Abstract
Nitric oxide (NO) plays a crucial role in muscle oxidative capacity, which predicts muscle strength. This study aimed to investigate whether different breathing techniques (nasal or oral breathing) affect muscle performance during acute exhaustive exercise. In our study, 49 healthy individuals (24♀/25♂; age 22.8 ± 3.4 years) performed two Wingate anaerobic tests in a counterbalanced order. Although perceived exertion was significantly higher with oral breathing (Borg Scale: 9.0 ± 1.1 vs. 8.0 ± 1.3, p = 0.04), breathing mode did not impact power output (peak: 749 ± 290 vs. 728 ± 284 W; average: 576 ± 217 vs. 575 ± 216 W, p = 0.2). NIRS data indicated no significant differences in muscle desaturation between the two breathing modes; however, nasal breathing resulted in significantly faster (0.45 ± 0.4 vs. 0.23 ± 0.12%/s, p = 0.02) and greater (75.2 ± 4.0 vs. 73.1 ± 3.6%, p = 0.04) post-exercise muscle recovery. As an indirect marker of NO bioavailability, flow-mediated dilation (FMD) was associated with a significant improvement (Pre: 107.4 ± 3.0% vs. Post: 110.3 ± 3.6%, p < 0.001) via nasal breathing only, with a significant difference between the two breathing modes (p < 0.0001). Therefore, we suggest that the nitrate–nitrite–NO pathway enhances muscle energy and function, which highlights the importance of nasal breathing.
1. Introduction
During physical activity, muscle performance depends on numerous factors [1,2], for example, muscle composition (fiber type, size and length, architecture, fascicle pennation angle, etc.), muscular adipose tissue, metabolic supply, insulin sensitivity, neuromuscular activation, and muscle oxidative capacity. In a recent review [1], only muscle oxidative capacity was found to be a predictor of muscle strength. Indeed, it has been demonstrated that skeletal muscle bioenergetics is a major determinant of functional mobility, defined by walking speed. The more demanding the walking task, the more significant the direct role of bioenergetics [3]. This has crucial clinical implications. Indeed, aging has been associated with a decline in skeletal muscle mitochondrial oxidative capacity, something that negatively affects one’s ability to walk [4], an essential ability for daily activities and for older adults to maintain their independence [5]. Therefore, interventions aimed at improving and maintaining muscle strength through muscle mitochondrial function—mainly, if not exclusively, physical exercise—are mandatory to reduce, postpone, or prevent mobility loss [6,7].
Among the different pathways that underpin skeletal muscle metabolism, nitric oxide (NO) has a functionally relevant role [8]. Indeed, it regulates mitochondrial respiration and biogenesis and can inhibit the electron transport that regulates oxygen consumption and ATP generation. At the same time, it can induce an increase in reactive oxygen and nitrogen species [9,10]. NO is also a major contributor to exercise hyperemia, a coping mechanism to meet increased demand for oxygen and energy substrates [11].
NO cannot be stored inside cells; therefore, it requires multiple controlling mechanisms to regulate its functions. In muscular cells, two main sources of NO exist: the nitric oxide synthase (NOS)-dependent pathway and the NOS-independent pathway. The latter is where nitrate (NO3) is reduced to nitrite (NO2) and further reduced to NO [12]; that being said, the main source of NO is the NOS-dependent pathway, which accounts for about two-thirds of NO production [13]. It involves a series of reactions catalyzed by NOS enzymes that oxidize L-arginine to NO, which requires the presence of many cofactors, including oxygen [11]. Although all NOS isoforms—neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS)—are found in muscular cells, in normal conditions, iNOS is hardly detectable and is mostly expressed in response to inflammatory insult and/or oxidative stress [14]. It is also important to note that there is no compensatory relationship between NOS isoforms; if one of them is lacking, this does not trigger an increased protein expression of the other forms—this is the case in animal studies, at least [15]. Although several mechanisms are involved in NO production, including shear stress, cardiac output, and arteriovenous difference, this study focused on the potential to increase NO production through iNOS stimulation in the nasal fossae and sinus mucosae. Indeed, nasal breathing promotes NO concentration up to ten times more than what has been observed in the bronchi [16,17]; meanwhile, oral breathing can significantly reduce circulating NO, which might promote vascular dysfunction [18]. Although the evidence for this is quite old, more recent data have also suggested that nasal breathing might significantly improve the maintenance of NO production and bioavailability in order to reduce the occurrence of vascular dysfunction while exposed to extreme environments [19].
Therefore, the objective of this exploratory study was to investigate the effects of nasal breathing on muscle performance during exhaustive exercise. Nasal breathing was hypothesized to enhance vessel diameter and blood flow during exercise and, consequently, muscle oxygenation. This might be relevant for strenuous exercise routines like kendo (Japanese fencing), which has been known to increase oxidative stress and cause cellular damage [20,21].
2. Materials and Methods
Experimental procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Academic Ethical Committee of Brussels (Belgium) (Ethic committee B 200-2022-038).
2.1. Experimental Protocol
After we received written informed consent and medical clearance, 49 healthy nonsmoking individuals (24 females and 25 males) volunteered for this study. They were selected from a larger population of students in physical and sports education (aged between 18 and 31 years); however, none of the participants trained in sports at a professional or elite level. They were not classified as being at risk of cardiovascular diseases according to the current Belgian recommendations on sports cardiology and exercise [22,23]. They were also instructed not to drink any alcoholic [24] or caffeinated beverages [25,26], smoke [27], or perform any kind of exercise [28,29] for 8 h prior to the experiment. These are all confounding factors of flow-mediated dilation (FMD) analysis.
