grouped analysis impossible. Figure 1 shows the top five most common match injuries in the English Premiership competition during 2011–2018, highlighting the frequency of ligament injuries.
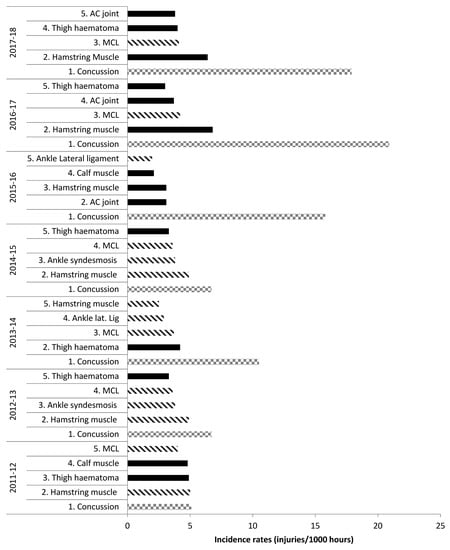
Figure 1.
Top five most common injuries: English Premiership Rugby. Adapted from the Professional Rugby Injury Surveillance Project (PRISP) annual reports 2011–2018 [38,39]. Key: Lined bars = ligament injuries; squared bars = concussion; filled bars = any other injury.
2.2. Tendon and Ligament Injury Severity and Burden in Rugby
The current literature is limited regarding the severity (days absence from full training or match play) of injuries at specific anatomical locations in elite RL. From the available data, Gibbs [40] found ankle ligament tears were the most severe, followed by MCL tears and groin muscle/tendon tears. More recently, Orchard [41] stated anterior cruciate ligament (ACL) tears were the most severe, followed by shoulder sprains and dislocations and MCL tears. This is supported by Fitzpatrick et al. [20], although that study calculated severity from date of occurrence until date of return to full training, which differs from the RU’s consensus statement on injury definitions and data collection procedures [19] and would increase severity data. These studies suggest that ligament/joint (non-bone) and muscle/tendon injuries are the main causes of RL players missing matches, thus impairing competitive success and player wellbeing.
Rugby union has similar but more consistent findings to RL, with muscle/tendon and ligament/joint (non-bone) injuries making up three of the top five most severe injuries for forwards; ACL, Achilles tendon and MCL injuries caused 988, 726 and 718 days absence, respectively [6]. For backs, three of the top five most severe were hamstring muscle, MCL, and ACL injuries causing 1176, 870 and 815 days absence, respectively [6]. Knee injuries in particular (ACL and MCL) resulted in the greatest absence for forwards and backs [6]. At the 2007 RU World Cup, muscle/tendon (mainly muscle) and ligament/joint (non-bone) were the third and fourth most severe injuries, with backs having a higher severity of both [14] (not tested statistically). At the 2011 RU World Cup, ligament/joint (non-bone) and muscle/tendon (mainly tendon) were the third and fourth most severe injuries with backs again having a higher severity of both [7] (not tested statistically). Fuller et al. [8] identified knee ligament injuries as the most severe and Achilles tendon injuries as the fourth most severe at the 2015 RU World Cup for all players. In Williams et al.’s [11] meta-analysis, a similar pattern was seen, with ligament/joint (non-bone) injuries the second most severe and muscle/tendon injuries the fourth. Table 2 summarises the severity of muscle/tendon and ligament/joint (non-bone) injuries for English Premiership and World Cup competitions. The large variability can be attributed to several factors such as different settings (league or cup tournament), cohort sizes and opportunities for data collection.

Table 2.
Muscle/tendon and ligament/joint (non-bone) injury severity rates in elite rugby union.
For injury burden (days absence/1000 h), in the English Premiership competition across 2011–2018, ligament/joint (non-bone) injuries dominated the top five highest risk match injuries. Three different ligament injuries were usually in the top five highest risk injuries (all except 2015–2016 and 2017–2018 when there were two), with ACL and MCL injuries included every season (apart from 2017–2018 when ACL was not) [15,16,37,38]. Figure 2 features the top five highest risk injuries during 2011–2018.
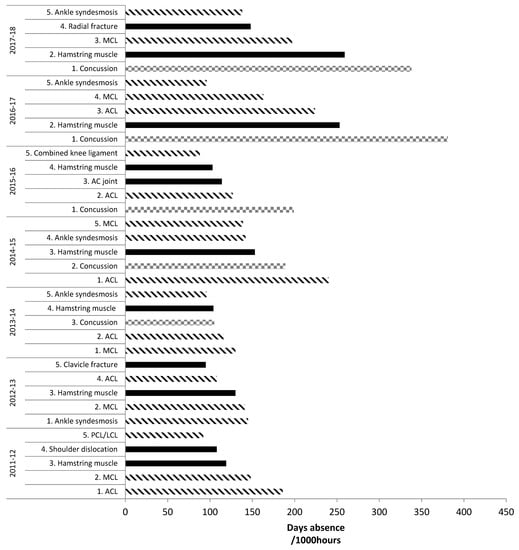
Figure 2.
Top five highest risk match injuries: English Premiership Rugby. Adapted from PRISP annual reports, 2011–2018 [38,39]. Key: lined bars = ligament injuries; squared bars = concussion; filled bars = any other injury.
In elite rugby, muscle/tendon and ligament/joint (non-bone) injuries are some of the most severe and frequently occurring injuries players receive and are, therefore, extremely debilitating to playing squads. Generally, in elite RU, there appears to be a trend towards more severe injuries [39]. Whether this is due to the more conservative approach to injury management or increased damage caused by larger collisions remains to be established. A deeper understanding of the potential causes and, subsequently, any preventative measures against these injuries would be of great value to both governing bodies and medical staff.
3. Risk Factors for Injury in Rugby
From the available literature, it is difficult to state exactly how each muscle/tendon and ligament/joint (non-bone) injury occurred during rugby matches or training. Nevertheless, the most common causes of injury in RL and RU are tackles and physical collisions [42], with the ball carrier generally at highest risk [43], though not for concussion [44]. Further risk factors for injury in rugby identified in previous literature are: playing position [5,6], level of play [11], training volume and load [45,46,47], ground conditions and playing surface [48], anthropometric characteristics [49,50], previous injury [51] including concussion [52,53], physiological characteristics [11,50] and age [54]. The precise mechanisms of tendon and ligament injury are not well understood [55,56], with multiple factors probably involved [55,56]. It has been suggested that interactions between genetic and environmental factors can amplify intrinsic risk factors (anthropometry, physiological characteristics, etc.) and place a predisposed athlete at higher risk of injury once an inciting event occurs [55,57,58]. For example, during typical physiological environments the matrix of ligaments and tendons will adapt in response to load [59]. However, variation in the loading pattern such as higher strains or a higher volume of low strains could lead to maladaptation, resulting in degeneration or a failed healing response [59], and thus injury (Figure 3). The tolerable load varies between individuals, resulting in large inter-individual variation in response to ligament and tendon tissue loading. This large inter-individual variation is thought to be partly due to a genetic component [60], meaning some individuals are more predisposed to ligament and tendon injury than others.
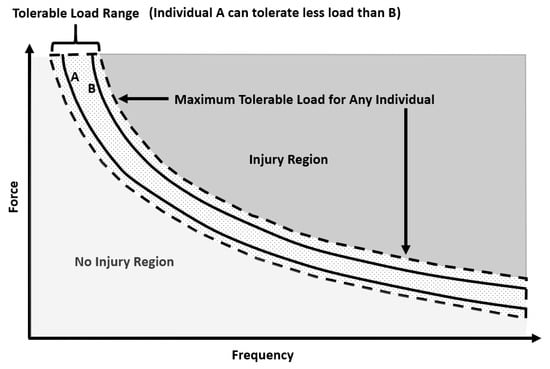
Figure 3.
Hypothetical curve illustrating the relationship between magnitude (force) and frequency of load, which can injure the tendon or ligament (Injury Region). The tolerable load range for a given population is indicated by the dashed lines. The tolerable load curves for two hypothetical individuals are indicated by the solid lines (A can tolerate less load than B). Adapted from Reference [61].
4. Tendon and Ligament Pathologies
4.1. Tendinopathy
Tendons, especially the Achilles, are designed to tolerate significant loads. Mechanical loading of tendon leads to an increase in collagen gene expression and an upturn in collagen protein complex synthesis, which is likely regulated by the strain experienced by local tenocytes [62]. The increased collagen formation peaks ~24 h after substantial mechanical loading, while the degradation of collagen proteins also increases after loading but appears to peak earlier [62]. Thus, maintaining tendon homeostasis is a finely tuned process, and despite a tendon’s ability to adjust to mechanical loading, overuse will potentially result in injury such as tendinopathy.
