Physiological Predictors of Peak Velocity in the VAM-EVAL Incremental Test and the Role of Kinematic Variables in Running Economy in Triathletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
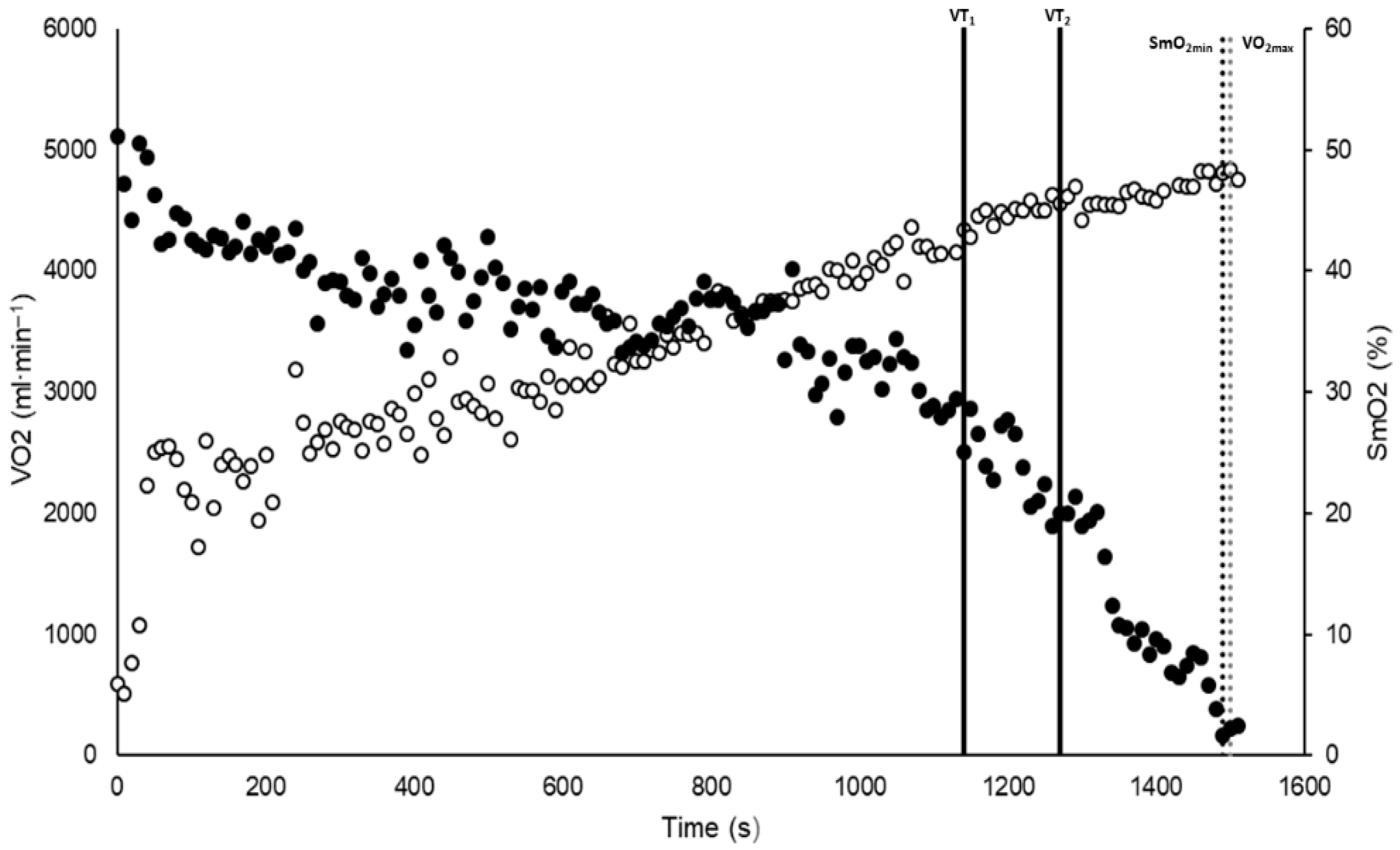
2.3. Instruments
2.4. Experimental Procedure
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef]
- Korhonen, M.T.; Mero, A.A.; Alén, M.; Sipilä, S.; Häkkinen, K.; Liikavainio, T.; Viitasalo, J.K.; Haverinen, M.T.; Suominen, H. Biomechanical and skeletal muscle determinants of maximum running speed with aging. Med. Sci. Sports Exerc. 2009, 41, 844–856. [Google Scholar] [CrossRef]
- McCormick, A.; Meijen, C.; Marcora, S. Psychological Determinants of Whole-Body Endurance Performance. Sports Med. 2015, 45, 997–1015. [Google Scholar] [CrossRef]
- Millet, G.P.; Vleck, V.E.; Bentley, D.J. Physiological differences between cycling and running: Lessons from triathletes. Sports Med. 2009, 39, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.E.; Howley, E.T.; Bassett, D.R.; Thompson, D.L.; Fitzhugh, E.C. Test of the classic model for predicting endurance running performance. Med. Sci. Sports Exerc. 2010, 42, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaard, S.; Brocherie, F.; Jaspers, R.T. Under the Hood: Skeletal Muscle Determinants of Endurance Performance. Front. Sports Act. Living 2021, 3, 719434. [Google Scholar] [CrossRef]
- Wagner, P.D. Determinants of maximal oxygen transport and utilization. Annu. Rev. Physiol. 1996, 58, 21–50. [Google Scholar] [CrossRef]
- Wagner, P.D. Determinants of maximal oxygen consumption. J. Muscle Res. Cell Motil. 2023, 44, 73–88. [Google Scholar] [CrossRef]
- Joyner, M.J.; Dominelli, P.B. Central cardiovascular system limits to aerobic capacity. Exp. Physiol. 2021, 106, 2299–2303. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Rasmussen, P.; Siebenmann, C.; Díaz, V.; Gassmann, M.; Pesta, D.; Gnaiger, E.; Nordsborg, N.B.; Robach, P.; Lundby, C. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J. Appl. Physiol. 2011, 111, 1422–1430. [Google Scholar] [CrossRef]
- Trapp, S.; Hayes, E.; Galpin, A.; Kaminsky, L.; Jemiolo, B.; Fink, W.; Trappe, T.; Jansson, A.; Gustafsson, T.; Tesch, P. New records in aerobic power among octogenarian lifelong endurance athletes. J. Appl. Physiol. 2013, 114, 3–10. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Coyle, E.F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef]
- Saunders, P.U.; Pyne, D.B.; Telford, R.D.; Hawley, J.A. Factors affecting running economy in trained distance runners. Sports Med. 2004, 34, 465–485. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.R.; Kilding, A.E. Running economy: Measurement, norms and determining factors. Sports Med. 2015, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Dolgan, J.; Griffin, J.; Bestwick, A. The physiological importance of preferred stride frequency during running at different speeds. J. Exerc. Physiol. 2008, 11, 26–32. [Google Scholar]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.; Vandekerckhove, K.; Coomans, I.; Prieur, F.; Bourgois, J.G. An integrated view on the oxygenation responses to incremental exercise at the brain, the locomotor and respiratory muscles. Eur. J. Appl. Physiol. 2016, 116, 2085–2102. [Google Scholar] [CrossRef]