Each participant, already familiarized with the procedure, completed two Wingate anaerobic tests (WAnTs) on an assault bike (Rogue Echo Bike V3.0, Rogue Fitness, Columbus, OH, USA), spaced two days apart. This air-resistance bike facilitated simultaneous engagement of both the upper and lower extremities. The test procedure we followed is well described in the literature [30]. In short, it included a warm-up of 5 min—pedaling at 50–70 rpm (approximately 50–70 watts). After warming up, the subjects rested in a sitting position on the bike for five minutes. Then, at the count of “3, 2, 1…Go!” the participants started to pedal as hard as possible for thirty seconds. Their task was to maintain the maximum cadence until the end of the test. Therefore, they were continuously motivated by verbal encouragement. Although durations such as 15, 20, or even 45 and 60 s have been described in the literature, we opted for the 30-s duration, which is the typical duration of the WAnT [31]. Afterwards, a 5 min recovery ride at a warm-up pace was carried out to help cool participants down.
Power output was recorded every 5 s. Each subject’s peak power output was defined as their highest value, and the average peak power output was calculated over the 30-s WAnT. These values were used to determine mean peak power output (PPO) and average peak power output (aPPO) across all 49 subjects for further analysis.
All procedures were performed in the Physiology Laboratory at the Haute Ecole Bruxelles Brabant. The WAnTs were performed under one of two conditions, either nasal (N) or oral breathing (O), performed in counterbalanced order. As each participant regularly engaged in sports, it is reasonable to assume they possessed enhanced respiratory control. Therefore, under the N condition, participants had athletic tape placed over their mouths to prevent any oral breathing, while for the O condition, a standard laboratory nose clip was used to prevent nasal breathing. These were placed immediately following the warm-up, approximately 20 s prior to the start of the WAnT, and removed when the recovery period began. Participants were randomly assigned to begin with either the N or O condition and vice versa for their second test. Also, the time of day for testing was kept consistent for all subjects during the second test [32], making each participant his or her own control.
2.2. Oxygenation: Pulse Oximetry (SPO2) and Near-Infrared Spectroscopy (NIRS)
Arterial oxygen saturation and vastus lateralis (VL) muscle tissue oxygenation were assessed by pulse oximetry (SPO2) and near-infrared spectroscopy (NIRS), respectively, as a reflection of macro- and microcirculation.
To avoid artifacts in SPO2 readings using a probe (Nellcor PM10N, Medtronic Canada, Brampton, ON, Canada), the probe’s placement area (second or third finger on the non-dominant hand) was cleaned with a cotton swab soaked in alcohol, and the probe was secured with hypoallergenic skin tape. The participants were also asked, as often as needed, not to squeeze this hand on the bike handle. After a minute of auto-calibration, the oximetry recording started and was maintained throughout the testing sessions, including the warm-up and recovery periods.
Muscle oxygenation of the VL was measured using a continuous-wave NIRS device (Train.Red Plus, Gelderland, The Netherlands). The probe was placed on the subject’s non-dominant leg, perpendicular to the estimated longitudinal axis of the VL, 15 cm above the superior border of the patella and 5 cm lateral to the midline of the thigh. The distance of the probe to the patella was outlined with a marker and measured to ensure consistent placement between sessions. It was attached to the skin with an adhesive patch that prevents interference from ambient light and ensures data quality; it was then secured around the thigh using a black elasticated strap. After each WAnT, we checked for a slightly depressed cutaneous area at the location of the probe to ensure that the proper contact between the probe and the skin was maintained. Detailed descriptions of the technology behind NIRS can be found in other studies that measure the light attenuation at wavelengths of 770 and 830 nm using algorithms based on a modified Beer–Lambert law [33,34]. The NIRS sampling rate was set at 1 Hz, and the device calculated the total saturation index (TSI), which is a measure of local oxygenation. All data were collected on a smart device and then transferred to a computer. The baseline TSI was determined to be the average of 30 s preceding the WAnT. The area under the curve over 30 s of exercise to exhaustion (AUC 30sec) and the difference between the baseline and the lowest TSI value (TSI) during the WAnT were utilized as a measure of the magnitude of oxygen desaturation. The downslope of the TSI calculated over the 30 sec of the WAnT was a measure of oxygen (O2) desaturation rate, while the upslope of the TSI signal was a measure of the O2 resaturation rate, calculated over a 10 s window immediately following the conclusion of the WAnT.
2.3. Flow-Mediated Dilation (FMD)
Flow-mediated dilation (FMD) is an established measure of the endothelium-dependent vasodilation mediated by nitric oxide (NO) [35]. Therefore, using a digital diagnostic ultrasound system (DP-30, Mindray, Echomedic, Ghent, Belgium), we assessed the effect of oral versus nasal breathing on main conduit arteries. We opted for the reactive hyperemia technique, whereby brachial artery diameter is measured before and 1 min after a 5 min ischemia induced by inflating a cuff placed on the forearm, as previously described [19,36,37]. The artery diameter was measured manually with an electronic caliper (provided by the ultrasonography software) in a three-fold repetition pattern. The average diameter over these three measurements was used to calculate the percent increase in arterial diameter from the resting state to maximal dilation. All measurements were performed by experienced operators who conduct more than 100 scans/year, which is a recommended criterion to maintain competency in the FMD method [38].
