Abstract
The application of natural juices in sports nutrition is attracting growing interest due to their potential antioxidant, anti-inflammatory, and ergogenic properties. Exercise, especially when prolonged or intense, increases oxidative stress and muscle damage, leading athletes to explore dietary strategies that support recovery and enhance performance. This systematic review investigates the effectiveness of five widely studied juices—beetroot, pomegranate, cherry, watermelon, and pickle juice—in the context of athletic supplementation and recovery. A thorough search of the PubMed, Scopus, and Web of Science databases was conducted to identify studies published between 2010 and 2025. Fifty peer-reviewed articles met the inclusion criteria, examining various physiological, biochemical, and performance-related outcomes linked to juice consumption. Given the methodological diversity among studies, a qualitative synthesis was employed. The juices were compared across four key outcomes—inflammation, oxidative stress, delayed onset of muscle soreness, and exercise performance—to determine their most consistent benefits. Beetroot juice, noted for its high nitrate content, consistently enhanced oxygen efficiency and submaximal endurance, although benefits in elite or sprint athletes were less evident. Both pomegranate and cherry juices were effective in reducing muscle soreness and inflammatory markers, particularly when consumed over several days surrounding exercise. Watermelon juice, primarily through its L-citrulline content, offered antioxidant and recovery support, although performance outcomes varied. Evidence for pickle juice was limited, with no notable ergogenic effects beyond anecdotal cramp relief. Overall, natural juices can support recovery and occasionally improve performance, depending on the specific juice, dosage, and athlete characteristics. Beetroot juice stands out as the most reliable in enhancing performance, while pomegranate and cherry juices are more beneficial for recovery. Future research with standardized protocols is essential to determine optimal application across diverse athletic contexts.
1. Introduction
The intersection of nutrition and athletic performance is drawing increasing attention, with a growing emphasis on food-based interventions that support recovery, reduce inflammation, and combat oxidative stress [1]. Among these, natural juices have emerged as a promising and accessible strategy, owing to their dense composition of bioactive compounds, such as polyphenols, flavonoids, betalains, vitamins, and amino acids [2]. These constituents are known to influence physiological mechanisms, including antioxidant activity, nitric oxide (NO) bioavailability, and inflammatory pathways—processes that are central to mitigating muscle damage, fatigue, and delayed recovery following intense or prolonged exercise [3,4,5,6].
Despite the proliferation of research into isolated nutrients, there remains significant interest in whole-food sources that offer synergistic effects through multiple compounds [7]. Fruit and vegetable juices—particularly beetroot, pomegranate, cherry, watermelon, and even unconventional options like pickle juice—have been studied in various athletic contexts. While differing in composition, these juices share common proposed mechanisms: the enhancement of endothelial function through NO pathways, the reduction of oxidative stress via radical scavenging, and the modulation of inflammation through cytokine regulation [8,9,10,11,12,13,14,15,16,17,18,19,20]. However, gaps persist in understanding their comparative effectiveness, the optimal dosing strategies, and the consistency of outcomes across populations and exercise modalities. Although they have been researched for some time, we are specifically interested in their current scientific status, including what has been confirmed through robust evidence and where uncertainty remains.
This systematic review seeks to synthesize existing evidence on five commonly studied juices—beetroot, pomegranate, tart cherry, watermelon, and pickle juice—to evaluate their roles in athletic recovery and performance. We aim to explore the extent to which these natural juices improve exercise recovery outcomes through shared or distinct physiological mechanisms. Specifically, we examine whether their bioactive profiles contribute meaningfully to reductions in muscle soreness, inflammation, or oxidative stress and whether this evidence supports their practical use in sports nutrition. Beetroot juice (BRJ), for instance, is rich in dietary nitrates that enhance NO-mediated vasodilation, mitochondrial efficiency, and oxygen delivery to muscles [13,16,19]. Pomegranate juice (POMj) offers potent polyphenols such as ellagitannins, which reduce oxidative stress and modulate cytokines like interleukin-6 (IL-6) and tumor necrosis factor (TNF) [11,14,20]. Tart cherry juice (TCJ), particularly the Montmorency variety, is concentrated in anthocyanins and melatonin, compounds that may reduce muscle damage and promote recovery and sleep [8,9,10,12,15,17,18]. Pickle juice (PJ) has gained attention for its possible neuromuscular effects through transient receptor potential channel activation, which may help relieve exercise-induced muscle cramps via the reflex inhibition of alpha motor neurons [21,22,23]. Finally, watermelon juice (WJ), as a natural source of L-citrulline, supports NO synthesis and improves vascular function while also providing lycopene, beta-carotene, and vitamins A and C [24,25,26,27,28,29,30,31,32,33]. By uniting these interventions under a single analytical framework, this review aims to clarify their physiological underpinnings, assess the strength and limitations of current evidence, and identify where further investigation is warranted. In doing so, we aim to offer both researchers and practitioners a clearer understanding of how these juices may serve as effective tools in enhancing recovery and performance in athletic settings.
2. Materials and Methods
2.1. Search Strategy
A comprehensive literature search was conducted in early May 2025 across three major databases: PubMed, Scopus, and Web of Science. The search strategy utilized a combination of keywords and Boolean operators (AND/OR), incorporating terms such as (Supplements OR Juices) AND (Athletes OR Recovery OR Exercise Performance), (Beetroot OR Pomegranate OR Cherry OR Pickle OR Watermelon), and (Antioxidants OR Inflammation OR Muscle Soreness OR Cramp).
The search covered the publication period from 2010 to 2025, with the aim of identifying studies that investigated the effects of beetroot, pomegranate, cherry, pickle, and watermelon juices on athletic recovery, exercise performance, muscle soreness, and inflammation.
2.2. Inclusion and Exclusion Criteria
The inclusion and exclusion criteria were structured in accordance with the PICOS (Population, Intervention, Comparison, Outcome, Study Design) framework and are presented in Table 1, with the exception of “C” (Comparison), which was not explicitly required, as many nutrition/exercise studies compare outcomes to a placebo or baseline, and our review allowed for heterogeneous comparators [34].

Table 1.
Inclusion and exclusion criteria used during full-text screening.
2.3. Data Extraction
The study selection process was documented using the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flowchart, which outlined the number of records identified, screened, excluded, and ultimately included in the review [35]. Data extraction was carried out using a standardized form that captured key information, including the first author(s); study design; participant characteristics (age, gender, and training status); type of juice, dosage, duration, and frequency of supplementation; measured outcomes (muscle soreness, inflammation, and performance indicators); and main findings.
This systematic review was reported according to the PRISMA (see Supplementary File S1) and registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) under the registration number INPLASY202560053 (see Supplementary File S2).
The methodological quality of the included studies was evaluated using the Physiotherapy Evidence Database (PEDro) scale, a validated tool for assessing the quality of clinical research [36]. All authors (B.V., M.F., L.P., K.S.-P., and T.P.-I.) independently performed the quality assessments, with any disagreements resolved through discussion and consensus.
Given the substantial heterogeneity across studies in terms of design, participant characteristics, interventions, and outcomes, a meta-analysis was not feasible. Therefore, a qualitative synthesis was conducted to analyze and compare the findings.
3. Results
To ensure methodological transparency and reproducibility, the study identification and selection process was conducted in accordance with the PRISMA guidelines and included two distinct screening phases. A total of 84 records were initially identified through systematic searches of three major databases: PubMed (n = 43), Scopus (n = 33), and Web of Science (n = 8). After removing 12 duplicates, 72 unique records remained and were screened based on their titles and abstracts for relevance to the research topic. At this stage, 10 records were excluded: four involved non-exercising populations, four examined unrelated outcomes, and two focused on elderly or clinical groups rather than healthy, physically active individuals. The remaining 62 articles underwent full-text assessment.
During the full-text screening, 12 additional articles were excluded due to not meeting the inclusion criteria. The most common reason was the use of synthetic supplements in non-liquid forms—primarily powders, capsules, or multi-ingredient formulations not specific to the targeted juices (n = 8). The other four exclusions were due to irrelevant outcome measures (e.g., lacking data on physical performance, muscle recovery, inflammation, or oxidative stress). Several of these excluded studies also involved elderly or clinical populations using the supplements for disease-related recovery, rather than exercise-induced stress in athletic or physically active individuals. This two-phase exclusion process ensured that the final qualitative synthesis included only studies strictly aligned with the PICOS criteria (n = 50). The full process of study selection is presented in Figure 1.
