Isocaloric High-Intensity Interval and Circuit Training Increases Excess Post-Exercise Oxygen Consumption and Lipid Oxidation Compared to Moderate-Intensity Continuous Training
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Experimental Design
2.2. Participants
2.3. Anthropometric Assessments
2.4. Cardiopulmonary Exercise Test (CPET)
2.5. Determination of Ventilatory Threshold (VT) and Respiratory Compensation Point (RCP)
2.6. Calculation of Substrate Metabolism
2.7. Monitoring of Internal Training Load
2.8. Isocaloric Exercise Protocols
2.9. Equalization of Training Overload
2.10. Statistical Analysis
3. Results
3.1. Comparison Between Training Overload and Isocaloric Training Session
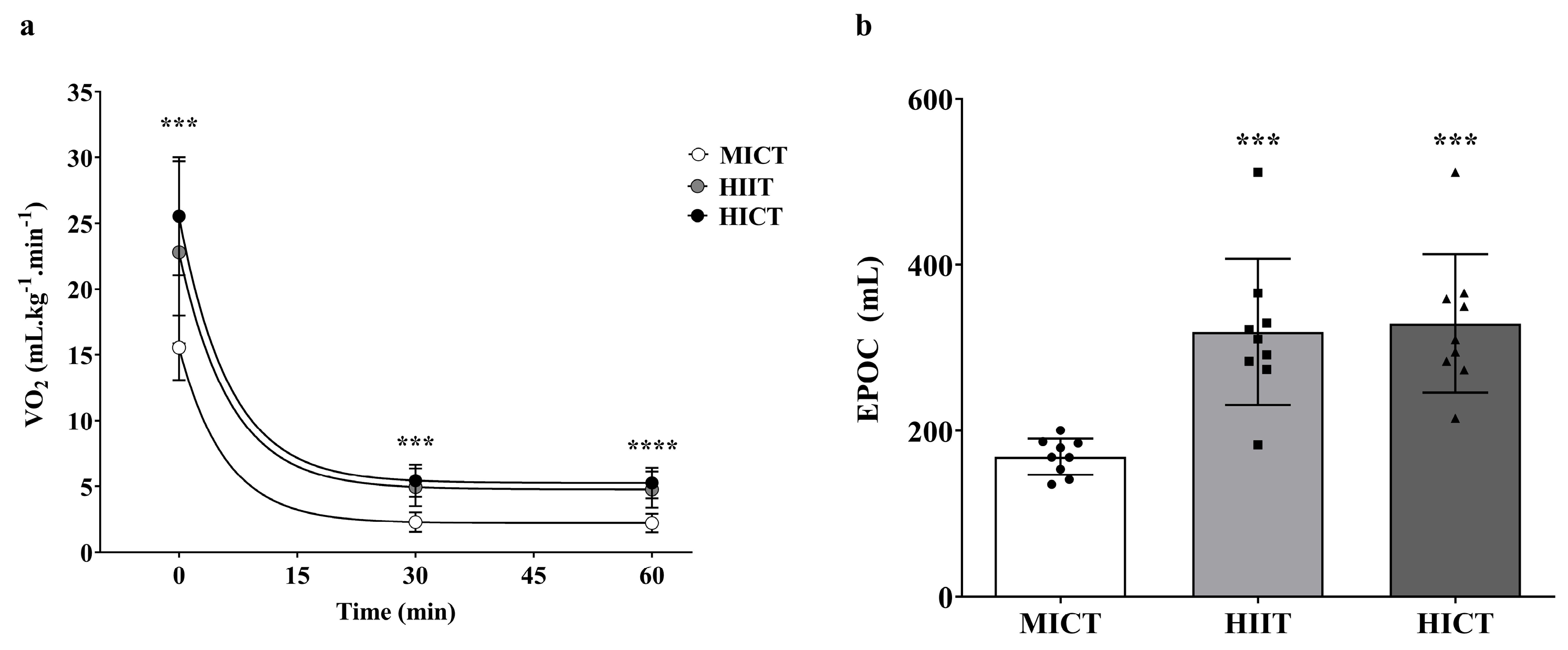
3.2. EPOC During MICT, HIIT, and HICT
3.3. Substrate Metabolism During and After MICT, HIIT and HICT