Traditionally, “tendinitis” was the preferred term to describe chronic pain in a symptomatic tendon, which implied that inflammatory processes played a central role in the disease aetiology. However, treatment protocols designed to modify inflammation had limited success [63,64] and few or no inflammatory cells were found in symptomatic tissue [65,66]. Therefore, the terms “tendinosis” or more generally “tendinopathy” are now preferred [67]. Tendinopathy is a diverse clinical syndrome associated with swelling, pain, impaired tissue healing and decreased performance [68]. There appears to be a continuum between physiology and pathology; as such, overuse (e.g., excessive repetitive loading of the tendon) could be considered the primary cause of disease [67].
Biomolecular studies of tendinopathy are relatively sparse although some observations have been made. Increased expression of messenger RNA (mRNA) has been found for type I and III collagens within symptomatic tendons [62,69]. This could reflect decreases in total collagen content (and a biological attempt to compensate) and an increased ratio of type III collagen relative to type I [70,71,72]. This increased proportion of type III collagen within the main fiber bundles appears to reduce fibril diameter [73], probably weakening the tendon and increasing risk of rupture [74].
Tendinopathies are caused by multiple intrinsic and/or extrinsic risk factors [55]. Common intrinsic risk factors include age, anthropometry, sex, anatomical factors, hyperthermia, previous injury and systemic diseases [75,76], with genetic variation also recently proposed [77]. Common extrinsic risk factors include environmental conditions, shoes/surface, training errors, nutrition, medication and mechanical loading [75,76,77]. Anatomical factors such as alignment and suboptimal biomechanics could contribute to two-thirds of Achilles tendon disorders among athletes [78]. Low-level highly repetitive strains below the failure threshold, or high strains even without great repetition, cause tendon degeneration [79]. Thus, excessive loading during physical training is considered the primary extrinsic determinant of tendon degeneration [80]. In the presence of intrinsic risk factors such as genetic predisposition, excessive loading may therefore further increase the risk of tendinopathy. There is no direct evidence of what causes tendinopathy in rugby players, although potential causes include: differing ground conditions that change the magnitude and temporal characteristics of the loads experienced; running and certain contact situations that elevate low-level repetitive loading; tackling, scrums, and mauls that elicit high strain; excessive training and match volume (insufficient recovery and/or excessive loading).
4.2. Tendon Rupture
Tendon rupture is an acute injury where partial or complete tearing of the tendon occurs. This is observed at the microscopic and macroscopic level, whereas tendinopathy occurs without macroscopic tearing [81]. Partial or complete rupture will inhibit tendon continuity, limiting range of motion and force-generating capabilities. Extrinsic risk factors are thought to dominate tendon rupture incidence, with intrinsic risk factors also considered important [82]. Intrinsic and extrinsic risk factors for tendon rupture are similar to those mentioned for tendinopathy, although rupture often follows one isolated overloading event [62,83,84,85,86,87]. In rugby, this is probably through high-loading scenarios such as scrums, mauls, sprinting, tackling and landing from jumps. During loading, the crimping formation of the collagen within the tendon is lost, and the collagen responds to the increasing load linearly [88]. Tendon strain >4% causes microscopic tearing of fibers and strain >8-10% causes macroscopic failure and rupture [88,89]. The aetiology of tendon rupture is not completely understood [90]. However, it appears to be multi-factorial, typically involving a combination of excessive loading and intrinsic risk factors [91]. Histologically, degenerative tendinopathy is the most frequent finding in acute tendon ruptures [92].
4.3. Molecular Changes in Tendinopathy and Tendon Rupture
Gene expression is altered in symptomatic tendons. Increased mRNA expression has been reported for proteoglycans such as aggrecan and biglycan [93], decorin and versican [94], glycoproteins such as tenascin-C and fibronectin [62], angiogenic factors such as vascular endothelial growth factor (VEGF) [95], collagen type I [94], tissue inhibitor of metallaoproteinase 1 (TIMP 1) and 2 [94], and proteolytic enzymes such as the disintegrin and metalloproteinase (ADAM-12) [96], plus several matrix metalloproteinases (MMPs 1, 2, 9, 13, and 23) [94,96]. Conversely, decreased mRNA expression has been reported for TIMP3 and MMPs 3, 10, and 12 [96]. However, the molecular signature of tendinopathy appears quite different from that of tendon rupture. Jones et al. [96] found lower mRNA expression in ruptured than tendinopathic tendons of ADAMTS 2, 3 and 17, MMP 7, 16, 23, 24 and 28, as well as TIMP 2, 3, and 4, and increased expression of ADAMs 8 and 12, A disintegrin and metalloproteinase with thrombospondin motifs 4, TIMP1, and MMPs 1, 8, 10, 12, 19, and 25. Such differences in gene expression potentially contribute to disease pathophysiology [97].
Alterations in gene expression in symptomatic tendons suggests there is an interaction between genes and environment and thus a genetic component to the aetiology of this disease. Indeed, in a twin study of tennis elbow (epicondylitis) in women [98], heritability was estimated at ~40%. Furthermore, several studies report associations between Achilles tendinopathy and several genetic variants, as discussed in Section 6.
4.4. ACL Tear and Rupture
Injuries to the ACL are among the most frequent knee ligament injuries in sport and usually require reconstruction [99,100]. In the RU, although ACL injuries are not the most frequent, they have been in the top five most severe injuries for six of the last seven seasons in English Premiership Rugby (2011–2018) [37,39], accounting for 224 days of absence/1000 h in 2016–2017 [37,39]. Frequently, ACL injuries lead to muscle weakness, altered movement, joint effusion, reduced functional performance, and have been associated with continuing clinical sequelae such as chondral lesions, meniscal tears and increased risk of early-onset post-traumatic osteoarthritis [101,102,103,104,105].
Dallalana [106] established that the primary mechanisms of ACL injury in RU are a player being tackled, tackling or in general collisions, accounting for 43%, 29% and 14% of all ACL injuries, respectively. However, the remaining 14% of ACL injuries occurred through non-contact mechanisms such as twisting and turning [106]. More recently, using video to analyze the mechanisms for ACL injury in RU showed that 57% occurred through contact [107]. Two main scenarios were identified: offensive running and being tackled, suggesting that the ball carrier is at increased risk of ACL injury. The remaining 43% were through non-contact mechanisms, mainly sidestepping maneuvers. There are numerous intrinsic and extrinsic risk factors for ACL injury, including age, anthropometry, sex, previous injury, anatomical variation, neuromuscular and cognitive factors, and genetics [56,108,109,110] for intrinsic risk factors. Whereas for extrinsic risk factors, environmental conditions, shoes/surfaces, training errors, and mechanical errors would be common [56,108,109,110]. However, trauma to the knee is a fundamental requirement [108,109].
It is possible for ACL injuries to be either partial tears or complete ruptures. Like tendon, when load is placed through the ACL the crimping formation of collagen will stretch linearly with increasing load [111]. Strains >4% cause microscopic tearing of the fibers and >8–10% strain cause macroscopic failure and rupture [111]. Though high-traumatic strains are a typical cause of ACL rupture, microscopic damage to ligament tissue occurs at relatively low levels of strain [112]. Furthermore, changes at the microscopic level such as extra-cellular matrix (ECM) alterations and cellular damage alter the mechanical properties of ligaments, thus when a ligament with microstructural alterations has strain applied, rupture can follow [112]. Thus, ACL rupture may occur in the same manner as tendon rupture, with prior degeneration of the tissue before the inciting event.
4.5. Molecular Characteristics of ACL Tear and Rupture
Over the last ~25 years, numerous studies have examined genetic factors that potentially predispose an individual to ACL injury [113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129]. Tears of the ACL seem at least twice as likely in individuals with a family history of ACL tear compared to those with no family history [113,114]. To the authors’ knowledge, there have been no twin studies estimating the heritability of ligament injury, unlike tendon [98]. In our opinion, this would be an extremely useful addition to the literature to develop an understanding in this area. The majority of research into the genetics of ACL injury has utilized gene association studies (GAS). From these studies, variants in several genes have been associated with altered risk of ACL injury, as detailed in Section 6.
5. Genetics of Tendon and Ligaments
Genetic variation may have a strong influence on tendon and ligament structure and function, which could alter an individual’s risk of injury. Inter-individual variability of tendon and ligament properties is likely to cause microtrauma and macrotrauma at differing strain levels among individuals, thus similar injury-inciting events amongst rugby players may have vastly different outcomes. Published associations exist between gene variants (of proteins that play structural and functional roles within tendons and ligaments) and susceptibility to injury for tendinopathy [130,131,132,133,134,135,136,137,138,139,140], tendon rupture [140] and ACL rupture [116,117,118,119,120,121,122,125,127,128,129,139]. Therefore, due to the high incidence and severity of tendon and ligament injuries within elite rugby, there is a potential future role for genetic screening of players to aid in injury risk management, but the practicalities are yet to be developed. In addition, the literature regarding genetic variants and tendon and ligament injuries is in its infancy, with little replication. Currently, there are no studies examining the genetics of tendon and ligament injuries within elite rugby.