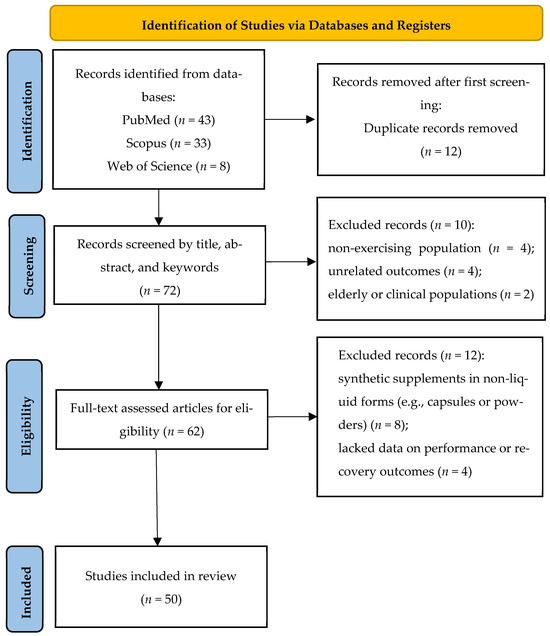
Figure 1.
PRISMA flow diagram of the study selection process.
3.1. Methodological Quality Assessment
The methodological quality and internal validity of the included RCTs were assessed using the PEDro scale. This tool examines 11 design-related criteria, such as random allocation, concealed allocation, baseline comparability, blinding procedures, and adequacy of follow-up. While the first item pertains to external validity and is not factored into the final score, the maximum attainable score is 10. Across the included studies, the average PEDro score was 7 out of 10, reflecting an overall good level of methodological quality (see Supplementary Table S1). According to the PEDro classification system, scores ranging from 6 to 8 denote good quality, while scores of 9 or 10 represent excellent quality. The results indicate that the majority of the studies reviewed were well designed, with a limited risk of bias, thereby enhancing the reliability of the findings.
3.2. Study Characteristics
Out of the 50 selected studies, 29 investigated the effects of BRJ, and six examined POMj, TCJ, or WJ, while only three focused on PJ’s impact on performance and recovery. The majority of the included studies were randomized, placebo-controlled trials, with many employing crossover or counterbalanced designs to minimize interindividual variability and reduce potential bias. Sample sizes ranged from 9 to 80 participants (a total of 788 participants), with an average age of approximately 20 years. Most study populations comprised young, recreationally active individuals, trained adults, and competitive athletes, with a predominance of male participants (M = 594, F = 194). Research was conducted in a diverse array of countries, including the United Kingdom, the United States, Canada, Iran, Tunisia, China, Australia, Spain, Latvia, the Netherlands, Denmark, Italy, and Poland. This wide geographic distribution underscores the global scientific interest in juice-based supplementation and enhances the generalizability of the findings.
A summary of the selected studies examining the effects of various juice types, ranked by relevance and organized for comparative synthesis, is presented in Table 2, Table 3, Table 4, Table 5 and Table 6. Due to the heterogeneity in terms of dosage, outcome measures, and participant characteristics, studies were divided by juice type to reflect the scope of the literature and support a comprehensive narrative analysis.

Table 2.
Comprehensive overview of studies about beetroot juice-based supplementation (n = 29).

Table 3.
Comprehensive overview of studies about pomegranate juice-based supplementation (n = 6).

Table 4.
Comprehensive overview of studies about tart cherry juice-based supplementation (n = 6).

Table 5.
Comprehensive overview of studies about pickle juice-based supplementation (n = 3).

Table 6.
Comprehensive overview of studies about watermelon juice-based supplementation (n = 6).
4. Discussion
This systematic review examined the role of various natural juices in sports supplementation and recovery, highlighting key trends and findings across the literature. The primary objective was to assess the scientific evidence supporting the use of natural juices as nutritional interventions among athletic populations. In light of the increasing interest in functional foods and natural alternatives to synthetic supplements, this topic holds both contemporary relevance and practical importance. Juices enriched with antioxidants and bioactive compounds are often promoted for their potential to reduce oxidative stress, support recovery, and enhance physical performance. However, the extent to which these benefits are supported by empirical evidence remains inconsistent. This review aimed to clarify which effects have been substantiated through scientific research, which expected outcomes have not been reliably demonstrated, and how these juices currently stand as evidence-based supplements within the context of athletic performance and recovery.
4.1. Beetroot Juice
Beetroot juice emerged as the most frequently studied supplement among the included articles. It is a rich source of dietary nitrate (NO3−), which is converted in the body into NO—a molecule recognized for its vasodilatory effects and potential to enhance athletic performance. Through multiple physiological mechanisms, NO enhances oxygen utilization during skeletal muscle contraction, facilitates more even oxygen distribution within muscle tissue, and supports cellular oxygen delivery through the endogenous oxidation of L-arginine [87,88]. Additionally, it has been associated with enhanced mitochondrial efficiency, increased glucose availability, immunomodulatory functions, and the stimulation of mitochondrial biogenesis [89]. Due to these wide-ranging physiological effects, BRJ has been extensively studied for its impacts on cardiovascular function. For instance, Zhang et al. [64] observed a significant reduction in both the average heart rate (HR) and rating of perceived exertion (RPE) following the acute consumption of BRJ containing 6.45 mmol of nitrate. Interestingly, higher doses did not yield further improvements. These results suggest enhanced left ventricular contractility, which may lead to an increased stroke volume. However, the study did not report significant changes in blood pressure (BP), in contrast to the findings of Esen et al. [53] and Wylie et al. [45], who noted BP reductions following BRJ supplementation. Such discrepancies may be attributed to variations in the timing and methodology of BP assessment.
With regard to metabolic markers, Zhang et al. [64] reported no significant changes in blood lactate levels at the maximal exercise intensity, contrasting with findings from other studies [38,45,61], which observed elevated lactate concentrations at supramaximal intensities. These findings suggest that the effect of BRJ on lactate production may be influenced by the degree of exercise-induced muscle hypoxia, which in turn modulates the NO metabolic pathway.
In the context of recovery, Daab et al. [50] found that daily supplementation with 2 × 150 mL of BRJ reduced delayed-onset muscle soreness (DOMS) in semi-professional soccer players following simulated match conditions, despite no corresponding changes in biochemical indicators of muscle damage. Similarly, Clifford et al. [42] reported decreased muscle pain and improved dynamic muscle function after sprint-induced muscle damage; however, there were no observed improvements in sprint performance or isometric strength. In a later study, Clifford et al. [90] found that BRJ had no effect on post-exercise recovery, with no improvements in markers of inflammation or muscle damage. Moreover, Clifford et al. [42] and Esen et al. [54] demonstrated that acute BRJ supplementation failed to reduce post-exercise creatine kinase (CK) and high-sensitivity C-reactive protein (hsCRP) concentrations, despite increasing the pain threshold and exerting analgesic effects—likely attributable to its phenolic compounds and betalain content. These findings support the emerging perspective that the recovery-related benefits of BRJ may involve complex interactions among multiple bioactive components, rather than nitrate metabolism alone.
Exercise performance was the most commonly investigated outcome in BRJ supplementation research. Multiple studies [38,40,42,45,48,49,50,52,53,55,57,58,60] reported improvements in maximal oxygen uptake (VO2max), time to exhaustion, and power output when BRJ doses containing 6.4–12.8 mmol of NO3− were ingested 2–3 h prior to exercise. However, several studies [38,40,55,59,63] found no significant effects on performance, particularly in sprint-based activities [39,42] and swimming disciplines [49,61].
