Impact of Isolated Exercise-Induced Small Airway Dysfunction on Exercise Performance in Professional Male Cyclists
Highlights
- Elite athletes may experience nonbeneficial effects on their respiratory system. This research aimed to examine the impact of exercise on small airways and to establish whether isolated exercise-induced small airway dysfunction hurts exercise performance.
- It is imperative to recognize that the small airways are affected in isolation or in combination with the reduction in forced expiratory volume at the first second (FEV1). This understand-ing is crucial for the accurate diagnosis and effective treatment of respiratory symptoms of elite athletes.
- Athletes with exercise-induced isolated small airway dysfunction have a lower exercise ca-pacity (VO2 max).
- Isolated exercise-induced small airway dysfunction may be considered an indicator of exercise-induced bronchoconstriction in professional athletes.
- The detection and diagnosis of small airway dysfunction are important because treatment may reverse the remodeling of small airways in professional athletes and improve their exercise capacity.
- However, there are many questions about nonbeneficial effects on respiratory function in professional endurance athletes. Because, in most cases, elite athletes with affected respiratory function are asymptomatic, consideration should be given to systematic control of the respiratory system, such as through regular cardiological control.
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, H.; Price, O.J.; Greenwell, J.; Hull, J.H. Respiratory impact of a grand tour: Insight from professional cycling. Eur. J. Appl. Physiol. 2021, 121, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Furber, M.J.; Van Someren, K.A.; Anton-Solanas, A.; Swart, J. The Physiological Profile of a Multiple Tour de France Winning Cyclist. Med. Sci. Sports Exerc. 2017, 49, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Pigakis, K.M.; Stavrou, V.T.; Pantazopoulos, I.; Daniil, Z.; Kontopodi-Pigaki, A.K.; Gourgoulianis, K. Effect of Hydration on Pulmonary Function and Development of Exercise-Induced Bronchoconstriction among Professional Male Cyclists. Adv. Respir. Med. 2023, 91, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, P.B.; Katayama, K.; Vermeulen, T.D.; Stuckless, T.J.R.; Brown, C.V.; Foster, G.E.; Shell, A.W. Work of breathing influences muscle sympathetic nerve activity during semi-recumbent cycle exercise. Acta Physiol. 2019, 225, e13212. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.R.; Satiroglu, R.; Fico, B.; Tanaka, H.; Vardarli, E.; Luci, J.; Coyle, E.F. Inertial Load Power Cycling Training Increases Muscle Mass, and Aerobic Power in Older Adults. Med. Sci. Sports Exerc. 