Juice-Based Supplementation Strategies for Athletic Performance and Recovery: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
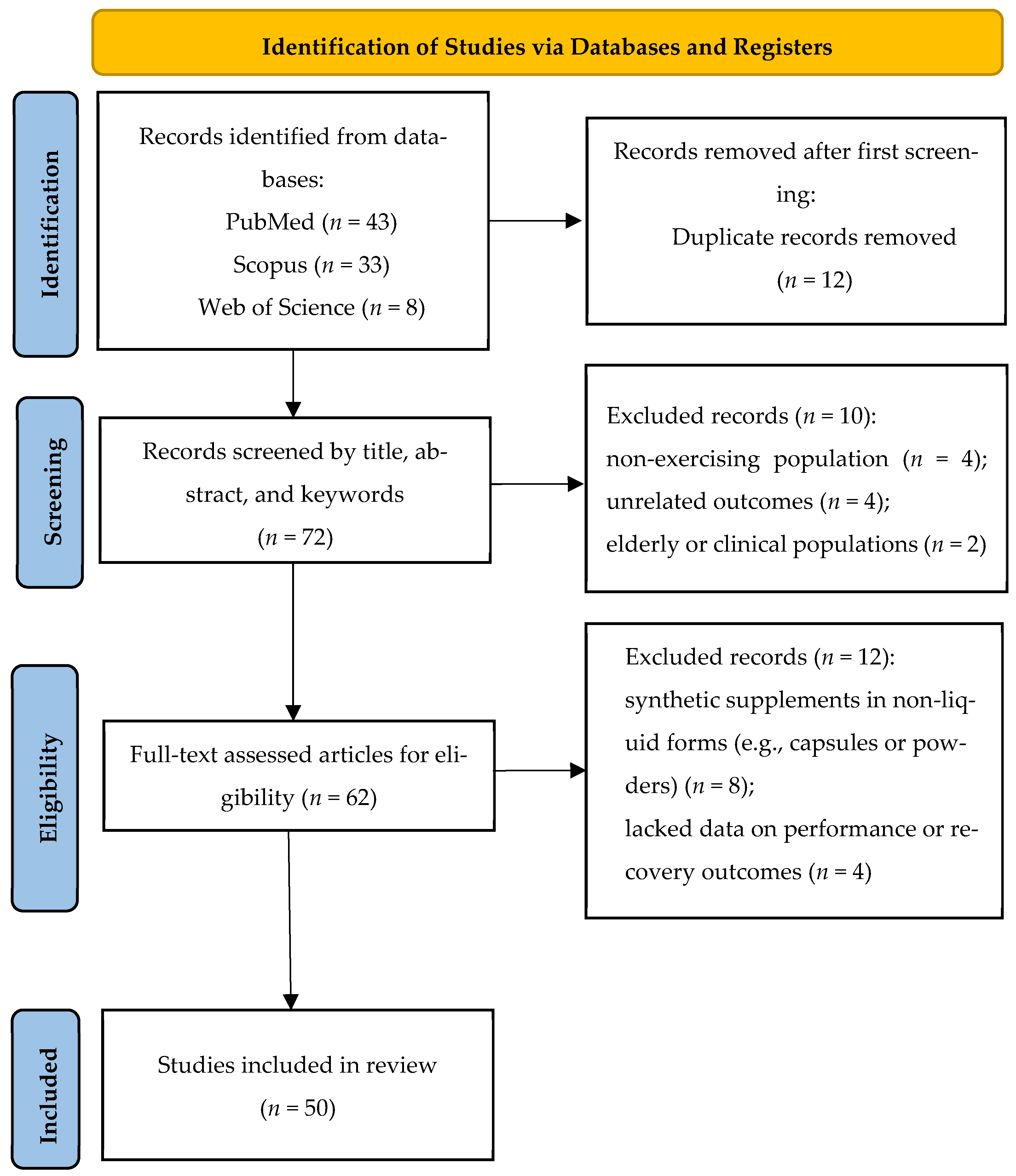
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. Methodological Quality Assessment
3.2. Study Characteristics
4. Discussion
4.1. Beetroot Juice
4.2. Pomegranate Juice
4.3. Cherry Juice
4.4. Pickle Juice
4.5. Watermelon Juice
4.6. Comparative Discussion of Physiological Outcomes
4.7. Practical Implications for Application
5. Conclusions
Limitations and Future Perspectives
- -
- Conducting well-powered RCTs with larger sample sizes and standardized supplementation protocols;
- -
- Designing comparative studies that directly assess different juice types using consistent methodologies;
- -
- Implementing long-term intervention studies to examine chronic adaptations and recovery processes;
- -
- Exploring the underlying cellular and molecular mechanisms, especially for lesser-understood juices like pomegranate and pickle juice;
- -
- Including female participants and athletes from a variety of backgrounds to assess sex- and population-specific responses;
- -
- Combination strategies (e.g., juice plus caffeine or other nutrients) to evaluate synergistic effects on performance and recovery.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| NO | Nitric Oxide |
| BRJ | Beetroot Juice |
| POMj | Pomegranate Juice |
| IL-6 | Interleukin-6 |
| TNF | Tumor Necrosis Factor |
| TCJ | Tart Cherry Juice |
| PJ | Pickle Juice |
| WJ | Watermelon Juice |
| RCTs | Randomized Controlled Trials |
| INPLASY | International Platform of Registered Systematic Review and Meta-Analysis Protocols |
| PEDro | Physiotherapy Evidence Database Scale |
| M | Male |
| F | Female |
| NO2− | Nitrite |
| NO3− | Nitrate |
| VO2 | Oxygen Uptake |
| TT | Time Trial |
| YYIR1 | Yo-Yo Intermittent Recovery level 1 |
| SD | Single-Dose |
| DD | Double-Dose |
| AEC | Aerobic Energy Cost |
| VCO2 | Carbon Dioxide production |
| VE | Minute Ventilation |
| MVC | Maximal Voluntary Contraction |
| MIVC | Maximal Isometric Voluntary Contraction |
| SJ | Squat Jump |
| CMJ | Countermovement Jump |
| RI | Reactive Strength Index |
| PPT | Pressure Pain Threshold |
| CK | Creatine Kinase |
| CRP | C-Reactive Protein |
| hsCRP | High-Sensitivity C-Reactive Protein |
| PC | Protein Carbonyls |
| LOOH | Lipid Hydroperoxides |
| SaO2 | Oxygen Saturation |
| HR | Heart Rate |
| DEXA | Dual X-Ray Absorptiometry |
| RPE | Rating of Perceived Exertion |
| HI | High |
| LO | Low |
| KNO3 | Potassium Nitrate |
| SIT | Sprint Interval Training |
| BP | Blood Pressure |
| SBP | Systolic Blood Pressure |
| GAE | Gallic Acid Equivalent |
| LDH | Lactate Dehydrogenase |
| DOMS | Delayed-Onset Muscle Soreness |
| TTE | Time to Exhaustion |
| XC | Cross-Country |
| RTF | Repetitions to Failure |
| TSI | Tissue Saturation Index |
| VAS | Visual Analog Scale |
| TQR | Total Quality Recovery |
| VO2max | Maximal Oxygen uptake |
| MBT | Medicine Ball Throw |
| VJH | Vertical Jump Height |
| BF% | Body Fat Percentage |
| POMx | Pomegranate Extract |
| ASAT | Aspartate Aminotransferase |
| TPC | Total Phenolic Content |
| o-DPO | Orthodiphenol |
| MDA | Malonaldehyde |
| UA | Uric Acid |
| TAC | Total Antioxidant Capacity |
| Hcy | Homocysteine |
| MCJ | Montmorency Cherry Juice |
| MTCJ | Montmorency Tart Cherry Juice |
| F2-IsoP | F2-Isoprostane |
| Na | Sodium |
| K | Potassium |
| IMTP | Isometric Mid-Thigh Pull |
| BMX | Bicycle Motocross |
| GPS | Global Positioning System |
References
- Wang, L.; Meng, Q.; Su, C.H. From Food Supplements to Functional Foods: Emerging Perspectives on Post-Exercise Recovery Nutrition. Nutrients 2024, 16, 4081. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; Shaw, C.S.; Stepto, N.K.; Levinger, I. Exercise and Glycemic Control: Focus on Redox Homeostasis and Redox-Sensitive Protein Signaling. Front. Endocrinol. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The Compelling Link Between Physical Activity and the Body’s Defense System. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Li, S.; Fasipe, B.; Laher, I. Potential Harms of Supplementation with High Doses of Antioxidants in Athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-Gonzáles, A.; Madrigal-Santillán, E.O.; Angeles-Valencia, M.; Anguiano-Robledo, L.; González-López, L.L.; Sosa-Gomez, A.; Fregoso-Aguilar, T.; Esquivel-Chirino, C.; Ruiz-Velazco-Benítez, Y.A.; et al. Phytochemicals and modulation of exercise-induced oxidative stress: A novel overview of antioxidants. Am. J. Transl. Res. 2022, 14, 8292–8314. [Google Scholar]
- Jacobs, D.R.; Tapsell, L.C. Food, Not Nutrients, Is the Fundamental Unit in Nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef]
- Connolly, D.A.; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a Tart Cherry Juice Blend in Preventing the Symptoms of Muscle Damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sports 2009, 20, 843–852. [Google Scholar] [CrossRef]
- Strobel, N.A.; Peake, J.M.; Matsumoto, A.; Marsh, S.A.; Coombes, J.S.; Wadley, G.D. Antioxidant Supplementation Reduces Skeletal Muscle Mitochondrial Biogenesis. Med. Sci. Sports Exerc. 2011, 43, 1017–1024. [Google Scholar] [CrossRef]
- Visioli, F.; Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.C.R.; Oliveira Assumpção, C.; Prestes, J.; Sérgio Denadai, B. Consumption of cherries as a strategy to attenuate exercise-induced muscle damage and inflamation in humans. Nutr. Hosp. 2015, 32, 1885–1893. [Google Scholar] [CrossRef]
- Van Hoorebeke, J.; Trias, C.; Davis, B.; Lozada, C.; Casazza, G. Betalain-Rich Concentrate Supplementation Improves Exercise Performance in Competitive Runners. Sports 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Crum, E.M.; Che Muhamed, A.M.; Barnes, M.; Stannard, S.R. The Effect of Acute Pomegranate Extract Supplementation on Oxygen Uptake in Highly-Trained Cyclists During High-Intensity Exercise in a High Altitude Environment. J. Int. Soc. Sports Nutr. 2017, 14, 14. [Google Scholar] [CrossRef]
