Cognitive Fitness: Harnessing the Strength of Exerkines for Aging and Metabolic Challenges
Abstract
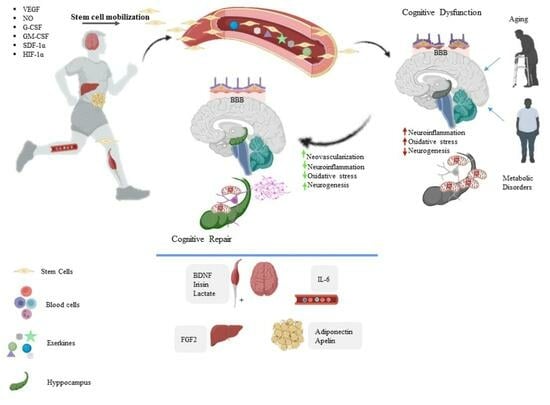
1. Introduction
- Genetic factors: genetic defects play a significant role in the development of cognitive disorders, with some genetic factors increasing the risk of cognitive impairment and dementia.
- Ethnicity: Different ethnic groups may have varying susceptibility to cognitive disorders due to genetic factors and cultural influences. For example, individuals of African descent are at a higher risk of developing Alzheimer’s disease (AD)‚ while individuals of Asian descent may have a lower risk [11].
- Geographical location: The prevalence of cognitive disorders can differ across regions due to factors such as access to healthcare, education, and lifestyle. For instance, cognitive disorders may be more common in urban areas with higher levels of pollution and stress [12].
- Gender: gender has been found to play a role in the prevalence of cognitive disorders, with some studies suggesting that women are at a higher risk of developing AD [15].
- 6.
- Nutrition: Dietary factors play a crucial role in cognitive function and prevention of cognitive disorders. A diet rich in anti-inflammatory, low-sugar, and minimally processed foods, as well as a proper omega-6 to omega-3 ratio, can help maintain cognitive health [11].
- 7.
- Metabolic diseases: suffering from metabolic diseases, such as diabetes and obesity, has been associated with an increased risk of CI and dementia [12].
1.1. Aging, Metabolic Disorders, and CI
1.1.1. Brain Atrophy
1.1.2. Neuroplasticity
1.1.3. Neurogenesis
1.1.4. Neurotransmitter Changes
1.1.5. Amyloid Plaques (Aβ) and Tau Tangles
1.1.6. Oxidative Stress and Inflammation
1.1.7. Vascular Changes and Dysfunction
1.1.8. Mitochondrial Dysfunction
1.1.9. Insulin Resistance
1.1.10. Brain Energy Metabolism
1.1.11. Advanced Glycation End Products (AGEs)
1.2. Management of CI
2. Exerkines and CI
2.1. IL-6
2.2. Cathepsin B
2.3. Brain-Derived Neurotrophic Factor (BDNF)
2.4. Irisin
2.5. Apelin
2.6. Clusterin
2.7. C-X3-C Motif Chemokine Ligand 1 (CX3CL1)
2.8. FGF2
2.9. FGF21
2.10. IGF-1
2.11. LIF
2.12. VEGF
2.13. Glial-Cell-Line-Derived Neurotrophic Factor (GDNF)
2.14. 3-Hydroxybutyrate (3OHB)
2.15. Lactate
2.16. PGC1-a
2.17. Neurotransmitters and Neuromodulators
2.18. Inflammation
2.19. Oxidative Stress
2.20. Beneficial Effect of Stem Cells
3. Exercise Prescription Guidelines
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petersen, R.C. Mild cognitive impairment. Contin. Lifelong Learn. Neurol. 2016, 22, 404. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I. Attention in Cognitive Neuroscience: An Overview; The MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Corbo, I.; Casagrande, M. Higher-level executive functions in healthy elderly and mild cognitive impairment: A systematic review. J. Clin. Med. 2022, 11, 1204. [Google Scholar] [CrossRef]
- Roark, B.; Mitchell, M.; Hosom, J.-P.; Hollingshead, K.; Kaye, J. Spoken language derived measures for detecting mild cognitive impairment. IEEE Trans. Audio Speech Lang. Process. 2011, 19, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Singleton, E.H.; Fieldhouse, J.L.; van’t Hooft, J.J.; Scarioni, M.; van Engelen, M.P.E.; Sikkes, S.A.; de Boer, C.; Bocancea, D.I.; van den Berg, E.; Scheltens, P.A.; et al. Social cognition deficits and biometric signatures in the behavioural variant of Alzheimer’s disease. Brain 2023, 146, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Maier, F.; Greuel, A.; Hoock, M.; Kaur, R.; Tahmasian, M.; Schwartz, F.; Csoti, I.; Jessen, F.; Drzezga, A.; van Eimeren, T.; et al. Impaired self-awareness of cognitive deficits in Parkinson’s disease relates to cingulate cortex dysfunction. Psychol. Med. 2023, 53, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cai, H.; Bai, W.; Su, Z.; Tang, Y.L.; Ungvari, G.S.; Ng, C.H.; Zhang, Q.; Xiang, Y.T. Global prevalence of mild cognitive impairment among older adults living in nursing homes: A meta-analysis and systematic review of epidemiological surveys. Transl. Psychiatry 2023, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Song, W.X.; Wu, W.W.; Zhao, Y.Y.; Xu, H.L.; Chen, G.C.; Jin, S.Y.; Chen, J.; Xian, S.X.; Liang, J.H. Evidence from a meta-analysis and systematic review reveals the global prevalence of mild cognitive impairment. Front. Aging Neurosci. 2023, 15, 1227112. [Google Scholar] [CrossRef]
- Orumiyehei, A.; Khoramipour, K.; Rezaei, M.H.; Madadizadeh, E.; Meymandi, M.S.; Mohammadi, F.; Chamanara, M.; Bashiri, H.; Suzuki, K. High-intensity interval training-induced hippocampal molecular changes associated with improvement in anxiety-like behavior but not cognitive function in rats with type 2 diabetes. Brain Sci. 2022, 12, 1280. [Google Scholar] [CrossRef]
- Rajizadeh, M.A.; Moslemizadeh, A.; Hosseini, M.S.; Rafiei, F.; Soltani, Z.; Khoramipour, K. Adiponectin receptor 1 could explain the sex differences in molecular basis of cognitive improvements induced by exercise training in type 2 diabetic rats. Sci. Rep. 2023, 13, 16267. [Google Scholar] [CrossRef]
- Kurczyńska, A.; Ślusarczyk, K. Dietary factors and controversies in dementia prevention. Sci. Pap. Witelon Coll. 2020, 4, 69–89. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.L.; Ellis, K.A. Physical activity for cognitive health: What advice can we give to older adults with subjective cognitive decline and mild cognitive impairment? Dialogues Clin. Neurosci. 2019, 21, 61–68. [Google Scholar] [CrossRef]
- Hsiao, C.; Wen, C.-J.; Yen, H.-Y.; Hsueh, M.-C.; Liao, Y. Association between accelerometer-measured light-intensity physical activity and cognitive function in older adults. J. Nutr. Health Aging 2022, 26, 230–235. [Google Scholar] [CrossRef]
- Huang, L.; Chen, H.; Gao, M.; Shen, J.; Tao, Y.; Huang, Y.; Lv, R.; Xie, R.; Lv, X.; Xu, X.; et al. Dietary factors in relation to the risk of cognitive impairment and physical frailty in Chinese older adults: A prospective cohort study. Eur. J. Nutr. 2024, 63, 267–277. [Google Scholar]
- Shaik, M.G.; Joshi, S.V.; Akunuri, R.; Rana, P.; Rahman, Z.; Polomoni, A.; Yaddanapudi, V.M.; Dandekar, M.P.; Srinivas, N. Small molecule inhibitors of NLRP3 inflammasome and GSK-3β in the management of traumatic brain injury: A review. Eur. J. Med. Chem. 2023, 9, 115718. [Google Scholar] [CrossRef]
- Burtscher, J.; Soltany, A.; Visavadiya, N.P.; Burtscher, M.; Millet, G.P.; Khoramipour, K.; Khamoui, A.V. Mitochondrial stress and mitokines in aging. Aging Cell 2023, 22, e13770. [Google Scholar] [CrossRef]
- Alsolami, K. Structural and Functional Changes in Cerebral Aging. Egypt. Acad. J. Biol. Sci. C Physiol. Mol. Biol. 2023, 15, 149–180. [Google Scholar] [CrossRef]
- Harada, C.N.; Love, M.C.N.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Murman, D.L. The impact of age on cognition. In Seminars in Hearing; Thieme Medical Publishers: Leipzig, Germany, 2015; Volume 36, pp. 111–121. [Google Scholar]
- Bennett, I.J.; Madden, D.J. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience 2014, 276, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Fernandes, H.M.; Magalhães, R.; Moreira, P.S.; Marques, P.; Soares, J.M.; Amorim, L.; Portugal-Nunes, C.; Castanho, T.; Santos, N.C. Signatures of white-matter microstructure degradation during aging and its association with cognitive status. Sci. Rep. 2021, 11, 4517. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Y.; Mok, V. Age-related white matter changes. J. Aging Res. 2011, 2011, 617927. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Hsu, C.-C.H.; Huang, W.-Y.; Chen, Y.-L.; Lin, C.; Chen, C.-K.; Lin, C.; Shyu, Y.-C.; Lin, C.-P. White matter integrity underlies the physical-cognitive correlations in subjective cognitive decline. Front. Aging Neurosci. 2021, 13, 700764. [Google Scholar] [CrossRef]