3.4. Total Substrate Metabolism During MICT, HIIT and HICT
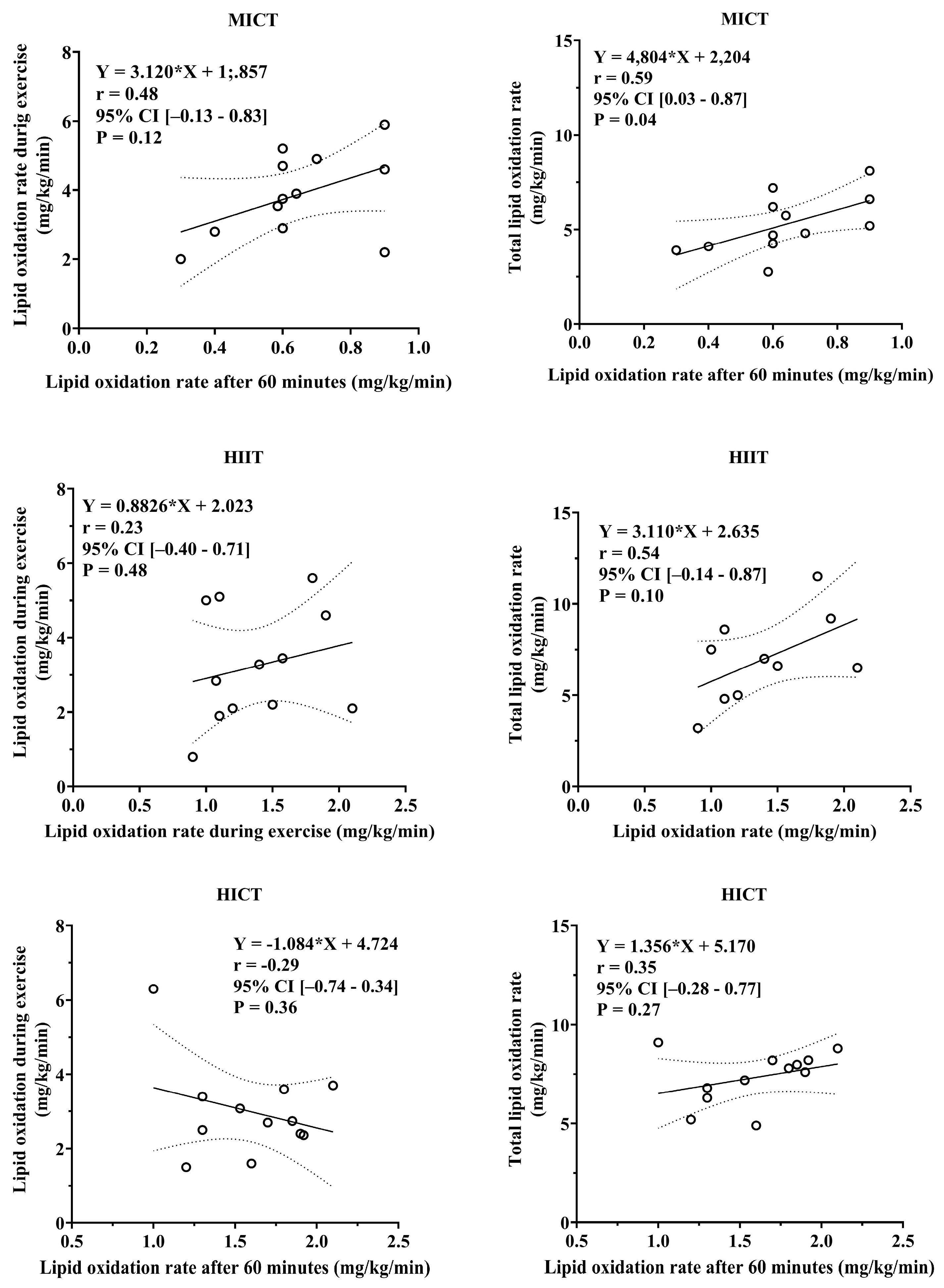
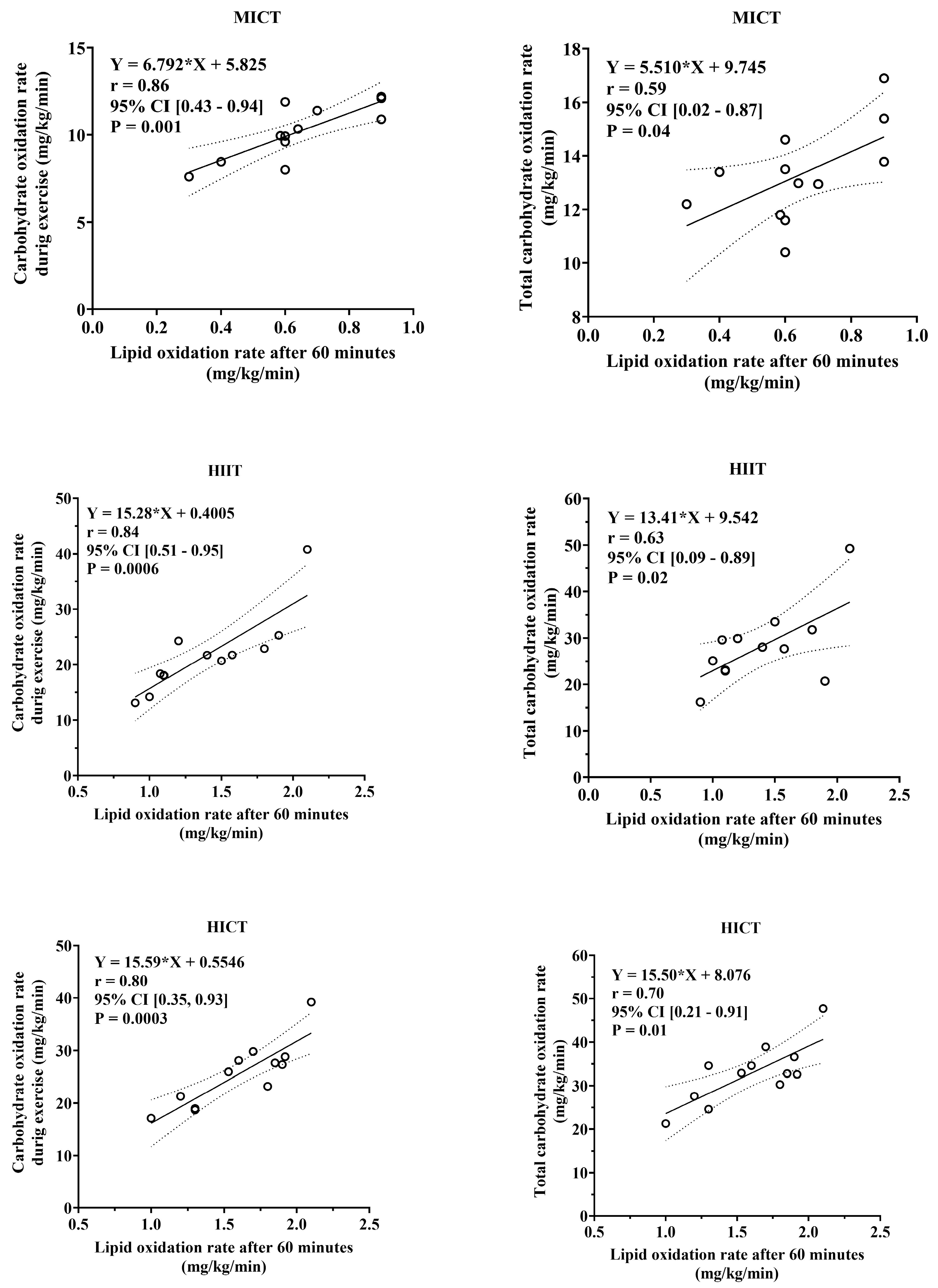
3.5. Association Between Total Carbohydrate and Lipid Oxidation During Exercise and Post-Exercise Lipid Utilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Average power |
| ATP | Adenosine triphosphate |
| BMI | Body mass index |
| BMR | Basal metabolic rate |
| CPET | Cardiopulmonary exercise test |
| EE | Energy expenditure |
| EPOC | Excess post-exercise oxygen consumption |
| FM | Fat mass |
| HICT | High-intensity circuit training |
| HIIT | High-intensity interval training |
| HR | Heart rate |
| kJ | kilojoules |
| LBM | Lean body mass |
| MICT | Moderate-intensity continuous training |
| One-Way | Unidirectional analysis of variance |
