Open Water Swimming: Swimmers’ Kinematical and Neuromuscular Characterisation in 5 km Swim
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
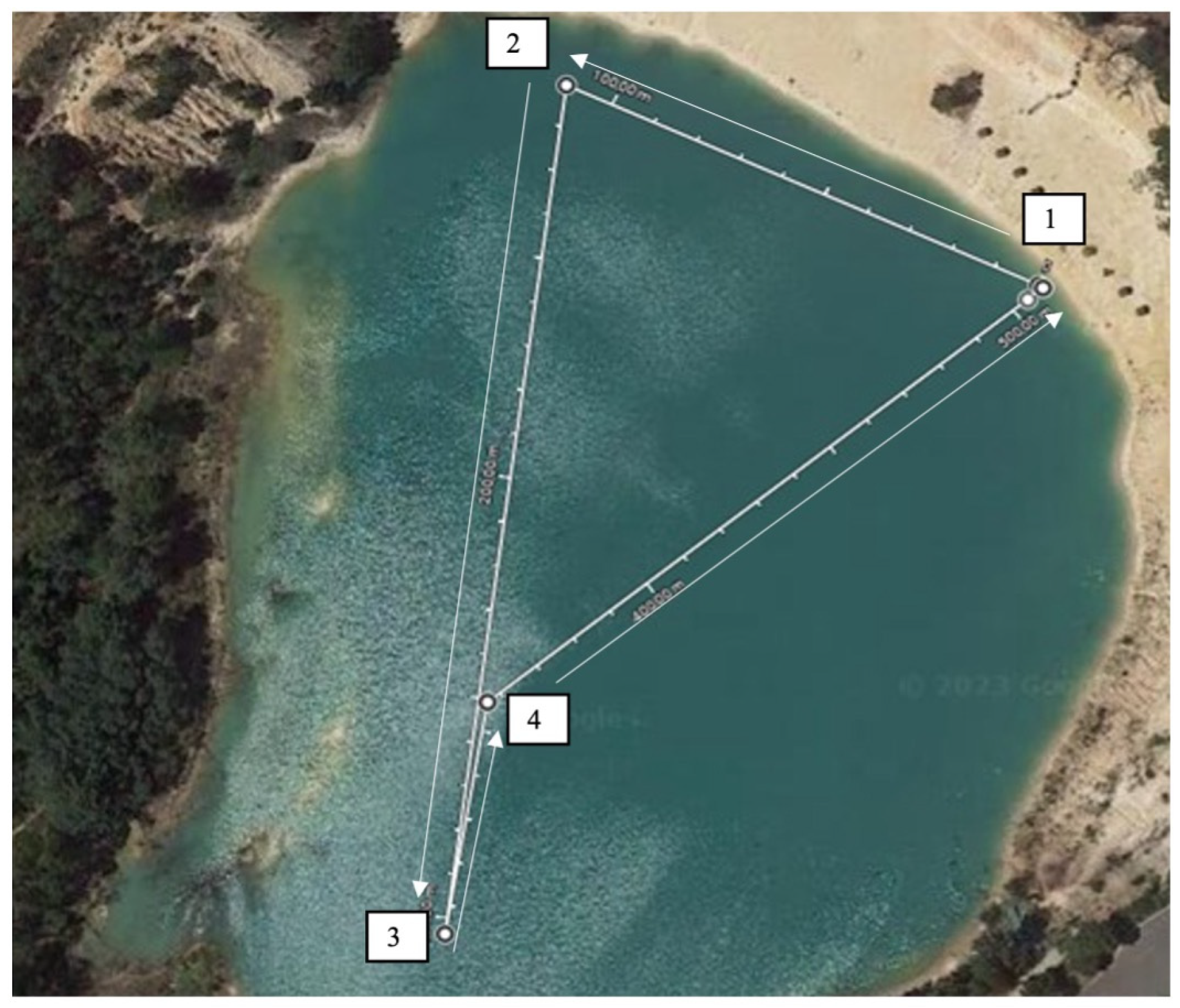
2.3. Design and Procedures
2.4. Statistical Analysis
3. Results
Eletromyography
4. Discussion
4.1. Kinematic
4.2. Neuromuscular Activity
4.3. Practical Applications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldassarre, R.; Bonifazi, M.; Zamparo, P.; Piacentini, M.F. Characteristics and Challenges of Open-Water Swimming Performance: A Review. Int. J. Sports Physiol. Perform. 2017, 12, 1275–1284. [Google Scholar] [CrossRef]
- Zacca, R.; Neves, V.; Da Silva Oliveira, T.; Soares, S.; Rama, L.M.P.L.; De Souza Castro, F.A.; Vilas-Boas, J.P.; Pyne, D.B.; Fernandes, R.J. 5 km front crawl in pool and open water swimming: Breath-by-breath energy expenditure and kinematic analysis. Eur. J. Appl. Physiol. 2020, 120, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- De Ioannon, G.; Cibelli, G.; Mignardi, S.; Antonelli, A.; Capranica, L.; Piacentini, M.F. Pacing and Mood Changes while Crossing the Adriatic Sea from Italy to Albania: A Case Study. Int. J. Sports Physiol. Perform. 2015, 10, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Matsuura, Y.; Baba, Y.; Hara, R. Thermal Sensation After the 10-km Open-Water Swimming in Cool Water Depends on the Skin’s Thermal Sensitivity Rather Than Core Temperature. Int. J. Sports Physiol. Perform. 2024, 19, 28–33. [Google Scholar] [CrossRef] [PubMed]
- López-Belmonte, Ó.; Ruiz-Navarro, J.J.; Gay, A.; Cuenca-Fernández, F.; Cejuela, R.; Arellano, R.; Cejuela, R. Swimming Performance in Elite Triathletes: Comparison Between Open Water and Pool Conditions. Scand. J. Med. Sci. Sports 2024, 34, e14702. [Google Scholar] [CrossRef]
- Pelarigo, J.G.; Greco, C.C.; Denadai, B.S.; Fernandes, R.J.; Vilas-Boas, J.P.; Pendergast, D.R. Do 5% changes around maximal lactate steady state lead to swimming biophysical modifications? Hum. Mov. Sci. 2016, 49, 258–266. [Google Scholar] [CrossRef]
- Rodriguez, L.; Veiga, S. Effect of the Pacing Strategies on the Open-Water 10-km World Swimming Championships Performances. Int. J. Sports Physiol. Perform. 2018, 13, 694–700. [Google Scholar] [CrossRef]