6. Identifying Candidate Genes
Traditionally, top-down or unmeasured genotype approaches have been utilized to identify the heritability of phenotypes. While these provide useful estimates for identifying the genetic influence of certain phenotypes, they offer no evidence of the specific genes or polygenic profiles that contribute to the phenotype. Furthermore, high-throughput approaches such as genome-wide association studies (GWAS) frequently identify a variety of candidate genes, of which only a small percentage are actually relevant to the phenotype of interest and validating all the identified candidate genes is not always possible [141]. Genome-wide association studies also require particularly large sample sizes to be effective and meet the generally accepted significance level of p < 5 × 10−8 to minimize the risk of false positives, but that is not yet feasible in rugby. Thus, there is a need to study candidate genes because an adequately powered GWAS is currently impossible, although judicious use of GWAS results from other relevant populations to identify candidate genes can be fruitful [142]. A strength of GAS is that selection of candidate genes is based on detailed knowledge of a protein and its role vis-à-vis the phenotype of interest. Once a candidate gene is identified, the next logical step is to find functionally significant polymorphisms, with priority given to non-synonymous (missense) single nucleotide polymorphisms (SNPs) that change an amino acid in a protein or a nonsense variation that creates a premature stop codon, as these are most likely to have substantial biological effects [143]. However, polymorphisms in regions of DNA that regulate the expression of genes have recently become more appreciated for their functional roles [144,145]. Thus, several genes have been identified that may influence injury risk and are worthy of study within elite rugby (Table 3).

Table 3.
Candidate genes, candidate proteins, and their abbreviations.
6.1. COL1A1 as a Candidate Gene
The gene COL1A1 codes for the α1 chain of Col I, which is responsible for the high tensile strength of tendons and ligaments via its strong parallel fiber bundles and cross-linking formation [146]. Several studies have investigated associations between the Sp1 polymorphism (rs1800012) and a variety of soft tissue injuries; including cruciate ligament ruptures, Achilles tendinopathy and rupture, shoulder dislocation and tennis elbow (Table 4). Individuals of TT genotype appear to be at lower risk of cruciate ligament injury, particularly the ACL [115,116,122]. In contrast, there seems to be no association between tendinopathies or tendon rupture and the Sp1 polymorphism [147].

Table 4.
COL1A1 rs1800012 genetic association studies with tendon and ligament injuries in humans.
6.2. COL3A1 as a Candidate Gene
The protein Col III is an important fibrillar collagen that is similar in structure to Col I. However, Col III is a homotrimeric molecule (three α1 (III) chains) as opposed to the heterotrimeric form of Col I [149,150]. Col III frequently mixes with Col I to form mixed fibrils and is also plentiful in elastic tissue [151]. Specifically, it is found in the solid component of tendons and ligaments [152], where it functions with Col I, V, and XII to enable normal collagen fibrillogenesis [153,154]. The pro-α1 chains of Col III are encoded by the COL3A1 gene. Three studies have investigated the association between COL3A1 and ACL rupture (Table 5), but none have examined tendon pathology. Stępień-Słodkowska et al. [129] found the AA genotype of the COL3A1 rs1800255 polymorphism was more common in male recreational Polish skiers with ACL rupture than apparently healthy skiers. Similar evidence was found in Polish professional footballers [127], but not replicated in a broader population [155]. Collectively, these results suggest that individuals involved in sport who carry the AA genotype may have increased risk of ACL rupture.

Table 5.
COL3A1 rs1800255 genetic association studies with tendon and ligament injuries in humans.
6.3. COL5A1 as a Candidate Gene
Probably the most explored gene regarding tendon and ligament injury is COL5A1 (Table 6), which encodes the α1 chains of type V collagen. The protein Col V is a minor fibrillar collagen that is known to associate with type I and III collagen [156]. Although Col V is a minor collagen in terms of content, research suggests that it functions as a major collagen in developing connective tissues [157]. Mokone et al. [131] were the first to associate the COL5A1 gene with Achilles tendon pathology, finding the C allele of the rs12722 polymorphism less common in those with injury. This association was replicated for Achilles tendinopathy [158] and ACL rupture in females [117], with the C allele also underrepresented in tennis elbow patients versus controls [159]. These findings suggest the C allele may be protective against tendon and ligament injuries. A recent investigation by the RugbyGene project [160] found differences in allele and genotype frequencies for the COL5A1 rs12722 and rs3196378 polymorphisms between elite rugby athletes (rs12722: CC genotype = 21%, C allele = 47%; rs3196378: CC genotype 23%, C allele = 48%) and non-athletes (rs12722: CC genotype: 16%, C allele = 41%; rs3196378: CC genotype = 16%, C allele = 41%, p ≤ 0.02) [161]. These findings suggest that elite rugby players may have an inherited resistance against soft-tissue injury.

Table 6.
COL5A1 rs12722 genetic association studies with tendon and ligament injuries in humans.
6.4. MIR608 as a Candidate Gene
MicroRNAs (miRNA) are a class of small non-coding RNAs that induce gene silencing and translational repression [164,165]. Allele-specific polymorphisms within miRNA target sites influence the tissue-specific miRNA regulation of hundreds of genes, which implies that their genetic variation may be a prevalent cause of inter-individual phenotypic variability [166]. This potential variance has been seen in the microRNA 608 (MIR608) gene, which was associated with altered risk of Achilles tendinopathy [135,155,163]. To date, three studies have investigated the link between the MIR608 rs4919510 polymorphism and Achilles tendon pathology (Table 7), with none examining ACL rupture. The latest investigation involved a genome-wide approach; Kim et al. [155] observed that although MIR608 rs4919510 did not approach genome-wide significance (p < 5 × 10−8), when covariates such as age, sex and ancestry were not used in analysis of a tentative association identified (p = 5.1 × 10−3). The combined results from the three studies suggest that MIR608 may have a role in altering tendon injury risk but the evidence is inconclusive.

Table 7.
MIR608 rs4919510 genetic association studies with tendon and ligament injuries in humans.
6.5. MMP3 as a Candidate Gene
The protein MMP3, encoded by the MMP3 gene, has a fundamental role in the regular development, repair, and remodeling of connective tissues, by regulating ECM homeostasis via proteolytic activity [167]. Several studies have examined the association between polymorphisms rs679620, rs591058 and rs650108 within MMP3 and Achilles tendon pathologies and ACL ruptures (Table 8). These three polymorphisms span most of the MMP3 gene as they are within all four major haploblocks (one exon SNP rs679620, two intron SNPs rs591058, rs650108) [167]. Raleigh et al. [132] first investigated the three polymorphisms, finding all three independently associated with increased risk of Achilles tendinopathy, specifically the GG genotype of rs679620, CC genotype of rs591058, and AA genotype of 650108. The GG genotype of rs679620 has also been associated with Achilles tendon rupture [140]. Conversely, Posthumus et al. [120] and Gibbon et al. [168] found no independent associations between any of these variants and Achilles tendinopathy [168] or ACL rupture [120,168]. However, when inferred haplotype was considered, Posthumus et al. [120] and Gibbon et al. [168] found they were associated with ACL rupture and Achilles tendinopathy, respectively. Interestingly, Gibbon et al. [168] found the G (rs679620), C (rs5901058) and G (rs650108) alleles were overrepresented in controls, which contrasts with previous findings [132] but aligns with a recent study of Achilles tendon rupture, ACL tears and tendinopathy in a broader population [155]. Therefore, the literature appears to suggest the chromosomal region 11q22 has some influence on musculoskeletal injuries, most likely polygenic in nature, and warrants further investigation.

Table 8.
MMP3 rs679620, rs591058 and rs650108 genetic association studies with tendon and ligament injuries in humans.
6.6. TIMP2 as a Candidate Gene
The TIMPs are natural inhibitors of MMPs, which they bind with in a 1:1 stoichiometry [169]. In pathological conditions such as Achilles tendinopathy where irregular MMP activity occurs, alterations in TIMP are important as they directly influence MMP activity [169]. The SNP TIMP2 rs4789932 was associated with Achilles tendon pathologies in two studies [137,140] (Table 9). However, they contain opposing findings with the CT genotype associated with Achilles tendon pathology one [137], but overrepresented in controls in another [140]. Recently, Kim et al. [155] reported no association after corrections for testing multiple hypotheses, but possibly adds a little support to the data of El Khoury et al. [140]. Thus, although at present it is unclear which genotype/allele within the TIMP2 polymorphism affects tendon injury risk, the evidence tentatively suggests that it may play a role.

Table 9.
TIMP2 rs4789932 genetic association studies with tendon and ligament injuries in humans.