- Kime, R.; Fujioka, M.; Osawa, T.; Takagi, S.; Niwayama, M.; Kaneko, Y.; Osada, T.; Murase, N.; Katsumura, T. Which is the best indicator of muscle oxygen extraction during exercise using NIRS?: Evidence that HHb is not the candidate. Adv. Exp. Med. Biol. 2013, 789, 163–169. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M. Muscle oxygenation by near-infrared-based tissue oximeters. J. Appl. Physiol. 2009, 107, 371–373. [Google Scholar] [CrossRef][Green Version]
- Perrey, S.; Quaresima, V.; Ferrari, M. Muscle Oximetry in Sports Science: An Updated Systematic Review. Sports Med. 2024, 54, 975–996. [Google Scholar] [CrossRef]
- Feldmann, A.; Schmitz, R.; Erlancher, D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0 to 100% scale: Reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 115001. [Google Scholar] [CrossRef]
- Batterson, P.M.; Kirby, B.S.; Hasselmann, G.; Feldmann, A. Muscle oxygen saturation rates coincide with lactate-based exercise thresholds. Eur. J. Appl. Physiol. 2023, 123, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Sendra-Pérez, C.; Encarnación-Martínez, A.; Murias, J.M.; De la Fuente, C.; Salvador-Palmer, R.; Martin-Rivera, F.; Priego-Quesada, J.I. Muscular excitation and oxygen extraction responses in power-generating and stabilizing muscles during a graded cycling test. J. Sports Sci. 2025, 43, 1675–1684. [Google Scholar] [CrossRef]
- McManus, C.J.; Collison, J.; Cooper, C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018, 23, 015007. [Google Scholar] [CrossRef] [PubMed]
- Tew, G.A.; Ruddock, A.D.; Saxton, J.M. Skin blood flow differentially affects near-infrared spectroscopy-derived measures of muscle oxygen saturation and blood volume at rest and during dynamic leg exercise. Eur. J. Appl. Physiol. 2010, 110, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Motobe, M.; Murase, N.; Osada, T. Non-invasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn. Med. 2004, 3, 2. [Google Scholar] [CrossRef]
- Ryan, T.E.; Erickson, M.L.; Brizendine, J.T.; Young, H.J.; McCully, K.K. Non-invasive evaluation of skeletal muscle mitochondrial capacity with near infrared spectroscopy: Correcting for blood volume changes. J. Appl. Physiol. 2012, 113, 175–183. [Google Scholar] [CrossRef]
- Pilotto, A.M.; Adami, A.; Mazzolari, R.; Brocca, L.; Crea, E.; Zuccarelli, L.; Pellegrino, M.A.; Bottinelli, R.; Grassi, B.; Rossiter, H.B.; et al. Near-infrared spectroscopy estimation of combined skeletal muscle oxidative capacity and O2 diffusion capacity in humans. J. Physiol. 2022, 600, 4153–4168. [Google Scholar] [CrossRef]