For example, Jonvik et al. [46] explored the influence of training status on the ergogenic response to BRJ supplementation in sprint athletes. The study included recreational, competitive, and Olympic-level elite participants, all of whom exhibited significant increases in plasma nitrate and nitrite concentrations after six days of BRJ intake. Despite this physiological response, no improvements were observed in peak or mean power output across three repeated Wingate tests. However, all groups experienced a consistent reduction of approximately 2.8% in the time taken to reach peak power, indicating an enhanced acceleration capacity. This benefit from BRJ appeared independent of the training level, suggesting that even elite sprint athletes—typically characterized by a high proportion of type II muscle fibers [91]—may experience performance gains in acceleration-specific contexts. Although the impact on overall power output was limited, improved acceleration may offer a competitive advantage in sports where explosive starts are critical, such as sprinting, speed skating, BMX racing, and various field-based sports. The study [46] concluded that BRJ may hold situational value in enhancing acceleration during high-intensity exercise, regardless of an athlete’s performance level. In a rigorous study involving elite professional tennis players, Fernández-Elías et al. [51] explored whether acute BRJ supplementation could enhance movement patterns and performance during competitive match play. Despite the promising nitrate content (6.4 mmol) in the beetroot shot, the results showed no significant improvements in running performance, serve speed, or grip strength compared to the placebo. The study’s use of GPS and accelerometry to measure real-time match dynamics was a methodological strength, offering the precise tracking of movement under competitive conditions. However, the lack of observed benefits may be due to the athletes’ high training status and possible ceiling effects in their physiological adaptations. Additionally, the study [51] highlights that acute nitrate supplementation may not be sufficient in intermittent sports like tennis, where performance is influenced by complex physical and technical demands.
Interestingly, performance improvements were more consistently observed in endurance-based activities over middle distances (e.g., 1–10 km), especially when BRJ was combined with caffeine. For example, a study combining 70 mL of BRJ (administered 120 min before exercise) with caffeine (6 mg/kg, taken 60 min prior) significantly improved 1000 m running performance and accelerated post-exercise recovery, suggesting a synergistic effect between the two supplements [65].
Christensen et al. [37] investigated the effects of six-day BRJ supplementation (0.5 L/day) in highly trained cyclists. Although the nitrate levels increased, no improvements were observed in oxygen uptake (VO2) kinetics, endurance capacity, or repeated sprint performance. The authors proposed that highly trained athletes may already possess optimized endogenous NO production due to the training-induced upregulation of NO synthase enzymes, which are known to increase with training [90]. This may also account for the limited ergogenic benefits reported in elite-level sports such as tennis and swimming.
Conversely, some studies demonstrated positive outcomes, including improved muscle oxygenation and reduced oxygen costs during submaximal exercise efforts [43,56]. Patrician and Schagatay [43] investigated the effects of dietary nitrate supplementation, in the form of concentrated BRJ (70 mL), on arterial oxygen saturation (SaO2) following dynamic apnea dives in trained male apnea divers. The study demonstrated that BRJ consumption significantly elevated post-dive SaO2 values compared to a placebo, indicating a more efficient oxygen-conserving response. These findings point out that dietary nitrate may enhance safety margins and performance in apnea by reducing the oxygen cost. The authors [43] further propose that such supplementation could benefit other sports involving restricted breathing, such as swimming, spearfishing, or synchronized swimming. Additionally, other research highlighted enhancements in cognitive performance under physical stress [48,63], indicating that BRJ’s benefits may extend beyond muscular function. However, gender-related differences have emerged, with less consistent effects observed among female participants [64].
In summary, BRJ appears to be most effective in supporting endurance and submaximal performance, especially when consumed at doses of 6.4–12.8 mmol of nitrate, approximately 2–3 h prior to exercise. Its efficacy, however, is influenced by factors such as the training status, the type of exercise, and individual variability in responsiveness. Limited or inconsistent effects have been reported in elite athletes and in short-duration, high-intensity exercise formats, such as repeated sprints with rest intervals, which may offset any ergogenic advantages of BRJ. While findings related to recovery and cardiovascular markers are promising, the overall evidence remains mixed, underscoring the need for more standardized, controlled research.
4.2. Pomegranate Juice
Pomegranate juice is another polyphenol-rich juice with strong antioxidant properties. In a study by Ammar et al. [69], natural POMj supplementation significantly attenuated acute oxidative stress following a weightlifting session. Compared to a placebo, POMj led to a smaller rise in malondialdehyde—a marker of lipid peroxidation—and a greater increase in enzymatic antioxidant activity immediately after exercise. These protective effects are likely due to POMj’s rich polyphenol profile, which contributes to antioxidant defense through mechanisms such as free radical scavenging, the modulation of antioxidant enzymes, metal ion chelation, and the recycling of vitamins C and E. Additionally, the study [69] noted that oxidative stress was typically more pronounced in the morning, yet POMj supplementation appeared to blunt this time-of-day-dependent response. Overall, POMj appears effective in minimizing oxidative damage and accelerating recovery after intensive resistance training. In a follow-up study, Ammar et al. [71] assessed hormonal and homocysteine (Hcy) responses to Olympic-style weightlifting. Compared to a placebo, POMj supplementation resulted in a lowered acute testosterone response and a significant 14% reduction in the plasma Hcy concentration during the recovery period. While both groups exhibited typical post-exercise hormonal fluctuations, only the POMj group showed a sustained decrease in Hcy, with an inverse correlation observed between the Hcy and testosterone concentrations. These findings suggest that POMj may play a role in modulating both oxidative and endocrine responses to strength-based exercise, although additional research is needed to clarify its broader physiological implications.
Furthermore, Urbaniak et al. [70] investigated the long-term effects of POMj supplementation (50 mL/day over two months) on antioxidant capacity and iron metabolism in elite rowers. The POMj group exhibited a significantly greater resting total antioxidant capacity (TAC) compared to the placebo. Post-exercise, both groups experienced a rise in IL-6 and a drop in TAC, but the POMj group consistently maintained higher antioxidant levels. However, POMj supplementation did not influence iron-related biomarkers, including soluble transferrin receptors, iron levels, or hepcidin. These results support the role of POMj in enhancing antioxidant defense in trained individuals, although it appears to have no effect on iron metabolism.
Trombold et al. [66] examined the impacts of an ellagitannin-rich pomegranate extract (POMx) on recovery following eccentric exercise. Participants who consumed POMx experienced benefits in recovery after isometric muscle strength exercise within 2–3 days compared to the placebo group. However, there were no significant differences in inflammatory or muscle damage biomarkers, such as IL-6, CK, or C-reactive protein (CRP), suggesting that POMx may improve functional muscle recovery, although the mechanisms remain unclear. In another study [67], the authors examined whether POMj supplementation could aid recovery after eccentric exercise in 17 resistance-trained men. Participants consumed either POMj or a placebo before performing intense eccentric elbow and knee exercises. The results showed significantly improved recovery of elbow flexor strength and reduced muscle soreness in the POMj group from 2 to 168 h after exercise, while no such benefits were observed for the knee extensors. Although the precise mechanism remains unclear, the high polyphenol content in POMj may have stabilized muscle cell membranes and preserved excitation–contraction coupling by scavenging peroxyl radicals and limiting lipid peroxidation [92]. This membrane-stabilizing effect could explain the early attenuation of muscle weakness observed at the 2 h mark. These findings reveal a targeted benefit of POMj for upper-body muscle recovery in trained individuals following eccentric loading.
Dosing protocols varied across studies, with pomegranate supplementation ranging from 50 to 1000 mg of extract or 250–500 mL of juice, typically administered over a period of 7 to 15 days. Despite the recognized antioxidant potential of anthocyanins and ellagitannins in pomegranate, the evidence for their effectiveness in supporting muscle recovery and enhancing performance remains mixed. While some studies confirm POMj’s role in mitigating reactive oxygen species, lipid peroxidation, and pro-inflammatory cytokines, the magnitude and consistency of these effects appear to be context-dependent. This variability may stem from differences in study protocols, supplementation strategies, and the training statuses of participants, all of which can modulate the physiological response to pomegranate supplementation.