- Dickstein, D.L.; Weaver, C.M.; Luebke, J.I.; Hof, P.R. Dendritic spine changes associated with normal aging. Neuroscience 2013, 251, 21–32. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, H.; Chen, K.; Chen, X.; Yang, G. Learning-dependent dendritic spine plasticity is reduced in the aged mouse cortex. Front. Neural Circuits 2020, 14, 581435. [Google Scholar] [CrossRef]
- Vázquez-Hernández, N.; Martínez-Torres, N.; González-Burgos, I. Plastic changes to dendritic spines in the cerebellar and prefrontal cortices underlie the decline in motor coordination and working memory during successful aging. Behav. Brain Res. 2021, 400, 113014. [Google Scholar] [CrossRef]
- Feldman, M.L.; Dowd, C. Loss of dendritic spines in aging cerebral cortex. Anat. Embryol. 1975, 148, 279–301. [Google Scholar] [CrossRef]
- Kirch, C.; Gollo, L.L. Single-neuron dynamical effects of dendritic pruning implicated in aging and neurodegeneration: Towards a measure of neuronal reserve. Sci. Rep. 2021, 11, 1309. [Google Scholar] [CrossRef]
- Kinnunen, K.M.; Cash, D.M.; Poole, T.; Frost, C.; Benzinger, T.L.; Ahsan, R.L.; Leung, K.K.; Cardoso, M.J.; Modat, M.; Malone, I.B. Presymptomatic atrophy in autosomal dominant Alzheimer’s disease: A serial magnetic resonance imaging study. Alzheimer’s Dement. 2018, 14, 43–53. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.-G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Chakravarthy, H.; Sharma, S.; Shree, S.; Bhat, A.R.; Pradeepkiran, J.A.; Devanathan, V. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Res. Rev. 2023, 89, 101994. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, J.; Zhou, P.; Li, J.; Gao, H.; Xia, Y.; Wang, Q. Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2012, 97, 1–13. [Google Scholar] [CrossRef]
- Borodinsky, L.N.; Belgacem, Y.H.; Swapna, I.; Sequerra, E.B. Dynamic regulation of neurotransmitter specification: Relevance to nervous system homeostasis. Neuropharmacology 2014, 78, 75–80. [Google Scholar] [CrossRef]
- Kittler, J.T.; Oliver, P.L. 15 Genomic and Post-Genomic Tools for Studying Synapse Biology. In The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2006; p. 279. [Google Scholar]
- Hansen, J.Y.; Shafiei, G.; Markello, R.D.; Smart, K.; Cox, S.M.; Nørgaard, M.; Beliveau, V.; Wu, Y.; Gallezot, J.-D.; Aumont, É. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat. Neurosci. 2022, 25, 1569–1581. [Google Scholar] [CrossRef]
- Hines, A.D.; McGrath, S.; Latham, A.S.; Kusick, B.; Mulligan, L.; Richards, M.L.; Moreno, J.A. Activated gliosis, accumulation of amyloid β, and hyperphosphorylation of tau in aging canines with and without cognitive decline. Front. Aging Neurosci. 2023, 15, 1128521. [Google Scholar] [CrossRef]
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global cognitive impairment prevalence and incidence in community dwelling older adults—A systematic review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Mimenza-Alvarado, A.; Granados-Domínguez, L.; Flores-López, A.; López-Barradas, A.; Ortiz, V.; Pérez-Cruz, C.; Sánchez-Vidal, H.; Hernández-Acosta, J.; Ávila-Funes, J.A. The Gut Microbiota–Brain Axis during Aging, Mild Cognitive Impairment and Dementia: Role of Tau Protein, β-Amyloid and LPS in Serum and Curli Protein in Stool. Nutrients 2023, 15, 932. [Google Scholar] [CrossRef]
- Gaikwad, S.; Senapati, S.; Haque, M.A.; Kayed, R. Senescence, brain inflammation, and oligomeric tau drive cognitive decline in Alzheimer’s disease: Evidence from clinical and preclinical studies. Alzheimer’s Dement. 2023, 20, 709–727. [Google Scholar] [CrossRef]