- Vitale, K.C.; Hueglin, S.; Broad, E. Tart Cherry Juice in Athletes: A Literature Review and Commentary. Curr. Sports Med. Rep. 2017, 16, 230–239. [Google Scholar] [CrossRef]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of Beetroot Juice Supplementation on Intermittent High-Intensity Exercise Efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflammation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef]
- McIlvenna, L.C.; Muggeridge, D.J.; Whitfield, J. Exploring the Mechanisms by Which Nitrate Supplementation Improves Skeletal Muscle Contractile Function: One Fibre at a Time. J. Physiol. 2020, 598, 25–27. [Google Scholar] [CrossRef]
- Bahari, H.; Rafiei, H.; Goudarzi, K.; Omidian, K.; Asbaghi, O.; Kolbadi, K.S.; Naderian, M.; Hosseini, A. The Effects of Pomegranate Consumption on Inflammatory and Oxidative Stress Biomarkers in Adults: A Systematic Review and Meta-Analysis. Inflammopharmacology 2023, 31, 2283–2301. [Google Scholar] [CrossRef]
- Dale, R.B.; Leaver-Dunn, D.; Bishop, P.A. Compositional Analysis of a Common Acetic Acid Solution with Practical Implications for Ingestion. J. Athl. Train. 2003, 38, 57–61. [Google Scholar]
- Miller, K.C.; Mack, G.W.; Knight, K.L.; Hopkins, J.T.; Draper, D.O.; Fields, P.J.; Hunter, I. Reflex Inhibition of Electrically Induced Muscle Cramps in Hypohydrated Humans. Med. Sci. Sports Exerc. 2010, 42, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.C. Exercise-Associated Muscle Cramps. In Exertional Heat Illness. A Clinical and Evidence-Based Guide; Adams, W.M., Jardine, J.F., Eds.; Springer: Cham, Switzerland, 2019; pp. 117–136. [Google Scholar] [CrossRef]
- Edwards, A.J.; Wiley, E.R.; Brown, E.D.; Clevidence, B.A.; Vinyard, B.T.; Collins, J.K.; Perkins-Veazie, P.; Baker, R.A. Consumption of Watermelon Juice Increases Plasma Concentrations of Lycopene and β-Carotene in Humans. J. Nutr. 2003, 133, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.J.; Pendleton, L.C.; Eichler, D.C. Argininosuccinate synthase: At the center of arginine metabolism. Int. J. Biochem. Mol. Biol. 2011, 2, 8–23. [Google Scholar] [PubMed]
- Pinto, M.P.; Santos, C.N.; Henriquesa, C.; Lima, G.; Quedas, F. Lycopene content and antioxidant capacity of Portuguese watermelon fruits. Electron. J. Environ. Agric. Food Chem. 2011, 10, 2090–2097. [Google Scholar]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Davis, A.R.; Webber, C.L.; Liu, W.; Perkins-Veazie, P.; Levi, A.; King, S. Watermelon Quality Traits as Affected by Ploidy. HortScience 2013, 48, 1113–1118. [Google Scholar] [CrossRef]
- Naz, A.; Sadiq Butt, M.; Pasha, I.; Nawaz, H. Antioxidant Indices of Watermelon Juice and Lycopene Extract. Pak. J. Nutr. 2013, 12, 255–260. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and Nitrogen Homeostasis: An Overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Wethington, L.N.; Stone, M.S.; Stewart, R.W.; Moyen, N.E. Acute Citrulline Malate Supplementation Improves Upper- and Lower-Body Submaximal Weightlifting Exercise Performance in Resistance-Trained Females. Eur. J. Nutr. 2015, 56, 775–784. [Google Scholar] [CrossRef]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in Health and Disease. Review on Human Studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- De Morton, N.A. The Pedro Scale Is a Valid Measure of the Methodological Quality of Clinical Trials: A Demographic Study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Christensen, P.M.; Nyberg, M.; Bangsbo, J. Influence of Nitrate Supplementation on VO2 Kinetics and Endurance of Elite Cyclists. Scand. J. Med. Sci. Sports 2012, 23, e21–e31. [Google Scholar] [CrossRef]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary Nitrate Supplementation Improves Team Sport-Specific Intense Intermittent Exercise Performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef]
- Martin, K.; Smee, D.; Thompson, K.G.; Rattray, B. No Improvement of Repeated-Sprint Performance with Dietary Nitrate. Int. J. Sports Physiol. Perform. 2014, 9, 845–850. [Google Scholar] [CrossRef]
- Pinna, M.; Roberto, S.; Milia, R.; Marongiu, E.; Olla, S.; Loi, A.; Migliaccio, G.; Padulo, J.; Orlandi, C.; Tocco, F.; et al. Effect of Beetroot Juice Supplementation on Aerobic Response During Swimming. Nutrients 2014, 6, 605–615. [Google Scholar] [CrossRef]
- Thompson, C.; Wylie, L.J.; Fulford, J.; Kelly, J.; Black, M.I.; McDonagh, S.T.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Dietary Nitrate Improves Sprint Performance and Cognitive Function During Prolonged Intermittent Exercise. Eur. J. Appl. Physiol. 2015, 115, 1825–1834. [Google Scholar] [CrossRef]
- Clifford, T.; Berntzen, B.; Davison, G.; West, D.; Howatson, G.; Stevenson, E. Effects of Beetroot Juice on Recovery of Muscle Function and Performance Between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8, 506. [Google Scholar] [CrossRef]
- Patrician, A.; Schagatay, E. Dietary Nitrate Enhances Arterial Oxygen Saturation After Dynamic Apnea. Scand. J. Med. Sci. Sports 2016, 27, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Vanhatalo, A.; Jell, H.; Fulford, J.; Carter, J.; Nyman, L.; Bailey, S.J.; Jones, A.M. Dietary Nitrate Supplementation Improves Sprint and High-Intensity Intermittent Running Performance. Nitric Oxide 2016, 61, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Bailey, S.J.; Kelly, J.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. Influence of Beetroot Juice Supplementation on Intermittent Exercise Performance. Eur. J. Appl. Physiol. 2016, 116, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Nyakayiru, J.; Van Dijk, J.W.; Maase, K.; Ballak, S.B.; Senden, J.M.G.; Van Loon, L.J.C.; Verdijk, L.B. Repeated-sprint Performance and Plasma Responses Following Beetroot Juice Supplementation Do Not Differ Between Recreational, Competitive and Elite Sprint Athletes. Eur. J. Sport Sci. 2018, 18, 524–533. [Google Scholar] [CrossRef]
- Richard, P.; Koziris, L.P.; Charbonneau, M.; Naulleau, C.; Tremblay, J.; Billaut, F. Time-Trial Performance in World-Class Speed Skaters After Chronic Nitrate Ingestion. Int. J. Sports Physiol. Perform. 2018, 13, 1317–1323. [Google Scholar] [CrossRef]