4.3. Cherry Juice
Tart cherries (particularly the Montmorency variety) contain significantly higher concentrations of bioactive compounds—notably anthocyanins and total polyphenols—than sweet cherries. Research demonstrates that TCJ possesses 2–3 times greater antioxidant capacity compared to its sweet counterpart, potentially explaining its more robust anti-inflammatory and recovery-enhancing effects in exercise contexts [15,17]. This enhanced antioxidant activity stems primarily from elevated levels of cyanidin-3-glucoside and cyanidin-3-rutinoside, two key anthocyanins that mediate free radical scavenging and inflammatory pathway modulation [92,93]. Connolly et al. [8] were the first to examine its effects on exercise-induced muscle damage, reporting that CJ significantly reduced strength loss and muscle pain, although it had no effect on tenderness or range of motion. These benefits were attributed to CJ’s ability to reduce secondary muscle damage by attenuating the inflammatory response. The juice administered in the study was equivalent to the consumption of 100–120 fresh cherries per day, which likely contributed to its efficacy. The proposed biological mechanism involves protecting against the secondary damage cascade that follows eccentric muscle contractions. Such contractions initially cause the mechanical disruption of myofibrils and cell membranes. If extensive, this damage triggers an inflammatory response involving leukotrienes, neutrophil infiltration, and free radical generation—factors that further exacerbate tissue injury. The bioactive compounds in TCJ may help interrupt this cycle, thereby preserving muscle structure and function.
Kuehl et al. [94] found that daily supplementation with 355 mL of Montmorency CJ for 7 days before and during a marathon significantly reduced post-race muscle soreness. Bell et al. [73] reported improvements in cycling economy and the preservation of muscle strength during a 72 h recovery period in trained cyclists, along with significant reductions in inflammatory markers such as IL-6 and hsCRP when compared to a placebo. Similarly, Bowtell et al. [72] observed the improved recovery of isometric muscle strength and reduced oxidative stress indicators (e.g., CRP and CK) following strenuous exercise. However, findings across the literature are not entirely consistent. Several studies found no significant effects on athletic performance, muscle soreness, or recovery outcomes [75,76,77]. Notably, a study involving professional soccer players [76] failed to demonstrate clear recovery benefits from TCJ supplementation, suggesting that the effects of CJ may vary depending on the sport, training status, or exercise protocol.
The dosages used across the reviewed studies varied considerably, typically ranging from 60 to 300 mL per day, which is approximately equivalent to 100–180 cherries, and were generally administered before and/or after exercise. However, inconsistencies in antioxidant-monitoring methods, differences in exercise intensity, and variations in the supplementation duration and timing of intake complicate cross-study comparisons.
In summary, although TCJ appears to offer potential benefits for recovery, which has already been established [95]—particularly in preserving muscle function and reducing inflammation when consumed for several days pre-exercise—the current body of evidence is not yet strong enough to classify it as consistently effective across all contexts or athletic populations.
4.4. Pickle Juice
Pickle juice, albeit an unconventional supplement, has garnered interest mainly for its potential role in muscle cramp prevention and its electrolyte replenishment properties. It is commonly believed that dehydration and sodium loss contribute to the onset of exercise-associated muscle cramps. McKenney et al. [80] examined the effects of consuming one or two boluses of PJ during exercise on plasma electrolytes and hydration status. The study involved nine euhydrated, physically active men exercising in a hot environment and compared the effects of no PJ intake, a single bolus (1 mL/kg), and two boluses. The results showed that the plasma sodium and potassium concentrations remained unaffected, with no signs of hyperkalemia or fluid imbalance. Additionally, plasma osmolality and volume also remained unchanged. These findings support the notion that consuming up to two small servings of PJ during exercise is safe and does not disrupt electrolyte or fluid homeostasis; however, as the study did not assess the muscle cramp incidence, its effectiveness for cramp prevention remains inconclusive.
Similarly, Miller et al. [78] assessed whether the ingestion of PJ, mustard, or deionized water under hypohydrated conditions affected plasma electrolyte levels or osmolality. After inducing approximately 2.9% hypohydration through two hours of vigorous exercise, participants consumed one of the test fluids. Despite the intake of around 1.5 g of sodium from PJ or mustard, no significant changes were observed in plasma sodium or potassium levels, osmolality, or plasma volume within 60 min. These results indicate that neither PJ nor mustard exacerbates exercise-induced hypertonicity or causes hyperkalemia, but they are also ineffective as rapid electrolyte or fluid replenishment strategies.
Peikert et al. [79] investigated the effects of the pre-exercise ingestion of PJ, hypertonic saline, and deionized water on aerobic performance and thermoregulatory responses. The study showed no significant differences among the three beverages in the time to exhaustion, core temperature, sweat volume, or plasma volume. While the core temperature increased during exercise, the rise was consistent across all groups. The authors [79] proposed that larger volumes of PJ might be required to elicit measurable physiological effects. Although concerns exist regarding hyperkalemia—given its association with fatigue and potential cardiac arrhythmias [96]—this study found no causal link between PJ intake and elevated potassium levels. Minor increases in plasma potassium were likely due to exercise-induced muscle contractions rather than the ingested fluids.
Overall, the current evidence does not support ergogenic or thermoregulatory benefits of PJ supplementation in athletic performance or recovery. Although anecdotal reports suggest a potential role in cramp relief, the scientific validation of this claim remains limited. Further research is warranted to clarify PJ’s mechanisms of action and its practical effectiveness, particularly in real-world athletic settings.
4.5. Watermelon Juice
Watermelon juice, recognized for its high L-citrulline content, has shown promising but varied effects on exercise performance and recovery. Although its influence on immediate performance enhancement remains uncertain, several studies suggest that regular consumption may contribute to faster recovery, reduced blood lactate concentrations, and the preservation of muscle function post-exercise. Multiple studies demonstrated that chronic supplementation, such as 500 mL over a six-week period, was associated with enhanced total antioxidant capacity, reduced muscle soreness, and improved VO2max [86]. Similarly, citrulline-enriched WJ was found to improve muscle oxygenation, maintain jump performance, and lower plasma lactate levels after a half-marathon [84], while Bailey et al. [83] observed improved oxygen delivery to the muscles, although no significant effect was found on time to exhaustion.
Acute or short-term supplementation, however, produced less consistent outcomes. Cutrufello et al. [82] found no improvements in VO2max, anaerobic threshold, or time to exhaustion following a single pre-exercise dose. Likewise, in another study [85], seven days of WJ supplementation did not enhance isometric strength, barbell velocity, muscle oxygenation, or vascular diameter during resistance exercise in trained men, although a small increase in the percentage change of muscle oxygenation was noted. The form in which the juice is consumed also appears to affect its efficacy. Pasteurization was found to reduce the bioavailability of citrulline, suggesting that fresh or minimally processed juice may be more effective [81].
In summary, WJ appears to be more effective as a recovery aid and antioxidant booster when consumed regularly and in sufficient quantities. Its effectiveness as a short-term ergogenic supplement in improving strength or endurance performance remains less certain.
4.6. Comparative Discussion of Physiological Outcomes
Each juice investigated in this review exhibits distinct strengths and limitations across key physiological domains relevant to athletic recovery and performance, spanning oxidative stress, inflammation, DOMS, and exercise capacity (see Table 7).

Table 7.
Comparative effects of beetroot, pomegranate, cherry, watermelon, and pickle juices on key physiological outcomes in athletes.
Due to its nitrate content, BRJ consistently supports submaximal performance and oxygen efficiency, particularly in endurance exercise. However, its effects on inflammation, DOMS, or recovery markers are inconsistent across studies and tend to diminish in elite populations with preexisting optimized NO pathways [37,45,46,61,64]. POMj shows greater consistency in reducing oxidative stress and inflammation, attributable to its high polyphenol and ellagitannin content. While the performance benefits are less robust, its ability to modulate hormonal and inflammatory responses after strength training is promising [66,68,69,71]. CJ shares a similar anti-inflammatory profile with POMj. Multiple studies report reductions in IL-6, CRP, and DOMS [73,74,75], particularly with pre- and post-exercise supplementation. However, its effects on objective performance metrics (e.g., sprint time or power output) are more variable [72,73,74]. WJ, rich in L-citrulline, can improve antioxidant capacity and reduce post-exercise lactate, although most benefits require chronic use rather than acute intake [83,84,86]. Its impact on actual performance outcomes remains less established. PJ, while historically used for cramp relief, lacks strong evidence in terms of inflammation, DOMS, or recovery benefits. Most studies confirm its safety in moderate doses but demonstrate no significant effects on electrolyte balance, plasma volume, or muscle function [78,79,80].