| ANOVA | |
| PAR-Q | Physical activity readiness questionnaire |
| PO | Power output |
| RCP | Respiratory compensation point |
| RER | Respiratory exchange ratio |
| RPE | Rating of perceived exertion |
| RT | Resistance training |
| SD | standard deviation |
| Two-Way | Bidirectional analysis of variance |
| ANOVA | |
| UNIVERSO | Salgado de Oliveira University |
| VCO2 | Carbon dioxide production |
| VE/VCO2 | Ventilatory equivalent for CO2 |
| VE/VO2 | Ventilatory equivalent for O2 |
| VO2 | Oxygen consumption |
| VT | Ventilatory threshold |
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exper. 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Willis, E.A.; Szabo-Reed, A.N.; Ptomey, L.T.; Honas, J.J.; Steger, F.L.; Washburn, R.A.; Donnelly, J.E. Energy Expenditure and Intensity of Group-Based High-Intensity Functional Training: A Brief Report. J. Phys. Act. Health 2019, 16, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.A.; Lee, D.-C.; Khan, A.; Wiesner, G.H.; Bauman, A.E.; Stamatakis, E.; Biddle, S.J.H. Muscle-strengthening exercise among 397,423 U.S. adults: Prevalence, correlates, and associations with health conditions. Am. J. Prev. Med. 2018, 55, 864–874. [Google Scholar] [CrossRef]
- DiPietro, L.; Buchner, D.M.; Marquez, D.X.; Pate, R.R.; Pescatello, L.S.; Whitt-Glover, M.C. New scientific basis for the 2018 U.S. Physical Activity Guidelines. J. Sport Health Sci. 2019, 8, 197–200. [Google Scholar] [CrossRef]
- Westerterp, K.R. Control of Energy Expenditure in Humans. [Updated 2022 Mar 21]. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278963 (accessed on 2 April 2023).
