Menstrual Dysfunction in Adolescent Female Athletes
Abstract
1. Introduction
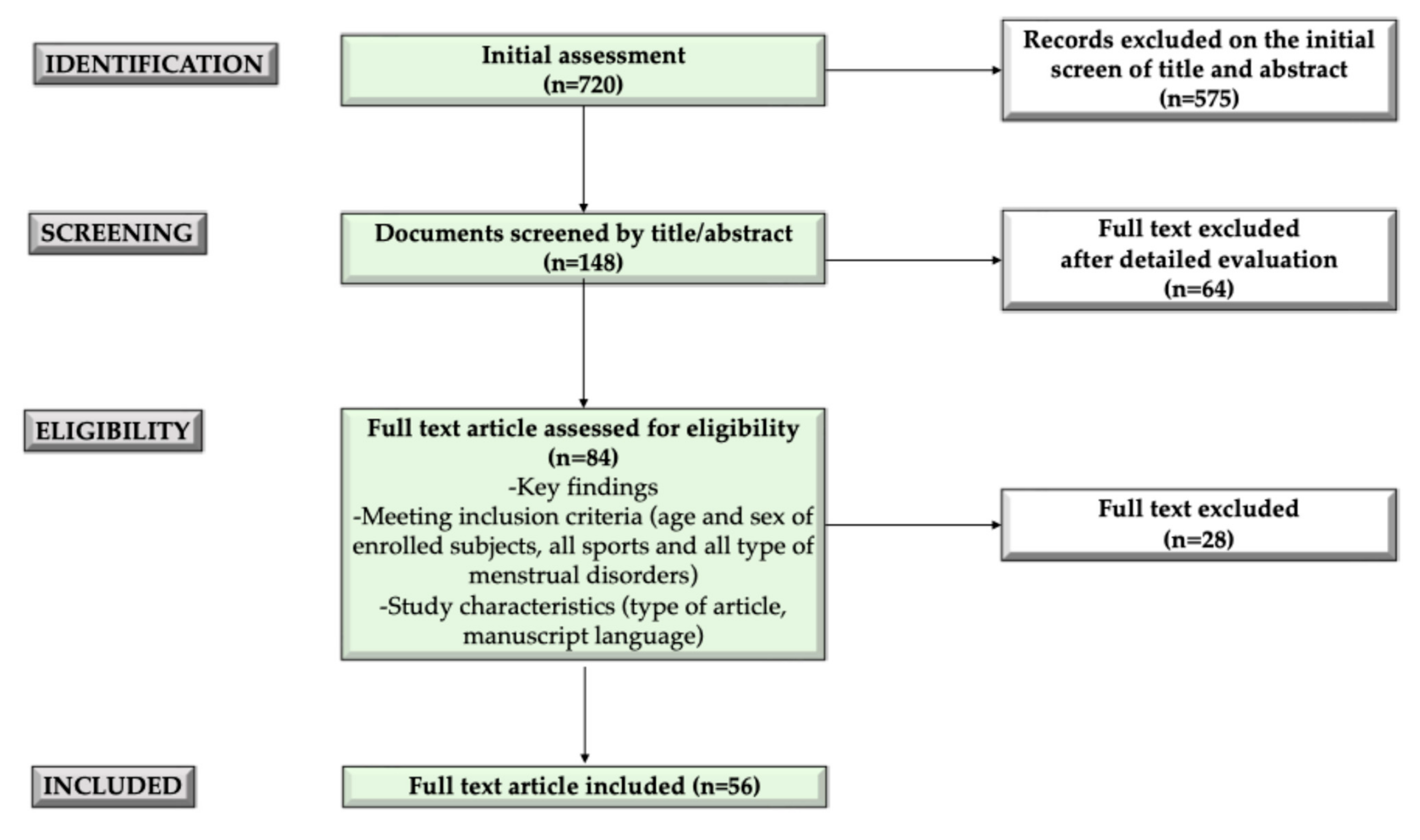
2. Methods
3. Menstrual Cycle and Menstrual Dysfunction in Adolescents
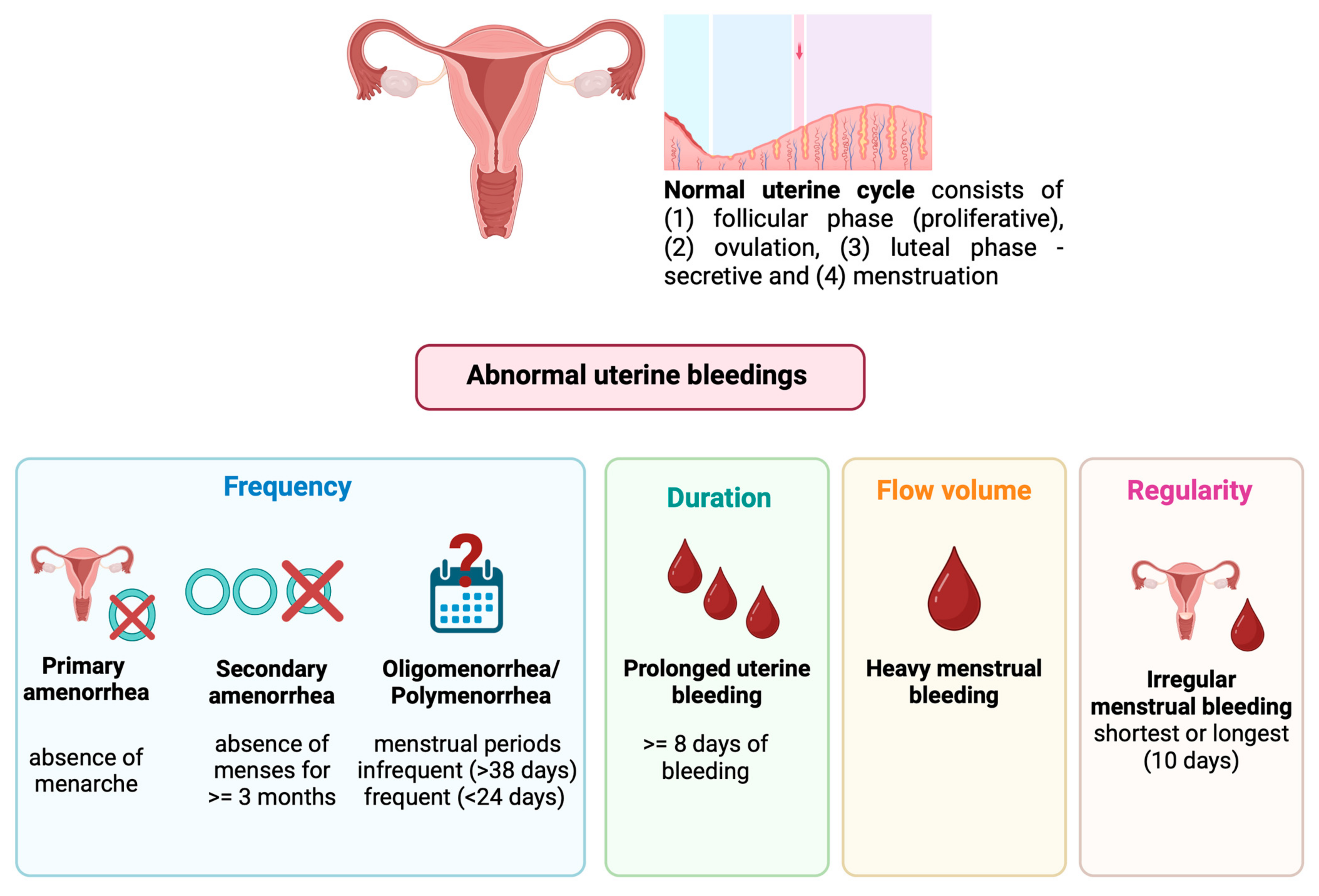
3.1. The Normal Menstrual Cycle
3.2. Menstrual Dysfunction in Adolescents
3.3. The Evaluation of Menstrual Dysfunction
4. The Interplay between Exercise and the Menstrual Cycle
5. Menstrual Dysfunction in Adolescent Female Athletes
5.1. Menstrual Dysfunction across Sports
5.2. Impact of Training Program on Menstrual Dysfunction
6. Limits
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abreu, A.P.; Kaiser, U.B. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Buyken, A.E.; Shi, L.; Karaolis-Danckert, N.; Kroke, A.; Wudy, S.A.; Degen, G.H.; Remer, T. Beyond overweight: Nutrition as an important lifestyle factor influencing timing of puberty. Nutr. Rev. 2012, 70, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, K. Menses Requires Energy: A Review of How Disordered Eating, Excessive Exercise, and High Stress Lead to Menstrual Irregularities. Clin. Ther. 2020, 42, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5-17 years: Summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 141. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc. Health 2020, 4, 23–35. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. RBE 2018, 16, 22. [Google Scholar] [CrossRef]
- Morrison, A.E.; Fleming, S.; Levy, M.J. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin. Endocrinol. 2021, 95, 229–238. [Google Scholar] [CrossRef]
- Roupas, N.; Georgopoulos, N. Menstrual function in sports. HORMONES 2011, 10, 104–116. [Google Scholar] [CrossRef]
- Redman, L.M.; Loucks, A.B. Menstrual Disorders in Athletes. Sports Med. 2005, 35, 747–755. [Google Scholar] [CrossRef]
- Chapa, D.A.N.; Johnson, S.N.; Richson, B.N.; Bjorlie, K.; Won, Y.Q.; Nelson, S.V.; Ayres, J.; Jun, D.; Forbush, K.T.; Christensen, K.A.; et al. Eating-disorder psychopathology in female athletes and non-athletes: A meta-analysis. Int. J. Eat. Disord. 2022, 55, 861–885. [Google Scholar] [CrossRef]
- Coelho, A.R.; Cardoso, G.; Brito, M.E.; Gomes, I.N.; Cascais, M.J. The Female Athlete Triad/Relative Energy Deficiency in Sports (RED-S). Rev. Bras. Ginecol. Obstet. Rev. Fed. Bras. Soc. Ginecol. Obstet. 2021, 43, 395–402. [Google Scholar] [CrossRef]
- Gould, R.J.; Ridout, A.J.; Newton, J.L. Relative Energy Deficiency in Sport (RED-S) in Adolescents—A Practical Review. Int. J. Sports Med. 2023, 44, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, H.; Sagar, S.S.; Stenling, A. Fear of failure, psychological stress, and burnout among adolescent athletes competing in high level sport. Scand. J. Med. Sci. Sports 2017, 27, 2091–2102. [Google Scholar] [CrossRef]
- Meczekalski, B.; Katulski, K.; Czyzyk, A.; Podfigurna-Stopa, A.; Maciejewska-Jeske, M. Functional hypothalamic amenorrhea and its influence on women’s health. J. Endocrinol. Investig. 2014, 37, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Committee on Adolescent Health Care. Committee Opinion No. 651: Menstruation in Girls and Adolescents Using the Menstrual Cycle as a Vital Sign. Obstet. Gynecol. 2015, 126, e143–e146. [Google Scholar] [CrossRef]