Overall, POMj and CJ demonstrate the strongest recovery-related benefits (including anti-inflammatory and antioxidant effects), whereas BRJ offers the most consistent ergogenic effects for endurance. WJ supports the antioxidant status with prolonged use, and PJ’s utility remains primarily anecdotal. The variability in the results highlights the importance of tailoring supplementation according to the exercise type, athlete training status, and timing protocols.
4.7. Practical Implications for Application
BRJ shows the most consistent performance-enhancing effects, especially for submaximal endurance exercise, with recommended doses between 6.4 and 12.8 mmol of nitrate, typically consumed 2–3 h before activity [38,45,64]. However, its efficacy may diminish in elite athletes, potentially due to ceiling effects in NO-related pathways, as demonstrated by Fernández-Elías et al. [51], who reported no benefit from acute BRJ use in professional tennis players. Moreover, nitrate supplementation may be less effective in intermittent sports (e.g., tennis, soccer), where performance depends not just on aerobic efficiency but also on technical–tactical factors.
To reduce inflammation and oxidative stress, POMj and TCJ are more reliable. POMj is typically consumed at 250–500 mL/day for 7–15 days and is particularly effective after resistance or strength-based training, where it modulates cytokines and oxidative markers [68,69,71]. TCJ, usually dosed at 60–300 mL/day for several days before and after exercise, optimally reduces DOMS and preserves muscle function following eccentric or prolonged efforts [72,73,74].
WJ, due to its L-citrulline content, supports antioxidant defenses and may reduce soreness, but its effects require chronic supplementation (≥1–2 weeks, 500–710 mL/day). Acute benefits on strength or endurance remain unclear [83,84,86]. PJ lacks evidence in terms of ergogenic or recovery benefits; its role in cramp relief remains anecdotal, and it is typically used in small, acute doses (1–2 mL/kg) during or after exercise [78,80].
Target populations differ: BRJ suits trained recreational or sub-elite endurance athletes, while POMj and TCJ benefit athletes in high-volume/-intensity resistance training. Supplementation strategies should align with the exercise type, training status, timing, and goals. Future research should address optimal protocols, gender-specific responses, and potential juice–nutrient synergies.
5. Conclusions
This systematic review emphasizes the diverse physiological effects and supplementation outcomes associated with various natural juices in athletic settings. A key shared feature among these juices is their rich content of bioactive compounds such as nitrates, polyphenols, anthocyanins, citrulline, and electrolytes. These components play a role in alleviating oxidative stress, mitigating muscle damage, and supporting post-exercise recovery.
Among the juices examined, BRJ consistently demonstrated ergogenic benefits, particularly for endurance and submaximal exercise, when consumed at optimal doses and timing. However, its effectiveness may diminish in elite athletes due to physiological ceiling effects, and its application appears limited in technically demanding, intermittent sports. POMj and TCJ showed the strongest evidence for anti-inflammatory and antioxidant effects, especially when consumed prophylactically over multiple days before and after exercise. These juices are particularly effective for recovery from resistance training and eccentric exercise. While some performance improvements were noted, they were less reliably observed across studies. Watermelon juice appeared more effective in enhancing recovery and boosting the antioxidant status when consumed over extended periods, rather than as an acute performance enhancer. Conversely, pickle juice did not show measurable benefits for performance, hydration, or electrolyte replacement, and current support for its role in preventing muscle cramps remains largely anecdotal.
The variability in study designs, participant demographics, supplementation protocols, and outcome measures highlights the need for more standardized and rigorous research to determine optimal supplementation strategies and their sport-specific applications. Given their rich profiles of bioactive compounds, natural fruit and vegetable juices offer promising benefits as dietary supplements for athletic performance and recovery. However, their application should be tailored based on the type, intensity, duration, and goals of the physical activity. Each juice contains a unique composition of phytonutrients that exert effects through specific metabolic pathways and mechanisms of action. Therefore, future research should focus on identifying the precise bioactive constituents responsible for the observed physiological outcomes, as well as understanding their pharmacokinetics and interactions. Moreover, the optimal dosing regimens and threshold levels required to elicit beneficial effects remain to be clearly defined. The potential synergistic or antagonistic effects of combining various juices or compounds should also be explored to better inform evidence-based supplementation strategies for athletes and physically active individuals. Finally, when tailored to individual needs, juice supplementation may offer a natural and functional strategy to enhance recovery and support athletic performance.
Limitations and Future Perspectives
Although interest in natural juice supplementation to enhance athletic performance and recovery continues to grow, this systematic review has several notable limitations. One major challenge was the heterogeneity among the included studies. Differences in dosage, duration of supplementation, juice formulation (e.g., concentrates vs. fresh juices), and the types of outcome measures used made it difficult to directly compare findings across studies. This variability also prevented the performance of a meta-analysis. Another limitation was the relatively small sample sizes in many of the studies, along with a lack of representation of female athletes and diverse athletic populations. This restricts the generalizability of the findings across sexes and training backgrounds. Moreover, the range of exercise modalities studied, including endurance, resistance, and sprint-based protocols, along with inconsistencies in supplementation timing (acute vs. chronic use), further challenged the ability to draw firm conclusions about the efficacy of each juice type. The absence of standardized approaches for the evaluation of recovery, oxidative stress, and performance outcomes also hindered the synthesis of results across trials.
To advance the field, future research should prioritize several key areas:
- -
- Conducting well-powered RCTs with larger sample sizes and standardized supplementation protocols;
- -
- Designing comparative studies that directly assess different juice types using consistent methodologies;
- -
- Implementing long-term intervention studies to examine chronic adaptations and recovery processes;
- -
- Exploring the underlying cellular and molecular mechanisms, especially for lesser-understood juices like pomegranate and pickle juice;
- -
- Including female participants and athletes from a variety of backgrounds to assess sex- and population-specific responses;
- -
- Combination strategies (e.g., juice plus caffeine or other nutrients) to evaluate synergistic effects on performance and recovery.
Clarifying dose–response relationships, identifying optimal timing strategies, and understanding formulation choices (e.g., fresh vs. pasteurized, juice vs. extract) will also be essential in developing evidence-based guidelines in sports nutrition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sports13080269/s1. Supplementary File S1. PRISMA 2020 checklist, Supplementary File S2. INPLASY protocol, Supplementary Table S1. PEDro scale evaluation.