6.7. VEGFA as a Candidate Gene
Angiogenesis is essential during the repair and remodeling of injured tendons, although it can also potentially reduce mechanical stability due to the proteolytic activity in the ECM by invading endothelial cells [170]. Vascular endothelial growth factor (VEGF) is an endothelial cell mitogen that stimulates angiogenesis [171,172]. It activates endothelial cells and vascular smooth muscle migration and proliferation, as well as enhancing endothelial cell survival and differentiation [173]. Vascular endothelial growth factor has a number of isoforms (A–D); the most relevant being VEGFA [173] encoded by the VEGFA gene. Variants within VEGFA have been associated with ACL rupture [125] and Achilles tendinopathy [174] (Table 10). Interestingly, a polymorphism appears to play a different role in acute (ACL rupture) and chronic (Achilles tendinopathy) injury. The CC variant of rs699947 was overrepresented in non-contact ACL ruptures compared to controls, suggesting a role in increased ACL rupture risk [125]. Yet the CC variant might protect against Achilles tendinopathy, being underrepresented in a control population versus an Achilles tendinopathy group [174]. Further investigation is needed to improve understanding of its role in musculoskeletal injury.

Table 10.
VEGFA rs699947 genetic association studies with tendon and ligament injuries in humans.
6.8. Additional Candidate Genes of Interest
Several genetic variants recently identified in a GWAS [155] are worthy of future study, such as COLGALT1 rs8090 and NID1 rs4660148. These had strongest associations with ACL rupture (p = 6 × 10−4) and Achilles tendon injury (p = 5 × 10−5), respectively, although none approached genome-wide significance (p < 5 × 10−8). Inevitably, there will be many other as yet unidentified genetic variants that emerge as research advances.
7. Future Directions/Conclusions
The exact pathophysiology of tendon and ligament injuries is yet to be fully elucidated, as they are complex multifactorial conditions. There appears to be growing evidence of a genetic influence, although much stronger evidence is needed. The genes mentioned within this text and many others should be explored further regarding their relevance to tendon and ligament injuries. This would be particularly useful in a sport such as rugby, due to its high incidence and severity of injury.
To be truly relevant to elite rugby, research must involve appropriate cohorts who possess the extreme phenotypes and behaviors only found at the elite level. Elite athletes undergo heavy training loads and are likely to exhibit characteristics near the limits of human physiological capability; indeed, elite rugby has one of the highest incidences of injury in sport, with tendon and ligament injuries some of the most frequent and severe. Regular participation at the elite level in rugby would mean players have been exposed to one of the highest levels of risk for tendon and ligament injury in any professional sporting environment, and at least to some extent, have been able to succeed in that sport despite that high environmental risk. This ability to recover from or withstand musculoskeletal soft tissue injury that is potentially performance-limiting or career-ending, but nevertheless achieve elite status, may be reflected in distinct genetic characteristics. Large sample sizes are required for genetic research to gain sufficient statistical power and reduce the likelihood of statistical errors. Additionally, the sample sizes should be in the hundreds and ideally thousands (especially if GWAS or other hypothesis-free approaches are to be used), which is extremely challenging due to the limited number of elite athletes in a given sport. Therefore, large international collaborations are required to achieve this aim within rugby [160]. Accordingly, genetic analyses of players already included in large rugby injury databases could prove fruitful in explaining some of the currently unexplained inter-individual variability in injury susceptibility and may provide new markers of injury risk within elite rugby. Such findings could then be applied alongside existing non-genetic data to aid the personalized management of playing load and injury risk amongst rugby players.
Author Contributions
Conceptualization J.B. and A.G.W; writing—original draft preparation J.B., A.G.W., and M.A.; writing—review and editing, M.A., G.K.S., S.H.D., S.M.H., M.J.C. and A.G.W.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gabbett, T.J. Incidence of injury in junior and senior rugby league players. Sports Med. 2004, 34, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, W.; Pollard, H.; Hough, K.; Tully, C. Injury in rugby league. J. Sci. Med. Sport. 2006, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.H.M.; Kemp, S.P. Recent trends in rugby union. Clin. Sports Med. 2008, 27, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, N. Injuries in professional rugby league: A three year prospective study of the South Sydney Professional Rugby League Football Club. Am. J. Sports Med. 1993, 21, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.J.; Jenkins, D.G.; Abernethy, B. Physical collisions and injury in professional rugby league match-play. J. Sci. Med. Sport 2011, 14, 210–215. [Google Scholar] [CrossRef]
- Brooks, J.H.M.; Fuller, C.W.; Kemp, S.P.T.; Reddin, D.B. Epidemiology of injuries in English professional rugby union: Part 1 match injuries. Br. J. Sports Med. 2005, 39, 757–766. [Google Scholar] [CrossRef]
- Fuller, C.W.; Sheerin, K.; Targett, S. Rugby World Cup 2011: International Rugby Board Injury Surveillance Study. Br. J. Sports Med. 2013, 47, 1184–1191. [Google Scholar] [CrossRef]
- Fuller, C.W.; Taylor, A.; Kemp, S.P.T.; Raftery, M. Rugby World Cup 2015: World Rugby injury surveillance study. Br. J. Sports Med. 2017, 51, 51–57. [Google Scholar] [CrossRef]
- Fuller, C.W.; Brooks, J.H.M.; Cancea, R.J.; Hall, J.; Kemp, S.P.T. Contact events in rugby union and their propensity to cause injury. Br. J. Sports Med. 2007, 41, 862–867. [Google Scholar] [CrossRef]
- Gabbett, T.J.; Hodgson, P. Incidence of injury in semi-professional rugby league players. Br. J. Sports Med. 2003, 37, 36–44. [Google Scholar] [CrossRef]
- Williams, S.; Trewartha, G.; Kemp, S.; Stokes, K. A Meta-Analysis of Injuries in Senior Men’s Professional Rugby Union. Sports Med. 2013, 43, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Trewartha, G.; Kemp, S.P.T.; Brooks, J.H.M.; Fuller, C.W.; Taylor, A.E.; Cross, M.J.; Stokes, K.A. Time loss injuries compromise team success in Elite Rugby Union: A 7-year prospective study. Br. J. Sports Med. 2016, 50, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Best, J.P.; McIntosh, A.S.; Savage, T. Rugby World Cup 2003 injury surveillance project. Br. J. Sports Med. 2005, 39, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.W.; Laborde, F.; Leather, R.J.; Molloy, M.G. International Rugby Board Rugby World Cup 2007 injury surveillance study. Br. J. Sports Med. 2008, 42, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Brooks, J.; Cross, M.; Morrow, P.; Williams, S.; Anstiss, T.; Smith, A.; Taylor, A.; Trewartha, G.; Widdowson, J.; et al. England Professional Rugby Injury Surveillance Project 2013–2014 Season Report; Rugby Football Union: Twickenham, UK, 2015. [Google Scholar]
- Kemp, S.; Brooks, J.; Cross, M.; Morrow, P.; Williams, S.; Anstiss, T.; Smith, A.; Taylor, A.; Palmer, C.; Bryan, R.; et al. England Professional Rugby Injury Surveillance Project 2014–2015 Season Report; Rugby Football Union: Twickenham, UK, 2016. [Google Scholar]
- Haseler, C.M.; Carmont, M.R.; England, M. The epidemiology of injuries in English youth community rugby union. Br. J. Sports Med. 2010, 44, 1093–1099. [Google Scholar] [CrossRef]
- Roberts, S.P.; Trewartha, G.; England, M.; Shaddick, G.; A Stokes, K. Epidemiology of time-loss injuries in English community-level rugby union. BMJ Open 2013, 3, e003998. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.W.; Molloy, M.G.; Bagate, C.; Bahr, R.; Brooks, J.H.; Donson, H.; Kemp, S.P.; McCrory, P.; McIntosh, A.S.; Meeuwisse, W.H.; et al. Consensus Statement on Injury Definitions and Data Collection Procedures for Studies of Injuries in Rugby Union. Clin. J. Sport Med. 2007, 17, 177–181. [Google Scholar] [CrossRef]