2.4. Statistical Analysis
The sample size was established a priori using G*Power 3.1 software (v.3.1.9.6, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany), and the expected effect size was set at 0.5, the α level was set at 0.05, and the power (1-β) was set at 0.95, leading to a sample size of 47 individuals.
The normality of the results was verified using the Kolmogorov–Smirnov test. Depending on the distribution, comparisons between oral versus nasal breathing were performed using either a paired t-test or a Wilcoxon signed-rank test; meanwhile, for comparisons between men and women, we used their equivalent for unpaired data, either an unpaired t-test or a Mann–Whitney U test, respectively. Finally, comparisons between results at different times and the baseline were carried out using repeated-measure one-way ANOVA tests or a Friedman test.
All statistical tests were performed using a standard computer statistical package, GraphPad Prism version 10.4.1 for Mac (GraphPad Software, San Diego, CA, USA). A threshold of p < 0.05 was considered statistically significant. All data are presented as means ± standard deviation (SD).
3. Results
The baseline demographics of the participants (24♀/25♂) are summarized in Table 1. Men were significantly taller and heavier than women, but their body compositions were similar.

Table 1.
Demographic variables of the study participants (***: p < 0.001; Mann–Whitney).
3.1. Power Output
A significant increase in PPO and aPPO was observed between the first and second sessions, from 703 ± 283 to 775 ± 287 W (p < 0.001, paired t-test) and from 539 ± 211 to 612 ± 216 W (p < 0.001, paired t-test), respectively (Figure 1A). This represents an increase of 9.9 ± 11.9% and 15.1 ± 19.2%, respectively. There is also a significant difference in both PPO and aPPO between females and males (Figure 1C) (♀: 8.5 ± 2.9 and 6.9 ± 2.2 W/kg; ♂: 13.2 ± 3.5 and 10.0 ± 2.8 W/kg, respectively, p < 0.001, Mann–Whitney). However, this gender difference remains constant across sessions (PPO: 0.8 ± 1.0 (♀) vs. 1.0 ± 1.1 (♂) W/kg, p = 0.06 Mann–Whitney; aPPO: 0.8 ± 1.0 (♀) vs. 1.3 ± 1.6 (♂) W/kg, p = 0.4, Mann–Whitney). When transformed as a percentage variation to evaluate the magnitude of the change, the increase in both PPO and aPPO is similar in females and males (PPO: 9.5 ± 12.5 (♀) vs. 10.2 ± 11.6 (♂) %; aPPO: 13.2 ± 16.0 (♀) vs. 17.0 ± 22.0 (♂) %, p = 0.3 and p = 0.68, respectively, Mann–Whitney).
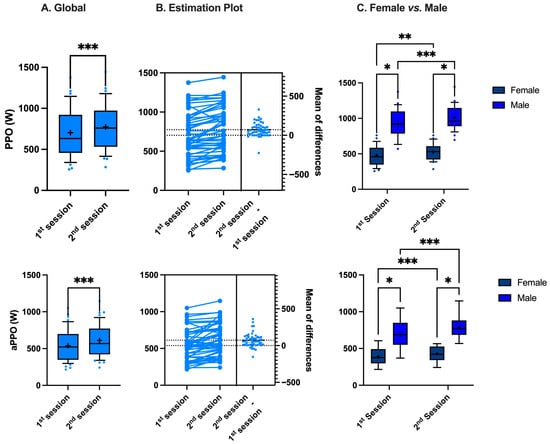
Figure 1.
Difference in peak power output (PPO) and average peak power output (aPPO) between sessions (A,B) and between female and male (C) (*: p < 0.05, **: p < 0.01, ***: p < 0.001; (A): paired t-test, (C): Mann–Whitney).
Although there are some gender differences and between-sessions differences, our counterbalanced crossover study design allows for a direct comparison of oral vs. nasal breathing by grouping all the results into a single analysis, showing that the respiratory mode has no effect on either the PPO or the aPPO (749 ± 290 vs. 728 ± 284 W, p = 0.2 paired t-test and 576 ± 217 vs. 575 ± 216 W, p = 0.2 paired t-test, respectively—Figure 2), which is consistent with the previous literature [39,40].
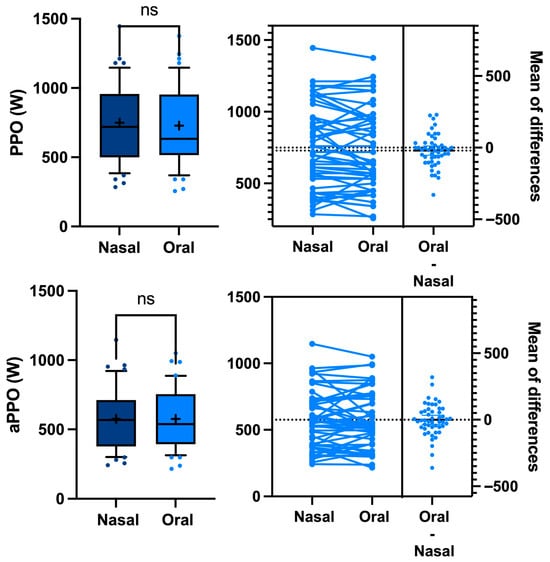
Figure 2.