- Baldassarre, R.; Pennacchi, M.; La Torre, A.; Bonifazi, M.; Piacentini, M.F. Do the Fastest Open-Water Swimmers have A Higher Speed in Middle- and Long-Distance Pool Swimming Events? J. Funct. Morphol. Kinesiol. 2019, 4, 15. [Google Scholar] [CrossRef]
- Puce, L.; Chamari, K.; Marinelli, L.; Mori, L.; Bove, M.; Faelli, E.; Fassone, M.; Cotellessa, F.; Bragazzi, N.L.; Trompetto, C. Muscle Fatigue and Swimming Efficiency in Behind and Lateral Drafting. Front. Physiol. 2022, 13, 835766. [Google Scholar] [CrossRef]
- Puce, L.; Biz, C.; Ruaro, A.; Mori, F.; Bellofiore, A.; Nicoletti, P.; Bragazzi, N.L.; Ruggieri, P. Analysis of Kinematic and Muscular Fatigue in Long-Distance Swimmers. Life 2023, 13, 2129. [Google Scholar] [CrossRef]
- Cohen, R.C.Z.; Cleary, P.W.; Mason, B.R.; Pease, D.L. Studying the effects of asymmetry on freestyle swimming using smoothed particle hydrodynamics. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 271–284. [Google Scholar] [CrossRef]
- Figueiredo, P.; Pendergast, D.R.; Vilas-Boas, J.P.; Fernandes, R.J. Interplay of Biomechanical, Energetic, Coordinative, and Muscular Factors in a 200 m Front Crawl Swim. BioMed Res. Int. 2013, 2013, 897232. [Google Scholar] [CrossRef]
- Costill, D.; Kovaleski, J.; Porter, D.; Kirwan, J.; Fielding, R.; King, D. Energy Expenditure During Front Crawl Swimming: Predicting Success in Middle-Distance Events. Int. J. Sports Med. 1985, 6, 266–270. [Google Scholar] [CrossRef]
- Zamparo, P.; Cortesi, M.; Gatta, G. The energy cost of swimming and its determinants. Eur. J. Appl. Physiol. 2020, 120, 41–66. [Google Scholar] [CrossRef]
- Morais, J.E.; Barbosa, T.M.; Forte, P.; Bragada, J.A.; Castro, F.A.D.S.; Marinho, D.A. Stability analysis and prediction of pacing in elite 1500 m freestyle male swimmers. Sports Biomech. 2023, 22, 1496–1513. [Google Scholar] [CrossRef] [PubMed]
- Conceição, A.; Silva, A.J.; Barbosa, T.; Karsai, I.; Louro, H. Neuromuscular Fatigue during 200 M Breaststroke. J. Sports Sci. Med. 2014, 13, 200–210. [Google Scholar] [PubMed]
- Afsharipour, B.; Soedirdjo, S.; Merletti, R. Two-dimensional surface EMG: The effects of electrode size, interelectrode distance and image truncation. Biomed. Signal Process. Control 2019, 49, 298–307. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Konrad, P. The ABC of EMG: A Practical Introduction to Kinesiological Electromyography; Noraxon Inc.: Scottsdale, AZ, USA, 2005. [Google Scholar]