- Fitzpatrick, A.C.; Naylor, A.S.; Myler, P.; Robertson, C. A three-year epidemiological prospective cohort study of rugby league match injuries from the European Super League. J. Sci. Med. Sport 2018, 21, 160–165. [Google Scholar] [CrossRef]
- Van Mechelen, W.; Hlobil, H.; Kemper, H.C. Incidence, severity, aetiology and prevention of sports injuries. A review of concepts. Sports Med. 1992, 14, 82. [Google Scholar] [CrossRef]
- Fuller, C.W.; Drawer, D. The Application of Risk Management in Sport. Sports Med. 2004, 34, 49–56. [Google Scholar] [CrossRef]
- Gissane, C.; Jennings, D.C.; Standing, P. Incidence of Injury in Rugby League Football. Physiotherapy 1993, 79, 305–310. [Google Scholar] [CrossRef]
- Seward, H.; Orchard, J.; Hazard, H.; Collinson, D. Football injuries in Australia at the élite level. Med. J. Aust. 1993, 159, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, S.; Gissane, C.; Jennings, D. Injury in rugby league: A four year prospective survey. Br. J. Sports Med. 1996, 30, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Gissane, C.; Jennings, D.; Kerr, K.; White, J. Injury Rates in Rugby League Football: Impact of Change in Playing Season. Am. J. Sports Med. 2003, 31, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Gissane, C.; Marshall, S.W.; King, D.; Clark, T. The Incidence of Match and Training Injuries in Rugby League: A Pooled Data Analysis of Published Studies. Int. J. Sports Sci. Coach. 2014, 9, 417–432. [Google Scholar]
- Cross, M.; Williams, S.; Kemp, S.P.; Fuller, C.; Taylor, A.; Brooks, J.; Trewartha, G.; Stokes, K. Does the Reliability of Reporting in Injury Surveillance Studies Depend on Injury Definition? Orthop. J. Sports Med. 2018, 6, 2325967118760536. [Google Scholar] [CrossRef] [PubMed]
- Orchard, J.; Hoskins, W. For Debate: Consensus Injury Definitions in Team Sports Should Focus on Missed Playing Time. Clin. J. Sport Med. 2007, 17, 192–196. [Google Scholar] [CrossRef]
- Hodgson, L.; Gissane, C.; Gabbett, T.J.; A King, D. For Debate: Consensus Injury Definitions in Team Sports Should Focus on Encompassing all Injuries. Clin. J. Sport Med. 2007, 17, 188–191. [Google Scholar] [CrossRef]
- King, D.; Gabbett, T.; Gissane, C.; Hodgson, L. Epidemiological studies of injuries in rugby league: Suggestions for definitions, data collection and reporting methods. J. Sci. Med. Sport 2009, 12, 12–19. [Google Scholar] [CrossRef]
- Moore, I.S.; Ranson, C.; Mathema, P. Injury Risk in International Rugby Union: Three-Year Injury Surveillance of the Welsh National Team. Orthop. J. Sports Med. 2015, 3, 2325967115596194. [Google Scholar] [CrossRef]
- Brooks, J.H.M.; Fuller, C.; Kemp, S.; Reddin, D. A prospective study of injuries and training amongst the England 2003 Rugby World Cup squad. Br. J. Sports Med. 2005, 39, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.H.M.; Fuller, C.W.; Kemp, S.P.T.; Reddin, D.B. Epidemiology of injuries in English professional rugby union: Part 2 training Injuries. Br. J. Sports Med. 2005, 39, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.W.; Raftery, M.; Readhead, C.; Targett, S.G.R.; Molloy, M.G. Impact of the International Rugby Board’s experimental law variations on the incidence and nature of match injuries in southern hemisphere professional rugby union. S. Afr. Med J. 2009, 99, 232. [Google Scholar] [PubMed]
- Fuller, C.W.; Clarke, L.; Molloy, M.G. Risk of injury associated with rugby union played on artificial turf. J. Sports Sci. 2010, 28, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Brooks, J.; West, S.; Cross, M.; Morrow, P.; Williams, S.; Anstiss, T.; Smith, A.; Taylor, A.; Palmer, C.; et al. English Professional Rugby Injury Surveillance Project 2015–2016 Season Report; Rugby Football Union: Twickenham, UK, 2017. [Google Scholar]
- Kemp, S.; West, S.; Brooks, J.; Cross, M.; Williams, S.; Anstiss, T.; Smith, A.; Bryan, R.; Hibbins-Butler, R.; O’Leary, B.; et al. English Professional Rugby Injury Surveillance Project 2016–2017 Season Report; Rugby Football Union: Twickenham, UK, 2018. [Google Scholar]
- Kemp, S.; West, S.; Brooks, J.; Cross, M.; Williams, S.; Anstiss, T.; Smith, A.; Bryan, R.; Henderson, L.; Locke, D.; et al. English Professional Rugby Injury Surveillance Project 2017–2018 Season Report; Rugby Football Union: Twickenham, UK, 2019. [Google Scholar]
- Gibbs, N. Common rugby league injuries. Recommendations for treatment and preventative measures. Sports Med. 1994, 18, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Orchard, J. Missed time through injury and injury management at an NRL club. Sport Health 2004, 22, 11–19. [Google Scholar]
- Bathgate, A.; Best, J.P.; Craig, G.; Jamieson, M. A prospective study of injuries to elite Australian rugby union players. Br. J. Sports Med. 2002, 36, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Quarrie, K.L.; Hopkins, W.G. Tackle Injuries in Professional Rugby Union. Am. J. Sports Med. 2008, 36, 1705–1716. [Google Scholar] [CrossRef]
- Cross, M.J.; Tucker, R.; Raftery, M.; Hester, B.; Williams, S.; A Stokes, K.; Ranson, C.; Mathema, P.; Kemp, S. Tackling concussion in professional rugby union: A case–control study of tackle-based risk factors and recommendations for primary prevention. Br. J. Sports Med. 2017. [Google Scholar] [CrossRef]
- Gabbett, T.J.; Jenkins, D.G. Relationship between training load and injury in professional rugby league players. J. Sci. Med. Sport 2011, 14, 204–209. [Google Scholar] [CrossRef]
- Cross, M.J.; Williams, S.; Trewartha, G.; Kemp, S.P.; Stokes, K.A. The Influence of In-Season Training Loads on Injury Risk in Professional Rugby Union. Int. J. Sports Physiol. Perform. 2016, 11, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Windt, J.; Gabbett, T.J.; Ferris, D.; Khan, K.M. Training load–injury paradox: Is greater preseason participation associated with lower in-season injury risk in elite rugby league players? Br. J. Sports Med. 2016, 51, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Hume, P.A.; Kara, S. A Review of Football Injuries on Third and Fourth Generation Artificial Turfs Compared with Natural Turf. Sports Med. 2011, 41, 903–923. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.W.; Ashton, T.; Brooks, J.H.M.; Cancea, R.J.; Hall, J.; Kemp, S.P.T. Injury risks associated with tackling in rugby union. Br. J. Sports Med. 2010, 44, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.J.; Ullah, S.; Finch, C.F. Identifying risk factors for contact injury in professional rugby league players – Application of a frailty model for recurrent injury. J. Sci. Med. Sport 2012, 15, 496–504. [Google Scholar] [CrossRef]
- Quarrie, K.L.; Alsop, J.; Waller, A.; Bird, Y.; Marshall, S.; Chalmers, D. The New Zealand rugby injury and performance project. VI. A prospective cohort study of risk factors for injury in rugby union football. Br. J. Sports Med. 2001, 35, 157–166. [Google Scholar] [CrossRef]
- Cross, M.; Kemp, S.; Smith, A.; Trewartha, G.; Stokes, K. Professional Rugby Union players have a 60% greater risk of time loss injury after concussion: A 2-season prospective study of clinical outcomes. Br. J. Sports Med. 2016, 50, 926–931. [Google Scholar] [CrossRef]
- Rafferty, J.; Ranson, C.; Oatley, G.; Mostafa, M.; Mathema, P.; Crick, T.; Moore, I.S. On average, a professional rugby union player is more likely than not to sustain a concussion after 25 matches. Br. J. Sports Med. 2018. [Google Scholar] [CrossRef]
- Brooks, J.H. The Epidemiology of Injuries in Professional Rugby; University of Leicester: Leicester, UK, 2004. [Google Scholar]
- Riley, G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A.; Bahr, R.; Beynnon, B.D.; DeMaio, M.; Dick, R.W.; Engebretsen, L.; Garrett, W.E.; Hannafin, J.A.; et al. Understanding and Preventing Noncontact Anterior Cruciate Ligament Injuries: A Review of the Hunt Valley II Meeting, January 2005. Am. J. Sports Med. 2006, 34, 1512–1532. [Google Scholar] [CrossRef]
- Meeuwise, W.H. Assessing causation in sport injury: A multifactorial model. Clin. J. Sport Med. 1994, 4, 166–170. [Google Scholar] [CrossRef]