- Thompson, C.; Vanhatalo, A.; Kadach, S.; Wylie, L.J.; Fulford, J.; Ferguson, S.K.; Blackwell, J.R.; Bailey, S.J.; Jones, A.M. Discrete Physiological Effects of Beetroot Juice and Potassium Nitrate Supplementation Following 4-WK Sprint Interval Training. J. Appl. Physiol. 2018, 124, 1519–1528. [Google Scholar] [CrossRef]
- Esen, O.; Nicholas, C.; Morris, M.; Bailey, S.J. No Effect of Beetroot Juice Supplementation on 100-m and 200-m Swimming Performance in Moderately Trained Swimmers. Int. J. Sports Physiol. Perform. 2019, 14, 706–710. [Google Scholar] [CrossRef]
- Daab, W.; Bouzid, M.A.; Lajri, M.; Bouchiba, M.; Saafi, M.A.; Rebai, H. Chronic Beetroot Juice Supplementation Accelerates Recovery Kinetics Following Simulated Match Play in Soccer Players. J. Am. Coll. Nutr. 2020, 40, 61–69. [Google Scholar] [CrossRef]
- Fernández-Elías, V.; Courel-Ibáñez, J.; Pérez-López, A.; Jodra, P.; Moreno-Pérez, V.; Coso, J.D.; López-Samanes, Á. Acute Beetroot Juice Supplementation Does Not Improve Match-Play Activity in Professional Tennis Players. J. Am. Nutr. Assoc. 2020, 41, 30–37. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Palau-Salvà, G.; Serra-Payá, N.; Ruiz-Hermosel, M.; Berbell, M.; Viñals, X.; Bataller, M.G.; Carbonell, T.; Vilches-Saez, S.; Cobo, E.P.; et al. Understanding the Effects of Beetroot Juice Intake on CrossFit Performance by Assessing Hormonal, Metabolic and Mechanical Response: A Randomized, Double-Blind, Crossover Design. J. Int. Soc. Sports Nutr. 2020, 17, 56. [Google Scholar] [CrossRef]
- Esen, O.; Domínguez, R.; Karayigit, R. Acute Beetroot Juice Supplementation Enhances Intermittent Running Performance but Does Not Reduce Oxygen Cost of Exercise Among Recreational Adults. Nutrients 2022, 14, 2839. [Google Scholar] [CrossRef]
- Esen, O.; Faisal, A.; Zambolin, F.; Bailey, S.J.; Callaghan, M.J. Effect of Nitrate Supplementation on Skeletal Muscle Motor Unit Activity During Isometric Blood Flow Restriction Exercise. J. Appl. Physiol. 2022, 122, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Giv, V.; Aminaei, M.; Nikoei, R. The Effect of Eight Weeks Beetroot Juice Supplement on Aerobic, Anaerobic Power, and Field Performance of Soccer Players. Res. Sports Med. 2022, 32, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Z.; Wang, X.; Wang, G.; Wang, Y.; Tang, K.; Gao, B. Influence of Chronic Nitrate-Rich Beetroot Juice Supplementation on the Endurance Performance of Active Winter Triathletes: A Randomized Controlled Trial. J. Am. Nutr. Assoc. 2022, 42, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Castro, J.M.; Campos-Perez, J.; Ranchal-Sanchez, A.; Durán-López, N.; Domínguez, R. Acute Effects of Beetroot Juice Supplements on Lower-Body Strength in Female Athletes: Double-Blind Crossover Randomized Trial. Sports Health 2022, 14, 812–821. [Google Scholar] [CrossRef]
- Tan, R.; Pennell, A.; Price, K.M.; Karl, S.T.; Seekamp-Hicks, N.G.; Paniagua, K.K.; Weiderman, G.D.; Powell, J.P.; Sharabidze, L.K.; Lincoln, I.G.; et al. Effects of Dietary Nitrate Supplementation on Performance and Muscle Oxygenation During Resistance Exercise in Men. Nutrients 2022, 14, 3703. [Google Scholar] [CrossRef]
- Esen, O.; Karayigit, R.; Peart, D.J. Acute Beetroot Juice Supplementation Did Not Enhance Intermittent Running Performance in Trained Rugby Players. Eur. J. Sport Sci. 2023, 23, 2321–2328. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of Beetroot Juice Supplementation on Muscle Soreness and Performance Recovery After Exercise-Induced Muscle Damage in Female Volleyball Players. Nutrients 2023, 15, 3763. [Google Scholar] [CrossRef]
- Moreno-Heredero, B.; Morencos, E.; Morais, J.; Barbosa, T.M.; Veiga, S. A Single Dose of Beetroot Juice Not Enhance Performance During Intervallic Swimming Efforts. J. Sports Sci. Med. 2024, 23, 228–235. [Google Scholar] [CrossRef]
- Neteca, J.; Veseta, U.; Liepina, I.; Volgemute, K.; Dzintare, M.; Babarykin, D. Effect of Beetroot Juice Supplementation on Aerobic Capacity in Female Athletes: A Randomized Controlled Study. Nutrients 2024, 17, 63. [Google Scholar] [CrossRef]
- Tan, R.; Merrill, C.; Riley, C.F.; Hammer, M.A.; Kenney, R.T.; Riley, A.A.; Li, J.; Zink, A.C.; Karl, S.T.; Price, K.M.; et al. Acute Inorganic Nitrate Ingestion Does Not Impact Oral Microbial Composition, Cognitive Function, or High-Intensity Exercise Performance in Female Team-Sport Athletes. Eur. J. Appl. Physiol. 2024, 124, 3511–3525. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, Z.; Heung-Sang Wong, S.; Zheng, C.; Tsz-Chun Poon, E. Acute Effects of Various Doses of Nitrate-Rich Beetroot Juice on High-Intensity Interval Exercise Responses in Women: A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial. J. Int. Soc. Sports Nutr. 2024, 21, 2334680. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huo, L.; Wang, F.; Wang, T.; Rong, W.; He, Y. Caffeine and Beetroot Juice Optimize 1,000-m Performance: Shapley Additive Explanations Analysis. Am. J. Mens. Health 2025, 19, 15579883251327907. [Google Scholar] [CrossRef]
- Trombold, J.R.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin Consumption Improves Strength Recovery 2–3 D After Eccentric Exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Trombold, J.R.; Reinfeld, A.S.; Casler, J.R.; Coyle, E.F. The Effect of Pomegranate Juice Supplementation on Strength and Soreness After Eccentric Exercise. J. Strength. Cond. Res. 2011, 25, 1782–1788. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate Supplementation Accelerates Recovery of Muscle Damage and Soreness and Inflammatory Markers After a Weightlifting Training Session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of Pomegranate Juice Supplementation on Oxidative Stress Biomarkers Following Weightlifting Exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef]