2021, 53, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Price, O.J.; Ansley, L.; Menzies-Gow, A.; Cullinan, P.; Hull, J.H. Airway dysfunction in elite athletes—An occupational lung disease? Allergy 2013, 68, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.W.; Whyte, G.P.; McConnell, A.K.; Harries, M.G. Impact of changes in the IOC-MC asthma criteria: A British perspective. Thorax 2005, 60, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.W.; Whyte, G.P.; McConnell, A.K.; Nevill, A.M.; Harries, M.G. Mid-expiratory flow versus FEV1 measurements in the diagnosis of exercise-induced asthma in elite athletes. Thorax 2006, 61, 111–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stensrud, T.; Rossvoll, Ø.; Mathiassen, M.; Melau, J.; Illidi, C.; Ostgaard, H.N.; Hisdal, J.; Stang, J. Lung function and oxygen saturation after participation in Norseman Xtreme Triathlon. Scand. J. Med. Sci. Sports 2020, 30, 1008–1016. [Google Scholar] [CrossRef]
- Aggarwal, B.; Mulgirigama, A.; Berend, N. Exercise-induced bronchoconstriction: Prevalence, pathophysiology, patient impact, diagnosis, and management. NPJ Prim. Care Respir. Med. 2018, 28, 31. [Google Scholar] [CrossRef]
- Weiler, J.M.; Bonini, S.; Coifman, R.; Craig, T.; Delgado, L.; Capao-Filipe, M.; Passali, D.; Randolph, C.; Storms, W.; Ad Hoc Committee of Sports Medicine Committee of American Academy of Allergy, Asthma & Immunology. American Academy of Allergy, Asthma & Immunology Work Group report: Exercise-induced asthma. J. Allergy Clin. Immunol. 2007, 119, 1349–1358. [Google Scholar] [PubMed]
- Pigakis, K.M.; Stavrou, V.T.; Pantazopoulos, I.; Daniil, Z.; Kontopodi, A.K.; Gourgoulianis, K.; Kontopodi, A. Exercise-Induced Bronchospasm in Elite Athletes. Cureus 2022, 14, e20898. [Google Scholar] [CrossRef] [PubMed]
- Holzer, K.; Douglass, J.A. Exercise-induced bronchoconstriction in elite athletes: Measuring the fall. Thorax 2006, 61, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Greiwe, J.; Cooke, A.; Nanda, A.; Epstein, S.Z.; Wasan, A.N.; Shepard, K.V., 2nd; Capao-Filipe, M.; Nish, A.; Rubin, M.; Gregory, K.L.; et al. Work Group Report: Perspectives in Diagnosis and Management of Exercise-Induced Bronchoconstriction in Athletes. J. Allergy Clin. Immunol. Pract 2020, 8, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.S.; Buston, M.H.; Wharton, M.J. The effect of exercise on ventilatory function in the child with asthma. Br. J. Dis. Chest 1962, 56, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Kurowski, M.; Moreira, A.; Bullens, D.M.A.; Carlsen, K.H.; Delgodo, L.; Kowalski, M.L.; Seys, S.F. Mechanism of exercise-induced bronchoconstriction in athletes: Current perspectives and future challenges. Allergy 2018, 73, 8–16. [Google Scholar] [CrossRef]