- September, A.V.; Mokone, G.G.; Schwellnus, M.P.; Collins, M. Genetic risk factors for Achilles tendon injuries. Int. Sports Med. J. 2006, 7, 201–215. [Google Scholar]
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252. [Google Scholar] [CrossRef]
- Collins, M.; Raleigh, S. Genetic Risk Factors for Musculoskeletal Soft Tissue Injuries. Elite Young Athlete 2009, 54, 136–149. [Google Scholar]
- September, A.V.; Posthumus, M. Application of Genomics in the Prevention, Treatment and Management of Achilles Tendinopathy and Anterior Cruciate Ligament Ruptures. Recent Patents DNA Gene Seq. 2012, 6, 216–223. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef]
- Almekinders, L.C.; Temple, J.D. Etiology, diagnosis, and treatment of tendonitis: An analysis of the literature. Med. Sci. Sports Exerc. 1998, 30, 1183–1190. [Google Scholar] [CrossRef]
- Bisset, L.; Beller, E.; Jull, G.; Brooks, P.; Darnell, R.; Vicenzino, B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: Randomised trial. BMJ 2006, 333, 939. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nobuhara, K.; Hamada, T. Pathologic Evidence of Degeneration as a Primary Cause of Rotator Cuff Tear. Clin. Orthop. Relat. Res. 2003, 415, 111–120. [Google Scholar] [CrossRef]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Morrey, M.E.; Dean, B.J.F.; Carr, A.J.; Morrey, B.F. Tendinopathy: Same Disease Different Results—Why? Oper. Tech. Orthop. 2013, 23, 39–49. [Google Scholar] [CrossRef]
- Pajala, A.; Melkko, J.; Leppilahti, J.; Ohtonen, P.; Soini, Y.; Risteli, J. Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol. Histopathol. 2009, 24, 1207. [Google Scholar] [PubMed]
- Riley, G.P.; Harrall, R.L.; Constant, C.R.; Chard, M.D.; E Cawston, T.; Hazleman, B.L.; Riley, G. Tendon degeneration and chronic shoulder pain: Changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994, 53, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Neto, J.; Witzel, S.; Teodoro, W.; Carvalho-Junior, A.; Fernandes, T.; Yoshinari, H. Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Jt. Bone Spine 2002, 69, 189–194. [Google Scholar] [CrossRef]
- Eriksen, H.A.; Pajala, A.; Leppilahti, J.; Risteli, J. Increased content of type III collagen at the rupture site of human Achilles tendon. J. Orthop. Res. 2002, 20, 1352–1357. [Google Scholar] [CrossRef]
- Lapiere, C.M.; Nusgens, B.; Piérard, G.E. Interaction between Collagen Type I and Type III in Conditioning Bundles Organization. Connect. Tissue Res. 1977, 5, 21–29. [Google Scholar] [CrossRef]
- Magnusson, S.; Qvortrup, K.; Larsen, J.; Rosager, S.; Hanson, P.; Aagaard, P.; Krogsgaard, M.; Kjaer, M. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Boil. 2002, 21, 369–377. [Google Scholar] [CrossRef]
- Nourissat, G. Use of autologous growth factors in aging tendon and chronic tendinopathy. Front. Biosci. 2013, 5, 911–921. [Google Scholar] [CrossRef]
- Magnan, B.; Bondi, M.; Pierantoni, S.; Samaila, E. The pathogenesis of Achilles tendinopathy: A systematic review. Foot Ankle Surg. 2014, 20, 154–159. [Google Scholar] [CrossRef]
- Ribbans, W.J.; Collins, M. Pathology of the tendo Achillis Do our genes contribute? Bone Jt. J. 2013, 95B, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kvist, M. Achilles Tendon Injuries in Athletes. Sports Med. 1994, 18, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Selvanetti, A.; Cipolla, M.; Puddu, G. Overuse tendon injuries: Basic science and classification. Oper. Tech. Sports Med. 1997, 5, 110–117. [Google Scholar] [CrossRef]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon injury: From biology to tendon repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181. [Google Scholar] [PubMed]
- Thornton, G.M.; A Hart, D. The interface of mechanical loading and biological variables as they pertain to the development of tendinosis. J. Musculoskelet. Neuronal Interact. 2011, 11, 94. [Google Scholar] [PubMed]
- Kaux, J.F.; Forthomme, B.; Goff, C.L.; Crielaard, J.M.; Croisier, J.L. Current opinions on tendinopathy. J. Sports Sci Med. 2011, 10, 238–253. [Google Scholar]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon Healing: Repair and Regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.C.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, W.B. Cell-matrix response in tendon injury. Clin. Sports Med. 1992, 11, 533. [Google Scholar] [PubMed]
- David, L.; Grood, E.S.; Noyes, F.R.; Zernicke, R.E. Biomechanics of ligaments and tendons. Exerc. Sport Sci. Rev. 1978, 6, 125. [Google Scholar]
- Longo, U.G.; Ronga, M.; Maffulli, N. Acute Ruptures of the Achilles Tendon. Sports Med. Arthrosc. Rev. 2009, 17, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Axibal, D.P.; Anderson, J.G. Multiple Tendon Ruptures of Unknown Etiology. Foot Ankle Spec. 2013, 6, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.W. Achilles tendon rupture: A review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Corps, A.N.; Robinson, A.H.N.; Movin, T.; Costa, M.L.; Hazleman, B.L.; Riley, G.P. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology 2006, 45, 291–294. [Google Scholar] [CrossRef]
- Karousou, E.; Ronga, M.; Vigetti, D.; Passi, A.; Maffulli, N. Collagens, Proteoglycans, MMP-2, MMP-9 and TIMPs in Human Achilles Tendon Rupture. Clin. Orthop. Relat. Res. 2008, 466, 1577–1582. [Google Scholar] [CrossRef]
- Pufe, T.; Petersen, W.; Tillmann, B.; Mentlein, R. The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons. Virchows Archiv. 2001, 439, 579–585. [Google Scholar] [CrossRef]
- Jones, G.C.; Corps, A.N.; Pennington, C.J.; Clark, I.M.; Edwards, D.R.; Bradley, M.M.; Hazleman, B.L.; Riley, G.P. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human Achilles tendon. Arthritis Rheum. 2006, 54, 832–842. [Google Scholar] [CrossRef]
- Xu, Y.l.; Murrell, G.A.C. The Basic Science of Tendinopathy. Clin. Orthop. Relat. Res. 2008, 466, 1528–1538. [Google Scholar] [CrossRef]
- Hakim, A.J.; Cherkas, L.F.; Spector, T.D.; MacGregor, A.J. Genetic associations between frozen shoulder and tennis elbow: A female twin study. Rheumatology 2003, 42, 739. [Google Scholar] [PubMed]
- Corry, I. Injuries of the sporting knee. Br. J. Sports Med. 2000, 34, 395. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Susanne, H.; Klaus, S. Epidemiology of athletic knee injuries: A 10-year study. Knee 2006, 13, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Von Porat, A.; Roos, E.M.; Roos, H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: A study of radiographic and patient relevant outcomes. Ann. Rheum. Dise. 2004, 63, 269–273. [Google Scholar] [CrossRef]
- Nebelung, W.; Wuschech, H. Thirty-five Years of Follow-up of Anterior Cruciate Ligament—Deficient Knees in High-Level Athletes. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Quatman, C.E.; Kiapour, A.; Myer, G.D.; Ford, K.R.; Demetropoulos, C.K.; Goel, V.K.; Hewett, T.E. Cartilage Pressure Distributions Provide a Footprint to Define Female Anterior Cruciate Ligament Injury Mechanisms. Am. J. Sports Med. 2011, 39, 1706–1713. [Google Scholar] [CrossRef]
- Chu, C.R.; Beynnon, B.D.; Buckwalter, J.A.; Garrett, J.W.E.; Katz, J.N.; Rodeo, S.A.; Spindler, K.P.; Stanton, R.A. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): Report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am. J. Sports Med. 2011, 39, 1569–1578. [Google Scholar] [CrossRef]
- Levine, J.W.; Kiapour, A.M.; Quatman, C.E.; Wordeman, S.C.; Goel, V.K.; Hewett, T.E.; Demetropoulos, C.K. Clinically Relevant Injury Patterns After an Anterior Cruciate Ligament Injury Provide Insight Into Injury Mechanisms. Am. J. sports Med. 2013, 41, 385–395. [Google Scholar] [CrossRef]
- Dallalana, R.J.; Brooks, J.H.M.; Kemp, S.P.T.; Williams, A.M. The Epidemiology of Knee Injuries in English Professional Rugby Union. Am. J. Sports Med. 2007, 35, 818–830. [Google Scholar] [CrossRef]
