Neuromuscular Responses to Unilateral and Bilateral Execution of Eccentric Exercises: A Multidimensional sEMG Study
Abstract
1. Introduction
- Does unilateral eccentric loading lead to greater regional activation than bilateral loading within the same exercise?
- Do sEMG-derived parameters reflect distinct neuromuscular strategies across different modes?
2. Materials and Methods
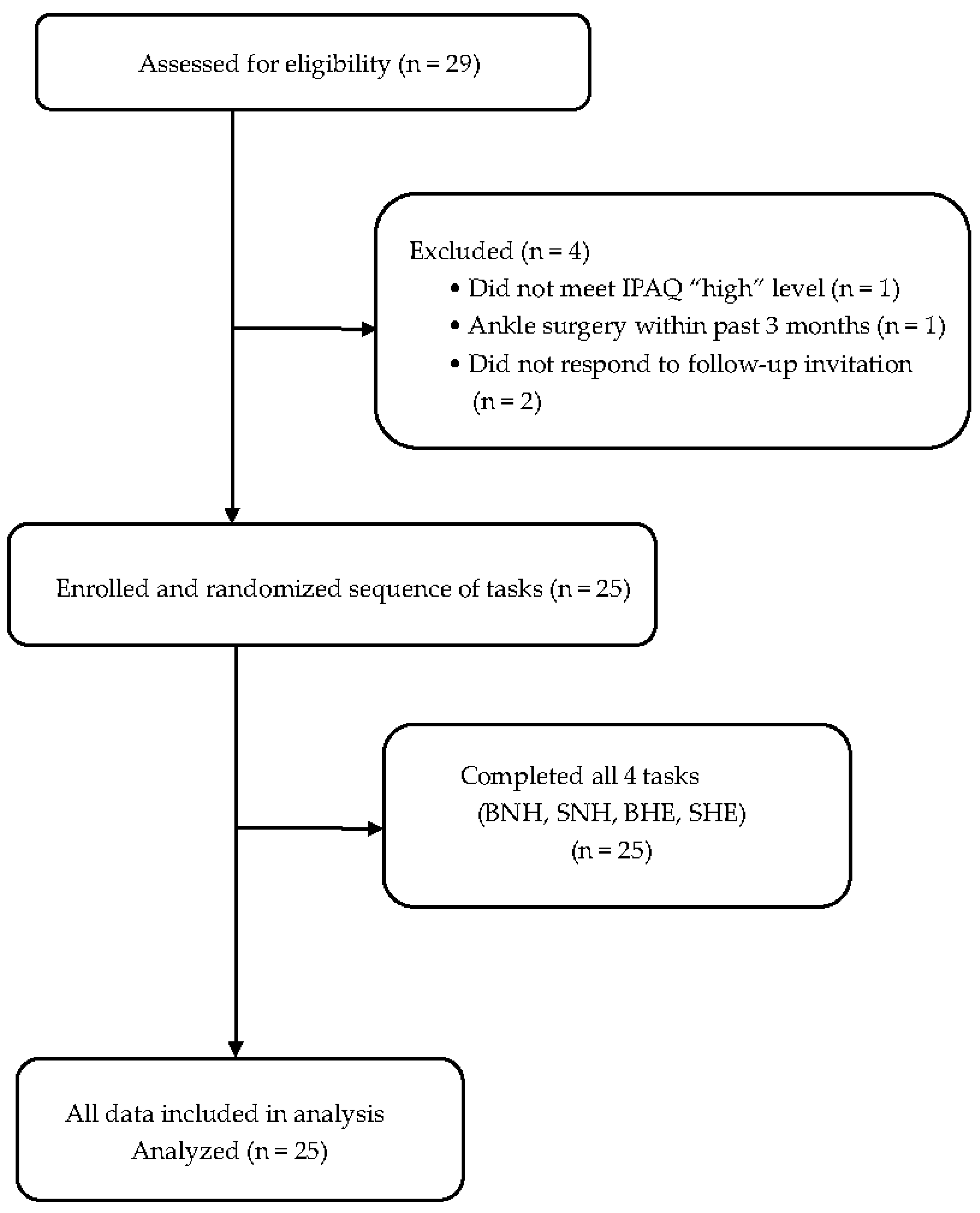
2.1. Study Design
2.2. Participants
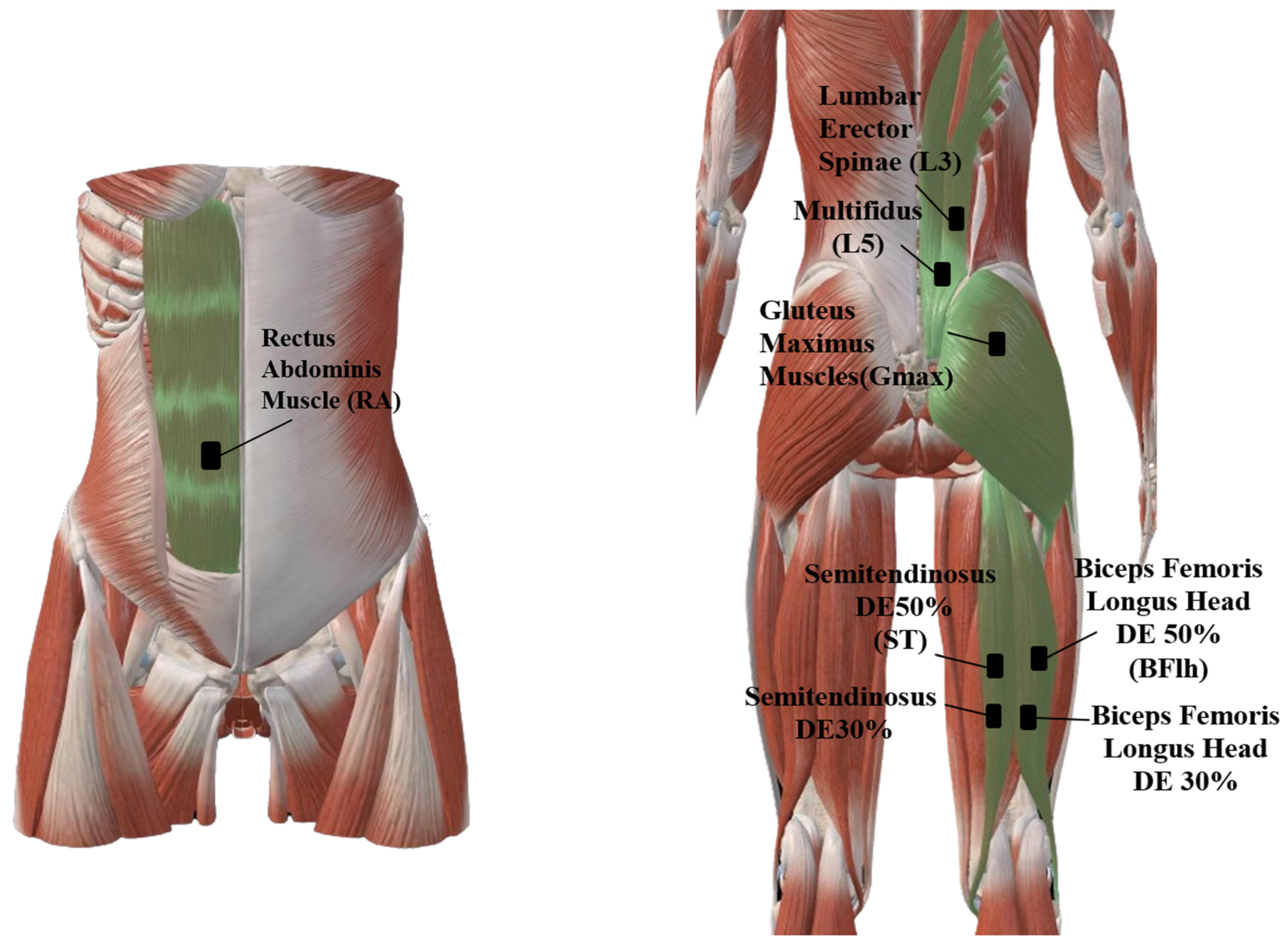
2.3. EMG Data Collection
2.4. Kinematic Analysis
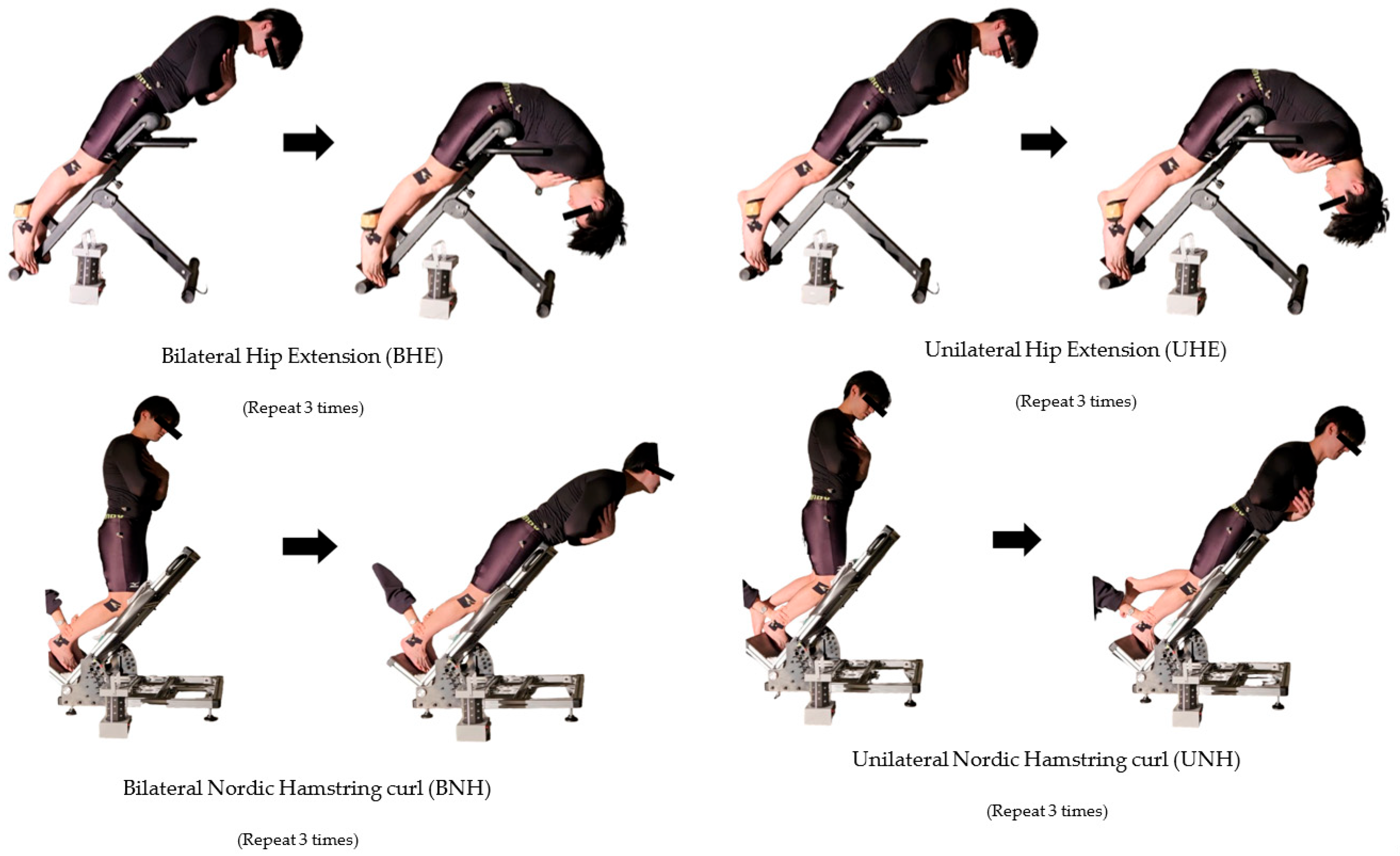
2.5. Testing Procedure
2.6. Signal Processing and Feature Extraction
2.7. Data Analysis
3. Results
3.1. Variations in Hamstring Regions and Preferences
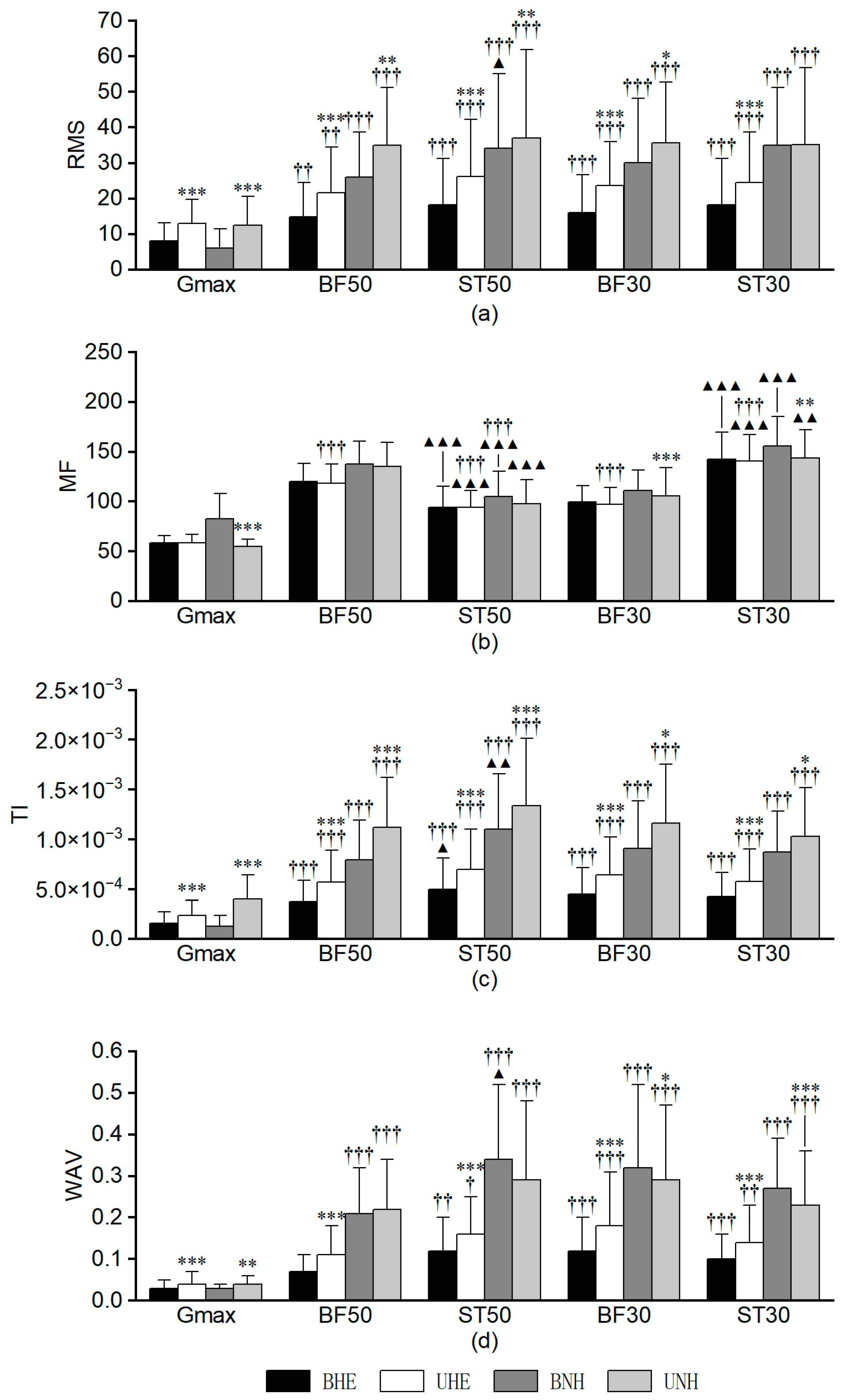
3.2. Gluteal–Hamstring Complex
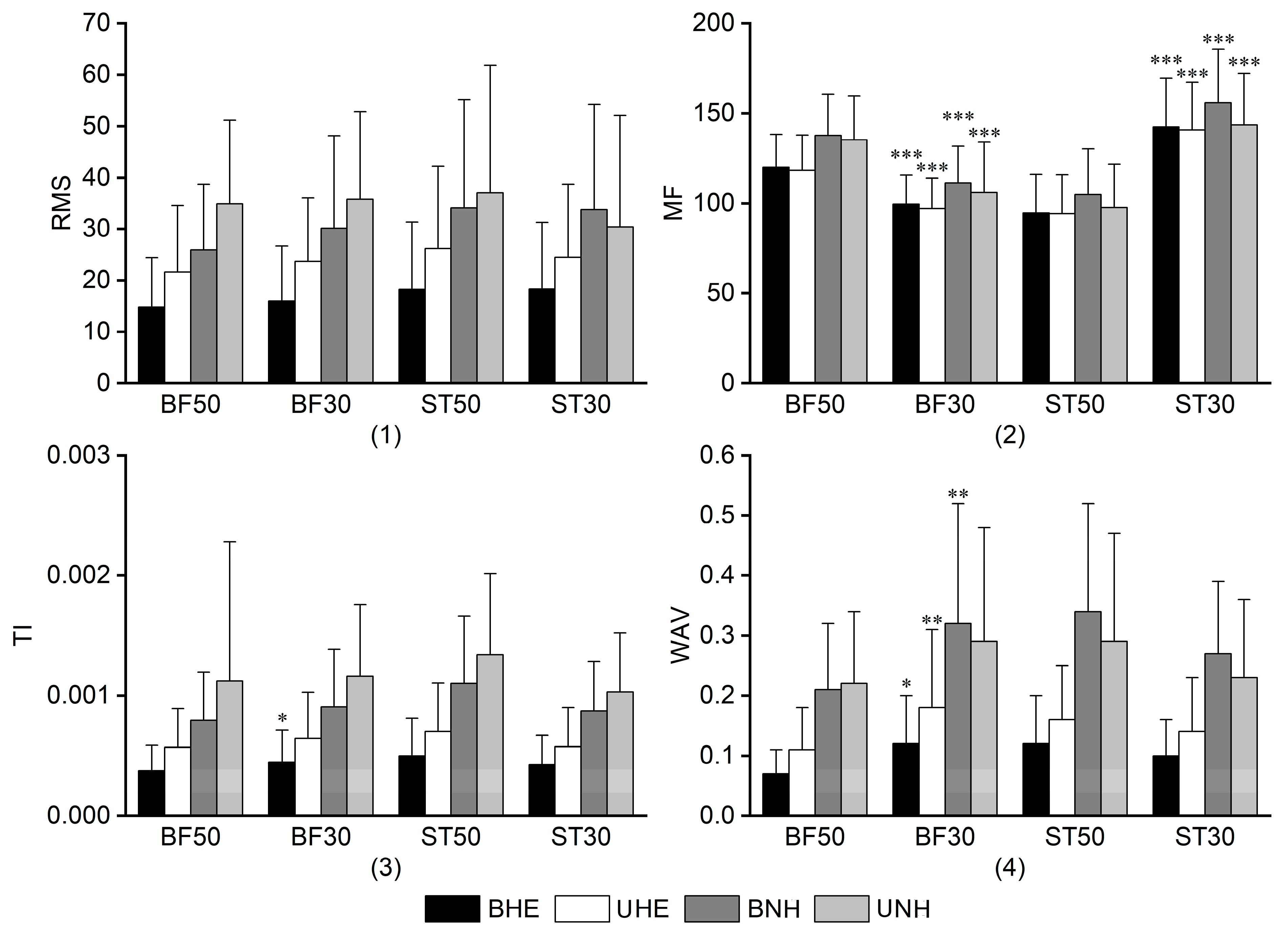
3.2.1. RMS
3.2.2. MF
3.2.3. TI
3.2.4. WAV
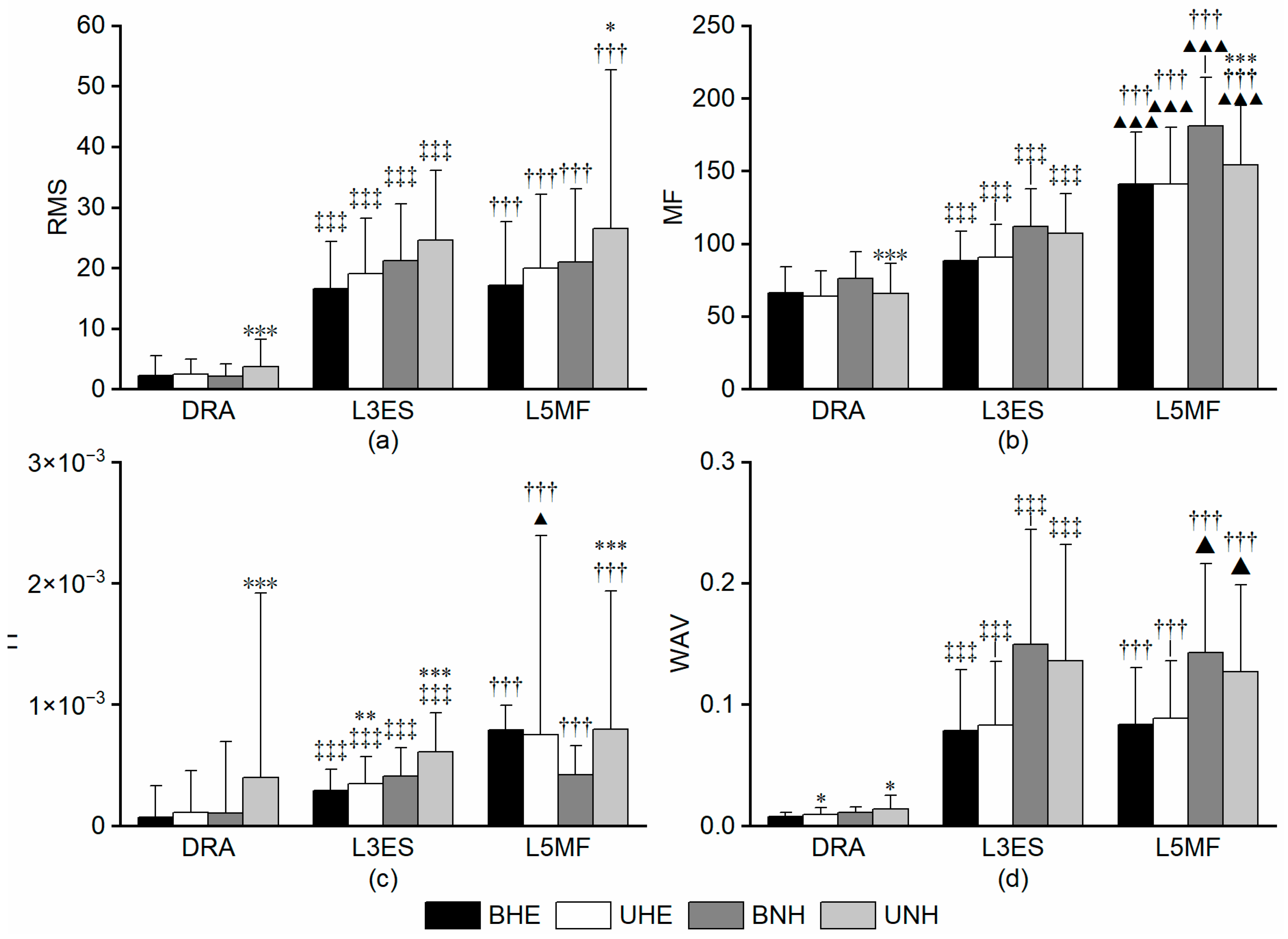
3.3. Trunk Stabiliser Muscles
3.3.1. RMS
3.3.2. MF
3.3.3. TI
3.3.4. WAV
4. Discussion
4.1. Neuromuscular Differences Between Unilateral and Bilateral Loading
4.2. Regional Functional Specialisation of the Hamstrings
4.3. Practical Applications
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BF | biceps femoris |
| BHE | bilateral hip extension |
| BNH | bilateral Nordic hamstring curl |