- Foster, C.; Al-Zubeidi, H. Menstrual Irregularities. Pediatr. Ann. 2018, 47, 23–28. [Google Scholar] [CrossRef]
- Klusmann, H.; Eisenlohr-Moul, T.; Baresich, K.; Schmalenberger, K.M.; Girdler, S.; Andersen, E. Analyzing the atypical–Methods for studying the menstrual cycle in adolescents. Psychoneuroendocrinology 2023, 158, 106389. [Google Scholar] [CrossRef]
- Yaşa, C.; Güngör Uğurlucan, F. Approach to Abnormal Uterine Bleeding in Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2020, 12 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Kabra, R.; Fisher, M. Abnormal uterine bleeding in adolescents. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101185. [Google Scholar] [CrossRef]
- Rivas Paz, M.; Torres Mendoza, B.M.; Torres Castillo, N. Age of the onset of menarche and its complications: A literature review. Int. J. Gynecol. Obstet. 2023, 162, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Itriyeva, K. The normal menstrual cycle. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101183. [Google Scholar] [CrossRef] [PubMed]
- Gunn, H.M.; Tsai, M.C.; McRae, A.; Steinbeck, K.S. Menstrual Patterns in the First Gynecological Year: A Systematic Review. J. Pediatr. Adolesc. Gynecol. 2018, 31, 557–565.e6. [Google Scholar] [CrossRef]
- Carlson, L.J.; Shaw, N.D. Development of Ovulatory Menstrual Cycles in Adolescent Girls. J. Pediatr. Adolesc. Gynecol. 2019, 32, 249–253. [Google Scholar] [CrossRef]
- Gray, S.H. Menstrual Disorders. Pediatr. Rev. 2013, 34, 6–18. [Google Scholar] [CrossRef]
- Balen, A.H.; Tamblyn, J.; Skorupskaite, K.; Munro, M.G. A comprehensive review of the new FIGO classification of ovulatory disorders. Hum. Reprod. Update 2024, 30, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, S.; Mok, E.; Frei, J.; Henderson, M.; Rahme, E.; Dasgupta, K.; Nakhla, M. Associations of Diabetes-related and Health-related Quality of Life with Glycemic Levels in Adolescents With Type 1 Diabetes Preparing to Transition to Adult Care. Can. J. Diabetes 2023, 47, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Seppä, S.; Kuiri-Hänninen, T.; Holopainen, E.; Voutilainen, R. MANAGEMENT OF ENDOCRINE DISEASE: Diagnosis and management of primary amenorrhea and female delayed puberty. Eur. J. Endocrinol. 2021, 184, R225–R242. [Google Scholar] [CrossRef]
- Gimunová, M.; Paulínyová, A.; Bernaciková, M.; Paludo, A.C. The Prevalence of Menstrual Cycle Disorders in Female Athletes from Different Sports Disciplines: A Rapid Review. Int. J. Environ. Res. Public Health 2022, 19, 14243. [Google Scholar] [CrossRef]
- Ko, J.K.Y.; Lao, T.T.; Cheung, V.Y.T. Pictorial Blood Loss Assessment Chart for Evaluating Heavy Menstrual Bleeding in Asian Women. Hong Kong Med. J. 2021, 27, 399–404. Available online: https://www.hkmj.org/abstracts/v27n6/399.htm (accessed on 27 May 2024). [CrossRef]