- Shimada, H.; Kitamura, S.; Shinotoh, H.; Endo, H.; Niwa, F.; Hirano, S.; Kimura, Y.; Zhang, M.-R.; Kuwabara, S.; Suhara, T. Association between Aβ and tau accumulations and their influence on clinical features in aging and Alzheimer’s disease spectrum brains: A [11C] PBB3-PET study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 6, 11–20. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, C.; Lee, M.J.; Jung, Y. Therapeutic strategies to improve liver regeneration after hepatectomy. Exp. Biol. Med. 2023, 248, 1313–1318. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Galea, I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular risk factors and dysfunction in aging and dementia. Veins Lymphat. 2022, 11. [Google Scholar] [CrossRef]
- Ghebre, Y.T.; Yakubov, E.; Wong, W.T.; Krishnamurthy, P.; Sayed, N.; Sikora, A.G.; Bonnen, M.D. Vascular aging: Implications for cardiovascular diseases and therapy. Transl. Med. 2016, 6, 183. [Google Scholar] [CrossRef]
- Inoue, Y.; Shue, F.; Bu, G.; Kanekiyo, T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 46. [Google Scholar] [CrossRef]
- Tobias-Wallingford, H.; Coppotelli, G.; Ross, J.M. Mitochondria in Ageing and Diseases: Partie Deux. Int. J. Mol. Sci. 2023, 24, 10359. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimer’s Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef]
- Ramya, C.; Rajalakshmi, R.; Vijayashankar, U.; Bhanukumar, M. Role of glycemic status and insulin resistance indices on cognition. Biomedicine 2023, 43, 718–721. [Google Scholar]
- Portero-Otin, M.; de la Maza, M.P.; Uribarri, J. Dietary Advanced Glycation End Products: Their Role in the Insulin Resistance of Aging. Cells 2023, 12, 1684. [Google Scholar] [CrossRef]
- Lu, Y.; Bu, F.Q.; Wang, F.; Liu, L.; Zhang, S.; Wang, G.; Hu, X.Y. Recent advances on the molecular mechanisms of exercise-induced improvements of cognitive dysfunction. Transl. Neurodegener. 2023, 12, 9. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.H.; Kramer, A.F. Physical activity, brain, and cognition. Curr. Opin. Behav. Sci. 2015, 4, 27–32. [Google Scholar] [CrossRef]
- Bidzan-Bluma, I.; Lipowska, M. Physical activity and cognitive functioning of children: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 800. [Google Scholar] [CrossRef]
- Kim, B.R.; Kwon, H.; Chun, M.Y.; Park, K.D.; Lim, S.M.; Jeong, J.H.; Kim, G.H. White matter integrity is associated with the amount of physical activity in older adults with super-aging. Front. Aging Neurosci. 2020, 12, 549983. [Google Scholar] [CrossRef]
- Luan, X.; Liu, J.; Luo, X. Examining the Link between physical activity and cognitive function: A parallel mediation model of Health and Wellbeing among Adolescents. Front. Psychol. 2022, 13, 764842. [Google Scholar] [CrossRef]
- Chaddock-Heyman, L.; Mackenzie, M.J.; Zuniga, K.; Cooke, G.E.; Awick, E.; Roberts, S.; Erickson, K.I.; McAuley, E.; Kramer, A.F. Higher cardiorespiratory fitness levels are associated with greater hippocampal volume in breast cancer survivors. Front. Hum. Neurosci. 2015, 9, 465. [Google Scholar] [CrossRef]
- Gupta, R.; Khan, R.; Cortes, C.J. Forgot to exercise? Exercise derived circulating myokines in alzheimer’s disease: A perspective. Front. Neurol. 2021, 12, 649452. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; Van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and specificity: Insights from the interleukin 6 family of cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Res. Ther. 2006, 8, 1–6. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Linge, I.; Tsareva, A.; Kondratieva, E.; Dyatlov, A.; Hidalgo, J.; Zvartsev, R.; Apt, A. Pleiotropic Effect of IL-6 produced by B-lymphocytes during early phases of adaptive immune responses against TB infection. Front. Immunol. 2022, 13, 750068. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Hekmatikar, A.A.; Sotvan, H. An overview of Fatmax and MFO in exercise. Razi J. Med. Sci. 2020, 27, 49–59. [Google Scholar]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ramírez-Vélez, R.; Díez, J.; González, A.; Izquierdo, M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J. Sport Health Sci. 2023, 12, 147–157. [Google Scholar] [CrossRef]