- Feldmann, A.M.; Erlacher, D.; Pfister, S. Muscle oxygen dynamics in elite climbers during finger hang tests at varying intensities. Sci. Rep. 2020, 10, 3040. [Google Scholar] [CrossRef]
- Baláš, J.; Michailov, M.; Giles, D.; Kodejška, J.; Panáčková, M.; Fryer, S. Active recovery of the finer flexors enhances intermittent handgrip performance in rock climbers. Eur. J. Sport Sci. 2016, 16, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Okushima, D.; Poole, D.C.; Barstow, T.J.; Rossiter, H.B.; Kondo, N.; Bowen, T.S.; Amano, T.; Koga, S. Greater VO2peak is correlated with greater skeletal muscle deoxygenation amplitude and haemoglobin concentration within individual muscles during ramp-incremental cycle exercise. Physiol. Resp. 2016, 4, e13065. [Google Scholar] [CrossRef]
- Feldmann, A.; Ammann, L.; Gätcher, F.; Zibung, M.; Erlacher, D. Muscle oxygen saturation breakpoints reflect ventilatory thresholds both in cycling and running. J. Hum. Kinet. 2022, 83, 87–97. [Google Scholar] [CrossRef]
- Fryer, S.; Stoner, L.; Stone, K.; Giles, D.; Sveen, J.; Garrido, I.; España-Romero, V. Forearm muscle oxidative capacity index predicts sport rock-climbing performance. Eur. J. Appl. Physiol. 2016, 116, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Furno Puglia, V.; Paquette, M.; Bergdahl, A. Characterization of muscle oxygenation response in well-trained hand cyclists. Eur. J. Appl. Physiol. 2024, 124, 3241–3251. [Google Scholar] [CrossRef]
- Paquette, M.; Bieuzen, F.; Billaut, F. Muscle oxygenation rather than VO2max as a Strong Predictor of Performance in Sprint Canoe-Kayak. Int. J. Sports Physiol. Perform. 2018, 13, 1299–1307. [Google Scholar] [CrossRef]
- Pallarés, J.G.; Cerezuela-Espejo, V.; Morán-Navarro, R.; Martínez-Cava, A.; Conesa, E.; Courel-Ibáñez, J. A New Short Track Test to Estimate the VO2max and Maximal Aerobic Speed in Well-Trained Runners. J. Strength Cond. Res. 2019, 33, 1216–1221. [Google Scholar] [CrossRef]
- Bosquet, L.; Léger, L.; Legros, P. Methods to determine aerobic endurance. Sports Med. 2002, 32, 675–700. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.W.; Rowell, A.L. Running velocity at VO2max. Med. Sci. Sports Exerc. 1996, 28, 114–119. [Google Scholar] [CrossRef]
- Dupont, G.; Akakpo, K.; Berthoin, S. The effect of in-season, high intensity interval training in soccer players. J. Strength Cond. Res. 2004, 18, 584–589. [Google Scholar] [CrossRef]
- Buchheit, M.; Chivot, A.; Parouty, J.; Mercier, D.; Haddad, H.; Laursen, P.B.; Ahmaidi, S. Monitoring endurance running performance using cardiac parasympathetic function. Eur. J. Appl. Physiol. 2010, 108, 1153–1167. [Google Scholar] [CrossRef]
- Racil, G.; Ben Ounis, O.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of hig vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obsese young females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef]
- Bertuzzi, R.; Nascimento, E.M.; Urso, R.P.; Damasceno, M.; Lima-Silva, A.E. Energy system contributions during incremental exercise test. J. Sports Sci. Med. 2013, 12, 454–460. [Google Scholar]