- Urbaniak, A.; Basta, P.; Ast, K.; Wołoszyn, A.; Kuriańska-Wołoszyn, J.; Latour, E.; Skarpańska-Stejnborn, A. The Impact of Supplementation with Pomegranate Fruit (Punica granatum L.) Juice on Selected Antioxidant Parameters and Markers of Iron Metabolism in Rowers. J. Int. Soc. Sports Nutr. 2018, 15, 35. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Trabelsi, K.; Bragazzi, N.L.; Boukhris, O.; Bouaziz, M.; Ayadi, F.; El Abed, K.; Driss, T.; Souissi, N.; et al. Effects of Natural Polyphenol-Rich Pomegranate Juice on the Acute and Delayed Response of Homocysteine and Steroidal Hormones Following Weightlifting Exercises: A Double-Blind, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2020, 17, 15. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency Cherry Juice Reduces Muscle Damage Caused by Intensive Strength Exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery Facilitation with Montmorency Cherries Following High-Intensity, Metabolically Challenging Exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef]
- Bell, P.; Stevenson, E.; Davison, G.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of Tart Cherry Juice on Recovery and Next Day Performance in Well-Trained Water Polo Players. J. Int. Soc. Sports Nutr. 2016, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.; Brashill, C.; Brett, A.; Clifford, T. Tart Cherry Juice: No Effect on Muscle Function Loss or Muscle Soreness in Professional Soccer Players After a Match. Int. J. Sports Physiol. Perform. 2020, 15, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Rapin, N.; Andrushko, J.W.; Farthing, J.P.; Gordon, J.; Chilibeck, P.D. The Effect of Tart Cherry Juice Compared to a Sports Drink on Cycling Exercise Performance, Substrate Metabolism, and Recovery. PLoS ONE 2024, 19, e0307263. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.C. Electrolyte and Plasma Responses After Pickle Juice, Mustard, and Deionized Water Ingestion in Dehydrated Humans. J. Athl. Train. 2014, 49, 360–367. [Google Scholar] [CrossRef]
- Peikert, J.; Miller, K.C.; Albrecht, J.; Tucker, J.; Deal, J. Pre-Exercise Ingestion of Pickle Juice, Hypertonic Saline, or Water and Aerobic Performance and Thermoregulation. J. Athl. Train. 2014, 49, 204–209. [Google Scholar] [CrossRef]
- McKenney, M.A.; Miller, K.C.; Deal, J.E.; Garden-Robinson, J.A.; Rhee, Y.S. Plasma and Electrolyte Changes in Exercising Humans After Ingestion of Multiple Boluses of Pickle Juice. J. Athl. Train. 2015, 50, 141–146. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Alacid, F.; Carrasco, M.; Martínez, I.; Aguayo, E. Watermelon Juice: Potential Functional Drink for Sore Muscle Relief in Athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef]
- Cutrufello, P.T.; Gadomski, S.J.; Zavorsky, G.S. The Effect Ofl-Citrulline and Watermelon Juice Supplementation on Anaerobic and Aerobic Exercise Performance. J. Sports Sci. 2014, 33, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two Weeks of Watermelon Juice Supplementation Improves Nitric Oxide Bioavailability but Not Endurance Exercise Performance in Humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Ramos-Campo, D.J.; Fernández-Lobato, B.; Rubio-Arias, J.A.; Alacid, F.; Aguayo, E. Biochemical, Physiological, and Performance Response of a Functional Watermelon Juice Enriched in L-Citrulline During a Half-Marathon Race. Food Nutr. Res. 2017, 61, 1330098. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Pinzone, A.G.; Lipes, S.E.; Mangine, G.T.; Townsend, J.R.; Allerton, T.D.; Sell, K.M.; Ghigiarelli, J.J. Effect of Watermelon Supplementation on Exercise Performance, Muscle Oxygenation, and Vessel Diameter in Resistance-Trained Men. Eur. J. Appl. Physiol. 2022, 122, 1627–1638. [Google Scholar] [CrossRef]
- AghabeigiAmin, P.; Azizi, M.; Tahmasebi, W. Effects of 6 Weeks of Watermelon Juice Supplementation on Total Antioxidant Capacity and VO2max in Elite Taekwondo Athletes. Sport Sci. Health 2025, 21, 923–929. [Google Scholar] [CrossRef]
- Erzurum, S.C.; Ghosh, S.; Janocha, A.J.; Xu, W.; Bauer, S.; Bryan, N.S.; Tejero, J.; Hemann, C.; Hille, R.; Stuehr, D.J.; et al. Higher Blood Flow and Circulating NO Products Offset High-Altitude Hypoxia Among Tibetans. Proc. Natl. Acad. Sci. USA 2007, 104, 17593–17598. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of Dietary Nitrate on Oxygen Cost During Exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Dejam, A.; Hunter, C.J.; Schechter, A.N.; Gladwin, M.T. Emerging Role of Nitrite in Human Biology. Blood Cells Mol. Dis. 2004, 32, 423–429. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. Beetroot Juice Is More Beneficial than Sodium Nitrate for Attenuating Muscle Pain After Strenuous Eccentric-Bias Exercise. Appl. Physiol. Nutr. Metab. 2017, 42, 1185–1191. [Google Scholar] [CrossRef]
- Bex, T.; Baguet, A.; Achten, E.; Aerts, P.; De Clercq, D.; Derave, W. Cyclic Movement Frequency is Associated with Muscle Typology in Athletes. Scand. J. Med. Sci. Sports 2017, 27, 223–229. [Google Scholar] [CrossRef]
- Seeram, N. Cyclooxygenase Inhibitory and Antioxidant Cyanidin Glycosides in Cherries and Berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.-C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and Antiinflammatory Activities of Anthocyanins and Their Aglycon, Cyanidin, from Tart Cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Kuehl, K.S.; Perrier, E.T.; Elliot, D.L.; Chesnutt, J.C. Efficacy of Tart Cherry Juice in Reducing Muscle Pain During Running: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2010, 7, 17. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of Pomegranate Supplementation on Exercise Performance and Post-Exercise Recovery in Healthy Adults: A Systematic Review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef]
- Ettinger, P.O.; Regan, T.J.; Oldewurtel, H.A. Hyperkalemia, Cardiac Conduction, and the Electrocardiogram: A Review. Am. Heart J. 1974, 88, 360–371. [Google Scholar] [CrossRef]
| Category | Criteria | Details |
|---|---|---|
| Inclusion | Population | Human participants who are athletes or physically active individuals. |
| Intervention | Supplementation with beetroot, pomegranate, cherry, watermelon, or pickle juice in liquid form. | |
| Study Design | Randomized controlled trials (RCTs), cohort studies, cross-sectional studies, case studies. | |
| Outcomes | Studies reporting at least one outcome related to exercise performance, muscle soreness, inflammation, or recovery. | |
| Language | Published in English. | |
| Exclusion | Population | Animal studies. |