- Burtscher, J.; Millet, G.P.; Place, N.; Kayser, B.; Zanou, N. The muscle-brain axis and neurodegenerative diseases: The key role of mitochondria in exercise-induced neuroprotection. Int. J. Mol. Sci. 2021, 22, 6479. [Google Scholar] [CrossRef]
- Bunk, J.; Prieto Huarcaya, S.; Drobny, A.; Dobert, J.P.; Walther, L.; Rose-John, S.; Arnold, P.; Zunke, F. Cathepsin D variants associated with neurodegenerative diseases show dysregulated functionality and modified α-synuclein degradation properties. Front. Cell Dev. Biol. 2021, 9, 581805. [Google Scholar]
- Zhang, M.; Jia, J.; Yang, Y.; Zhang, L.; Wang, X. Effects of exercise interventions on cognitive functions in healthy populations: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 92, 102116. [Google Scholar] [CrossRef]
- Sanders, L.M.; Hortobagyi, T.; la Bastide-van Gemert, S.; van der Zee, E.A.; van Heuvelen, M.J. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210036. [Google Scholar] [CrossRef]
- Kumar, M.; Srivastava, S.; Muhammad, T. Relationship between physical activity and cognitive functioning among older Indian adults. Sci. Rep. 2022, 12, 2725. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Dudai, Y.; Mayford, M.R. The molecular and systems biology of memory. Cell 2014, 157, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S. Physical activity for cognitive health promotion: An overview of the underlying neurobiological mechanisms. Ageing Res. Rev. 2023, 86, 101868. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Raz, N.; Lindenberger, U.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Williamson, A.; Dahle, C.; Gerstorf, D.; Acker, J.D. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb. Cortex 2005, 15, 1676–1689. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef]
- Tarassova, O.; Ekblom, M.M.; Moberg, M.; Lövdén, M.; Nilsson, J. Peripheral BDNF response to physical and cognitive exercise and its association with cardiorespiratory fitness in healthy older adults. Front. Physiol. 2020, 11, 1080. [Google Scholar] [CrossRef]
- Walsh, E.I.; Smith, L.; Northey, J.; Rattray, B.; Cherbuin, N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. Ageing Res. Rev. 2020, 60, 101044. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Panati, K.; Suneetha, Y.; Narala, V. Irisin/FNDC5—An updated review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 689–697. [Google Scholar]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, İ.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Lee, B.; Shin, M.; Park, Y.; Won, S.-Y.; Cho, K.S. Physical exercise-induced myokines in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 5795. [Google Scholar] [CrossRef]
- Brown, B.M.; Frost, N.; Rainey-Smith, S.R.; Doecke, J.; Markovic, S.; Gordon, N.; Weinborn, M.; Sohrabi, H.R.; Laws, S.M.; Martins, R.N. High-intensity exercise and cognitive function in cognitively normal older adults: A pilot randomised clinical trial. Alzheimer’s Res. Ther. 2021, 13, 33. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Besse-Patin, A.; Montastier, E.; Vinel, C.; Castan-Laurell, I.; Louche, K.; Dray, C.; Daviaud, D.; Mir, L.; Marques, M.; Thalamas, C. Effect of endurance training on skeletal muscle myokine expression in obese men: Identification of apelin as a novel myokine. Int. J. Obes. 2014, 38, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Merino-Zamorano, C.; Fernández-de Retana, S.; Montañola, A.; Batlle, A.; Saint-Pol, J.; Mysiorek, C.; Gosselet, F.; Montaner, J.; Hernández-Guillamon, M. Modulation of Amyloid-β 1–40 Transport by ApoA1 and ApoJ Across an in vitro Model of the Blood-Brain Barrier. J. Alzheimer’s Dis. 2016, 53, 677–691. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, Z.; Betley, M.J.; Willoughby, D.; Lehallier, B.; Olsson, N.; Bonanno, L.; Fairchild, K.J.; Contrepois, K.; Elias, J.E.; Rando, T.A. Exercise conditioned plasma dampens inflammation via clusterin and boosts memory. bioRxiv 2019. [Google Scholar] [CrossRef]