- Benhammou, S.; Mourot, L.; Mokkedes, M.I.; Bengoua, A.; Belkadi, A. Assessment of maximal aerobic speed in runners with different performance levels: Interest of a new intermittent running test. Sci. Sports 2021, 36, 413.e1–413.e9. [Google Scholar] [CrossRef]
- Schabort, E.J.; Killian, S.C.; Gibson, A.S.C.; Hawley, J.A.; Noakes, T.D. Prediction of triathlon race time from laboratory testing in national triathletes. Med. Sci. Sports Exerc. 2000, 32, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Wiecha, S.; Kasiak, P.S.; Cieśliński, I.; Maciejczyk, M.; Mamcarz, A.; Śliż, D. Modeling Physiological Predictors of Running Velocity for Endurance Athletes. J. Clin. Med. 2022, 11, 6688. [Google Scholar] [CrossRef]
- Pizzuto, F.; de Oliveira, C.F.; Soares, T.S.A.; Rago, V.; Silva, G.; Oliveira, J. Relationship Between Running Economy and Kinematic Parameters in Long-Distance Runners. J. Strength Cond. Res. 2019, 33, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Leite, O.H.C.; do Prado, D.M.L.; Rabelo, N.D.D.A.; Pires, L.; Barton, G.J.; Hespanhol, L.; Lucareli, P.R.G. Two sides of the same runner! The association between biomechanical and physiological markers of endurance performance in distance runners. Gait Posture 2024, 113, 252–257. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.J.; Butson, J.; Rogerson, M.; Waterworth, S.; Jones, B.; Cooper, C.E.; Sandercockm, G. The influence of full length compression tights during treadmill running at race speed. Int. J. Sports Sci. Coach. 2024, 19, 401–409. [Google Scholar] [CrossRef]
- Trang, S.; Mattioni Maturana, F.; Murias, J.M.; Herbert, M.R.; Keir, D.A. An undergraduate laboratory to study exercise thresholds. Adv. Physiol. Educ. 2023, 47, 604–614. [Google Scholar] [CrossRef]
- Okawara, H.; Iwasawa, Y.; Sawada, T.; Sugai, K.; Daigo, K.; Seki, Y.; Ichihara, G.; Nakashima, D.; Sano, M.; Nakamura, M.; et al. Anaerobic threshold using seat lactate sensor under hypoxia. Sci. Rep. 2023, 13, 22865. [Google Scholar] [CrossRef]
- Merni, F.; Di Michele, R.; Mantovani, J. A preliminary investigation of methods to determine running economy through a continuous incremental test. In New Ideas in Fundamentals of Human Movement and Sport Science: Current Issues and Perspective; IASK: Belgrado, Serbia, 2009. [Google Scholar]
- García-Pinillos, F.; Roche-Seruendo, L.E.; Marcén-Cinca, N.; Marco-Contreras, L.A.; Latorre-Román, P.A. Absolute Reliability and Concurrent Validity of the Stryd System for the Assessment of Running Stride Kinematics at Different Velocities. J. Strength Cond. Res. 2021, 35, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; García Marquéz, J.R.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2012, 36, 27–46. [Google Scholar] [CrossRef]