Difference in peak power output (PPO) and average peak power output (aPPO) between oral vs. nasal breathing during a Wingate anaerobic test. Every participant acts as his or her own control (ns: not significant, paired t-test).
3.2. Oxygenation
SPO2 was not significantly modified across the WAnT procedure (oral: 97.9 ± 0.9 (Pre) vs. 97.6 ± 1.0 (Nadir) vs. 98.0 ± 0.9 (Post) %, p = 0.12 Friedman; nasal: 98.2 ± 0.7 (Pre) vs. 97.0 ± 0.9 (Nadir) vs. 98.1 ± 0.9 (Post) %, p = 0.07 Friedman test). Also, breathing pattern did not significantly modify SPO2 (Pre: p = 0.15; Nadir: p = 0.08; Post: p = 0.31, Wilcoxon).
The average global TSI signal profile across the 49 subjects, the variables assessed, and the average of the TSI signal during WAnT are all shown in Figure 3.
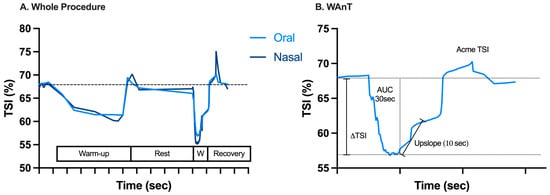
Figure 3.
Average time plot of near-infrared spectroscopy-derived tissue oxygen saturation (TSI) signal after elimination of artifacts measured across the 49 subjects through (A) the whole procedure and (B) the WAnT representative of oral breathing, as well as the variables assessed (AUC = area under the curve; ΔTSI = the difference between baseline TSI and the lowest TSI value). On the X-axis, each gradation corresponds to 60 s.
Table 2 shows NIRS parameter data between conditions (oral vs. nasal breathing). No significant differences were found in the desaturation rate and the magnitude of this desaturation. On the other hand, when it comes to resaturation of the VL muscle, nasal breathing was responsible for a significantly faster and greater compensation than oral breathing.

Table 2.
Tissue oxygen saturation (TSI) during a Wingate test while breathing either through the mouth or the nose (*: p < 0.05).
3.3. Flow-Mediated Dilation (FMD)
As said in the Introduction, nasal breathing seems to be a potent physiological strategy to increase circulating NO [19,41,42], potentially modulating the effects of exhaustive exercise on FMD. Accordingly, FMD variation values were not significantly modified while breathing through the mouth (Pre: 105.9 ± 3.6% vs. Post: 106.1 ± 3.6%, p = 0.81 paired t-test). On the other hand, nasal breathing is associated with a significant improvement in FMD (Pre: 107.4 ± 3.0% vs. Post: 110.3 ± 3.6%, p < 0.001 paired t-test). The difference between the two conditions is very significant (p < 0.0001, Wilcoxon) (Figure 4). FMD returned to normal values 2 h after the WAnT was executed while breathing through the nose at 101.6 ± 2.5%.
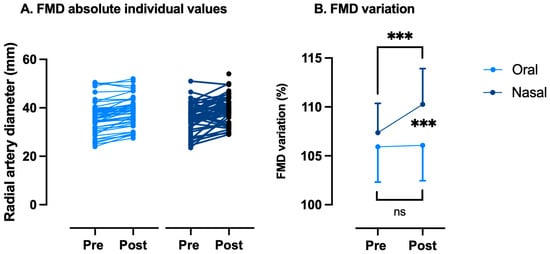
Figure 4.
Variations in flow-mediated dilation (FMD) between oral and nasal breathing after a Wingate anaerobic test. FMD changes are expressed as follows: (A) absolute individual values and (B) % of pre-exercise values. Every participant acts as his or her own control (ns: not significant, ***: p < 0.001; intragroup: paired t-Test; intergroup: Wilcoxon).
4. Discussion
The primary finding from this study was that exercise performance improved in the second session and was significantly higher in men than women, with men’s PPO and aPPO exceeding women’s by 36% and 31%, respectively. This gender gap is larger than reported in previous studies (10–20% [43,44]), possibly due to differences in body composition [45] or exercise modality. Indeed, gender differences in power output may be smaller when adjusted for fat-free mass rather than body mass [43]. Also, the use of an assault bike, which increased the involvement of the upper body, likely widened the gap [46]. Furthermore, the influence of external factors such as verbal encouragement, motivation, and the presence of an audience cannot be overlooked [47,48]. Indeed, the fluctuation in the WAnT test–retest variability was estimated to range from 0 to as high as 22%, with a global trend toward improvement [49], which calls into question the objective intragroup and intra-subject comparisons. However, the crossover design, with participants as their own controls and random allocations for breathing modes, should have mitigated any bias and improved reliability. Indeed, our results align with previous studies [50].
PPO results fall within the 50th to 75th percentile of the Wingate muscular power test [51]. This is relevant to participants involved in club or recreational sports who consistently exercise at moderate (3.0–5.9 METs) or strenuous (6 + METs) intensities, which aligns with our sample criteria. Indeed, the participants were sports practitioners, although not at an elite level. Nonetheless, it is reasonable to assume they have enhanced experience in respiratory control. This might have skewed our results. However, the counterbalanced crossover design over two days, as well as devices used to prevent mixed breathing, likely minimized this issue. Moreover, although nasal breathing enhances ventilatory efficiency [52], the mode of breathing did not have a significant impact on performance metrics during a high-intensity anaerobic test. This finding is corroborated by previous studies [53].