- Carlsen, K.H.; Anderson, S.D.; Bjermer, L.; Bonini, S.; Brusasco, V.; Canonica, W.; Cummiskey, J.; Delgado, L.; Del Giacco, S.R.; Drobnic, F.; et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: Epidemiology, mechanisms, and diagnosis: Part I of the report from the joint task force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy 2008, 63, 387–403. [Google Scholar] [PubMed]
- Parsons, J.P.; Hallstrand, T.S.; Mastronarde, J.G.; Kaminsky, D.A.; Rundel, K.W.; Hull, J.H.; Storms, W.W.; Welier, J.M.; Cheek, F.M.; Wilson, K.C.; et al. An Official American Thoracic Society Clinical Practice Guideline: Exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2013, 197, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P.; O’Byrne, P.M. Asthma, and exercise-induced bronchoconstriction in athletes. N. Engl. J. Med. 2015, 372, 641–648. [Google Scholar] [CrossRef]
- Messan, F.; Marqueste, T.; Akplogan, B.; Decherchi, P.; Grélot, L. Exercise-Induced Bronchospasm Diagnosis in Sportsmen and Sedentary. Int. Sch. Res. Not. 2012, 2012, 314583. [Google Scholar] [CrossRef]
- Bonini, M.; Palange, P. Exercise-induced bronchoconstriction: New evidence in pathogenesis, diagnosis, and treatment. Asthma Res. Pract. 2015, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.D. An overview of asthma and airway hyperresponsiveness on Olympic athletes. Br. J. Sports Med. 2012, 46, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Hallstrand, T.S.; Moody, M.W.; Wurfel, M.M.; Schwartz, L.B.; Henderson, W.R., Jr.; Aitken, M.L. Inflammatory basis of exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2005, 172, 679–686. [Google Scholar] [CrossRef]
- Anderson, S.D.; Holzer, K. Exercise-induced asthma: Is the right diagnosis in elite athletes. J. Allergy Clin. Immunol. 2000, 106, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Kippelen, P.; Anderson, S.D. Airway injury during high–level exercise. Br. J. Sports Med. 2012, 46, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Zarqa, A.; Norsk, P.; Ulrik, C.S. Mechanism and Management of Exercise Induced Asthma in Elite Athletes. J. Asthma 2012, 49, 480–486. [Google Scholar]
- Mead, J. The lungs “quiet zone”. N. Engl. J. Med. 1970, 282, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Pare, P.D.; Hackett, T.L. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol. Rev. 2017, 97, 529–552. [Google Scholar] [CrossRef]
- Petsonk, E.; Stansbury, R.; Beekman-Wagner, L.A.; Long, J.L.; Wang, M.L. Small Airway Dysfunction and Abnormal Exercise Responses. A Study in Coal Miners. Ann. Am. Thorac. Soc. 2016, 13, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Elbehairy, A.F.; Ciavaglia, C.E.; Webb, K.A.; Guenette, J.A.; Jensen, D.; Mourad, S.M.; Neder, J.A.; O’Donnell, D.E.; Canadian Respiratory Research Network. Pulmonary Gas Exchange Abnormalities in Mild Chronic Obstructive Pulmonary Disease. Implications for Dyspnea and Exercise Intolerance. Am. J. Respir. Crit. Care Med. 2015, 191, 1384–1394. [Google Scholar] [CrossRef]