- Craig, A.; Pendeegast, D. Relationships of stroke rate, distance per stroke, and velocity in competitive swimming. Med. Sci. Sports 1979, 11, 278–283. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Zingg, M.A.; Rüst, C.A.; Rosemann, T.; Lepers, R.; Knechtle, B. Analysis of swimming performance in FINA World Cup long-distance open water races. Extrem. Physiol. Med. 2014, 3, 2. [Google Scholar] [CrossRef]
- Craig, A.B.; Skehan, P.L.; Pawelczyk, J.A.; Boomer, W.L. Velocity, stroke rate, and distance per stroke during elite swimming competition. Med. Sci. Sports Exerc. 1985, 17, 625–634. [Google Scholar] [CrossRef]
- Oliveira, T.; Fernandes, R.; Vilas-Boas, J. Caraterização Biofísica dos 5000 m Crol em Águas Abertas. Master’s Thesis, University of Porto, Porto, Portugal, 2018. [Google Scholar]
- Stirn, I.; Jarm, T.; Kapus, V.P.; Strojnik, V. Evaluation of mean power spectral frequency of EMG signal during 100 metre crawl. Eur. J. Sport Sci. 2011, 13, 164–173. [Google Scholar] [CrossRef]
- Pink, M.; Perry, J.; Browne, A.; Scovazzo, M.L.; Kerrigan, J. The normal shoulder during freestyle swimming: An electromyographic and cinematographic analysis of twelve muscles. Am. J. Sports Med. 1991, 19, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.; Figueiredo, P.; Daly, D. Electromyography in the four competitive swimming strokes: A systematic review. J. Electromyogr. Kinesiol. 2015, 25, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Rouard, A.H.; Clarys, J.P. Cocontraction in the elbow and shoulder muscles during rapid cyclic movements in an aquatic environment. J. Electromyogr. Kinesiol. 1995, 5, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lauer, J.; Figueiredo, P.; Vilas-Boas, J.P.; Fernandes, R.J.; Rouard, A.H. Phase-dependence of elbow muscle coactivation in front crawl swimming. J. Electromyogr. Kinesiol. 2013, 23, 820–825. [Google Scholar] [CrossRef]
- Laudner, K.G.; Williams, J.G. The relationship between latissimus dorsi stiffness and altered scapular kinematics among asymptomatic collegiate swimmers. Phys. Ther. Sport 2013, 14, 50–53. [Google Scholar] [CrossRef]
- Lim, B.; Swafford, A.; Conroy, K.; Mercer, J. Shoulder Muscle Activity While Swimming in Different Wetsuits and Across Different Paces. Int. J. Exerc. Sci. 2023, 16, 172–181. [Google Scholar] [CrossRef]