| Publication type | Meta-analyses, systematic reviews, narrative reviews, or other secondary research. | |
| Supplement type | Studies using synthetic or encapsulated supplements (e.g., pills, powders) or multi-ingredient formulations unrelated to the juice itself. | |
| Outcomes | Studies that did not report performance or recovery-related outcomes. |
| Study | Study Design | Participants’ Characteristics | Juice Type | Dosage | Measured Outcomes | Findings |
|---|---|---|---|---|---|---|
| Christensen et al. (2012) [37] | Randomized, crossover | 10M elite endurance cyclists | BRJ (nitrate-rich) | 6 days, 500 mL/day, 0.5 g of NO3− | VO2 kinetics, endurance TT, repeated sprint capacity | Plasma nitrate levels were significantly elevated in BRJ conditions. No significant improvements in VO2 kinetics, TT performance, or repeated sprint capacity. The findings suggest that nitrate supplementation may not confer ergogenic effects in highly trained athletes. |
| Wylie et al. (2013) [38] | Randomized, double-blind, crossover | 14M recreational team-sport players | BRJ (nitrate-rich) | 490 mL, 4.1 mmol of NO3− per 70 mL, ~30 h before exercise | YYIR1, blood lactate, plasma nitrite concentration, muscle glucose uptake, muscle excitability | BRJ supplementation resulted in a 4.2% improvement in YYIR1 performance. Blood lactate was not significantly different between groups. Mean blood glucose was lower and plasma nitrite was higher in the BRJ group compared to placebo. |
| Martin et al. (2014) [39] | Randomized, double-blind, crossover | 16 team-sport athletes (9M + 7F) | BRJ (nitrate-rich) | 70 mL, 0.3 g of NO3−, SD, 2 h before repeated sprint protocol | Sprint performance, total work done, power output | BRJ supplementation did not improve repeated sprint performance, total work done, or power output. The study suggests that nitrate supplementation may not be beneficial for near-maximal and frequent sprint efforts. |
| Pinna et al. (2014) [40] | Randomized, crossover design | 14M moderately trained master swimmers | BRJ | 6 days, 0.5 L/day, 5.5 mmol of NO3− | Workload at anaerobic threshold, AEC, VO2, VCO2, VE | The workload at the anaerobic threshold was significantly increased after BRJ supplementation. The aerobic energy cost was significantly reduced in the BRJ supplementation test. Other variables did not show statistically significant differences between the BRJ and control tests, suggesting that it positively affects swimming performance by reducing AEC and increasing the anaerobic workload. |
| Thompson et al. (2015) [41] | Randomized, double-blind, crossover | 16M team-sport players | BRJ | 7 days, 140 mL/day, 6.4 mmol of NO3− | Intermittent sprint performance, cognitive reaction time, total work done | BRJ improved work done during sprints and maintained cognitive reaction time. |
| Clifford et al. (2016) [42] | Independent groups, randomized | 20M team-sport players | BRJ | 250 mL, twice a day, 3 days post-exercise | MIVC, CMJ, RI, PPT, CK, hsCRP, PC, LOOH | BRJ reduced decrements in CMJ and RI but had no effect on sprint performance or oxidative stress markers. |
| Patrician and Schagatay (2016) [43] | Randomized, crossover | 14M healthy apnea divers | BRJ (nitrate-rich) | 70 mL, ~5.0 mmol of NO3−, SD, 2.5 h before dynamic apnea performance | Arterial SaO2, HR | BRJ supplementation elevated SaO2 after 75 m dynamic apnea dives, indicating a potential oxygen-conserving effect. No significant effect on HR. |
| Thompson et al. (2016) [44] | Randomized, double-blind, crossover | 36M team-sport players | BRJ (nitrate-rich) | 5 days, 70 mL/day, 6.4 mmol of NO3− | Sprint performance (5 m, 10 m, 20 m), YYIR1 performance, cognitive reaction time | BRJ supplementation improved sprint split times and distance covered in the YYIR1 test. Reaction time to cognitive tasks was also shorter after BRJ compared to placebo. |
| Wylie et al. (2016) [45] | Randomized, placebo-controlled, crossover | 10M team-sport players | BRJ (nitrate-rich) | 5 days, 1 or 2 × 70 mL, 8.2 mmol NO3− per day (~4.1 mmol of NO3− per 70 mL) | Power output during 24 × 6 s sprints, 7 × 30 s sprints, and 6 × 60 s efforts; blood lactate | BRJ improved power output during short, high-intensity sprints but not during longer intervals or with longer recovery periods. Blood lactate levels increased significantly in the short-duration sprint protocol. |
| Jonvik et al. (2018) [46] | Randomized, double-blind, crossover | 52M: 20 recreational cyclists, 22 national talent speed skaters, 10 Olympic-level track cyclists | BRJ (nitrate-rich) | 140 mL/day, ~800 mg/d of NO3− | DEXA, Wingate test, plasma nitrate and nitrite concentrations, RPE, HR | BRJ significantly increased plasma nitrate and nitrite concentrations in all groups, regardless of training status. No improvement in peak or mean power output across three 30 s Wingate sprints. The only performance benefit observed was a 2.8% reduction in time to reach peak power, suggesting improved acceleration across all athletic levels and sprints. |
| Richard et al. (2018) [47] | Double-blind, placebo-controlled, crossover | 9 international-level short-track speed skaters (4M + 5F) | BRJ | HI: 115 mL, ~6.5 mmol of NO3− or LO: 115 mL, ~0.9 mmol of NO3−, 5 days with DD on day 5 | 1000 m TT performance; plasma nitrate and nitrite, lactate concentrations | Nitrate supplementation increased plasma nitrate and nitrite but did not improve single or repeated 1000 m TT performance in elite speed skaters. No effect on lactate concentration or recovery. |
| Thompson et al. (2018) [48] | Randomized, crossover, placebo-controlled | 30 recreationally active subjects (18M + 12F) | BRJ | 70 mL (~6.4 mmol of NO3− per 70 mL) in the morning and in the evening | VO2 peak, time to task failure, lactate, phosphocreatine recovery | BRJ enhanced exercise capacity adaptations more than KNO3 and SIT alone. |
| Esen et al. (2019) [49] | Randomized, double-blind, crossover | 10 moderately trained swimmers (5M + 5F) | BRJ (nitrate-rich) | 3 days, 140 mL/day, ~8 mmol/day of NO3− | SBP, blood lactate concentration, plasma nitrate and nitrite concentrations, 200 m and 100 m swimming TT | BRJ supplementation increased plasma nitrite concentration and lowered SBP, but had no effect on swim performance. BRJ does not show an ergogenic effect in moderately trained swimmers. |