- Sposato, B.; Scalese, M.; Migliorini, M.G.; Di Tomassi, M.; Scala, R. Small airway impairment and bronchial hyperresponsiveness in asthma onset. Allergy Asthma Immunol. Res. 2014, 6, 242–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majak, P.; Cichalewski, L.; Ożarek-Hanc, A.; Stelmach, W.; Jerzyńska, J.; Stelmach, I. Airway response to exercise measured by area under the expiratory flow-volume curve in children with asthma. Ann. Allergy Asthma Immunol. 2013, 111, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.D.; McKenzie, A.S.; Zach, J.A.; Wilson, C.G.; Curran-Everett, D.; Stinson, D.S.; Newell, J.D.; Lynch, D.A. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am. J. Roentgenol. 2013, 201, W460–W470. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, M.F. Review Article Vital capacity derived spirometric measurements. Sudan Med. J. 2012, 48, 86–100. [Google Scholar]
- Lutfi, M.F.; Sukkar, M.Y. Reliability of spirometric measurements in assessing asthma severity. Kuwait Med. J. 2010, 3, 433–439. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Halstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Morris, Z.Q.; Coz, A.; Starosta, D. An isolated reduction of the FEV3/FVC ratio is an indicator of mild lung injury. Chest 2013, 144, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Borekci, S.; Demir, T.; Gorek, A.; Uygun, M.; Yildirim, N. A simple measure to assess hyperinflation and air trapping: 1-Forced expiratory volume in three seconds/forced vital capacity. Balkan Med. J. 2017, 34, 113–118. [Google Scholar] [CrossRef]
- Li, K.; Gao, Y.; Pan, Z.; Jia, X.; Yan, Y.; Min, X.; Huang, K.; Jiang, T. Influence of emphysema and air trapping heterogeneity on pulmonary function in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2863–2872. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Chicago, IL, USA, 1988. [Google Scholar]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Heart 2007, 93, 1285–1292. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casubari, R.; Coates, A. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef]
- Hansen, J.E. Clinical Function Testing and Interpretation, 1st ed.; Clinical Focus Series; Jaypee Brothers Medical Publishers: London, UK, 2011. [Google Scholar]
- Hansen, J.E.; Sun, X.G.; Wasserman, K. Discriminating measures and normal values for expiratory obstruction. Chest 2006, 129, 369–377. [Google Scholar] [CrossRef]
- Crapo, R.O.; Morris, A.H.; Gardner, R.M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am. Respir. Dis. 1981, 123, 659–664. [Google Scholar]
- Miller, M.O.; Grove, D.M.; Pinnock, A.C. Time domain spirogram indices: Their variability and reference values in nonsmokers. Am. Rev. Respir. Dis. 1985, 132, 1041–1048. [Google Scholar] [PubMed]