- Montgomery, C.; Blackburn, J.; Withers, D.; Tierney, G.; Moran, C.; Simms, C. Mechanisms of ACL injury in professional rugby union: A systematic video analysis of 36 cases. Br. J. Sports Med. 2016, 52, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.C.; Vacek, P.; Johnson, R.J.; Slauterbeck, J.R.; Hashemi, J.; Shultz, S.; Beynnon, B.D. Risk Factors for Anterior Cruciate Ligament Injury: A Review of the Literature—Part 1: Neuromuscular and Anatomic Risk. Sports Health A Multidiscip. Approach. 2012, 4, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.C.; Vacek, P.; Johnson, R.J.; Slauterbeck, J.R.; Hashemi, J.; Shultz, S.; Beynnon, B.D. Risk Factors for Anterior Cruciate Ligament Injury: A Review of the Literature—Part 2: Hormonal, Genetic, Cognitive Function, Previous Injury, and Extrinsic Risk Factors. Sports Health A Multidiscip. Approach 2012, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Alentorn-Geli, E.; Myer, G.D.; Silvers, H.J.; Samitier, G.; Romero, D.; Lázaro-Haro, C.; Cugat, R. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 1: Mechanisms of injury and underlying risk factors. Knee Surgery Sports Traumatol. Arthrosc. 2009, 17, 705–729. [Google Scholar] [CrossRef] [PubMed]
- Abramowitch, S.D.; Saw, M.S.S.C.; Fenwick, J.A.; Woo, S.L.-Y.; Debski, R.E.; Zeminski, J. Injury and Repair of Ligaments and Tendons. Annu. Rev. Biomed. Eng. 2000, 2, 83–118. [Google Scholar]
- Provenzano, P.P.; Heisey, D.; Hayashi, K.; Lakes, R.; Vanderby, R. Subfailure damage in ligament: A structural and cellular evaluation. J. Appl. Physiol. 2002, 92, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Harner, C.D.; Paulos, L.E.; Greenwald, A.E.; Rosenberg, T.D.; Cooley, V.C. Detailed Analysis of Patients with Bilateral Anterior Cruciate Ligament Injuries. Am. J. Sports Med. 1994, 22, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.K.; Pedersen, C.L.; Birmingham, T.B.; Kirkley, A.; Jackowski, D.; Fowler, P.J. The familial predisposition toward tearing the anterior cruciate ligament: A case control study. Am. J. Sports Med. 2005, 33, 23. [Google Scholar] [CrossRef]
- Khoschnau, S.; Melhus, H.; Jacobson, A.; Rahme, H.; Bengtsson, H.; Ribom, E.; Grundberg, E.; Mallmin, H.; Michaëlsson, K. Type I collagen alpha1 Sp1 polymorphism and the risk of cruciate ligament ruptures or shoulder dislocations. Am. J. Sports Med. 2008, 36, 2432. [Google Scholar] [CrossRef]
- Posthumus, M.; September, A.V.; Keegan, M.; O’Cuinneagain, D.; Van Der Merwe, W.; Schwellnus, M.; Collins, M. Genetic risk factors for anterior cruciate ligament ruptures: COL1A1 gene variant. Br. J. Sports Med. 2009, 43, 352–356. [Google Scholar] [CrossRef]
- O’Cuinneagain, D.; Posthumus, M.; September, A.V.; Collins, M.; Van Der Merwe, W.; Schwellnus, M.P. The COL5A1 Gene Is Associated With Increased Risk of Anterior Cruciate Ligament Ruptures in Female Participants. Am. J. Sports Med. 2009, 37, 2234–2240. [Google Scholar]
- Posthumus, M.; September, A.V.; O’Cuinneagain, D.; van der Merwe, W.; Schwellnus, M.P.; Collins, M. The association between the COL12A1 gene and anterior cruciate ligament ruptures. Br. J. Sports Med. 2010, 44, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Malila, S.; Yuktanandana, P.; Saowaprut, S.; Jiamjarasrangsi, W.; Honsawek, S. Association between matrix metalloproteinase-3 polymorphism and anterior cruciate ligament ruptures. Genet. Mol. Res. 2011, 10, 4158–4165. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M.; Collins, M.; van der Merwe, L.; O’Cuinneagain, D.; van der Merwe, W.; Ribbans, W.J.; Schwellnus, M.P.; Raleigh, S.M. Matrix metalloproteinase genes on chromosome 11q22 and the risk of anterior cruciate ligament (ACL) rupture. Scand. J. Med. Sci. Sports 2012, 22, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Stępien-Słodkowska, M.; Ficek, K.; Eider, J.; Leońska-Duniec, A.; Maciejewska-Karłowska, A.; Sawczuk, M.; Zarębska, A.; Jastrzębski, Z.; Grenda, A.; Kotarska, K.; et al. The +1245g/t polymorphisms in the collagen type I alpha 1 (col1a1) gene in polish skiers with anterior cruciate ligament injury. Biol. Sport 2013, 30, 57. [Google Scholar] [CrossRef] [PubMed]
- Ficek, K.; Cięszczyk, P.; Kaczmarczyk, M.; Maciejewska-Karłowska, A.; Sawczuk, M.; Cholewiński, J.; Leońska-Duniec, A.; Stepien-Slodkowska, M.; Zarebska, A.; Stepto, N.K.; et al. Gene variants within the COL1A1 gene are associated with reduced anterior cruciate ligament injury in professional soccer players. J. Sci. Med. Sport 2013, 16, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Ficek, K.; Stepien-Slodkowska, M.; Kaczmarczyk, M.; Maciejewska-Karlowska, A.; Sawczuk, M.; Cholewinski, J.; Leonska-Duniec, A.; Zarebska, A.; Cieszczyk, P.; Zmijewski, P.; et al. Does the A9285G Polymorphism in Collagen Type XII α1 Gene Associate with the Risk of Anterior Cruciate Ligament Ruptures? Balk. J. Med Genet. 2014, 17, 41–46. [Google Scholar]
- Mannion, S.; Mtintsilana, A.; Posthumus, M.; Van Der Merwe, W.; Hobbs, H.; Collins, M.; September, A.V. Genes encoding proteoglycans are associated with the risk of anterior cruciate ligament ruptures. Br. J. Sports Med. 2014, 48, 1640–1646. [Google Scholar] [CrossRef]
- Rahim, M.; Gibbon, A.; Hobbs, H.; van der Merwe, W.; Posthumus, M.; Collins, M.; September, A.V. The association of genes involved in the angiogenesis-associated signaling pathway with risk of anterior cruciate ligament rupture: Angiogenesis and acl rupture risk. J. Orthop. Res. 2014, 32, 1612–1618. [Google Scholar] [CrossRef]
- Johnson, J.S.; Morscher, M.A.; Jones, K.C.; Moen, S.M.; Klonk, C.J.; Jacquet, R.; Landis, W.J. Gene expression differences between ruptured anterior cruciate ligaments in young male and female subjects. J. Bone Jt. Surg. 2015, 97, 71. [Google Scholar] [CrossRef]
- O’Connell, K.; Knight, H.; Ficek, K.; Leonska-Duniec, A.; Maciejewska-Karlowska, A.; Sawczuk, M.; Stepien-Slodkowska, M.; O’Cuinneagain, D.; van der Merwe, W.; Posthumus, M.; et al. Interactions between collagen gene variants and risk of anterior cruciate ligament rupture. Eur. J. Sport Sci. 2015, 15, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Stępień-Słodkowska, M.; Ficek, K.; Kaczmarczyk, M.; Maciejewska-Karłowska, A.; Sawczuk, M.; Leońska-Duniec, A.; Stępiński, M.; Ziętek, P.; Król, P.; Chudecka, M.; et al. The Variants within the COL5A1 Gene are Associated with Reduced Risk of Anterior Cruciate Ligament Injury in Skiers. J. Hum. Kinet. 2015, 45, 103–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stępień-Słodkowska, M.; Ficek, K.; Maciejewska-Karłowska, A.; Sawczuk, M.; Ziętek, P.; Król, P.; Zmijewski, P.; Pokrywka, A.; Cięszczyk, P. Overrepresentation of the COL3A1 AA genotype in Polish skiers with anterior cruciate ligament injury. Boil. Sport 2015, 32, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Mokone, G.G.; Gajjar, M.; September, A.V.; Schwellnus, M.P.; Greenberg, J.; Noakes, T.D.; Collins, M. The Guanine-Thymine Dinucleotide Repeat Polymorphism within the Tenascin-C Gene is Associated with Achilles Tendon Injuries. Am. J. Sports Med. 2005, 33, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Mokone, G.G.; Schwellnus, M.P.; Noakes, T.D.; Collins, M.; Schwellnus, M. The COL5A1 gene and Achilles tendon pathology. Scand. J. Med. Sci. Sports 2006, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, S.M.; Van Der Merwe, L.; Ribbans, W.J.; Smith, R.K.W.; Schwellnus, M.P.; Collins, M. Variants within the MMP3 gene are associated with Achilles tendinopathy: Possible interaction with the COL5A1 gene. Br. J. Sports Med. 2009, 43, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M.; Collins, M.; Cook, J.; Handley, C.J.; Ribbans, W.J.; Smith, R.K.W.; Schwellnus, M.P.; Raleigh, S.M. Components of the transforming growth factor-β family and the pathogenesis of human achilles tendon pathology-a genetic association study. Rheumatology 2010, 49, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Nell, E.-M.; Van Der Merwe, L.; Cook, J.; Handley, C.J.; Collins, M.; September, A.V. The apoptosis pathway and the genetic predisposition to Achilles tendinopathy. J. Orthop. Res. 2012, 30, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, Y.; Laguette, M.-J.; Prince, S.; Collins, M. Polymorphisms within the COL5A1 3′-UTR That Alters mRNA Structure and the MIR608 Gene are Associated with Achilles Tendinopathy. Ann. Hum. Genet. 2013, 77, 204–214. [Google Scholar] [CrossRef]