- Sokkary, N.A.; Venkateswaran, L.; Dietrich, J.E.; Teruya, J. Platelet function disorders and menorrhagia in adolescents: A review of laboratory diagnosis. J. Pediatr. Adolesc. Gynecol. 2012, 2, 233–237. [Google Scholar] [CrossRef]
- Bennett, A.R.; Gray, S.H. What to do when she’s bleeding through: The recognition, evaluation, and management of abnormal uterine bleeding in adolescents. Curr. Opin. Pediatr. 2014, 26, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Madeira, T.; Gama, A. Menstrual cycle among adolescents: Girls’ awareness and influence of age at menarche and overweight. Rev. Paul. Pediatr. 2022, 40, e2020494. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Berz, K.; McCambridge, T. Amenorrhea in the Female Athlete: What to Do and When to Worry. Pediatr. Ann. 2016, 45, e97–e102. [Google Scholar] [CrossRef]
- Cravo, R.M.; Frazao, R.; Perello, M.; Osborne-Lawrence, S.; Williams, K.W.; Zigman, J.M.; Vianna, C.; Elias, C.F. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS ONE 2013, 8, e58698. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Dominguez, C.E.; Yen, S.S. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 1998, 83, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.E.; Laughlin, G.A.; Nelson, J.C.; Yen, S.S. Altered binding of serum thyroid hormone to thyroxine-binding globulin in women with functional hypothalamic amenorrhea. Fertil. Steril. 1997, 68, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyn, K.; Smolinska, N.; Kiezun, M.; Szeszko, K.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Kaminski, T. Adiponectin: A New Regulator of Female Reproductive System. Int. J. Endocrinol. 2018, 2018, 7965071. [Google Scholar] [CrossRef]
- Męczekalski, B.; Niwczyk, O.; Battipaglia, C.; Troia, L.; Kostrzak, A.; Bala, G.; Maciejewska-Jeske, M.; Genazzani, A.D.; Luisi, S. Neuroendocrine disturbances in women with functional hypothalamic amenorrhea: An update and future directions. Endocrine 2024, 84, 769–785. [Google Scholar] [CrossRef]
- Kluge, M.; Schüssler, P.; Schmidt, D.; Uhr, M.; Steiger, A. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J. Clin. Endocrinol. Metab. 2012, 97, E448–E451. [Google Scholar] [CrossRef]
- Russell, M.; Stark, J.; Nayak, S.; Miller, K.K.; Herzog, D.B.; Klibanski, A.; Misra, M. Peptide YY in adolescent athletes with amenorrhea, eumenorrheic athletes and non-athletic controls. Bone 2009, 45, 104–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbagallo, F.; Cannarella, R.; Garofalo, V.; Marino, M.; La Vignera, S.; Condorelli, R.A.; Tiranini, L.; Nappi, R.E.; Calogero, A.E. The Role of Irisin throughout Women’s Life Span. Biomedicines 2023, 11, 3260. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef]
- Notaristefano, G.; Merola, A.; Scarinci, E.; Ubaldi, N.; Ranalli, M.; Tropea, A.; Diterlizzi, A.; Fabozzi, S.M.; Alesiani, O.; Silvestrini, A.; et al. Circulating irisin levels in functional hypothalamic amenorrhea: A new bone damage index? A pilot study. Endocrine 2022, 77, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Pauli, S.A.; Berga, S.L. Athletic amenorrhea: Energy deficit or psychogenic challenge? Ann. N. Y. Acad. Sci. 2010, 1205, 33–38. [Google Scholar] [CrossRef]