- Miller, M.O.; Grove, D.M.; Pinnock, A.C. Patterns of spirometric abnormality in individual smokers. Am. Rev. Respir. Dis. 1985, 132, 1034–1040. [Google Scholar] [PubMed]
- US Department of Health and Human Services [DHHS]; National Center for Health Statistics. Third National Health and Nutrition Examination Survey, 1988–1994: NHANES III Raw Spirometry Data File; Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2001. [Google Scholar]
- Deng, Z.; Li, X.; Li, C.; Zheng, Y.; Wu, F.; Wanz, Z.; Liu, S.; Tian, H.; Zheng, J.; Peng, J.; et al. Impaired exercise capacity in individuals with non-obstructive small airway dysfunction. J. Thorac. Dis. 2023, 15, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Elbehairy, A.; Elbehairy, J.; Guenette, J.; Faisal, A.; Faisal, A.; Casey, E.; Ciavaglia, C.E.; Weeb, K.; Jensen, D.; Ramsook, A.; et al. Mechanisms of exertional dyspnoea in symptomatic smokers without COPD. Eur. Respir. J. 2016, 48, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ofir, D.; Lavenezianna, P.; Webb, K.A.; Lam, Y.M.; O’Donnell, D.E. Mechanisms of dyspnoea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 622–629. [Google Scholar] [CrossRef]
- Di Marco, F.; Terrano, S.; Job, S.; Rinaldo, R.F.; Sferrazza Papa, G.F.; Roggi, M.A.; Santus, P.; Centanni, S. Cardiopulmonary exercise testing and second-line pulmonary function: Tests to detect obstructive pattern in symptomatic smokers with borderline spirometry. Respir. Med. 2017, 127, 7–13. [Google Scholar] [CrossRef]
- American Thoracic Society. American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. Respir. Crit. Care 2003, 167, 211–277, Erratum in Am. J. Respir. Crit. Care Med. 2003, 1451–1452. [Google Scholar] [CrossRef]
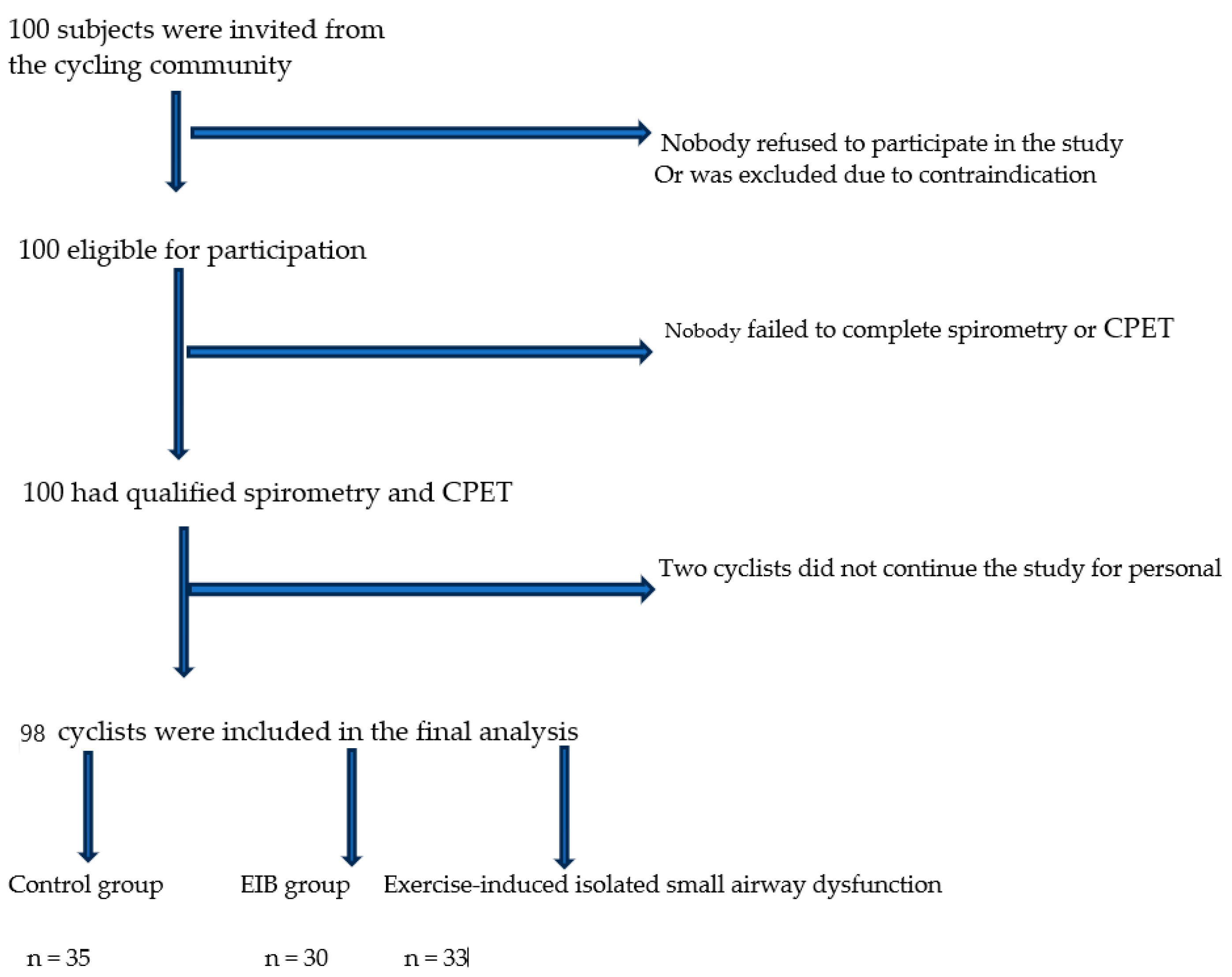
| Age, years | 27.0 ± 5.0 |
| Training age, years | 12.0 ± 5.0 |
| Body mass index, kg/m2 | 23.8 ± 1.4 |
| Hemoglobin, g/dL | 14.8 ± 1.1 |
| FeNO, ppb | 11.0 ± 3.0 |
| IgE, UI/mL | 53.0 ± 7.0 |
| Baseline Characteristics (before the CPET) n = 100 | Control Group (after the CPET) n = 35 | Isolated Exercise-Induced SAD Group (after the CPET) n = 33 | EIB Group (after the CPET) n = 30 | |
|---|---|---|---|---|
| FVC, L | 6.40 ± 0.6 | 6.10 ± 0.7 | 6.10 ± 0.70 | 6.0 ± 0.72 |
| FVC, % | 117.1 ± 6.7 | 112.4 ± 8.9 | 112.0 ± 4.4 | 110.0 ± 4.6 |
| FEV1, L | 5.20 ± 0.4 | 4.90 ± 0.5 | 4.73 ± 0.47 | 4.28 ± 0.47 *† |
| FEV1, % | 120.0 ± 6.9 | 118.0 ± 12 | 117.0 ± 14 | 109.0 ± 10 *† |
| FEV3, L | 5.85 ± 0.5 | 5.65 ± 0.55 | 5.32 ± 0.4 * | 5.10 ± 0.6 *† |
| FEV3, % | 120.0 ± 6.5 | 119.0 ± 11.5 | 115.0 ± 8.5 * | 110.0 ± 9.2 *† |
| FEV1/FVC | 0.81 ± 0.1 | 0.80 ± 0.7 | 0.77 ± 0.65 | 0.71 ± 0.45 *† |
| FEV3/FVC | 0.91 ± 0.2 | 0.93 ± 0.8 | 0.87 ± 0.3 * | 0.85 ± 0.6 * |