To further discuss our results, we must acknowledge some limitations. Indeed, NIRS recordings were performed on the lower limbs, while SPO2 and FMD were measured on upper limbs. We chose the quadriceps due to their higher oxygen consumption during cycling [54] and used the brachial artery for FMD because of operator expertise. While a positive correlation was observed between NIRS-derived oxygen resaturation rate and percent change in FMD for both the lower limbs (tibialis anterior muscle/popliteal artery) [55] and upper limbs (flexor digitorum superficialis muscle/brachial artery) [56], studies comparing both limbs are lacking. However, establishing a correlation between FMD and NIRS was not the objective of this study. In the present setting, FMD was employed as an indirect method to evaluate NO bioavailability.
Indeed, numerous studies have examined the effects of various NO-related supplements—including inorganic nitrate, nitrite, L-arginine, and L-citrulline—on exercise performance. These dietary supplements have been linked to improved performance, reduced oxygen consumption during exercise, enhanced vessel diameter and blood flow, and delayed onset of fatigue in highly active individuals [57]. Nasal breathing serves a similar function. In the context of a non-provocative 20-min dive to 10 m, whether using a half-mask (which bypasses the nose) or a full-face mask (allowing for nasal breathing only), it has been demonstrated that such conditions significantly enhance nitric oxide (NO) production [19]. Our results show enhanced vasodilation but no improved performance, which partially supports previous research on the NO hypothesis. This lack of performance improvement could be due to NO-related supplementation being primarily effective in untrained and older individuals [58], which do not match our sample criteria.
TSI also supports the involvement of NO. Previous studies have demonstrated the following: (1) a notable correlation between peak lactate levels and mean power in the WAnT, indicating that glycolytic metabolism is primarily activated, and (2) a significant increase of 78% in reactive oxygen species (ROS) free radical scavenging activities compared to pre-exercise values [59]. This suggests an increase in ROS production, as various proteins involved in glycolysis, mitochondrial electron transport, β-oxidation, and the tricarboxylic acid cycle can generate superoxide, hydrogen peroxide, and other ROS [60]. This high-intensity exercise-related oxidative stress caused by excessive ROS production may be counteracted by several NO-related mechanisms, whose bioavailability increases with nasal breathing [19]. Firstly, NO can react with superoxide to form peroxynitrite. While this nitrosative stress may be potentially damaging, it effectively removes superoxide from circulation, thereby mitigating its harmful impact on endothelial cells [61]. Secondly, NO triggers protective cellular signaling through soluble guanylate cyclase, thereby producing cGMP. This mediator activates protein kinases, safeguarding cells from oxidative damage and promoting vasodilation [62]. This process improves oxygen delivery while also accelerating the removal of metabolic by-products such as lactate, thereby reducing further oxidative stress [63]. Finally, NO regulates mitochondria, which affects both their function and cellular activity, by inhibiting respiratory chain complexes through competition with oxygen or modifying mitochondrial components, ultimately reducing oxidative stress [10]. Hypothetically—since we measured neither NO nor ROS—these mechanisms, either alone or together, might account for our results, mainly a significant increase in the resaturation rate and a stronger compensatory response when breathing through the nose. While the complete spectrum of processes involving NO is still debated, it is clear that NO interactions within metabolic networks are intricate and influenced by various factors, particularly the concentration of NO at the site of action [10].
5. Conclusions
The available data on oral-only or nasal-only breathing during anaerobic exercise is limited. However, we found that breathing mode did not impact power output or performance in high-intensity anaerobic exercise tests. Despite the advantages and disadvantages of each breathing mode, participant preference should not be the deciding factor [53]. Indeed, our findings also show that the nitrate–nitrite–NO pathway might enhance muscle energy and function, which highlights the importance of nasal breathing. Therefore, we call for further research into the effectiveness of nasal breathing during exercise. This research should focus on optimizing NO bioavailability for muscle recovery in both athletes and the elderly.
Author Contributions
Conceptualization, P.L., C.B. and F.G.; methodology, P.L., C.B., F.G., M.L. and C.L. (Clément Lévêque); formal analysis, P.L., M.L., C.L. (Clément Lévêque) and C.L. (Capucine Lafère); investigation, M.L., C.L. (Clément Lévêque) and C.L. (Capucine Lafère); data curation, P.L.; writing—original draft preparation, P.L., M.L., C.L. (Clément Lévêque) and C.L. (Capucine Lafère); writing—review and editing, P.L., C.B. and F.G.; supervision, P.L. and C.B.; project administration, P.L.; funding acquisition, P.L. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of the DELTO2X Project and was funded by WBE (Wallonia-Brussels Education, Belgium) and the Environmental, Occupational, and Aging (Integrative) Physiology Laboratory, Haute Ecole Bruxelles-Brabant (HE2B), Belgium.
Institutional Review Board Statement
All experimental procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Academic Ethical Committee of Brussels (Belgium) (Ethic committee B 200-2022-038). (Approval Date: 25 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to restriction related to data protection of government employee.