- Maïmoun, L.; Georgopoulos, N.A.; Sultan, C. Endocrine disorders in adolescent and young female athletes: Impact on growth, menstrual cycles, and bone mass acquisition. J. Clin. Endocrinol. Metab. 2014, 99, 4037–4050. [Google Scholar] [CrossRef]
- Martin, D.; Sale, C.; Cooper, S.B.; Elliott-Sale, K.J. Period Prevalence and Perceived Side Effects of Hormonal Contraceptive Use and the Menstrual Cycle in Elite Athletes. Int. J. Sports Physiol. Perform. 2018, 13, 926–932. [Google Scholar] [CrossRef]
- Dusek, T. Influence of high intensity training on menstrual cycle disorders in athletes. Croat. Med. J. 2001, 42, 79–82. [Google Scholar]
- Meng, K.; Qiu, J.; Benardot, D.; Carr, A.; Yi, L.; Wang, J.; Liang, Y. The risk of low energy availability in Chinese elite and recreational female aesthetic sports athletes. J. Int. Soc. Sports Nutr. 2020, 17, 13. [Google Scholar] [CrossRef]
- Wodarska, M.; Witkoś, J.; Drosdzol-Cop, A.; Dąbrowska, J.; Dąbrowska-Galas, M.; Hartman, M.; Plinta, R.; Skrzypulec-Plinta, V. Menstrual cycle disorders in female volleyball players. J. Obstet. Gynaecol. 2013, 33, 484–488. [Google Scholar] [CrossRef]
- Haakonssen, E.C.; Martin, D.T.; Jenkins, D.G.; Burke, L.M. Race Weight: Perceptions of Elite Female Road Cyclists. Int. J. Sports Physiol. Perform. 2015, 10, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hoch, A.Z.; Stavrakos, J.E.; Schimke, J.E. Prevalence of Female Athlete Triad Characteristics in a Club Triathlon Team. Arch. Phys. Med. Rehabil. 2007, 88, 681–682. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Coelho, G.M.; de Farias, M.L.F.; de Mendonça, L.M.C.; de Mello, D.B.; Lanzillotti, H.S.; Ribeiro, B.G.; de Abreu Soares, E. The prevalence of disordered eating and possible health consequences in adolescent female tennis players from Rio de Janeiro, Brazil. Appetite 2013, 64, 39–47. [Google Scholar] [CrossRef]
- De Souza, M.J.; Lee, D.K.; VanHeest, J.L.; Scheid, J.L.; West, S.L.; Williams, N.I. Severity of energy-related menstrual disturbances increases in proportion to indices of energy conservation in exercising women. Fertil. Steril. 2007, 88, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.C.; Williams, N.I.; Scheid, J.L.; Toombs, R.J.; De Souza, M.J. The association of a high drive for thinness with energy deficiency and severe menstrual disturbances: Confirmation in a large population of exercising women. Int. J. Sport. Nutr. Exerc. Metab. 2011, 21, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Deligeoroglou, E.; Tsimaris, P. Menstrual disturbances in puberty. Best. Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Barrack, M.T.; Rauh, M.J.; Nichols, J.F. Prevalence of and Traits Associated with Low BMD among Female Adolescent Runners. Med. Sci. Sports Exerc. 2008, 40, 2015–2021. [Google Scholar] [CrossRef]
- Thompson, S.H. Characteristics of the female athlete triad in collegiate cross-country runners. J. Am. Coll. Health J. ACH 2007, 56, 129–136. [Google Scholar] [CrossRef]