- De Miguel, Z.; Khoury, N.; Betley, M.J.; Lehallier, B.; Willoughby, D.; Olsson, N.; Yang, A.C.; Hahn, O.; Lu, N.; Vest, R.T. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 2021, 600, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-W.; Tsai, S.-F.; Kuo, Y.-M. Physical exercise enhances neuroplasticity and delays Alzheimer’s disease. Brain Plast. 2018, 4, 95–110. [Google Scholar] [CrossRef]
- Peake, J.; Della Gatta, P.; Suzuki, K.; Nieman, D. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Strömberg, A.; Olsson, K.; Dijksterhuis, J.P.; Rullman, E.; Schulte, G.; Gustafsson, T. CX3CL1—A macrophage chemoattractant induced by a single bout of exercise in human skeletal muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R297–R304. [Google Scholar] [CrossRef]
- Lee, C.C.; Wu, D.Y.; Chen, S.y.; Lin, Y.P.; Lee, T.M. Exercise intensities modulate cognitive function in spontaneously hypertensive rats through oxidative mediated synaptic plasticity in hippocampus. J. Cell. Mol. Med. 2021, 25, 8546–8557. [Google Scholar] [CrossRef]
- Singh, M.; Kakkar, A.; Sharma, R.; Kharbanda, O.; Monga, N.; Kumar, M.; Chowdhary, S.; Airan, B.; Mohanty, S. Synergistic effect of BDNF and FGF2 in efficient generation of functional dopaminergic neurons from human mesenchymal stem cells. Sci. Rep. 2017, 7, 10378. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Rahmaty, S.; Dehghan, P.; Khoramipour, K.; Saboory, M. The effect of listening to brain waves’ relaxing and exciting music during intense endurance training on blood cortisol levels of adult men. Am. J. Sports Sci. Med. 2015, 3, 77–81. [Google Scholar]
- Lewis, J.E.; Ebling, F.J.; Samms, R.J.; Tsintzas, K. Going back to the biology of FGF21: New insights. Trends Endocrinol. Metab. 2019, 30, 491–504. [Google Scholar] [CrossRef]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Wang, X.; Liang, G.; Li, X.; Jiang, C.; Pratchayasakul, W.; Chattipakorn, N. FGF21 and DPP-4 inhibitor equally prevents cognitive decline in obese rats. Biomed. Pharmacother. 2018, 97, 1663–1672. [Google Scholar] [CrossRef]
- Basereh, A.; Ebrahim, K.; Hovanloo, F.; Dehghan, P.; Khoramipour, K. Effect of blood flow restriction deal during isometric exercise on growth hormone and testosterone active males. Sport Physiol. 2017, 9, 51–68. [Google Scholar]
- Jiang, Y.; Lin, L.; Liu, N.; Wang, Q.; Yuan, J.; Li, Y.; Chung, K.K.; Guo, S.; Yu, Z.; Wang, X. FGF21 protects against aggravated blood-brain barrier disruption after ischemic focal stroke in diabetic db/db male mice via cerebrovascular PPARγ activation. Int. J. Mol. Sci. 2020, 21, 824. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Chen, S.-T.; Chen, Y.-J.; Shen, J.; Yao, W.-B.; Gao, X.-D.; Chen, S. Modulation of the astrocyte-neuron lactate shuttle system contributes to neuroprotective action of fibroblast growth factor 21. Theranostics 2020, 10, 8430. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Wang, C.-H.; Pan, C.-Y.; Chen, F.-C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.A.; Padovan, C.M.; Pereira, A.C.; Moraes, C.d. Moderate-intensity exercise training improves long-term memory in fructose-fed rats. Mot. Rev. Educ. Fís. 2020, 26, e10200081. [Google Scholar] [CrossRef]
- Broholm, C.; Mortensen, O.H.; Nielsen, S.; Akerstrom, T.; Zankari, A.; Dahl, B.; Pedersen, B.K. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J. Physiol. 2008, 586, 2195–2201. [Google Scholar] [CrossRef]