| Daab et al. (2020) [50] | Randomized, double-blind, crossover | 13M soccer players | BRJ | 150 mL, 250 mg of NO3−, total phenolic content: 385 mg/GAE/L, twice a day for 7 days | SJ, CMJ, MVC, 20 m sprint, CK, LDH, CRP, DOMS | BRJ supplementation reduced perceived muscle soreness and maintained better performance during recovery in CMJ, MVC, and sprint/placebo. No significant effect on biochemical markers of muscle damage. |
| Fernández-Elías et al. (2020) [51] | Randomized, double-blind | 9M professional tennis players | BRJ | 70 mL, 6.4 mmol of NO3−, 3 h before match | Match-play running performance, serve speed, isometric handgrip strength | No significant differences were found between the BRJ and placebo trials in match-play running performance, serve speed, or isometric handgrip strength. Acute BRJ supplementation did not produce any performance benefit in professional tennis players. |
| Garnacho-Castaño et al. (2020) [52] | Randomized, double-blind, crossover | 12M well-trained CrossFit practitioners | BRJ (nitrate-rich) | 140 mL, ~12.8 mmol of NO3−, SD | Number of repetitions, cortisol response, SaO2, muscular fatigue | BRJ increased the number of repetitions only in the exercise routine with a 3 min rest between sets. It also caused a greater increase in cortisol and a significant drop in SaO2, indicating increased muscular fatigue. |
| Esen et al. (2022) [53] | Randomized, double-blind, crossover | 12M recreationally active | BRJ (nitrate-rich) | 140 mL, 12.8 mmol of NO3−, SD | YYIR1 performance, VO2 at sub-maximal and peak levels | BRJ supplementation improved YYIR1 performance but did not significantly affect VO2 at sub-maximal or peak levels. |
| Esen et al. (2022) [54] | Randomized, double-blind, crossover | 16 healthy active young adults (10M + 6F) | BRJ (nitrate-rich) | 5 days, 2 × 70 mL/day,~12.8 mmol NO3−/day) | Plasma NO2−, MVC, RPE | Nitrate-rich BRJ increased plasma NO2− concentration and improved RPE in comparison to placebo group, while there was no significant difference in the MVC. BRJ may have implications in enhancing skeletal muscle contractile function. |
| Giv et al. (2022) [55] | Experimental, control group in pre- and post-test | 40M soccer players | BRJ | 8 weeks, 100 mL (300 mg of NO3−) | Aerobic power, respiratory exchange ratio, anaerobic threshold, anaerobic power, field performance, fatigue index | Soccer training combined with BRJ supplementation significantly improved aerobic power, respiratory exchange ratio, anaerobic threshold, anaerobic power, and field performance. |
| Huang et al. (2022) [56] | Randomized, double-blind, crossover | 80 young active winter triathletes (44M + 36F) | BRJ | 7 days, 3 doses per day, 6.5 mmol of NO3− per 70 mL | VO2, respiratory exchange ratio, blood lactic acid, TTE, 10 km XC skiing performance | BRJ supplementation decreased VO2, respiratory exchange ratio, and blood lactic acid levels during high-speed running. Increased TTE during cycling exhaustion testing but did not improve 10 km XC skiing performance. BRJ may enhance running economy and cycling TTE but not XC skiing performance. |
| Jurado-Castro et al. (2022) [57] | Randomized, double-blind, crossover | 14F physically active | BRJ | 70 mL, 400 mg of NO3− | CMJ, back squat test, muscular endurance test | Greater CMJ, increased performance. Confirmed ergogenic effect of BRJ on muscular endurance in the lower limbs. |
| Tan et al. (2022) [58] | Randomized, double-blind, crossover | 14M recreationally active | BRJ | 4 days, 2 × 70 mL, ~5.9 mmol of NO3− per 70 mL | Bench press, back squat, RTF, TSI | Acute BRJ ingestion increased RTF in bench press but not in back squat. No significant change in muscle oxygenation. |
| Esen et al. (2023) [59] | Randomized, counterbalanced, double-blind, placebo-controlled, crossover | 12M trained rugby players | BRJ (nitrate-rich) | 140 mL, 12.8 mmol of NO3−, SD | Modified YYIR1, CMJ, plasma NO3− and NO2− concentrations | Acute BRJ supplementation increased plasma NO3− and NO2− but did not improve intermittent running performance or CMJ performance in trained rugby players. |
| Hemmatinafar et al. (2023) [60] | Randomized, double-blind, crossover | 12F young volleyball players | BRJ | 50 mL, 4.1 mmol of NO3−, 8 servings over 2 days | Wall-sit performance, V sit and reach, vertical jump height, pressure pain threshold, thigh swelling, perceived muscle soreness, VAS | BRJ supplementation improved wall-sit performance, reduced swelling, and perceived muscle soreness but did not significantly affect vertical jump height or V sit and reach performance. |
| Moreno-Heredero et al. (2024) [61] | Randomized, placebo-controlled, double-blind, crossover | 18 competitive swimmers (9M + 9F) | BRJ | 70 mL, 6.4 mmol/400 mg of NO3−, SD | 100 m swim time, lactate, RPE, TQR | No significant improvement in swimming performance, lactate, or subjective measures. Possible positive effect on exercise. |
| Neteca et al. (2024) [62] | RCT study | 18F endurance athletes | BRJ (nitrate-rich) | 50 mL, ~6.2 mmol of NO3− | VO2max, HR, VE, ventilation to oxygen consumption ratio (VE/VO2) (VE/VCO2) | Significant improvements in VE, respiratory equivalents, and HR that confirm the effective ergogenic potential of BRJ. |
| Tan et al. (2024) [63] | Randomized, double-blind, crossover | 15F team-sport athletes | BRJ (nitrate-rich) | 140 mL, 12 mmol of NO3−, SD | 10 m and 20 m sprints, isokinetic handgrip dynamometry, MBT, horizontal CMJ, YYIR1, oral microbiota composition, cognitive flexibility | Acute BRJ ingestion increased plasma NO3− and NO2− but did not impact exercise performance, cognitive flexibility, or oral microbiota composition. |
| Zhang et al. (2024) [64] | Randomized, double-blinded, placebo-controlled, crossover | 13F recreationally active | BRJ (nitrate-rich) | SD: 6.45 mmol of NO3−, DD: 12.9 mmol of NO3− | HR, BP, blood lactate, oxygen saturation, RPE | Acute nitrate ingestion reduced HR and RPE during high-intensity interval exercise but no additional benefit observed with higher nitrate content. |