- Noakes, T.D.; Myburgh, K.H.; Schall, R. Peak treadmill running velocity during the VO2max test predicts running performance. J. Sports Sci. 1990, 8, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Thomason, H.; Roberts, E. Fractional utilization of aerobic capacity during distance running. Med. Sci. Sports 1973, 5, 248–252. [Google Scholar] [CrossRef]
- Farrell, P.A.; Wilmore, J.H.; Coyle, E.F.; Billing, J.E.; Costill, D.L. Plasma lactate accumulation and distance running performance. Med. Sci. Sports 1979, 11, 338–344. [Google Scholar] [CrossRef]
- Stratton, E.; O’Brien, B.J.; Harvey, J.; Blitvich, J.; McNicol, A.J.; Janissen, D.; Paton, C.; Knez, W. Treadmill velocity best predicts 5000-m run performance. Int. J. Sports Med. 2009, 30, 40–45. [Google Scholar] [CrossRef]
- Spencer, M.D.; Murias, J.M.; Paterson, D.H. Characterizing the profile of muscle deoxygenation during ramp incremental exercise in Young men. Eur. J. Appl. Physiol. 2012, 112, 3349–3360. [Google Scholar] [CrossRef]
- Batterson, P.M.; Kirby, B.S.; Feldmann, A. Response to: The remarkably tight relationship between blood lactate concentration and muscle oxygen saturation. Eur. J. Appl. Physiol. 2024, 124, 381–382. [Google Scholar] [CrossRef]
- Kirby, B.S.; Clark, D.A.; Bradley, E.M.; Wilkins, B.W. The balance of muscle oxygen supply and demand reveals critical metabolic rate and predicts time to exhaustion. J. Appl. Physiol. 2021, 130, 1915–1927. [Google Scholar] [CrossRef]
- Paquette, M.; Bieuzen, F.; Billaut, F. Effect of a 3-weeks training camp on muscle oxygenation, VO2 and performance in elite sprint kayakers. Front. Sports Act. Living 2020, 2, 47. [Google Scholar] [CrossRef]
- Villanova, S.; Pastorio, E.; Pilotto, A.M.; Marciano, A.; Quaresima, V.; Adami, A.; Rossiter, H.B.; Cardinale, D.A.; Porcelli, S. Oxidative and O2 diffusive function in triceps brachii of recreational to world class swimmers. Exp. Physiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Kitada, T.; Machida, S.; Naito, H. Influence of muscle fibre composition on muscle oxygenation during maximal running. BMJ Open Sport Exerc. Med. 2015, 1, e000062. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.; Barstow, T.J.; Celie, B.; Prieur, F.; Bourgois, J. The interrelationship between muscle oxygenation, muscle activation, and pulmonary oxygen uptake to incremental ramp exercise: Influence of aerobic fitness. Appl. Physiol. Nutr. Metab. 2016, 41, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Van Beekvelt, M.C.; Borghuis, M.S.; Van Engelen, B.G.; Wevers, R.A.; Colier, W.N. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin. Sci. 2001, 101, 21–28. [Google Scholar] [CrossRef]
- Davis, S.L.; Fadel, P.J.; Cui, J.; Thomas, G.D.; Crandall, C.G. Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J. Appl. Physiol. 2006, 100, 221–224. [Google Scholar] [CrossRef]
- Skotzke, P.; Schwindling, S.; Meyer, T. Side differences and reproducibility of the Moxy muscle oximeter during cycling in trained men. Eur. J. Appl. Physiol. 2024, 124, 3075–3083. [Google Scholar] [CrossRef]