| EHE | eccentric hip extension |
| EMG | electromyography |
| Gmax | gluteus maximus |
| INHE | inclined Nordic hamstring exercise |
| L3ES | erector spinae at the level of the third lumbar vertebra |
| L5MF | multifidus at the level of the fifth lumbar vertebra |
| MF | median frequency |
| MVIC | maximal voluntary isometric contractions |
| NHE | Nordic hamstring exercise |
| UHE | unilateral hip extension |
| UNH | unilateral Nordic hamstring curl |
| RA | rectus abdominis |
| RMS | root mean square |
| sEMG | surface electromyography |
| ST | semitendinosus |
| TI | torque index |
| WAV | waveform length |
References
- Ekstrand, J.; Waldén, M.; Hägglund, M. Hamstring injuries have increased by 4% annually in men’s professional football, since 2001: A 13-year longitudinal analysis of the UEFA Elite Club injury study. Br. J. Sports Med. 2016, 50, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Bourne, M.N.; van Dyk, N.; Pizzari, T. Recalibrating the risk of hamstring strain injury (HSI): A 2020 systematic review and meta-analysis of risk factors for HSI in sport. Br. J. Sports Med. 2020, 54, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Schache, A.G.; Dorn, T.W.; Wrigley, T.V.; Brown, N.A.; Pandy, M.G. Stretch and activation of the human biarticular hamstrings across a range of running speeds. Eur. J. Appl. Physiol. 