- Beals, K.A. Eating behaviors, nutritional status, and menstrual function in elite female adolescent volleyball players. J. Am. Diet. Assoc. 2002, 102, 1293–1296. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Torii, S.; Yamasawa, F.; Iwamoto, J.; Otsuka, T.; Goto, H.; Kusakabe, T.; Matsumoto, H.; Akama, T. Bone parameters of elite athletes with oligomenorrhea and prevalence seeking medical attention: A cross-sectional study. J. Bone Miner. Metab. 2021, 39, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Helge, E.W.; Kanstrup, I.L. Bone density in female elite gymnasts: Impact of muscle strength and sex hormones. Med. Sci. Sports Exerc. 2002, 34, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M.J.; Barrack, M.; Nichols, J.F. Associations between the female athlete triad and injury among high school runners. Int. J. Sports Phys. Ther. 2014, 9, 948–958. [Google Scholar] [PubMed]
- Feldmann, J.M.; Belsha, J.P.; Eissa, M.A.; Middleman, A.B. Female Adolescent Athletes’ Awareness of the Connection between Menstrual Status and Bone Health. J. Pediatr. Adolesc. Gynecol. 2011, 24, 311–314. [Google Scholar] [CrossRef]
- Czajkowska, M.; Drosdzol-Cop, A.; Gałązka, I.; Naworska, B.; Skrzypulec-Plinta, V. Menstrual Cycle and the Prevalence of Premenstrual Syndrome/Premenstrual Dysphoric Disorder in Adolescent Athletes. J. Pediatr. Adolesc. Gynecol. 2015, 28, 492–498. [Google Scholar] [CrossRef]
- Cristina-Souza, G.; Santos-Mariano, A.C.; Souza-Rodrigues, C.C.; Osiecki, R.; Silva, S.F.; Lima-Silva, A.E.; De Oliveira, F.R. Menstrual cycle alters training strain, monotony, and technical training length in young. J. Sports Sci. 2019, 37, 1824–1830. [Google Scholar] [CrossRef]
- Constantini, N.W.; Warren, M.P. Menstrual dysfunction in swimmers: A distinct entity. J. Clin. Endocrinol. Metab. 1995, 80, 2740–2744. [Google Scholar] [CrossRef]
- Coste, O.; Paris, F.; Galtier, F.; Letois, F.; Maïmoun, L.; Sultan, C. Polycystic ovary–like syndrome in adolescent competitive swimmers. Fertil. Steril. 2011, 96, 1037–1042. [Google Scholar] [CrossRef]
- Schtscherbyna, A.; Soares, E.A.; De Oliveira, F.P.; Ribeiro, B.G. Female athlete triad in elite swimmers of the city of Rio de Janeiro, Brazil. Nutrition 2009, 25, 634–639. [Google Scholar] [CrossRef]
- Muia, E.N.; Wright, H.H.; Onywera, V.O.; Kuria, E.N. Adolescent elite Kenyan runners are at risk for energy deficiency, menstrual dysfunction and disordered eating. J. Sports Sci. 2016, 34, 598–606. [Google Scholar] [CrossRef]
- Salbach, H.; Klinkowski, N.; Pfeiffer, E.; Lehmkuhl, U.; Korte, A. Body Image and Attitudinal Aspects of Eating Disorders in Rhythmic Gymnasts. Psychopathology 2007, 40, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Klentrou, P.; Plyley, M. Onset of puberty, menstrual frequency, and body fat in elite rhythmic gymnasts compared with normal controls. Br. J. Sports Med. 2003, 37, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, A.; Marchetti, M.; Battaglia, C.; Giombini, A.; Calcagno, G.; Fiorilli, G.; Piazza, M.; Pigozzi, F.; Borrione, P. Is menstrual delay a serious problem for elite rhythmic gymnasts? J. Sports Med. Phys. Fitness 2012, 52, 647–653. [Google Scholar]