Author Contributions
Conceptualization, B.V. and M.F.; methodology, B.V.; software, M.F.; validation, T.P.-I., L.P. and B.V.; formal analysis, K.S.-P. and T.P.-I.; investigation, B.V. and M.F.; resources, B.V.; data curation, B.V. and L.P.; writing—original draft preparation, B.V.; writing—review and editing, M.F., T.P.-I. and L.P.; visualization, M.F. and K.S.-P.; supervision, T.P.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NO | Nitric Oxide |
| BRJ | Beetroot Juice |
| POMj | Pomegranate Juice |
| IL-6 | Interleukin-6 |
| TNF | Tumor Necrosis Factor |
| TCJ | Tart Cherry Juice |
| PJ | Pickle Juice |
| WJ | Watermelon Juice |
| RCTs | Randomized Controlled Trials |
| INPLASY | International Platform of Registered Systematic Review and Meta-Analysis Protocols |
| PEDro | Physiotherapy Evidence Database Scale |
| M | Male |
| F | Female |
| NO2− | Nitrite |
| NO3− | Nitrate |
| VO2 | Oxygen Uptake |
| TT | Time Trial |
| YYIR1 | Yo-Yo Intermittent Recovery level 1 |
| SD | Single-Dose |
| DD | Double-Dose |
| AEC | Aerobic Energy Cost |
| VCO2 | Carbon Dioxide production |
| VE | Minute Ventilation |
| MVC | Maximal Voluntary Contraction |
| MIVC | Maximal Isometric Voluntary Contraction |
| SJ | Squat Jump |
| CMJ | Countermovement Jump |
| RI | Reactive Strength Index |
| PPT | Pressure Pain Threshold |
| CK | Creatine Kinase |
| CRP | C-Reactive Protein |
| hsCRP | High-Sensitivity C-Reactive Protein |
| PC | Protein Carbonyls |
| LOOH | Lipid Hydroperoxides |
| SaO2 | Oxygen Saturation |
| HR | Heart Rate |
| DEXA | Dual X-Ray Absorptiometry |
| RPE | Rating of Perceived Exertion |
| HI | High |
| LO | Low |
| KNO3 | Potassium Nitrate |
| SIT | Sprint Interval Training |
| BP | Blood Pressure |
| SBP | Systolic Blood Pressure |
| GAE | Gallic Acid Equivalent |
| LDH | Lactate Dehydrogenase |
| DOMS | Delayed-Onset Muscle Soreness |
| TTE | Time to Exhaustion |
| XC | Cross-Country |
| RTF | Repetitions to Failure |
| TSI | Tissue Saturation Index |
| VAS | Visual Analog Scale |
| TQR | Total Quality Recovery |
| VO2max | Maximal Oxygen uptake |
| MBT | Medicine Ball Throw |
| VJH | Vertical Jump Height |
| BF% | Body Fat Percentage |
| POMx | Pomegranate Extract |
| ASAT | Aspartate Aminotransferase |
| TPC | Total Phenolic Content |
| o-DPO | Orthodiphenol |
| MDA | Malonaldehyde |
| UA | Uric Acid |
| TAC | Total Antioxidant Capacity |
| Hcy | Homocysteine |
| MCJ | Montmorency Cherry Juice |
| MTCJ | Montmorency Tart Cherry Juice |
| F2-IsoP | F2-Isoprostane |
| Na | Sodium |
| K | Potassium |
| IMTP | Isometric Mid-Thigh Pull |
| BMX | Bicycle Motocross |
| GPS | Global Positioning System |
References
- Wang, L.; Meng, Q.; Su, C.H. From Food Supplements to Functional Foods: Emerging Perspectives on Post-Exercise Recovery Nutrition. Nutrients 2024, 16, 4081. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; Shaw, C.S.; Stepto, N.K.; Levinger, I. Exercise and Glycemic Control: Focus on Redox Homeostasis and Redox-Sensitive Protein Signaling. Front. Endocrinol. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The Compelling Link Between Physical Activity and the Body’s Defense System. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Li, S.; Fasipe, B.; Laher, I. Potential Harms of Supplementation with High Doses of Antioxidants in Athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-Gonzáles, A.; Madrigal-Santillán, E.O.; Angeles-Valencia, M.; Anguiano-Robledo, L.; González-López, L.L.; Sosa-Gomez, A.; Fregoso-Aguilar, T.; Esquivel-Chirino, C.; Ruiz-Velazco-Benítez, Y.A.; et al. Phytochemicals and modulation of exercise-induced oxidative stress: A novel overview of antioxidants. Am. J. Transl. Res. 2022, 14, 8292–8314. [Google Scholar]
- Jacobs, D.R.; Tapsell, L.C. Food, Not Nutrients, Is the Fundamental Unit in Nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef]
- Connolly, D.A.; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a Tart Cherry Juice Blend in Preventing the Symptoms of Muscle Damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sports 2009, 20, 843–852. [Google Scholar] [CrossRef]
- Strobel, N.A.; Peake, J.M.; Matsumoto, A.; Marsh, S.A.; Coombes, J.S.; Wadley, G.D. Antioxidant Supplementation Reduces Skeletal Muscle Mitochondrial Biogenesis. Med. Sci. Sports Exerc. 2011, 43, 1017–1024. [Google Scholar] [CrossRef]
- Visioli, F.; Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.C.R.; Oliveira Assumpção, C.; Prestes, J.; Sérgio Denadai, B. Consumption of cherries as a strategy to attenuate exercise-induced muscle damage and inflamation in humans. Nutr. Hosp. 2015, 32, 1885–1893. [Google Scholar] [CrossRef]
- Van Hoorebeke, J.; Trias, C.; Davis, B.; Lozada, C.; Casazza, G. Betalain-Rich Concentrate Supplementation Improves Exercise Performance in Competitive Runners. Sports 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Crum, E.M.; Che Muhamed, A.M.; Barnes, M.; Stannard, S.R. The Effect of Acute Pomegranate Extract Supplementation on Oxygen Uptake in Highly-Trained Cyclists During High-Intensity Exercise in a High Altitude Environment. J. Int. Soc. Sports Nutr. 2017, 14, 14. [Google Scholar] [CrossRef]
- Vitale, K.C.; Hueglin, S.; Broad, E. Tart Cherry Juice in Athletes: A Literature Review and Commentary. Curr. Sports Med. Rep. 2017, 16, 230–239. [Google Scholar] [CrossRef]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of Beetroot Juice Supplementation on Intermittent High-Intensity Exercise Efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflammation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef]
- McIlvenna, L.C.; Muggeridge, D.J.; Whitfield, J. Exploring the Mechanisms by Which Nitrate Supplementation Improves Skeletal Muscle Contractile Function: One Fibre at a Time. J. Physiol. 2020, 598, 25–27. [Google Scholar] [CrossRef]
- Bahari, H.; Rafiei, H.; Goudarzi, K.; Omidian, K.; Asbaghi, O.; Kolbadi, K.S.; Naderian, M.; Hosseini, A. The Effects of Pomegranate Consumption on Inflammatory and Oxidative Stress Biomarkers in Adults: A Systematic Review and Meta-Analysis. Inflammopharmacology 2023, 31, 2283–2301. [Google Scholar] [CrossRef]
- Dale, R.B.; Leaver-Dunn, D.; Bishop, P.A. Compositional Analysis of a Common Acetic Acid Solution with Practical Implications for Ingestion. J. Athl. Train. 2003, 38, 57–61. [Google Scholar]
- Miller, K.C.; Mack, G.W.; Knight, K.L.; Hopkins, J.T.; Draper, D.O.; Fields, P.J.; Hunter, I. Reflex Inhibition of Electrically Induced Muscle Cramps in Hypohydrated Humans. Med. Sci. Sports Exerc. 2010, 42, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.C. Exercise-Associated Muscle Cramps. In Exertional Heat Illness. A Clinical and Evidence-Based Guide; Adams, W.M., Jardine, J.F., Eds.; Springer: Cham, Switzerland, 2019; pp. 117–136. [Google Scholar] [CrossRef]
- Edwards, A.J.; Wiley, E.R.; Brown, E.D.; Clevidence, B.A.; Vinyard, B.T.; Collins, J.K.; Perkins-Veazie, P.; Baker, R.A. Consumption of Watermelon Juice Increases Plasma Concentrations of Lycopene and β-Carotene in Humans. J. Nutr. 2003, 133, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.J.; Pendleton, L.C.; Eichler, D.C. Argininosuccinate synthase: At the center of arginine metabolism. Int. J. Biochem. Mol. Biol. 2011, 2, 8–23. [Google Scholar] [PubMed]
- Pinto, M.P.; Santos, C.N.; Henriquesa, C.; Lima, G.; Quedas, F. Lycopene content and antioxidant capacity of Portuguese watermelon fruits. Electron. J. Environ. Agric. Food Chem. 2011, 10, 2090–2097. [Google Scholar]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Davis, A.R.; Webber, C.L.; Liu, W.; Perkins-Veazie, P.; Levi, A.; King, S. Watermelon Quality Traits as Affected by Ploidy. HortScience 2013, 48, 1113–1118. [Google Scholar] [CrossRef]