| Liu et al. (2025) [65] | Randomized, crossover | 20M participants | BRJ | 70 mL, 6.4 mmol of NO3− | Left-hand grip strength, average VJH, HR, blood lactate, BF% | BRJ supplementation along with caffeine may improve 1000 m performance. |
| Study | Study Design | Participants’ Characteristics | Juice Type | Dosage | Measured Outcomes | Findings |
|---|---|---|---|---|---|---|
| Trombold et al. (2010) [66] | Randomized, crossover | 16M recreationally active | POMj (POMx, ellagitannin-rich) | 9 days, 500 mL twice a day, 650 mg of polyphenols: 95.5% ellagitannins, 3.5% ellagic acid, and 1% anthocyanins | Isometric strength, muscle soreness, serum markers (CK, myoglobin, IL-6, CRP) | POMx significantly improved recovery of isometric strength at 48 and 72 h post-exercise compared to placebo. Serum markers of inflammation and muscle damage did not differ significantly between conditions. |
| Trombold et al. (2011) [67] | Randomized, crossover | 17M resistance-trained | POMj | 250 mL before eccentric exercise, 1.979 mg/L of tannins, 384 mg/L of anthocyanins, and 121 mg/L of ellagic acid derivatives | Isometric strength, muscle soreness (elbow flexors, knee extensors) | POMj reduced soreness and maintained strength in elbow flexors but had no effect on knee extensors. |
| Ammar et al. (2016) [68] | Crossover design with placebo, clinical trials | 9M elite weightlifters | POMj | 3 × 1500 mL per day in 48 h, 2.56 g of polyphenols in each 500 mL | Weightlifting performance, RPE, DOMS, HR, SBP, CK, LDH, ASAT, CRP | POMj supplementation reduced muscle soreness, inflammation, and muscle damage responses, while also enhancing performance and accelerating recovery of several biological markers. However, CK, LDH, and ASAT remained elevated, indicating incomplete recovery. |
| Ammar et al. (2017) [69] | Randomized, crossover | 9M elite weightlifters | POMj | 500 mL, 2.56 g of TPC, 1.08 g of o-DPO, 292.6 mg of flavonoids, and 46.75 mg of flavonols | MDA, catalase, glutathione peroxidase, UA, bilirubin | POMj supplementation attenuated oxidative stress (UA) and enhanced antioxidant enzyme activity after intensive weightlifting training session. |
| Urbaniak et al. (2018) [70] | Double-blind, placebo-controlled | 19M well-trained rowers | POMj | 2 months, 50 mL daily, total polyphenol content equal to 220 mg/100 g | Antioxidant parameters (TAC), iron metabolism markers (transferrin receptors, iron), inflammation marker (IL-6) | POMj supplementation increased plasma antioxidant potential (TAC) but had no significant effect on iron metabolism markers. Post-exercise IL-6 concentrations increased in both groups but were attenuated by POMj supplementation. |
| Ammar et al. (2020) [71] | Double-blind, placebo-controlled | 9M elite weightlifters | POMj (polyphenol-rich) | 250 mL (2.56 g of TPC, 1.08 g of o-DPO), 3 times a day, and 500 mL before the weightlifting session | Hcy, steroidal hormones (testosterone, cortisol), testosterone/cortisol ratio | POMj supplementation reduced post-exercise testosterone and attenuated the increase in Hcy during the 48 h recovery period. No effect on testosterone/cortisol ratio. |
| Study | Study Design | Participants’ Characteristics | Juice Type | Dosage | Measured Outcomes | Findings |
|---|---|---|---|---|---|---|
| Bowtell et al. (2011) [72] | Crossover, randomized | 10M athletes (rugby, football, and taekwondo) | MCJ | 10 days, 30 mL twice a day, total anthocyanin content was 9.117 mg/mL | MVC, CK, PC, hsCRP, total nitrotyrosine, antioxidant capacity | MCJ improved recovery of MVC and reduced oxidative damage, i.e., PC. |
| Bell et al. (2015) [73] | Randomized, double-blind, crossover | 16M trained cyclists | MTCJ | 8 days, 30 mL twice a day, 9.2 mg/mL of anthocyanins | MIVC, cycling efficiency, 6 s peak cycling power, DOMS, inflammation markers (IL-1β, IL-6, TNF-α, hsCRP), oxidative stress (LOOH), muscle damage (CK) | MTCJ supplementation prevented decline in MIVC, improved cycling efficiency, and attenuated IL-6 and hsCRP responses to high-intensity cycling. No significant effect on LOOH or CK. |
| Bell et al. (2016) [74] | Randomized, double-blind, crossover | 16M semi-professional soccer players | MTCJ | 8 days, 30 mL twice a day, 9.2 mg/mL of anthocyanins | MIVC, 20 m sprint, CMJ, agility, DOMS, inflammation markers (IL-1-β, IL-6, IL-8, TNF-α, hsCRP), muscle damage (CK), oxidative stress (LOOH) | MTCJ supplementation accelerated recovery of MIVC, CMJ, and agility and reduced DOMS. Acute IL-6 response was attenuated, but no significant effect on CK or LOOH levels. |
| McCormick et al. (2016) [75] | Randomized, double-blind, crossover | 9M water polo athletes | MTCJ | 90 mL daily concentrate diluted with water (30 mL serving, i.e., 200 mL beverage), 9.117 mg/mL of anthocyanins | IL-6, CRP, UA, F2-IsoP, DOMS, performance | No significant differences in blood markers, performance, or recovery measures between MTCJ and placebo. |
| Abbott et al. (2020) [76] | Double-blind, placebo-controlled, crossover | 10M professional soccer players | TCJ | 2 × 30 mL before and after a 90 min match and 12 and 36 h post-match | CMJ height, RI, DOMS, subjective well-being | TCJ supplementation did not significantly affect muscle function, RI, muscle soreness, or well-being after a soccer match. These findings cast doubt on the efficacy of TCJ as a recovery aid in professional soccer players. |
| Gao et al. (2024) [77] | Randomized, crossover, counterbalanced, placebo-controlled | 12 cyclists (8M + 4F) | TCJ | 300 mL/day, twice a day, 4 days before and 2 days after exercise (~9.2 mg/mL of anthocyanins) | MVC, low-frequency fatigue, cycling, performance, substrate metabolism | No significant differences between TCJ and sports drink in time trial performance, muscle soreness, or substrate metabolism. |
| Study | Study Design | Participants’ Characteristics | Juice Type | Dosage | Measured Outcomes | Findings |
|---|---|---|---|---|---|---|