- Klinkowski, N.; Korte, A.; Pfeiffer, E.; Lehmkuhl, U.; Salbach-Andrae, H. Psychopathology in elite rhythmic gymnasts and anorexia nervosa patients. Eur. Child. Adolesc. Psychiatry 2008, 17, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Reina, F.; Montivero, A.D.; Colodrón, M.; Vanrell, J.A. Influence of high-intensity training and of dietetic and anthropometric factors on menstrual cycle disorders in ballet dancers. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2006, 22, 31–35. [Google Scholar] [CrossRef]
- Liu, Z.; Gong, Y.; Nagamoto, H.; Okunuki, T.; Yamaguchi, R.; Kobayashi, Y.; Li, Y.; Maemichi, T.; Kumai, T. Low Body Fat Percentage and Menstrual Cycle Disorders in Female Elite Adolescent Dancers. J. Dance Med. Sci. Off. Publ. Int. Assoc. Dance Med. Sci. 2024, 28, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Prather, H.; Hunt, D.; McKeon, K.; Simpson, S.; Meyer, E.B.; Yemm, T.; Brophy, R. Are Elite Female Soccer Athletes at Risk for Disordered Eating Attitudes, Menstrual Dysfunction, and Stress Fractures? PM R 2016, 8, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Barrack, M.T.; Van Loan, M.D.; Rauh, M.J.; Nichols, J.F. Physiologic and behavioral indicators of energy deficiency in female adolescent runners with elevated bone turnover. Am. J. Clin. Nutr. 2010, 92, 652–659. [Google Scholar] [CrossRef]
- Czajkowska, M.; Plinta, R.; Rutkowska, M.; Brzęk, A.; Skrzypulec-Plinta, V.; Drosdzol-Cop, A. Menstrual Cycle Disorders in Professional Female Rhythmic Gymnasts. Int. J. Environ. Res. Public Health 2019, 16, 1470. [Google Scholar] [CrossRef]
- Czajkowska, M.; Drosdzol-Cop, A.; Naworska, B.; Galazka, I.; Gogola, C.; Rutkowska, M.; Skrzypulec-Plinta, V. The impact of competitive sports on menstrual cycle and menstrual disorders, including premenstrual syndrome, premenstrual dysphoric disorder and hormonal imbalances. Ginekol. Pol. 2020, 91, 503–512. [Google Scholar] [CrossRef]
- Egan, E.; Reilly, T.; Whyte, G.; Giacomoni, M.; Cable, N.T. Disorders of the Menstrual Cycle in Elite Female Ice Hockey Players and Figure Skaters. Biol. Rhythm. Res. 2003, 34, 251–264. [Google Scholar] [CrossRef]
- Rauh, M.J.; Tenforde, A.S.; Barrack, M.T.; Rosenthal, M.D.; Nichols, J.F. Sport Specialization and Low Bone Mineral Density in Female High School Distance Runners. J. Athl. Train. 2020, 55, 1239–1246. [Google Scholar] [CrossRef]
- Roupas, N.D.; Maïmoun, L.; Mamali, I.; Coste, O.; Tsouka, A.; Mahadea, K.K.; Mura, T.; Philibert, P.; Gaspari, L.; Mariano-Goulart, D.; et al. Salivary adiponectin levels are associated with training intensity but not with bone mass or reproductive function in elite Rhythmic Gymnasts. Peptides 2014, 51, 80–85. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Fredericson, M.; Sayres, L.C.; Cutti, P.; Sainani, K.L. Identifying Sex-Specific Risk Factors for Low Bone Mineral Density in Adolescent Runners. Am. J. Sports Med. 2015, 43, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.; Fahey, T.; White, T. Exercise Physiology: Human Bioenergetics and Its Applications; Mayfield: Mountain View, CA, USA, 1996. [Google Scholar]