- Naz, A.; Sadiq Butt, M.; Pasha, I.; Nawaz, H. Antioxidant Indices of Watermelon Juice and Lycopene Extract. Pak. J. Nutr. 2013, 12, 255–260. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and Nitrogen Homeostasis: An Overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Wethington, L.N.; Stone, M.S.; Stewart, R.W.; Moyen, N.E. Acute Citrulline Malate Supplementation Improves Upper- and Lower-Body Submaximal Weightlifting Exercise Performance in Resistance-Trained Females. Eur. J. Nutr. 2015, 56, 775–784. [Google Scholar] [CrossRef]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in Health and Disease. Review on Human Studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- De Morton, N.A. The Pedro Scale Is a Valid Measure of the Methodological Quality of Clinical Trials: A Demographic Study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Christensen, P.M.; Nyberg, M.; Bangsbo, J. Influence of Nitrate Supplementation on VO2 Kinetics and Endurance of Elite Cyclists. Scand. J. Med. Sci. Sports 2012, 23, e21–e31. [Google Scholar] [CrossRef]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary Nitrate Supplementation Improves Team Sport-Specific Intense Intermittent Exercise Performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef]
- Martin, K.; Smee, D.; Thompson, K.G.; Rattray, B. No Improvement of Repeated-Sprint Performance with Dietary Nitrate. Int. J. Sports Physiol. Perform. 2014, 9, 845–850. [Google Scholar] [CrossRef]
- Pinna, M.; Roberto, S.; Milia, R.; Marongiu, E.; Olla, S.; Loi, A.; Migliaccio, G.; Padulo, J.; Orlandi, C.; Tocco, F.; et al. Effect of Beetroot Juice Supplementation on Aerobic Response During Swimming. Nutrients 2014, 6, 605–615. [Google Scholar] [CrossRef]
- Thompson, C.; Wylie, L.J.; Fulford, J.; Kelly, J.; Black, M.I.; McDonagh, S.T.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Dietary Nitrate Improves Sprint Performance and Cognitive Function During Prolonged Intermittent Exercise. Eur. J. Appl. Physiol. 2015, 115, 1825–1834. [Google Scholar] [CrossRef]
- Clifford, T.; Berntzen, B.; Davison, G.; West, D.; Howatson, G.; Stevenson, E. Effects of Beetroot Juice on Recovery of Muscle Function and Performance Between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8, 506. [Google Scholar] [CrossRef]
- Patrician, A.; Schagatay, E. Dietary Nitrate Enhances Arterial Oxygen Saturation After Dynamic Apnea. Scand. J. Med. Sci. Sports 2016, 27, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Vanhatalo, A.; Jell, H.; Fulford, J.; Carter, J.; Nyman, L.; Bailey, S.J.; Jones, A.M. Dietary Nitrate Supplementation Improves Sprint and High-Intensity Intermittent Running Performance. Nitric Oxide 2016, 61, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Bailey, S.J.; Kelly, J.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. Influence of Beetroot Juice Supplementation on Intermittent Exercise Performance. Eur. J. Appl. Physiol. 2016, 116, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Nyakayiru, J.; Van Dijk, J.W.; Maase, K.; Ballak, S.B.; Senden, J.M.G.; Van Loon, L.J.C.; Verdijk, L.B. Repeated-sprint Performance and Plasma Responses Following Beetroot Juice Supplementation Do Not Differ Between Recreational, Competitive and Elite Sprint Athletes. Eur. J. Sport Sci. 2018, 18, 524–533. [Google Scholar] [CrossRef]
- Richard, P.; Koziris, L.P.; Charbonneau, M.; Naulleau, C.; Tremblay, J.; Billaut, F. Time-Trial Performance in World-Class Speed Skaters After Chronic Nitrate Ingestion. Int. J. Sports Physiol. Perform. 2018, 13, 1317–1323. [Google Scholar] [CrossRef]
- Thompson, C.; Vanhatalo, A.; Kadach, S.; Wylie, L.J.; Fulford, J.; Ferguson, S.K.; Blackwell, J.R.; Bailey, S.J.; Jones, A.M. Discrete Physiological Effects of Beetroot Juice and Potassium Nitrate Supplementation Following 4-WK Sprint Interval Training. J. Appl. Physiol. 2018, 124, 1519–1528. [Google Scholar] [CrossRef]
- Esen, O.; Nicholas, C.; Morris, M.; Bailey, S.J. No Effect of Beetroot Juice Supplementation on 100-m and 200-m Swimming Performance in Moderately Trained Swimmers. Int. J. Sports Physiol. Perform. 2019, 14, 706–710. [Google Scholar] [CrossRef]
- Daab, W.; Bouzid, M.A.; Lajri, M.; Bouchiba, M.; Saafi, M.A.; Rebai, H. Chronic Beetroot Juice Supplementation Accelerates Recovery Kinetics Following Simulated Match Play in Soccer Players. J. Am. Coll. Nutr. 2020, 40, 61–69. [Google Scholar] [CrossRef]
- Fernández-Elías, V.; Courel-Ibáñez, J.; Pérez-López, A.; Jodra, P.; Moreno-Pérez, V.; Coso, J.D.; López-Samanes, Á. Acute Beetroot Juice Supplementation Does Not Improve Match-Play Activity in Professional Tennis Players. J. Am. Nutr. Assoc. 2020, 41, 30–37. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Palau-Salvà, G.; Serra-Payá, N.; Ruiz-Hermosel, M.; Berbell, M.; Viñals, X.; Bataller, M.G.; Carbonell, T.; Vilches-Saez, S.; Cobo, E.P.; et al. Understanding the Effects of Beetroot Juice Intake on CrossFit Performance by Assessing Hormonal, Metabolic and Mechanical Response: A Randomized, Double-Blind, Crossover Design. J. Int. Soc. Sports Nutr. 2020, 17, 56. [Google Scholar] [CrossRef]
- Esen, O.; Domínguez, R.; Karayigit, R. Acute Beetroot Juice Supplementation Enhances Intermittent Running Performance but Does Not Reduce Oxygen Cost of Exercise Among Recreational Adults. Nutrients 2022, 14, 2839. [Google Scholar] [CrossRef]
- Esen, O.; Faisal, A.; Zambolin, F.; Bailey, S.J.; Callaghan, M.J. Effect of Nitrate Supplementation on Skeletal Muscle Motor Unit Activity During Isometric Blood Flow Restriction Exercise. J. Appl. Physiol. 2022, 122, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Giv, V.; Aminaei, M.; Nikoei, R. The Effect of Eight Weeks Beetroot Juice Supplement on Aerobic, Anaerobic Power, and Field Performance of Soccer Players. Res. Sports Med. 2022, 32, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Z.; Wang, X.; Wang, G.; Wang, Y.; Tang, K.; Gao, B. Influence of Chronic Nitrate-Rich Beetroot Juice Supplementation on the Endurance Performance of Active Winter Triathletes: A Randomized Controlled Trial. J. Am. Nutr. Assoc. 2022, 42, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Castro, J.M.; Campos-Perez, J.; Ranchal-Sanchez, A.; Durán-López, N.; Domínguez, R. Acute Effects of Beetroot Juice Supplements on Lower-Body Strength in Female Athletes: Double-Blind Crossover Randomized Trial. Sports Health 2022, 14, 812–821. [Google Scholar] [CrossRef]
- Tan, R.; Pennell, A.; Price, K.M.; Karl, S.T.; Seekamp-Hicks, N.G.; Paniagua, K.K.; Weiderman, G.D.; Powell, J.P.; Sharabidze, L.K.; Lincoln, I.G.; et al. Effects of Dietary Nitrate Supplementation on Performance and Muscle Oxygenation During Resistance Exercise in Men. Nutrients 2022, 14, 3703. [Google Scholar] [CrossRef]
- Esen, O.; Karayigit, R.; Peart, D.J. Acute Beetroot Juice Supplementation Did Not Enhance Intermittent Running Performance in Trained Rugby Players. Eur. J. Sport Sci. 2023, 23, 2321–2328. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of Beetroot Juice Supplementation on Muscle Soreness and Performance Recovery After Exercise-Induced Muscle Damage in Female Volleyball Players. Nutrients 2023, 15, 3763. [Google Scholar] [CrossRef]
- Moreno-Heredero, B.; Morencos, E.; Morais, J.; Barbosa, T.M.; Veiga, S. A Single Dose of Beetroot Juice Not Enhance Performance During Intervallic Swimming Efforts. J. Sports Sci. Med. 2024, 23, 228–235. [Google Scholar] [CrossRef]
- Neteca, J.; Veseta, U.; Liepina, I.; Volgemute, K.; Dzintare, M.; Babarykin, D. Effect of Beetroot Juice Supplementation on Aerobic Capacity in Female Athletes: A Randomized Controlled Study. Nutrients 2024, 17, 63. [Google Scholar] [CrossRef]