- Ma, X.; Cao, Z.; Zhu, Z.; Chen, X.; Wen, D.; Cao, Z. VO2max (VO2peak) in elite athletes under high-intensity interval training: A meta-analysis. Heliyon 2023, 9, e16663. [Google Scholar] [CrossRef]
- Rosenblat, M.A.; Watt, J.A.; Arnold, J.I.; Treff, G.; Sandbackk, Ø.B.; Esteve-Lanao, J.; Festa, L.; Filipas, L.; Galloway, S.D.; Muñoz, I.; et al. Which Training Intensity Distribution Intervention will Produce the Greatest Improvements in Maximal Oxygen Uptake and Time-Trial Performance in Endurance Athletes? A Systematic Review and Network Meta-analysis of Individual Participant Data. Sports Med. 2025, 55, 655–673. [Google Scholar] [CrossRef]
- Yogev, A.; Arnold, J.I.; Nelson, H.; Rosenblat, M.A.; Clarke, D.C.; Guenette, J.A.; Sporer, B.C.; Koehle, M. The effects of endurance training on muscle oxygen desaturation during incremental exercise tests: A systematic review and meta-analysis. Front. Sports Act. Living 2024, 6, 1406987. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Bieuzen, F.; Billaut, F. The effect of HIIT vs. SIT on muscle oxygenation in trained sprint kayakers. Eur. J. Appl. Physiol. 2021, 121, 2743–2759. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.R.; Cavanagh, P.R. Relationship between distance running mechanics, running economy, and performance. J. Appl. Physiol. 1987, 63, 1236–1245. [Google Scholar] [CrossRef]
- Cavanagh, P.R.; Williams, K.R. The effect of stride length variation on oxygen uptake during distance running. Med. Sci. Sports Exerc. 1982, 14, 30–35. [Google Scholar] [CrossRef]
- Cavagna, G.A.; Heglund, N.C.; Willems, P.A. Effect of an increase in gravity on the power output and the rebound of the body in human running. J. Exp. Biol. 2005, 208, 2333–2346. [Google Scholar] [CrossRef][Green Version]
- Tartaruga, M.P.; Brisswalter, J.; Peyré-Tartaruga, L.A.; Vargas, A.O.; Alberton, C.L.; Coertjens, M.; Cadore, E.L.; Tiggeman, C.L.; Silva, E.M.; Kroil, L.F.M. The relationship between running economy and biomechanical variables in distance runners. Res. Q. Exerc. Sport 2012, 83, 367–375. [Google Scholar] [CrossRef]
- Di Michele, R.; Merni, F. The concurrent effects of strike pattern and ground-contact time on running economy. J. Sci. Med. Sport 2014, 17, 414–418. [Google Scholar] [CrossRef]
- Suriano, R.; Bishop, D. Physiological attributes of triathletes. J. Sci. Med. Sport 2010, 13, 340–347. [Google Scholar] [CrossRef] [PubMed]
| Mean | SD | CV (%) | Range | |
|---|---|---|---|---|
| Age (years) | 24.3 | 5.3 | 22 | 19–33 |
| Body weight (kg) | 67.5 | 6.5 | 9.7 | 56–78 |
| Body height (cm) | 176.6 | 8.5 | 4.8 | 167–196 |
| BMI (kg·m−2) | 21.6 | 1.3 | 5.8 | 19.4–23.4 |
| ATT (mm) | 3.6 | 1.5 | 41.7 | 1.5–6.9 |
| TTE (s) | 1375.5 | 93.8 | 6.8 | 1250–1530 |
| TTSmO2min (s) | 1333.6 | 92.3 | 6.9 | 1230–1490 |
| VO2max (mL·min−1·kg−1) | 60.6 | 8.2 | 13.5 | 48–72.7 |
| HRmax (bpm) | 185.5 | 15.7 | 8.4 | 159–205 |
| SmO2min (%) | 15.1 | 13.3 | 88.4 | 0–38.6 |