- Ikuta, Y.; Matsuda, Y.; Yamada, Y.; Kida, N.; Oda, S.; Moritani, T. Relationship between decreased swimming velocity and muscle activity during 200-m front crawl. Eur. J. Appl. Physiol. 2012, 112, 3417–3429. [Google Scholar] [CrossRef]
- Murphy, M.; Polston, K.; Carroll, M.; Alm, J. Heat injury in open-water swimming: A narrative review. Curr. Sports Med. Rep. 2021, 20, 193–198. [Google Scholar] [CrossRef]
- Fernández-Galván, L.M.; Alcain Sein, J.; López-Nuevo, C.; Sánchez-Sierra, A.; Ladrián-Maestro, A.; Sánchez-Infante, J. Injury Patterns and Frequency in Swimming: A Systematic Review. Appl. Sci. 2025, 15, 1643. [Google Scholar] [CrossRef]
- De Martino, I.; Rodeo, S.A. The Swimmer’s Shoulder: Multi-directional Instability. Curr. Rev. Musculoskelet. Med. 2018, 11, 167–171. [Google Scholar] [CrossRef]
| 1000 m | 2000 m | 3000 m | 4000 m | 5000 m | |||
|---|---|---|---|---|---|---|---|
| Kinematic Variables | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | F-Ratio (p) | η2 |
| v [m/s] | 1.39 ± 0.18 | 1.38 ± 0.15 | 1.38 ± 0.14 | 1.37 ± 0.16 | 1.39 ± 0.18 | 1.94 (0.18) | 0.19 |
| SR [cycles-min−1] | 31.4 ± 3.04 | 31.1 ± 3.88 | 31.2 ± 3.29 f | 32.1 ± 2.76 | 33.0 ± 3.36 | 1.54 (0.24) | 0.16 |
| SL [m-cycle−1] | 2.72 ± 0.35 c | 2.67 ± 0.34 d | 2.65 ± 0.33 e | 2.60 ± 0.33 | 2.58 ± 0.40 | 3.34 (0.06) * | 0.29 |
| SI [m2s−1cycle−1] | 3.90 ± 0.82 abc | 3.71 ± 0.78 | 3.68 ± 0.75 | 3.61 ± 0.83 | 3.64 ± 0.94 | 3.29 (0.08) * | 0.29 |
| Distance | 1000 m | 2000 m | 3000 m | 4000 m | 5000 m | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscles | Phase | Mean ± SD | p-Value | d | Mean ± SD | p-Value | d | Mean ± SD | p-Value | d | Mean ± SD | p-Value | d | Mean ± SD | p-Value | d |
| UT | UW | 14.96 ± 15.09 | 0.06 | −0.78 | 14.74 ± 6.78 | 0.02 * | −1.15 | 15.44 ± 9.89 | 0.02* | −1.23 | 13.92 ± 9.08 | 0.01* | −1.22 | 13.25 ± 7.32 | 0.00 * | −1.41 |
| REC | 37.46 ± 38.09 | 32.18 ± 20.29 | 34.28 ± 19.28 | 30.73 ± 17.19 | 31.25 ± 16.57 | |||||||||||
| PD | UW | 16.98 ± 4.63 | 0.51 | −0.41 | 12.58 ± 8.59 | 0.89 | 0.31 | 8.93 ± 4.99 | 0.69 | 0.03 | 11.86 ± 6.77 | 0.97 | −0.11 | 11.60 ± 7.80 | 0.97 | −0.16 |
| REC | 19.56 ± 7.52 | 10.41 ± 4.63 | 8.77 ± 6.77 | 12.77 ± 9.29 | 13.13 ± 10.75 | |||||||||||