- Fox, E.L.; Mathews, D.K. Interval Training: Conditioning for Sports and General Fitness; Saunders College Publishing: Orlando, FL, USA, 1974. [Google Scholar]
- Amoruso, I.; Fonzo, M.; Barro, A.; Scardina, C.; Titton, F.; Bertoncello, C.; Baldovin, T. Determinants of menstrual dysfunction in the female athlete triad: A cross-sectional study in Italian athletes. Psychol. Sport Exerc. 2024, 73, 102653. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.; Gerlach, D.H.; Wilhite, A.L. Menstrual dysfunction in distance runners. Obstet. Gynecol. 1979, 54, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Passoni, P.; Inzoli, A.; De Ponti, E.; Polizzi, S.; Ceccherelli, A.; Fantauzzi, M.; Procaccianti, C.; Cattoni, A.; Villa, S.; Riva, A.; et al. Association between Physical Activity and Menstrual Cycle Disorders in Young Athletes. Int. J. Sports Med. 2024, 45, 543–548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dupuit, M.; Meignié, A.; Chassard, T.; Blanquet, L.; LeHeran, J.; Delaunay, T.; Bernardeau, E.; Toussaint, J.-F.; Duclos, M.; Antero, J. On-Field Methodological Approach to Monitor the Menstrual Cycle and Hormonal Phases in Elite Female Athletes. Int. J. Sports Physiol. Perform. 2023, 18, 1169–1178. [Google Scholar] [CrossRef]
- Roffler, A.; Fleddermann, M.T.; de Haan, H.; Krüger, K.; Zentgraf, K. Menstrual cycle tracking in professional volleyball athletes. Front. Sports Act. Living 2024, 6, 1408711. [Google Scholar] [CrossRef]
| Amenorrhea | |
|---|---|
| Disorders of Outflow Tract and/or Uterus |
|
| Ovary Disorders |
|
| Anterior Pituitary Disorders |
|
| Hypothalamus Disorders |
|
| Other menstrual abnormalities | |
| Oligomenorrhea/ Polymenorrhea |
|
| Heavy Menstrual Bleeding |
|
| Abnormal Uterine Bleeding |
|
| Author | Sample | Age Range | Sport | Type of Menstrual Dysfunction |
|---|---|---|---|---|
| Barrack MT, 2008 [58] | 93 | 16.1 ± 0.1 16 | Cross-country running | Primary amenorrhea, Secondary amenorrhea, Oligomenorrhea |
| Barrack MT, 2010 [78] | 39 | 15.7 ± 0.2 15–16 | Cross-country running | Primary amenorrhea, Secondary amenorrhea, Oligomenorrhea |
| Beals KA, 2002 [60] | 23 | 15.8 ± 1.1 14–16 | Volleyball | Primary amenorrhea, Oligomenorrhea, Menstrual cycle irregularities |
| Castelo-Branco, 2006 [75] | 38 | 14.8 ± 1.7 15–17 | Ballet | Oligomenorrhea, Menstrual cycle irregularities |
| de Oliveira Coelho GM, 2013 [54] | 24 | 14.77 ± 2.16 13–17 | Tennis | Secondary amenorrhea, Oligomenorrhea, Menstrual cycle irregularities |
| Coste O, 2011 [68] | 18 | 15.2 ± 1.1 14–16 | Swimming | Secondary amenorrhea, Oligomenorrhea |
| Cristina-Souza G, 2019 [66] | 12 | 16.5 ±1.6 14–18 | Track and field | Dysmenorrhea |
| Czajkowska M, 2019 [79] | 45 | 16.28 ± 0.84 15–16 | Rhythmic gymnastics | Secondary amenorrhea, Oligomenorrhea, Hypomenorrhea |
| Czajkowska M, 2020 [80] | 75 | 18.6 ± 1.9 17–20 | Middle- and long-distance running | Secondary amenorrhea, Oligomenorrhea, |