| Miller et al. (2014) [78] | Crossover study | 9 physically active individuals (7M + 2F) | PJ | 1 mL per kg of body mass, ~0.09 g of acetic acid | Plasma Na+, K+ concentrations, plasma osmolality, plasma volume changes | Ingesting PJ did not significantly alter plasma Na+, K+, or osmolality levels. Plasma volume changes were negligible. The study concludes that consuming small amounts of PJ does not fully replenish electrolyte losses after exercise. |
| Peikert et al. (2014) [79] | Crossover study | 9M physically active and euhydrated | PJ | 2 mL per kg of body mass, Na+: 395 mmol/L, K+: 29.5 mmol/L | TTE, core temperature, plasma volume, sweat volume | No significant differences were observed in TTE, core temperature, or sweat volume. Small amounts of PJ do not significantly impact aerobic performance or thermoregulation. |
| McKenney et al. (2015) [80] | Crossover study | 9M euhydrated, physically active | PJ | 1 or 2 boluses (1 mL/kg), Na+: 530 ± 14 mmol/L, K+: 28.8 mmol/L | Plasma Na+ and K+, plasma osmolality, plasma volume changes | Ingesting up to 2 boluses of PJ during and after exercise caused negligible changes in plasma Na+, K+, osmolality, and volume. No hyperkalemia occurred. |
| Study | Study Design | Participants’ Characteristics | Juice Type | Dosage | Measured Outcomes | Findings |
|---|---|---|---|---|---|---|
| Tarazona-Díaz et al. (2013) [81] | In vivo and in vitro study | 7M recreational athletes | WJ (natural and enriched) | 500 mL (6 g/500 mL = 1.17 g citrulline from watermelon + 4.83 g of citrulline added) | Muscle soreness, recovery HR | Both natural and enriched WJ reduced muscle soreness and recovery HR 24 h post-exercise. The study concludes that WJ may be a potential functional drink for post-exercise recovery. |
| Cutrufello et al. (2014) [82] | Randomized, double-blind study | 22 participants (11M + 11F) | WJ | 710 mL, ~1 g of citrulline or 6 g of L-citrulline, SD | Repetitions completed, time to exhaustion, VO2max, anaerobic threshold | No significant effects of L-citrulline or WJ on anaerobic or aerobic exercise performance. The study concludes that a single dose of WJ or L-citrulline is not effective in enhancing performance. |
| Bailey et al. (2016) [83] | Crossover study | 8M healthy recreationally active adults | WJ | 16 days, 300 mL/day (11.4 g of L-citrulline and 1.39 g of L-arginine per L) | Plasma NO, BP, muscle oxygenation, TTE | WJ increased plasma NO levels and muscle oxygenation during moderate-intensity exercise but did not improve TTE during severe-intensity exercise. The study concludes that WJ may not effectively enhance endurance performance. |
| Martínez-Sánchez et al. (2017) [84] | Randomized, double-blind, crossover design | 21M amateur runners | WJ (enriched in L-citrulline) | 500 mL, 3.45 g of L-citrulline | Muscle soreness, plasma lactate, glucose, lactate dehydrogenase, L-arginine, jump height, HR, perceived exertion | Muscle soreness significantly lower post-race. Plasma lactate concentrations were lower and lactate dehydrogenase and L-arginine concentrations were higher immediately after the race. Jump heights were maintained after WJ. |
| Gonzalez et al. (2022) [85] | Crossover design | 15M resistance-trained | WJ | 7 days, 104 mL of WJ concentrate mixed with 355 mL of water, twice a day (2.2 g of L-citrulline) | IMTP test and acute bench press protocol, vessel diameter, muscle oxygenation, and subjective perception | Short-term WJ supplementation resulted in a modest increase in skeletal muscle oxygenation (+4.1%) compared to placebo. However, it did not lead to significant improvements in isometric strength, bench press performance, vascular diameter, or overall muscle oxygenation in resistance-trained men. |
| Aghabeighiamin and Azizi (2025) [86] | RCT | 25F elite taekwondo athletes | WJ | 6 weeks, 500 mL daily, lycopene: 3.38–11.34 mg | TAC | WJ supplementation significantly increased TAC after 6 weeks. The findings suggest that WJ may be beneficial in reducing oxidative stress in elite athletes. |
| Juice Type | Inflammation | Oxidative Stress | DOMS | Performance | Key Notes |
|---|---|---|---|---|---|
| BRJ | Mixed (↑ NO may help, but inconsistent) [37,39,45] | Limited evidence [42] | Modest relief in some trials [50] | Strong for endurance, weaker for sprinting/elite [38,45,46] | Dose-dependent; best when preloaded 2–3 h before |
| POMj | ↓ IL-6, ↓ CRP, ↓ Hcy [68,69,71] | ↓ MDA, ↑ catalase, ↑ TAC [69,70] | Effective in upper-body eccentric soreness [66,67] | Mixed; mainly recovery-focused [68,71] | Strongest for oxidative and endocrine modulation |
| CJ | ↓ IL-6, CRP in many studies [72,73,74] | ↓ Lipid peroxidation [72] | ↓ Soreness, especially preloading [73,74] | Inconsistent across sports [76] | Best when consumed on days before and after exercise |
| PJ | No measurable effect [78,80] | Not assessed | Not directly measured | No significant impact [78,79] | Anecdotal relief of cramps not yet confirmed |
| WJ | Limited evidence [86] | ↑ TAC, ↓ lactate (chronic use) [83,86] | ↓ Soreness with enriched formulations [81] | Inconsistent, no VO2max changes [82,85] | Pasteurization reduces citrulline bioavailability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitošević, B.; Filipović, M.; Popović, L.; Sterkowicz-Przybycień, K.; Purenović-Ivanović, T. Juice-Based Supplementation Strategies for Athletic Performance and Recovery: A Systematic Review. Sports 2025, 13, 269. https://doi.org/10.3390/sports13080269
Vitošević B, Filipović M, Popović L, Sterkowicz-Przybycień K, Purenović-Ivanović T. Juice-Based Supplementation Strategies for Athletic Performance and Recovery: A Systematic Review. Sports. 2025; 13(8):269. https://doi.org/10.3390/sports13080269
Chicago/Turabian StyleVitošević, Biljana, Milica Filipović, Ljiljana Popović, Katarzyna Sterkowicz-Przybycień, and Tijana Purenović-Ivanović. 2025. "Juice-Based Supplementation Strategies for Athletic Performance and Recovery: A Systematic Review" Sports 13, no. 8: 269. https://doi.org/10.3390/sports13080269
APA StyleVitošević, B., Filipović, M., Popović, L., Sterkowicz-Przybycień, K., & Purenović-Ivanović, T. (2025). Juice-Based Supplementation Strategies for Athletic Performance and Recovery: A Systematic Review. Sports, 13(8), 269. https://doi.org/10.3390/sports13080269