Acknowledgments
The authors are grateful to all volunteer participants, especially students of the Haute Ecole Bruxelles-Brabant (Belgium), Motor Sciences Department (Physiotherapy).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| aPPO | Average peak power output |
| AUC30 | Area under the curve over 30 s |
| FMD | Flow-mediated dilation |
| NIRS | Near-infrared spectroscopy |
| NO | Nitric oxide |
| NO2 | Nitrite |
| NO3 | Nitrate |
| NOS | Nitric oxide synthase and isoforms: neural nNOS, endothelial eNOS, and inducible iNOS |
| O2 | Oxygen |
| PPO | Peak power output |
| SPO2 | Pulse oximetry |
| TSI | Total saturation index |
| VL | Vastus lateralis muscle |
| WAnT | Wingate anaerobic test |
References
- Kuschel, L.B.; Sonnenburg, D.; Engel, T. Factors of Muscle Quality and Determinants of Muscle Strength: A Systematic Literature Review. Healthcare 2022, 10, 1937. [Google Scholar] [CrossRef]
- Fitts, R.H.; McDonald, K.S.; Schluter, J.M. The determinants of skeletal muscle force and power: Their adaptability with changes in activity pattern. J. Biomech. 1991, 24 (Suppl. S1), 111–122. [Google Scholar] [CrossRef]
- Zane, A.C.; Reiter, D.A.; Shardell, M.; Cameron, D.; Simonsick, E.M.; Fishbein, K.W.; Studenski, S.A.; Spencer, R.G.; Ferrucci, L. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell 2017, 16, 461–468. [Google Scholar] [CrossRef]
- Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am. J. Phys. Med. Rehabil. 2013, 92, 28–35. [Google Scholar] [CrossRef][Green Version]
- Lombard, D.B.; Miller, R.A.; Pletcher, S.D. Biology of Aging and Longevity. In Hazzard’s Geriatric Medicine and Gerontology, 8th ed.; Halter, J.B., Ouslander, J.G., Studenski, S., High, K.P., Asthana, S., Supiano, M.A., Ritchie, C.S., Schmader, K., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar][Green Version]
- Izquierdo, M.; de Souto Barreto, P.; Arai, H.; Bischoff-Ferrari, H.A.; Cadore, E.L.; Cesari, M.; Chen, L.K.; Coen, P.M.; Courneya, K.S.; Duque, G.; et al. Global consensus on optimal exercise recommendations for enhancing healthy longevity in older adults (ICFSR). J. Nutr. Health Aging 2025, 29, 100401. [Google Scholar] [CrossRef] [PubMed]
- Nejatian Hoseinpour, A.; Bassami, M.; Ahmadizad, S.; Donath, L.; Setayesh, S.; Mirzaei, M.; Mohammad Rahimi, G.R. The influence of resistance training on inflammatory markers, body composition and functional capacity in healthy older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2025, 130, 105731. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef]
- Tengan, C.H.; Moraes, C.T. NO control of mitochondrial function in normal and transformed cells. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 573–581. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Melvin, M.N.; Roelofs, E.J.; Wingfield, H.L. Effects of pomegranate extract on blood flow and running time to exhaustion. Appl. Physiol. Nutr. Metab. 2014, 39, 1038–1042. [Google Scholar] [CrossRef]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A. Type 2 diabetes-related sarcopenia: Role of nitric oxide. Nutr. Metab. 2024, 21, 107. [Google Scholar] [CrossRef]
- Villanueva, C.; Giulivi, C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic. Biol. Med. 2010, 49, 307–316. [Google Scholar] [CrossRef]
- Upanan, S.; Lee, J.; Tunau-Spencer, K.J.; Rajvanshi, P.K.; Wright, E.C.; Noguchi, C.T.; Schechter, A.N. High nitrate levels in skeletal muscle contribute to nitric oxide generation via a nitrate/nitrite reductive pathway in mice that lack the nNOS enzyme. Front. Physiol. 2024, 15, 1352242. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Kawasumi, T.; Takeno, S.; Ishikawa, C.; Takahara, D.; Taruya, T.; Takemoto, K.; Hamamoto, T.; Ishino, T.; Ueda, T. The Functional Diversity of Nitric Oxide Synthase Isoforms in Human Nose and Paranasal Sinuses: Contrasting Pathophysiological Aspects in Nasal Allergy and Chronic Rhinosinusitis. Int. J. Mol. Sci. 2021, 22, 7561. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nasal nitric oxide in man. Thorax 1999, 54, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Levenez, M.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Germonpré, P.; Pique, H.; Virgili, F.; Bosco, G.; Lafère, P.; Balestra, C. Full-Face Mask Use during SCUBA Diving Counters Related Oxidative Stress and Endothelial Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 965. [Google Scholar] [CrossRef]
- Imai, H.; Hayashi, T.; Negawa, T.; Nakamura, K.; Tomida, M.; Koda, K.; Tajima, T.; Koda, Y.; Suda, K.; Era, S. Strenuous exercise-induced change in redox state of human serum albumin during intensive kendo training. Jpn. J. Physiol. 2002, 52, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Tanabe, K.; Akimoto, T.; Kimura, F.; Tanimura, Y.; Shimizu, K.; Okamoto, T.; Kono, I. Reducing exercise-induced muscular injury in kendo athletes with supplementation of coenzyme Q10. Br. J. Nutr. 2008, 100, 903–909. [Google Scholar] [CrossRef]