| Di Cagno A, 2012 [73] | 46 | 17.4 ± 3.0 14–20 | Rhythmic gymnasts | Secondary amenorrhea, Menstrual cycle irregularities |
| Dusek T, 2001 [49] | 72 | 15–19 | Basketball, volleyball, ballet, track and field | Primary amenorrhea, Secondary amenorrhea |
| Egan E, 2003 [81] | 37 | 17.5 ± 3.4 15–21 | Figure skating | Secondary amenorrhea, Oligomenorrhea |
| Felmann JM, 2011 [64] | 103 | 15–17 | Track and field | Secondary amenorrhea, Oligomenorrhea |
| Helge EW, 2002 [62] | 6 | 17.9 ± 1.5 17–19 | Artistic gymnastics | Primary amenorrhea, Secondary amenorrhea, Oligomenorrhea |
| Klentrou P, 2003 [72] | 23 | 14.7 ± 0.4 14–15 | Rhythmic gymnastics | Secondary amenorrhea, Oligomenorrhea |
| Klinkowski N, 2008 [74] | 51 | 15.2 ± 1.8 | Rhythmic gymnastics | Secondary amenorrhea |
| Liu Z, 2024 [76] | 131 | 15.9 ± 1.5 | Elite dance | Primary and Secondary amenorrhea |
| Muia EN, 2016 [70] | 56 | 16.0 | Middle-distance running | Primary amenorrhea, Secondary amenorrhea |
| Prather H, 2016 [77] | 220 | 16.4 ± 4 | Soccer | Primary amenorrhea |
| Rauh MJ, 2014 [63] | 89 | 15.5 ± 1.3 | Middle-distance running | Menstrual cycle irregularities |
| Rauh MJ, 2020 [82] | 64 | 15.6 ± 1.4 | Running | Heavy menstrual bleeding or longer breaks between menstrual bleeds |
| Roupas ND, 2014 [83] | 77 | 18.3 ± 2.6 | Rhythmic gymnastics | Primary amenorrhea, Oligomenorrhea |
| Salbach H, 2007 [71] | 50 | 14.8 ± 2.1 | Rhythmic gymnastics | Primary amenorrhea, Secondary amenorrhea |
| Schtscherbyna A, 2009 [69] | 78 | 16.7 ± 1.2 | Swimming | Oligomenorrhea |
| Tenforde AS, 2015 [84] | 91 | 16.9 ± 1.3 | Middle-distance running | Primary amenorrhea, Secondary amenorrhea |
| Thompson SH, 2007 [59] | 300 | 19.64 ± 1.56 | Cross-country running | Secondary amenorrhea, Oligomenorrhea |
| Tsukahara Y, 2021 [61] | 91 | 18.10 ± 0.37 | Track and field | Secondary amenorrhea, Oligomenorrhea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Vandoni, M.; Bianchi, A.; Pirazzi, A.; Tiranini, L.; Baldassarre, P.; Diotti, M.; Cavallo, C.; Nappi, R.E.; Zuccotti, G. Menstrual Dysfunction in Adolescent Female Athletes. Sports 2024, 12, 245. https://doi.org/10.3390/sports12090245
Calcaterra V, Vandoni M, Bianchi A, Pirazzi A, Tiranini L, Baldassarre P, Diotti M, Cavallo C, Nappi RE, Zuccotti G. Menstrual Dysfunction in Adolescent Female Athletes. Sports. 2024; 12(9):245. https://doi.org/10.3390/sports12090245
Chicago/Turabian StyleCalcaterra, Valeria, Matteo Vandoni, Alice Bianchi, Agnese Pirazzi, Lara Tiranini, Paola Baldassarre, Marianna Diotti, Caterina Cavallo, Rossella Elena Nappi, and Gianvincenzo Zuccotti. 2024. "Menstrual Dysfunction in Adolescent Female Athletes" Sports 12, no. 9: 245. https://doi.org/10.3390/sports12090245
APA StyleCalcaterra, V., Vandoni, M., Bianchi, A., Pirazzi, A., Tiranini, L., Baldassarre, P., Diotti, M., Cavallo, C., Nappi, R. E., & Zuccotti, G. (2024). Menstrual Dysfunction in Adolescent Female Athletes. Sports, 12(9), 245. https://doi.org/10.3390/sports12090245