2013, 113, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Garrett, W.E.; Moorman, C.T.; Yu, B. Injury rate, mechanism, and risk factors of hamstring strain injuries in sports: A review of the literature. J. Sport Health Sci. 2012, 1, 92–101. [Google Scholar] [CrossRef]
- van Dyk, N.; Behan, F.P.; Whiteley, R. Including the Nordic hamstring exercise in injury prevention programmes halves the rate of hamstring injuries: A systematic review and meta-analysis of 8459 athletes. Br. J. Sports Med. 2019, 53, 1362–1370. [Google Scholar] [CrossRef]
- Paton, B.M.; Read, P.; van Dyk, N.; Wilson, M.G.; Pollock, N.; Court, N.; Giakoumis, M.; Head, P.; Kayani, B.; Kelly, S.; et al. London International Consensus and Delphi study on hamstring injuries part 3: Rehabilitation, running and return to sport. Br. J. Sports Med. 2023, 57, 278–291. [Google Scholar] [CrossRef]
- Timmins, R.G.; Shield, A.J.; Williams, M.D.; Lorenzen, C.; Opar, D.A. Architectural adaptations of muscle to training and injury: A narrative review outlining the contributions by fascicle length, pennation angle and muscle thickness. Br. J. Sports Med. 2016, 50, 1467–1472. [Google Scholar] [CrossRef]
- Al Attar, W.S.A.; Soomro, N.; Sinclair, P.J.; Pappas, E.; Sanders, R.H. Effect of injury prevention programs that include the Nordic hamstring exercise on hamstring injury rates in soccer players: A systematic review and meta-analysis. Sports Med. 2017, 47, 907–916. [Google Scholar] [CrossRef]