| AD | UW | 6.55 ± 5.85 | 0.01 * | −1.16 | 4.72 ± 3.79 | 0.04 * | −1.08 | 4.88 ± 3.99 | 0.04 * | 1.07 | 3.49 ± 2.20 | 0.02 * | −0.98 | 3.75 ± 2.58 | 0.03 * | −0.93 |
| REC | 16.46 ± 10.55 | 11.84 ± 8.54 | 12.22 ± 8.89 | 9.47 ± 8.36 | 9.37 ± 8.14 | |||||||||||
| BB | UW | 11.55 ± 3.40 | 0.31 | −0.55 | 11.01 ± 4.15 | 0.31 | −0.49 | 10.43 ± 3.24 | 0.17 | −0.79 | 10.04 ± 2.78 | 0.20 | −0.69 | 10.03 ± 2.61 | 0.27 | −0.67 |
| REC | 15.31 ± 9.09 | 13.84 ± 7.13 | 15.52 ± 9.64 | 14.25 ± 8.11 | 13.19 ± 6.19 | |||||||||||
| TB | UW | 22.80 ± 7.10 | 0.00 * | 2.02 | 21.19 ± 7.53 | 0.00 * | 1.84 | 19.71 ± 6.87 | 0.00 * | 1.74 | 18.80 ± 8.09 | 0.01 * | 1.56 | 19.16 ± 9.09 | 0.03 * | 1.24 |
| REC | 10.22 ± 5.24 | 8.97 ± 5.60 | 9.04 ± 5.26 | 8.52 ± 4.60 | 9.87 ± 5.44 | |||||||||||
| PM | UW | 15.20 ± 8.33 | 0.12 | 0.74 | 13.13 ± 6.14 | 0.06 | 0.93 | 13.30 ± 6.12 | 0.09 | 0.86 | 12.12 ± 5.18 | 0.15 | 0.77 | 12.02 ± 5.26 | 0.45 | 0.30 |
| REC | 9.21 ± 7.97 | 8.28 ± 4.13 | 8.63 ± 4.71 | 8.33 ± 4.63 | 10.12 ± 7.12 | |||||||||||
| LD | UW | 10.51 ± 4.36 | 0.00 * | 2.34 | 9.87 ± 4.67 | 0.00 * | 2.01 | 9.75 ± 4.48 | 0.00 * | 2.10 | 9.00 ± 4.04 | 0.01 * | 1.74 | 8.91 ± 3.91 | 0.01 * | 1.57 |
| REC | 2.96 ± 1.35 | 2.94 ± 1.37 | 2.77 ± 1.50 | 3.30 ± 2.26 | 3.83 ± 2.38 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição, A.; Marinho, D.; Stastny, J.; Gonçalves, C.; Freitas, J.; da Costa-Machado, R.; Louro, H. Open Water Swimming: Swimmers’ Kinematical and Neuromuscular Characterisation in 5 km Swim. Sports 2025, 13, 335. https://doi.org/10.3390/sports13100335
Conceição A, Marinho D, Stastny J, Gonçalves C, Freitas J, da Costa-Machado R, Louro H. Open Water Swimming: Swimmers’ Kinematical and Neuromuscular Characterisation in 5 km Swim. Sports. 2025; 13(10):335. https://doi.org/10.3390/sports13100335
Chicago/Turabian StyleConceição, Ana, Daniel Marinho, Jan Stastny, Carlos Gonçalves, João Freitas, Renato da Costa-Machado, and Hugo Louro. 2025. "Open Water Swimming: Swimmers’ Kinematical and Neuromuscular Characterisation in 5 km Swim" Sports 13, no. 10: 335. https://doi.org/10.3390/sports13100335
APA StyleConceição, A., Marinho, D., Stastny, J., Gonçalves, C., Freitas, J., da Costa-Machado, R., & Louro, H. (2025). Open Water Swimming: Swimmers’ Kinematical and Neuromuscular Characterisation in 5 km Swim. Sports, 13(10), 335. https://doi.org/10.3390/sports13100335