| 1-FEV3/FVC, % | 8.6 ± 2.3 | 7.4 ± 2.5 | 12.8 ± 3.2 * | 15.0 ± 2.8 *† |
| MVV, L | 182.0 ± 34.0 | 171.5 ± 18.9 | 165.5 ± 16.4 * | 149.8 ± 15.2 *† |
| FEF25–75, L/s | 5.0 ± 1.1 | 4.40 ± 1.2 | 1.96 ± 1.4 * | 1.76 ± 1.35 * |
| FEF25–75, % | 103.1 ± 8.3 | 90.8 ± 11.5 | 65.0 ± 10 * | 63.0 ± 9.0 * |
| FEF50, L/s | 6.20 ± 0.7 | 7.60 ± 0.7 | 3.51 ± 0.4 * | 3.49 ± 0.3 * |
| FEF50, % | 112.0 ± 4.5 | 138.0 ± 5.3 | 63.0 ± 3.5 * | 63.0 ± 6.0 * |
| FEF75, L/s | 3.30 ± 0.4 | 3.47 ± 0.7 | 1.62 ± 0.4 * | 1.58 ± 0.4 * |
| FEF75, % | 127.0 ± 8.0 | 134.0 ± 7.0 | 63.0 ± 4.0 * | 61.0 ± 7.0 * |
| Age, years | 27.0 ± 5.0 | 26.2 ± 5.1 | 29.2 ± 6.1 * | 32.0 ± 4.3 * |
| BMI, kg/m2 | 23.8 ± 1.4 | 21.9 ± 3.2 | 22.3 ± 3.1 | 23.2 ± 3.4 |
| Overall | Controls | SAD | EIB | |
|---|---|---|---|---|
| VO2 max, mL/kg/min | 65.0 ± 4.4 | 69.0 ± 2.0 | 62.0 ± 2.3 * | 61.0 ± 2.35 * |
| Respiratory exchange ratio (RER) | 1.20 ± 0.1 | 1.22 ± 0.1 | 1.18 ± 0.09 * | 1.15 ± 0.11 * |
| VO2 AT% predicted VO2 max, % | 72.4 ± 5.2 | 75.5 ± 6.8 | 72.0 ± 2.8 | 70.0 ± 6.15 |
| O2-pulse (VO2/HR), mL/beats/min | 19.2 ± 2.5 | 19.5 ± 2.7 | 19.2 ± 2.2 | 18.9 ± 2.5 |
| VE, L/min | 120.0 ± 26.0 | 113.0 ± 28 | 122.0 ± 27 * | 125.0 ± 27.5 * |
| VE/VCO2 ratio (ventilatory efficiency) | 27.5 ± 1.3 | 26.5 ± 1.0 | 27.0 ± 1.5 | 29.0 ± 1.5 *† |
| Respiratory reserve (VE/MVV, %) | 64.0 ± 1.3 | 62.0 ± 1.0 | 74.0 ± 2.0 * | 83.0 ± 1.0 *† |
| Variables | Control Group | ISAD Group | EIB Group | ||
|---|---|---|---|---|---|
| Β (95% CI) | p | Β (95% CI) | p | ||
| VO2 peak | Ref. | −2.4 (−6.2 to −1.4) | 0.025 | −4.0 (−7.7 to −0.2) | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigakis, K.M.; Stavrou, V.T.; Kontopodi, A.K.; Pantazopoulos, I.; Daniil, Z.; Gourgoulianis, K. Impact of Isolated Exercise-Induced Small Airway Dysfunction on Exercise Performance in Professional Male Cyclists. Sports 2024, 12, 112. https://doi.org/10.3390/sports12040112
Pigakis KM, Stavrou VT, Kontopodi AK, Pantazopoulos I, Daniil Z, Gourgoulianis K. Impact of Isolated Exercise-Induced Small Airway Dysfunction on Exercise Performance in Professional Male Cyclists. Sports. 2024; 12(4):112. https://doi.org/10.3390/sports12040112
Chicago/Turabian StylePigakis, Konstantinos M., Vasileios T. Stavrou, Aggeliki K. Kontopodi, Ioannis Pantazopoulos, Zoe Daniil, and Konstantinos Gourgoulianis. 2024. "Impact of Isolated Exercise-Induced Small Airway Dysfunction on Exercise Performance in Professional Male Cyclists" Sports 12, no. 4: 112. https://doi.org/10.3390/sports12040112
APA StylePigakis, K. M., Stavrou, V. T., Kontopodi, A. K., Pantazopoulos, I., Daniil, Z., & Gourgoulianis, K. (2024). Impact of Isolated Exercise-Induced Small Airway Dysfunction on Exercise Performance in Professional Male Cyclists. Sports, 12(4), 112. https://doi.org/10.3390/sports12040112