- Higashihara, A.; Nagano, Y.; Takahashi, K.; Fukubayashi, T. Effects of forward trunk lean on hamstring muscle kinematics during sprinting. J. Sports Sci. 2015, 33, 1366–1375. [Google Scholar] [CrossRef]
- Mendiguchia, J.; Garrues, M.A.; Cronin, J.B.; Contreras, B.; Los Arcos, A.; Malliaropoulos, N.; Maffulli, N.; Idoate, F. Nonuniform changes in MRI measurements of the thigh muscles after two hamstring strengthening exercises. J. Strength Cond. Res. 2013, 27, 574–581. [Google Scholar] [CrossRef]
- Chumanov, E.S.; Heiderscheit, B.C.; Thelen, D.G. Hamstring musculotendon dynamics during stance and swing phases of high-speed running. Med. Sci. Sports Exerc. 2011, 43, 525–532. [Google Scholar] [CrossRef]
- Presland, J.D.; Timmins, R.G.; Bourne, M.N.; Opar, D.A.; Williams, M.D.; Hickey, J.T. The effect of Nordic hamstring exercise training volume on biceps femoris long head architectural adaptation. Scand. J. Med. Sci. Sports 2018, 28, 1775–1783. [Google Scholar] [CrossRef]
- Hegyi, A.; Csala, D.; Péter, A.; Finni, T.; Cronin, N.J. Region-dependent hamstrings activity in Nordic hamstring exercise and stiff-leg deadlift defined with high-density electromyography. Scand. J. Med. Sci. Sports 