- Børsheim, E.; Bahr, R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003, 33, 1037–1060. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Sun, Y.; Park, H.Y.; Kim, S.-W.; Jung, H.; Park, S.-A.; Kim, J.; Lim, K. Comparison of energy consumption and excess post-exercise oxygen consumption according to Taekwondo Taegeuk Poomsae performance in Taekwondo players. Phys. Act. Nutr. 2023, 27, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism During Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Pilon, R.; Matos-Santos, L.; Matlez, M.P.; Rodrigues, G.; Amorim, F.; Lattari, E.; Farinatti, P.; Monteiro, W. Effects of Isocaloric Resistance, Aerobic, and Concurrent Exercise on Excess Postexercise Oxygen Consumption in Older Adults. J. Strength Cond. Res. 2024, 38, 755–761. [Google Scholar] [CrossRef]
- Brisebois, M.F.; Biggerstaff, K.D.; Nichols, D.L. Cardiorespiratory responses to acute bouts of high-intensity functional training and traditional exercise in physically active adults. J. Sports Med. Phys. Fit. 2022, 62, 199–206. [Google Scholar] [CrossRef]
- Sturdy, R.E.; Astorino, T.A. Post-exercise metabolic response to kettlebell complexes vs. high intensity functional training. Eur. J. Appl. Physiol. 2024, 124, 3755–3766. [Google Scholar] [CrossRef]
- Warren, A.; Howden, E.J.; Williams, A.D.; Fell, J.W.; Johnson, N.A. Postexercise fat oxidation: Effect of exercise duration, intensity, and modality. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Celik, O.; Koşar, S.N.; Korkusuz, F.; Bozkurt, M. Reliability and validity of the modified Conconi test on concept II rowing ergometers. J. Strength Cond. Res. 2005, 19, 871–877. [Google Scholar] [CrossRef]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Bondareva, E.A.; Parfenteva, O.I.; Troshina, E.A.; Ershova, E.V.; Mazurina, N.V.; Komshilova, K.A.; Kulemin, N.A.; Ahmetov, I.I. Agreement between bioimpedance analysis and ultrasound scanning in body composition assessment. Am. J. Hum. Biol. 2024, 36, e24001. [Google Scholar] [CrossRef]
- Marques-Neto, S.R.; Maior, A.S.; Maranhão Neto, G.A.; Santos, E.L. Analysis of heart rate deflection points to predict the anaerobic threshold by a computerized method. J. Strength Cond. Res. 2012, 26, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Péronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar] [PubMed]
- Foster, C. Monitoring training in athletes with reference to overtraining syndrome. Med. Sci. Sports Exerc. 1998, 30, 1164–1168. [Google Scholar] [CrossRef]
- Guio de Prada, V.; Ortega, J.F.; Ramirez-Jimenez, M.; Morales-Palomo, F.; Pallares, J.G.; Mora-Rodriguez, R. Training intensity relative to ventilatory thresholds determines cardiorespiratory fitness improvements in sedentary adults with obesity. Eur. J. Sport Sci. 2019, 19, 549–556. [Google Scholar] [CrossRef]
- Haddock, C.K.; Poston, W.S.; Heinrich, K.M.; Jahnke, S.A.; Jitnarin, N. The Benefits of High-Intensity Functional Training Fitness Programs for Military Personnel. Mil. Med. 2016, 181, e1508–e1514. [Google Scholar] [CrossRef]
- Viana, R.B.; de Lira, C.A.B.; Naves, J.P.A.; Coswig, V.S.; Del Vecchio, F.B.; Gentil, P. Tabata protocol: A review of its application, variations and outcomes. Clin. Physiol. Funct. Imaging 2019, 39, 1–8. [Google Scholar] [CrossRef]