- Desomer, A.; Gerkens, S.; Vinck, I.; Léonard, C.; Neyt, M.; Paulus, D.; Van Brabandt, H. Cardiovascular pre-participation screening in young athletes. In Health Technology Assessment (HTA); Belgian Health Care Knowledge Centre (KCE): Brussels, Belgium, 2015; p. 151. [Google Scholar]
- Zujko, K.; Małek, Ł.A. How to Unmask Hidden Cardiovascular Diseases through Preparticipation Screening in Master Athletes? Rev. Cardiovasc. Med. 2022, 23, 405. [Google Scholar] [CrossRef] [PubMed]
- Hampton, S.M.; Isherwood, C.; Kirkpatrick, V.J.; Lynne-Smith, A.C.; Griffin, B.A. The influence of alcohol consumed with a meal on endothelial function in healthy individuals. J. Hum. Nutr. Diet. 2010, 23, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Naylor, L.H.; Zimmermann, D.; Guitard-Uldry, M.; Poquet, L.; Lévêques, A.; Eriksen, B.; Bel Rhlid, R.; Galaffu, N.; D’Urzo, C.; De Castro, A.; et al. Acute dose-response effect of coffee-derived chlorogenic acids on the human vasculature in healthy volunteers: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Tesselaar, E.; Nezirevic Dernroth, D.; Farnebo, S. Acute effects of coffee on skin blood flow and microvascular function. Microvasc. Res. 2017, 114, 58–64. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, P.; Meng, L.; Tang, W.; Peng, F. The association between smoking exposure and endothelial function evaluated using flow-mediated dilation values: A meta-analysis. BMC Cardiovasc. Disord. 2024, 24, 292. [Google Scholar] [CrossRef]
- Rognmo, O.; Bjørnstad, T.H.; Kahrs, C.; Tjønna, A.E.; Bye, A.; Haram, P.M.; Stølen, T.; Slørdahl, S.A.; Wisløff, U. Endothelial function in highly endurance-trained men: Effects of acute exercise. J. Strength Cond. Res. 2008, 22, 535–542. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, S.; Dai, H.; Chen, X.; Meng, Z.; Ying, X. Vascular endothelial function and its response to moderate-intensity aerobic exercise in trained and untrained healthy young men. Sci. Rep. 2024, 14, 20450. [Google Scholar] [CrossRef]
- Zupan, M.F.; Arata, A.W.; Dawson, L.H.; Wile, A.L.; Payn, T.L.; Hannon, M.E. Wingate Anaerobic Test peak power and anaerobic capacity classifications for men and women intercollegiate athletes. J. Strength Cond. Res. 2009, 23, 2598–2604. [Google Scholar] [CrossRef]
- Castañeda-Babarro, A. The Wingate Anaerobic Test, a Narrative Review of the Protocol Variables That Affect the Results Obtained. Appl. Sci. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Ravindrakumar, A.; Bommasamudram, T.; Tod, D.; Edwards, B.J.; Chtourou, H.; Pullinger, S.A. Daily variation in performance measures related to anaerobic power and capacity: A systematic review. Chronobiol. Int. 2022, 39, 421–455. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, L.; Herman, P.; Eke, A. The modified Beer-Lambert law revisited. Phys. Med. Biol. 2006, 51, N91–N98. [Google Scholar] [CrossRef]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef]
- Tremblay, J.C.; Pyke, K.E. Flow-mediated dilation stimulated by sustained increases in shear stress: A useful tool for assessing endothelial function in humans? Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H508–H520. [Google Scholar] [CrossRef] [PubMed]
- Germonpré, P.; Pontier, J.M.; Gempp, E.; Blatteau, J.E.; Deneweth, S.; Lafère, P.; Marroni, A.; Balestra, C. Pre-dive vibration effect on bubble formation after a 30-m dive requiring a decompression stop. Aviat. Space Environ. Med. 2009, 80, 1044–1048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lambrechts, K.; Germonpré, P.; Vandenheede, J.; Delorme, M.; Lafère, P.; Balestra, C. Mini Trampoline, a New and Promising Way of SCUBA Diving Preconditioning to Reduce Vascular Gas Emboli? Int. J. Environ. Res. Public Health 2022, 19, 5410. [Google Scholar] [CrossRef]
- Areas, G.P.T.; Mazzuco, A.; Caruso, F.R.; Jaenisch, R.B.; Cabiddu, R.; Phillips, S.A.; Arena, R.; Borghi-Silva, A. Flow-mediated dilation and heart failure: A review with implications to physical rehabilitation. Heart Fail. Rev. 2019, 24, 69–80. [Google Scholar] [CrossRef]
- Chinevere, T.D.; Faria, E.W.; Faria, I.E. Nasal splinting effects on breathing patterns and cardiorespiratory responses. J. Sports Sci. 1999, 17, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.R.; King, K.; Papalia, S.; Goodman, C.; Turley, K.R.; Wilmore, J.H. Comparison of maximal oxygen consumption with oral and nasal breathing. Aust. J. Sci. Med. Sport 1995, 27, 51–55. [Google Scholar]
- Balestra, C.; Cimino, F.; Theunissen, S.; Snoeck, T.; Provyn, S.; Canali, R.; Bonina, A.; Virgili, F. A red orange extract modulates the vascular response to a recreational dive: A pilot study on the effect of anthocyanins on the physiological consequences of scuba diving. Nat. Prod. Res. 2016, 30, 2101–2106. [Google Scholar] [CrossRef]