2018, 28, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Opar, D.A.; Williams, M.D.; Timmins, R.G.; Hickey, J.; Duhig, S.J.; Shield, A.J. Eccentric hamstring strength and hamstring injury risk in Australian footballers. Med. Sci. Sports Exerc. 2015, 47, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Alvares, J.B.; Marques, V.B.; Vaz, M.A.; Baroni, B.M. Four weeks of Nordic hamstring exercise reduce muscle injury risk factors in young adults. J. Strength Cond. Res. 2018, 32, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.N.; Opar, D.A.; Williams, M.D.; Shield, A.J. Eccentric knee flexor strength and risk of hamstring injuries in rugby union: A prospective study. Am. J. Sports Med. 2015, 43, 2663–2670. [Google Scholar] [CrossRef]
- Vaisman, A.; Guiloff, R.; Rojas, J.; Delgado, I.; Figueroa, D.; Calvo, R. Lower Limb Symmetry: Comparison of Muscular Power Between Dominant and Nondominant Legs in Healthy Young Adults Associated With Single-Leg-Dominant Sports. Orthop. J. Sports Med. 2017, 5, 2325967117744240. [Google Scholar] [CrossRef]
- Soga, T.; Keerasomboon, T.; Akiyama, K.; Hirose, N. Difference of hamstring activity between bilateral and unilateral nordic hamstring exercises with a sloped platform. Int. J. Sport Rehabil. 2022, 31, 325–330. [Google Scholar] [CrossRef]