- Saunders, C.J.; van der Merwe, L.; Posthumus, M.; Cook, J.; Handley, C.J.; Collins, M.; September, A.V. Investigation of variants within the COL27A1 and TNC genes and Achilles tendinopathy in two populations. J. Orthop. Res. 2013, 31, 632–637. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, L.; Posthumus, M.; Collins, M.; Handley, C.J.; Cook, J.; Raleigh, S.M. Polymorphic variation within the ADAMTS2, ADAMTS14, ADAMTS5, ADAM12 and TIMP2 genes and the risk of Achilles tendon pathology: A genetic association study. J. Sci. Med. Sport 2013, 16, 493–498. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, L.; Posthumus, M.; Collins, M.; Ribbans, W.; Raleigh, S. 82 TheCOL5A1Gene and Risk of Achilles Tendon Pathology in a British Cohort. Br. J. Sports Med. 2014, 48, A54. [Google Scholar] [CrossRef]
- El Khoury, L.; Posthumus, M.; Collins, M.; van der Merwe, W.; Handley, C.; Cook, J.; Raleigh, S.M. ELN and FBN2 Gene Variants as Risk Factors for Two Sports-related Musculoskeletal Injuries. Int. J. Sports Med. 2015, 36, 333–337. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, L.; Ribbans, W.J.; Raleigh, S.M. MMP3 and TIMP2 gene variants as predisposing factors for Achilles tendon pathologies: Attempted replication study in a British case–control cohort. Meta Gene 2016, 9, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Tranchevent, L.-C.; Ardeshirdavani, A.; ElShal, S.; Alcaide, D.; Aerts, J.; Auboeuf, D.; Moreau, Y. Candidate gene prioritization with Endeavour. Nucleic Acids Res. 2016, 44, W117–W121. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; Stebbings, G.K.; Kilduff, L.P.; Erskine, R.M.; Day, S.H.; Morse, C.I.; McPhee, J.S.; Cook, C.J.; Vance, B.; Ribbans, W.J.; et al. Fat mass and obesity associated (FTO) gene influences skeletal muscle phenotypes in non-resistance trained males and elite rugby playing position. BMC Genet. 2017, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Tabor, H.K.; Risch, N.J.; Myers, R.M. Candidate-gene approaches for studying complex genetic traits: Practical considerations. Nat. Rev. Genet. 2002, 3, 391–397. [Google Scholar] [CrossRef]
- Drysdale, C.M.; McGraw, D.W.; Stack, C.B.; Stephens, J.C.; Judson, R.S.; Nandabalan, K.; Arnold, K.; Ruano, G.; Liggett, S.B. Complex promoter and coding region β2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc. Natl. Acad. Sci. USA 2000, 97, 10483–10488. [Google Scholar] [CrossRef]
- Newton-Cheh, C.; Hirschhorn, J.N. Genetic association studies of complex traits: Design and analysis issues. Mutat. Res. Mol. Mech. Mutagen. 2005, 573, 54–69. [Google Scholar] [CrossRef]
- I Thompson, J.; Czernuszka, J.T. The effect of two types of cross-linking on some mechanical properties of collagen. Bio-Med. Mater. Eng. 1995, 5, 37. [Google Scholar]
- Posthumus, M.; September, A.V.; Schwellnus, M.P.; Collins, M. Investigation of the Sp1-binding site polymorphism within the COL1A1 gene in participants with Achilles tendon injuries and controls. J. Sci. Med. Sport 2009, 12, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Erduran, M.; Altinisik, J.; Meric, G.; Ates, O.; Ulusal, A.E.; Akseki, D. Is Sp1 binding site polymorphism within COL1A1 gene associated with tennis elbow? Gene 2014, 537, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Banos, C.C.; Thomas, A.H.; Kuo, C.K. Collagen fibrillogenesis in tendon development: Current models and regulation of fibril assembly. Birth Defects Res. Part C Embryo Today Rev. 2008, 84, 228–244. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K. Localization of collagen types in tissues. Int. Rev. Connect. Tissue Res. 1981, 9, 265. [Google Scholar] [PubMed]
- Frank, C.B. Ligament structure, physiology and function. J. Musculoskelet. Neuronal Interact. 2004, 4, 199. [Google Scholar]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef]
- Minamitani, T.; Ikuta, T.; Saito, Y.; Takebe, G.; Sato, M.; Sawa, H.; Nishimura, T.; Nakamura, F.; Takahashi, K.; Ariga, H.; et al. Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp. Cell Res. 2004, 298, 305–315. [Google Scholar] [CrossRef]
- Kim, S.K.; Roos, T.R.; Kleimeyer, J.P.; Ahmed, M.A.; Goodlin, G.T.; Fredericson, M.; Ioannidis, J.P.A.; Avins, A.L.; Dragoo, J.L. Genome-wide association screens for Achilles tendon and ACL tears and tendinopathy. PLoS ONE 2017, 12, e0170422. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and Function of the Skeletal Muscle Extracellular Matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Roulet, M.; Ruggiero, F.; Karsenty, G.; LeGuellec, D. A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: A clue for understanding collagen V function in developing connective tissues. Cell Tissue Res. 2007, 327, 323–332. [Google Scholar] [CrossRef] [PubMed]
- September, A.V.; Cook, J.; Handley, C.J.; Van Der Merwe, L.; Schwellnus, M.P.; Collins, M. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br. J. Sports Med. 2009, 43, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Altinisik, J.; Meric, G.; Erduran, M.; Ates, O.; Ulusal, A.E.; Akseki, D. The BstUI and DpnII Variants of the COL5A1 Gene Are Associated With Tennis Elbow. Am. J. Sports Med. 2015, 43, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; Kilduff, L.P.; Day, S.H.; Pitsiladis, Y.P.; Williams, A.G. Genomics in rugby union: A review and future prospects. Eur. J. Sport Sci. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; Kilduff, L.P.; Erskine, R.M.; Day, S.H.; Stebbings, G.K.; Cook, C.J.; Raleigh, S.M.; Bennett, M.A.; Wang, G.; Collins, M.; et al. COL5A1 gene variants previously associated with reduced soft tissue injury risk are associated with elite athlete status in rugby. BMC Genom. 2017, 18, 820. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M.; September, A.; O’Cuinneagain, D.; Van Der Merwe, W.; Schwellnus, M.; Collins, M. The COL5A1 Gene Is Associated With Increased Risk Of Anterior Cruciate Ligament Rupture In Female Participants: 611. Med. Sci. Sports Exerc. 2009, 41, 2234–2240. [Google Scholar] [CrossRef]
- Brown, K.L.; Seale, K.B.; El Khoury, L.Y.; Posthumus, M.; Ribbans, W.J.; Raleigh, S.M.; Collins, M.; September, A.V. Polymorphisms within the COL5A1 gene and regulators of the extracellular matrix modify the risk of Achilles tendon pathology in a British case-control study. J. Sports Sci. 2017, 35, 1475–1483. [Google Scholar] [CrossRef]
- Lau, N.C.; Lai, E.C. Diverse roles for RNA in gene regulation. Genome Biol. 2005, 6, 315. [Google Scholar] [CrossRef]
- Matzke, M.A.; Birchler, J.A. RNAi-mediated pathways in the nucleus. Nat. Rev. Genet. 2005, 6, 24–35. [Google Scholar] [CrossRef]
- Kim, J.; Bartel, D.P. Allelic imbalance sequencing reveals that single-nucleotide polymorphisms frequently alter microRNA-directed repression. Nat. Biotechnol. 2009, 27, 472–477. [Google Scholar] [CrossRef]
- Foster, B.P. Genetic Variation, Protein Composition and Potential Influences on Tendon Properties in Humans. Open Sports Med. J. 2012, 6, 8–21. [Google Scholar] [CrossRef]
- Gibbon, A.; Hobbs, H.; Van Der Merwe, W.; Raleigh, S.M.; Cook, J.; Handley, C.J.; Posthumus, M.; Collins, M.; September, A.V. The MMP3 gene in musculoskeletal soft tissue injury risk profiling: A study in two independent sample groups. J. Sports Sci. 2016, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. J. Am. Heart Assoc. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Pufe, T.; Zantop, T.; Tillmann, B.; Tsokos, M.; Mentlein, R. Expression of VEGFR-1 and VEGFR-2 in Degenerative Achilles Tendons. Clin. Orthop. Relat. Res. 2004, 420, 286–291. [Google Scholar] [CrossRef]
- Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999, 77, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Egginton, S. Invited review: Activity-induced angiogenesis. Pflüg. Archiv-Eur. J. Physiol. 2009, 457, 963–977. [Google Scholar] [CrossRef]
- Rahim, M.; El Khoury, L.Y.; Raleigh, S.M.; Ribbans, W.J.; Posthumus, M.; Collins, M.; September, A.V. Human Genetic Variation, Sport and Exercise Medicine, and Achilles Tendinopathy: Role for Angiogenesis-Associated Genes. OMICS J. Integr. Boil. 2016, 20, 520–527. [Google Scholar] [CrossRef]
- Rahim, M.; Hobbs, H.; Van Der Merwe, W.; Posthumus, M.; Collins, M.; September, A.V. Investigation of angiogenesis genes with anterior cruciate ligament rupture risk in a South African population. J. Sports Sci. 2017, 36, 551–557. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).