| VT1 (mL·min−1·kg−1) | 51 | 6.5 | 12.8 | 39.6–64.8 |
| VT2 (mL·min−1·kg−1) | 57.9 | 7.8 | 13.5 | 45.4–69.9 |
| RE12 (mL·min−1·kg−1) | 42.3 | 3.5 | 8.4 | 34.7–46.1 |
| CAD12 (spm) | 164.6 | 5.3 | 3.2 | 156.3–177.1 |
| VO12 (cm) | 82.8 | 7 | 8.4 | 67.6–93.4 |
| CT12 (ms) | 243 | 7 | 2.9 | 232.1–252.6 |
| SL12 (cm) | 1255.8 | 38.9 | 3.1 | 1168.9–1304.4 |
| RE16 (mL·min−1·kg−1) | 55.1 | 6.4 | 11.7 | 44–64.4 |
| CAD16 (spm) | 174.1 | 5.6 | 3.2 | 164.6–185.4 |
| VO216 (cm) | 83.3 | 7.3 | 8.7 | 71.8–98.9 |
| CT16 (ms) | 199.3 | 5.6 | 2.8 | 191.9–206.9 |
| SL16 (cm) | 1568.2 | 59.9 | 3.8 | 1494.6–1706.6 |
| Vpeak (km·h−1) | 18.8 | 0.8 | 4 | 18–20 |
| r | 90% CI | p | |
|---|---|---|---|
| Vpeak—VO2max | 0.76 | [0.38, 0.92] | 0.007 * |
| Vpeak—SmO2min | −0.68 | [−0.89, −0.25] | 0.020 * |
| Vpeak—VT1 | 0.82 | [0.51, 0.94] | 0.002 * |
| Vpeak—VT2 | 0.70 | [0.28, 0.90] | 0.016 * |
| Vpeak—RE12 | 0.16 | [−0.39, 0.63] | 0.631 |
| Vpeak—RE16 | 0.54 | [0.03, 0.83] | 0.083 |
| Vpeak—HRmax | 0.02 | [−0.51, 0.54] | 0.952 |
| RE12—CAD12 | 0.24 | [−0.33, 0.68] | 0.483 |
| RE12—VO12 | −0.33 | [−0.73, 0.23] | 0.321 |
| RE12—CT12 | 0.37 | [−0.20, 0.75] | 0.268 |
| RE12—SL12 | −0.19 | [−0.65, 0.37] | 0.573 |
| RE16—CAD16 | 0.21 | [−0.36, 0.66] | 0.541 |
| RE16—VO16 | −0.26 | [−0.69, 0.31] | 0.445 |
| RE16—CT16 | 0.38 | [−0.18, 0.75] | 0.250 |
| RE16—SL16 | −0.28 | [−0.70, 0.29] | 0.412 |
| TTE—TTSmO2min | 0.89 | [0.70, 0.97] | <0.001 * |
| SmO2min—VO2max | −0.22 | [−0.67, 0.35] | 0.521 |
| Dependent Variable | R2 | p | Indicator | β | p |
|---|---|---|---|---|---|
| Vpeak (km·h−1) | 0.76 | 0.007 | VO2max (mL·min−1·kg−1) | 0.64 | 0.002 |
| −0.68 | 0.020 | SmO2min (%) | −0.55 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montraveta, J.; Fernández-Jarillo, I.; Iglesias, X.; Feldmann, A.; Chaverri, D. Physiological Predictors of Peak Velocity in the VAM-EVAL Incremental Test and the Role of Kinematic Variables in Running Economy in Triathletes. Sports 2025, 13, 316. https://doi.org/10.3390/sports13090316
Montraveta J, Fernández-Jarillo I, Iglesias X, Feldmann A, Chaverri D. Physiological Predictors of Peak Velocity in the VAM-EVAL Incremental Test and the Role of Kinematic Variables in Running Economy in Triathletes. Sports. 2025; 13(9):316. https://doi.org/10.3390/sports13090316
Chicago/Turabian StyleMontraveta, Jordi, Ignacio Fernández-Jarillo, Xavier Iglesias, Andri Feldmann, and Diego Chaverri. 2025. "Physiological Predictors of Peak Velocity in the VAM-EVAL Incremental Test and the Role of Kinematic Variables in Running Economy in Triathletes" Sports 13, no. 9: 316. https://doi.org/10.3390/sports13090316
APA StyleMontraveta, J., Fernández-Jarillo, I., Iglesias, X., Feldmann, A., & Chaverri, D. (2025). Physiological Predictors of Peak Velocity in the VAM-EVAL Incremental Test and the Role of Kinematic Variables in Running Economy in Triathletes. Sports, 13(9), 316. https://doi.org/10.3390/sports13090316