- Keerasomboon, T.; Jamkrajang, P.; Limroongreungrat, W.; Chrunarm, T.; Soga, T.; Hirose, N. Comparing the hamstring muscle activity between injured and non-injured sides during a variety of Nordic hamstring exercises. PLoS ONE 2025, 20, e0325392. [Google Scholar] [CrossRef]
- Staudenmann, D.; Roeleveld, K.; Stegeman, D.F.; van Dieën, J.H. Methodological aspects of SEMG recordings for force estimation—A tutorial and review. J. Electromyogr. Kinesiol. 2010, 20, 375–387. [Google Scholar] [CrossRef]
- Vieira, T.M.; Botter, A. The accurate assessment of muscle excitation requires the detection of multiple surface electromyograms. Exerc. Sport Sci. Rev. 2021, 49, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kellis, E.; Katis, A. Biomechanical characteristics and determinants of instep soccer kicking. Sports Med. 2007, 6, 154–165. [Google Scholar]
- Held, S.; Siebert, T.; Donath, L.; Drobisch, S. Electromyographic activity of the vastus medialis and gastrocnemius implicates a slow stretch-shortening cycle during rowing in the field. Sci. Rep. 2020, 10, 9451. [Google Scholar] [CrossRef]
- Merletti, R.; Parker, P.J. Electromyography: Physiology, Engineering, and Noninvasive Applications; Wiley-IEEE Press: Hoboken, NJ, USA, 2004. [Google Scholar]