- Theunissen, S.; Guerrero, F.; Sponsiello, N.; Cialoni, D.; Pieri, M.; Germonpré, P.; Obeid, G.; Tillmans, F.; Papadopoulou, V.; Hemelryck, W.; et al. Nitric oxide-related endothelial changes in breath-hold and scuba divers. Undersea Hyperb. Med. 2013, 40, 135–144. [Google Scholar]
- Murphy, M.M.; Patton, J.F.; Frederick, F.A. Comparative anaerobic power of men and women. Aviat. Space Environ. Med. 1986, 57, 636–641. [Google Scholar]
- Sauvé, B.; Haugan, M.; Paulsen, G. Physical and Physiological Characteristics of Elite CrossFit Athletes. Sports 2024, 12, 162. [Google Scholar] [CrossRef]
- Serresse, O.; Ama, P.F.; Simoneau, J.A.; Lortie, G.; Bouchard, C.; Boulay, M.R. Anaerobic performances of sedentary and trained subjects. Can. J. Sport Sci. 1989, 14, 46–52. [Google Scholar]
- Hegge, A.M.; Bucher, E.; Ettema, G.; Faude, O.; Holmberg, H.C.; Sandbakk, Ø. Gender differences in power production, energetic capacity and efficiency of elite cross-country skiers during whole-body, upper-body, and arm poling. Eur. J. Appl. Physiol. 2016, 116, 291–300. [Google Scholar] [CrossRef]
- Van Hooren, B.; Van Der Lee, P.; Plasqui, G.; Bongers, B.C. The effect of a standardized verbal encouragement protocol on peak oxygen uptake during incremental treadmill testing in healthy individuals: A randomized cross-over trial. Eur. J. Sport Sci. 2024, 24, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wann, D.L.; Hackathorn, J. Audience effects in sport: The reciprocal flow of influence between athletes and spectators. In APA Handbook of Sport and Exercise Psychology: Sport Psychology; Anshel, M.H., Petrie, T.A., Steinfeldt, J.A., Eds.; American Psychological Association: Washington, DC, USA, 2019; pp. 469–488. [Google Scholar]
- Roessel, E.L.; Delgado, E.N.; Darling, M.L.; Holmgren, N.J.; Jensen, C.D.; VanNess, J.M. Wingate Test-Retest Variability in Healthy Subjects: 2737 Board #20 June 12:00 PM–3:30 PM. Med. Sci. Sports Exerc. 2018, 50, 666. [Google Scholar] [CrossRef]
- Leicht, A.S.; Sealey, R.M.; Sinclair, W.H. Influence of cycle ergometer type and sex on assessment of 30-second anaerobic capacity and power. Int. J. Sports Med. 2011, 32, 688–692. [Google Scholar] [CrossRef]
- Maud, P.J.; Shultz, B.B. Norms for the Wingate anaerobic test with comparison to another similar test. Res. Q. Exerc. Sport 1989, 60, 144–151. [Google Scholar] [CrossRef]
- Dallam, G.; Kies, B. The effect of nasal breathing versus oral and oronasal breathing during exercise: A review. J. Sports Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Recinto, C.; Efthemeou, T.; Boffelli, P.T.; Navalta, J.W. Effects of Nasal or Oral Breathing on Anaerobic Power Output and Metabolic Responses. Int. J. Exerc. Sci. 2017, 10, 506–514. [Google Scholar] [CrossRef]
- Rasdal, V. Oxygen Consumption in Cycling: The Relationship Between Whole Body Pulmonary O2 Consumption and Muscle Oxygenation in Different Muscles During Constant-Load Cycling. Master’s Thesis, NTNU—Norwegian University of Science and Technology, Trondheim, Norway, 2013. [Google Scholar]
- McLay, K.M.; Fontana, F.Y.; Nederveen, J.P.; Guida, F.F.; Paterson, D.H.; Pogliaghi, S.; Murias, J.M. Vascular responsiveness determined by near-infrared spectroscopy measures of oxygen saturation. Exp. Physiol. 2016, 101, 34–40. [Google Scholar] [CrossRef]
- Soares, R.N.; Somani, Y.B.; Proctor, D.N.; Murias, J.M. The association between near-infrared spectroscopy-derived and flow-mediated dilation assessment of vascular responsiveness in the arm. Microvasc. Res. 2019, 122, 41–44. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 2022, 63, E239–E245. [Google Scholar] [CrossRef]
- Shannon, O.M.; Clifford, T.; Seals, D.R.; Craighead, D.H.; Rossman, M.J. Nitric oxide, aging and aerobic exercise: Sedentary individuals to Master’s athletes. Nitric Oxide 2022, 125–126, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Ichikawa, H.; Ebine, N.; Minamiyama, Y.; Alharbi, A.A.D.; Iwamoto, N.; Fukuoka, Y. Effects of High-Intensity Anaerobic Exercise on the Scavenging Activity of Various Reactive Oxygen Species and Free Radicals in Athletes. Nutrients 2023, 15, 222. [Google Scholar] [CrossRef]
- Liemburg-Apers, D.C.; Willems, P.H.; Koopman, W.J.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zheng, X.; Song, Y.; Wu, L.; Li, L.; Tong, R.; Han, L.; Bian, Y. Decoding signaling mechanisms: Unraveling the targets of guanylate cyclase agonists in cardiovascular and digestive diseases. Front. Pharmacol. 2023, 14, 1272073. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Vanhatalo, A. The ‘Critical Power’ Concept: Applications to Sports Performance with a Focus on Intermittent High-Intensity Exercise. Sports Med. 2017, 47, 65–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).