- Marston, K.J.; Peiffer, J.J.; Newton, M.J.; Scott, B.R. A comparison of traditional and novel metrics to quantify resistance training. Sci. Rep. 2017, 7, 5606. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Wang, Z.; Wang, Y. Acute interval running induces greater excess post-exercise oxygen consumption and lipid oxidation than isocaloric continuous running in men with obesity. Sci. Rep. 2024, 14, 9178. [Google Scholar] [CrossRef]
- Panissa, V.L.G.; Fukuda, D.H.; Staibano, V.; Marques, M.; Franchini, E. Magnitude and duration of excess of post-exercise oxygen consumption between high-intensity interval and moderate-intensity continuous exercise: A systematic review. Obes. Rev. 2021, 22, e13099. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, C.; Cheng, L.; Wang, Y.; Peng, D.; Li, F.; Han, Y.; Wang, H. A comparative analysis of energy expenditure and substrate metabolism in male university students with overweight/obesity: Tabata vs HIIT and MICT. Front. Endocrinol. 2024, 15, 1323093. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Ulupınar, S.; Özbay, S.; Gençoğlu, C.; Ouergui, I.; Öget, F.; Kishalı, N.F.; Kıyıcı, F.; Yılmaz, H.H.; Ardigò, L.P. Evaluating bioenergetic pathway contributions from single to multiple sprints. Sci. Rep. 2024, 14, 27295. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Gomes, G.; Diniz Magalhães, C.O.; Queiroz, I.P.; de Andrade, J.A.; Garcia, B.C.C.; Pereira, R.R.d.S.; Costa, J.M.M.; Cassilhas, R.C.; Villela, D.C.; de Magalhães, F.C.; et al. Multiple Shorter High-Intensity Interval Exercise Sessions During the Day Result in Greater Energy Expenditure With Less Exertion Than a Longer Single Session: A Randomized Crossover Clinical Trial. Eur. J. Sport Sci. 2025, 25, e12302. [Google Scholar] [CrossRef]
- Jensen, T.E.; Richter, E.A. Regulation of glucose and glycogen metabolism during and after exercise. J. Physiol. 2012, 590, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Verdejo, R.; Bajpeyi, S.; Ravussin, E.; Galgani, J.E. Metabolic flexibility to lipid availability during exercise is enhanced in individuals with high insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E715–E722. [Google Scholar] [CrossRef] [PubMed]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.C.; Islam, H.; Hazell, T.J. Mechanistic and methodological perspectives on the impact of intense interval training on post-exercise metabolism. Scand. J. Med. Sci.Sports 2020, 30, 638–651. [Google Scholar] [CrossRef]
- Cunha, F.A.; Midgley, A.W.; McNaughton, L.R.; Farinatti, P.T. Effect of continuous and intermittent bouts of isocaloric cycling and running exercise on excess postexercise oxygen consumption. J. Sci. Med. Sport 2016, 19, 187–192. [Google Scholar] [CrossRef]
- Greer, B.K.; Sirithienthad, P.; Moffatt, R.J.; Marcello, R.T.; Panton, L.B. EPOC Comparison Between Isocaloric Bouts of Steady-State Aerobic, Intermittent Aerobic, and Resistance Training. Res. Q. Exerc. Sport 2015, 86, 190–195. [Google Scholar] [CrossRef] [PubMed]

| Variables | Healthy Trained Males (n = 12) |
|---|---|
| Age (years) | 30.4 ± 4.0 |
| Height (cm) | 175.7 ± 8.5 |
| Weight (kg) | 82.3 ± 8.6 |
| BMI (kg/m2) | 26.8 ± 1.6 |
| % FM | 8.6 ± 1.9 |
| VT (ml.kg.min−1) | 30.6 ± 5.2 |
| RCP (ml.kg.min−1) | 43.2 ± 6.1 |
| VO2max (ml.kg.min−1) | 52.9 ± 6.3 |
| VT (Watts) | 175.0 ± 15.1 |
| RCP (Watts) | 239.6 ± 19.8 |
| Max power (Watts) | 289.6 ± 24.9 |
| FC max (bpm) | 190.7 ± 5.5 |