- Kim, C.-Y.; Choi, J.-D.; Kim, S.-Y.; Oh, D.-W.; Jin, M.J. Comparison between muscle activation measured by electromyography and muscle thickness measured using ultrasonography for effective muscle assessment. J. Electromyogr. Kinesiol. 2014, 24, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. The Lancet 2025, 389, e081123. [Google Scholar] [CrossRef]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part I: Ankle, hip, and spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Robertson, D.G.E.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S.N. Research Methods in Biomechanics, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Banaśkiewicz, J.; Kasiak, P.; Chomiuk, T.; Mamcarz, A.; Śliż, D. Self-myofascial release therapy moderately influence electromyography of longissimus dorsi muscle in golfers. Biomed. Hum. Kinet. 2024, 16, 295–304. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Inappropriate interpretation of surface EMG signals and muscle fiber characteristics impedes understanding of the control of neuromuscular function. J. Appl. Physiol. 2015, 119, 1516–1518. [Google Scholar] [CrossRef]
- de Keijzer, K.L.; McErlain-Naylor, S.A.; Beato, M. Six Weeks of Unilateral Flywheel Hip-Extension and Leg-Curl Training Improves Flywheel Eccentric Peak Power but Does Not Enhance Hamstring Isokinetic or Isometric Strength. Int. J. Sports Physiol. Perform. 2024, 19, 34–43. [Google Scholar] [CrossRef]