- Tan, R.; Merrill, C.; Riley, C.F.; Hammer, M.A.; Kenney, R.T.; Riley, A.A.; Li, J.; Zink, A.C.; Karl, S.T.; Price, K.M.; et al. Acute Inorganic Nitrate Ingestion Does Not Impact Oral Microbial Composition, Cognitive Function, or High-Intensity Exercise Performance in Female Team-Sport Athletes. Eur. J. Appl. Physiol. 2024, 124, 3511–3525. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, Z.; Heung-Sang Wong, S.; Zheng, C.; Tsz-Chun Poon, E. Acute Effects of Various Doses of Nitrate-Rich Beetroot Juice on High-Intensity Interval Exercise Responses in Women: A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial. J. Int. Soc. Sports Nutr. 2024, 21, 2334680. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huo, L.; Wang, F.; Wang, T.; Rong, W.; He, Y. Caffeine and Beetroot Juice Optimize 1,000-m Performance: Shapley Additive Explanations Analysis. Am. J. Mens. Health 2025, 19, 15579883251327907. [Google Scholar] [CrossRef]
- Trombold, J.R.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin Consumption Improves Strength Recovery 2–3 D After Eccentric Exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Trombold, J.R.; Reinfeld, A.S.; Casler, J.R.; Coyle, E.F. The Effect of Pomegranate Juice Supplementation on Strength and Soreness After Eccentric Exercise. J. Strength. Cond. Res. 2011, 25, 1782–1788. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate Supplementation Accelerates Recovery of Muscle Damage and Soreness and Inflammatory Markers After a Weightlifting Training Session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of Pomegranate Juice Supplementation on Oxidative Stress Biomarkers Following Weightlifting Exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef]
- Urbaniak, A.; Basta, P.; Ast, K.; Wołoszyn, A.; Kuriańska-Wołoszyn, J.; Latour, E.; Skarpańska-Stejnborn, A. The Impact of Supplementation with Pomegranate Fruit (Punica granatum L.) Juice on Selected Antioxidant Parameters and Markers of Iron Metabolism in Rowers. J. Int. Soc. Sports Nutr. 2018, 15, 35. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Trabelsi, K.; Bragazzi, N.L.; Boukhris, O.; Bouaziz, M.; Ayadi, F.; El Abed, K.; Driss, T.; Souissi, N.; et al. Effects of Natural Polyphenol-Rich Pomegranate Juice on the Acute and Delayed Response of Homocysteine and Steroidal Hormones Following Weightlifting Exercises: A Double-Blind, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2020, 17, 15. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency Cherry Juice Reduces Muscle Damage Caused by Intensive Strength Exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery Facilitation with Montmorency Cherries Following High-Intensity, Metabolically Challenging Exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef]
- Bell, P.; Stevenson, E.; Davison, G.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of Tart Cherry Juice on Recovery and Next Day Performance in Well-Trained Water Polo Players. J. Int. Soc. Sports Nutr. 2016, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.; Brashill, C.; Brett, A.; Clifford, T. Tart Cherry Juice: No Effect on Muscle Function Loss or Muscle Soreness in Professional Soccer Players After a Match. Int. J. Sports Physiol. Perform. 2020, 15, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Rapin, N.; Andrushko, J.W.; Farthing, J.P.; Gordon, J.; Chilibeck, P.D. The Effect of Tart Cherry Juice Compared to a Sports Drink on Cycling Exercise Performance, Substrate Metabolism, and Recovery. PLoS ONE 2024, 19, e0307263. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.C. Electrolyte and Plasma Responses After Pickle Juice, Mustard, and Deionized Water Ingestion in Dehydrated Humans. J. Athl. Train. 2014, 49, 360–367. [Google Scholar] [CrossRef]
- Peikert, J.; Miller, K.C.; Albrecht, J.; Tucker, J.; Deal, J. Pre-Exercise Ingestion of Pickle Juice, Hypertonic Saline, or Water and Aerobic Performance and Thermoregulation. J. Athl. Train. 2014, 49, 204–209. [Google Scholar] [CrossRef]
- McKenney, M.A.; Miller, K.C.; Deal, J.E.; Garden-Robinson, J.A.; Rhee, Y.S. Plasma and Electrolyte Changes in Exercising Humans After Ingestion of Multiple Boluses of Pickle Juice. J. Athl. Train. 2015, 50, 141–146. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Alacid, F.; Carrasco, M.; Martínez, I.; Aguayo, E. Watermelon Juice: Potential Functional Drink for Sore Muscle Relief in Athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef]
- Cutrufello, P.T.; Gadomski, S.J.; Zavorsky, G.S. The Effect Ofl-Citrulline and Watermelon Juice Supplementation on Anaerobic and Aerobic Exercise Performance. J. Sports Sci. 2014, 33, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two Weeks of Watermelon Juice Supplementation Improves Nitric Oxide Bioavailability but Not Endurance Exercise Performance in Humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Ramos-Campo, D.J.; Fernández-Lobato, B.; Rubio-Arias, J.A.; Alacid, F.; Aguayo, E. Biochemical, Physiological, and Performance Response of a Functional Watermelon Juice Enriched in L-Citrulline During a Half-Marathon Race. Food Nutr. Res. 2017, 61, 1330098. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Pinzone, A.G.; Lipes, S.E.; Mangine, G.T.; Townsend, J.R.; Allerton, T.D.; Sell, K.M.; Ghigiarelli, J.J. Effect of Watermelon Supplementation on Exercise Performance, Muscle Oxygenation, and Vessel Diameter in Resistance-Trained Men. Eur. J. Appl. Physiol. 2022, 122, 1627–1638. [Google Scholar] [CrossRef]
- AghabeigiAmin, P.; Azizi, M.; Tahmasebi, W. Effects of 6 Weeks of Watermelon Juice Supplementation on Total Antioxidant Capacity and VO2max in Elite Taekwondo Athletes. Sport Sci. Health 2025, 21, 923–929. [Google Scholar] [CrossRef]
- Erzurum, S.C.; Ghosh, S.; Janocha, A.J.; Xu, W.; Bauer, S.; Bryan, N.S.; Tejero, J.; Hemann, C.; Hille, R.; Stuehr, D.J.; et al. Higher Blood Flow and Circulating NO Products Offset High-Altitude Hypoxia Among Tibetans. Proc. Natl. Acad. Sci. USA 2007, 104, 17593–17598. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of Dietary Nitrate on Oxygen Cost During Exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Dejam, A.; Hunter, C.J.; Schechter, A.N.; Gladwin, M.T. Emerging Role of Nitrite in Human Biology. Blood Cells Mol. Dis. 2004, 32, 423–429. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. Beetroot Juice Is More Beneficial than Sodium Nitrate for Attenuating Muscle Pain After Strenuous Eccentric-Bias Exercise. Appl. Physiol. Nutr. Metab. 2017, 42, 1185–1191. [Google Scholar] [CrossRef]
- Bex, T.; Baguet, A.; Achten, E.; Aerts, P.; De Clercq, D.; Derave, W. Cyclic Movement Frequency is Associated with Muscle Typology in Athletes. Scand. J. Med. Sci. Sports 2017, 27, 223–229. [Google Scholar] [CrossRef]
- Seeram, N. Cyclooxygenase Inhibitory and Antioxidant Cyanidin Glycosides in Cherries and Berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.-C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and Antiinflammatory Activities of Anthocyanins and Their Aglycon, Cyanidin, from Tart Cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Kuehl, K.S.; Perrier, E.T.; Elliot, D.L.; Chesnutt, J.C. Efficacy of Tart Cherry Juice in Reducing Muscle Pain During Running: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2010, 7, 17. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of Pomegranate Supplementation on Exercise Performance and Post-Exercise Recovery in Healthy Adults: A Systematic Review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef]
- Ettinger, P.O.; Regan, T.J.; Oldewurtel, H.A. Hyperkalemia, Cardiac Conduction, and the Electrocardiogram: A Review. Am. Heart J. 1974, 88, 360–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).