| Period | Carbohydrate Oxidation Rate (mg/kg/min) | Lipid Oxidation Rate (mg/kg/min) | EE (cal/kg/min) | Percentage of Energy from Carbohydrate (%) | Percentage of Energy from Lipid (%) | |
|---|---|---|---|---|---|---|
| During exercise | MICT | 10.34 ± 1.77 | 3.90 ± 1.43 | 76.4 ± 11.01 | 55.06 ± 12.57 | 44.94 ± 12.57 |
| HIIT | 21.73 ± 8.50 ** | 3.28 ± 1.77 | 116.44 ± 35.97 ** | 74.62 ± 12.31 * | 25.38 ± 12.31 * | |
| HICT | 25.95 ± 6.75 **** | 3.08 ± 1.46 | 131.47 ± 26.13 ** | 78.49 ± 10.88 ** | 21.51 ± 10.88 ** | |
| 30 after exercise | MICT | 1.42 ± 0.58 | 0.61 ± 0.20 | 11.19 ± 3.71 | 50.31 ± 7.32 | 49.69 ± 7.32 |
| HIIT | 3.70 ± 1.04 **** | 1.08 ± 0.41 ** | 24.57 ± 7.00 *** | 60.79 ± 8.06 * | 39.21 ± 8.06 * | |
| HICT | 4.06 ± 1.03 **** | 1.20 ± 0.24 *** | 27.03 ± 6.16 *** | 59.77 ± 3.76 ** | 40.23 ± 3.75 ** | |
| 60 after exercise | MICT | 1.25 ± 0.41 | 0.64 ± 0.22 | 10.63 ± 3.48 | 46.22 ± 4.24 | 53.78 ± 4.24 |
| HIIT | 2.57 ± 0.81 *** | 1.42 ± 0.43 *** | 22.94 ± 6.61 *** | 45.55 ± 3.85 | 54.45 ± 3.85 | |
| HICT | 2.89 ± 0.78 **** | 1.54 ± 0.35 **** | 25.36 ± 5.64 **** | 45.76 ± 3.83 | 54.24 ± 3.83 |
| Total Substrate Metabolism | MICT | HIIT | HICT |
|---|---|---|---|
| Total lipid oxidation rate (mg/kg/min) | 5.16 ± 1.68 | 5.77 ± 2.26 | 5.81 ± 1.47 |
| Total lipid energy output (cal/kg/min) | 46.40 ± 15.10 | 51.90 ± 20.36 | 52.29 ± 13.26 |
| Total carbohydrate oxidation rate (mg/kg/min) | 12.98 ± 2.33 | 28.05 ± 9.69 ** | 32.92 ± 8.01 *** |
| Total carbohydrate energy output (cal/kg/min) | 51.93 ± 9.33 | 112.19 ± 38.75 ** | 131.68 ± 32.06 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faleiro, V.; Gurgel, A.V.; Guimarães, T.T.; Figueiredo, T.C.; Teixeira, F.G.; Jotta, B.; Monteiro, E.R.; Meirelles, A.G.; Caldas, C.C.A.; de Almeida, M.T.; et al. Isocaloric High-Intensity Interval and Circuit Training Increases Excess Post-Exercise Oxygen Consumption and Lipid Oxidation Compared to Moderate-Intensity Continuous Training. Sports 2025, 13, 355. https://doi.org/10.3390/sports13100355
Faleiro V, Gurgel AV, Guimarães TT, Figueiredo TC, Teixeira FG, Jotta B, Monteiro ER, Meirelles AG, Caldas CCA, de Almeida MT, et al. Isocaloric High-Intensity Interval and Circuit Training Increases Excess Post-Exercise Oxygen Consumption and Lipid Oxidation Compared to Moderate-Intensity Continuous Training. Sports. 2025; 13(10):355. https://doi.org/10.3390/sports13100355
Chicago/Turabian StyleFaleiro, Viviane, Alexandre V. Gurgel, Thiago T. Guimarães, Tiago C. Figueiredo, Felipe G. Teixeira, Bruno Jotta, Estêvão R. Monteiro, Alexandre G. Meirelles, Carla C. A. Caldas, Maicon T. de Almeida, and et al. 2025. "Isocaloric High-Intensity Interval and Circuit Training Increases Excess Post-Exercise Oxygen Consumption and Lipid Oxidation Compared to Moderate-Intensity Continuous Training" Sports 13, no. 10: 355. https://doi.org/10.3390/sports13100355
APA StyleFaleiro, V., Gurgel, A. V., Guimarães, T. T., Figueiredo, T. C., Teixeira, F. G., Jotta, B., Monteiro, E. R., Meirelles, A. G., Caldas, C. C. A., de Almeida, M. T., Castiglione, R. C., & Marques-Neto, S. R. (2025). Isocaloric High-Intensity Interval and Circuit Training Increases Excess Post-Exercise Oxygen Consumption and Lipid Oxidation Compared to Moderate-Intensity Continuous Training. Sports, 13(10), 355. https://doi.org/10.3390/sports13100355