| Mean ± Standard Deviation | |
|---|---|
| Age (years) | 24.52 ± 3.74 |
| Height (cm) | 175.53 ± 5.33 |
| Weight (kg) | 72.06 ± 7.29 |
| BMI | 23.13 ± 2.57 |
| BHE | UHE | BNH | UNH | |
|---|---|---|---|---|
| BF50/ST50 | 0.81 | 0.82 | 0.76 | 0.94 |
| BF30/ST30 | 0.87 | 0.97 | 0.89 | 1.02 |
| BF50/BF30 | 0.93 | 0.91 | 0.86 | 0.98 |
| ST50/ST30 | 1.00 | 1.07 | 1.01 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Sugimoto, D.; Hirose, N. Neuromuscular Responses to Unilateral and Bilateral Execution of Eccentric Exercises: A Multidimensional sEMG Study. Sports 2025, 13, 364. https://doi.org/10.3390/sports13100364
You Y, Sugimoto D, Hirose N. Neuromuscular Responses to Unilateral and Bilateral Execution of Eccentric Exercises: A Multidimensional sEMG Study. Sports. 2025; 13(10):364. https://doi.org/10.3390/sports13100364
Chicago/Turabian StyleYou, Yanan, Dai Sugimoto, and Norikazu Hirose. 2025. "Neuromuscular Responses to Unilateral and Bilateral Execution of Eccentric Exercises: A Multidimensional sEMG Study" Sports 13, no. 10: 364. https://doi.org/10.3390/sports13100364
APA StyleYou, Y., Sugimoto, D., & Hirose, N. (2025). Neuromuscular Responses to Unilateral and Bilateral Execution of Eccentric Exercises: A Multidimensional sEMG Study. Sports, 13(10), 364. https://doi.org/10.3390/sports13100364