- Jia, D.; Cai, M.; Xi, Y.; Du, S. Interval exercise training increases LIF expression and prevents myocardial infarction-induced skeletal muscle atrophy in rats. Life Sci. 2018, 193, 77–86. [Google Scholar] [CrossRef]
- Hill, E.J.; Vernallis, A.B. Polarized secretion of leukemia inhibitory factor. BMC Cell Biol. 2008, 9, 53. [Google Scholar] [CrossRef]
- Broholm, C.; Pedersen, B.K. Leukaemia inhibitory factor-an exercise-induced myokine. Exerc. Immunol. Rev. 2010, 16, 77–85. [Google Scholar]
- Kapilevich, L.V.; Zakharova, A.N.; Kabachkova, A.V.; Kironenko, T.A.; Orlov, S.N. Dynamic and static exercises differentially affect plasma cytokine content in elite endurance-and strength-trained athletes and untrained volunteers. Front. Physiol. 2017, 8, 35. [Google Scholar] [CrossRef]
- Barha, C.K.; Falck, R.S.; Best, J.R.; Nagamatsu, L.S.; Hsiung, G.Y.R.; Sheel, A.W.; Hsu, C.L.; Kramer, A.F.; Voss, M.W.; Erickson, K.I.; et al. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 2011, 111, 251–259. [Google Scholar]
- Rich, B.; Scadeng, M.; Yamaguchi, M.; Wagner, P.D.; Breen, E.C. Skeletal myofiber vascular endothelial growth factor is required for the exercise training-induced increase in dentate gyrus neuronal precursor cells. J. Physiol. 2017, 595, 5931–5943. [Google Scholar] [CrossRef]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Hohman, T.J.; Bell, S.P.; Jefferson, A.L.; Initiative, A.s.D.N. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: Exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015, 72, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical exercise and health: A focus on its protective role in neurodegenerative diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- VanGyseghem, J.M.; Spitsbergen, J.M. Voluntary Exercise Increases Gdnf Protein Content and Endplate Area in Hindlimb Muscle of Male and Female Rats. SSRN 2023. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef]
- Khoury, R. Transfusion of Plasma from Young Exercise Mice Ameliorates Aging-Associated Cognitive Impairments on Learning and Memory through Activation of Autophagy. Ph.D. Thesis, Lebanese American University, Beirut, Lebanon, 2022. [Google Scholar]
- Xue, X.; Liu, B.; Hu, J.; Bian, X.; Lou, S. The potential mechanisms of lactate in mediating exercise-enhanced cognitive function: A dual role as an energy supply substrate and a signaling molecule. Nutr. Metab. 2022, 19, 52. [Google Scholar] [CrossRef]
- Sudo, M.; Costello, J.T.; McMorris, T.; Ando, S. The effects of acute high-intensity aerobic exercise on cognitive performance: A structured narrative review. Front. Behav. Neurosci. 2022, 16, 957677. [Google Scholar] [CrossRef]
- Ben Ayed, I.; Castor-Guyonvarch, N.; Amimour, S.; Naija, S.; Aouichaoui, C.; Ben Omor, S.; Tabka, Z.; El Massioui, F. Acute exercise and cognitive function in alzheimer’s disease. J. Alzheimer’s Dis. 2021, 82, 749–760. [Google Scholar] [CrossRef]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Hao, Q.; Zhou, H.; Zong, Y. IDO-Kynurenine pathway mediates NLRP3 inflammasome activation-induced postoperative cognitive impairment in aged mice. Int. J. Neurosci. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids: Modulators of brain function? Br. J. Nutr. 2008, 99, ES60–ES77. [Google Scholar] [CrossRef] [PubMed]
- Krugers, H.J.; Karst, H.; Joels, M. Interactions between noradrenaline and corticosteroids in the brain: From electrical activity to cognitive performance. Front. Cell. Neurosci. 2012, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Budde, H.; Velasques, B.; Ribeiro, P.; Soya, H. Neuromodulation of Exercise: Impact on Different Kinds of Behavior. Front Neurosci. 2020, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Castrillon, G.; Epp, S.; Bose, A.; Fraticelli, L.; Hechler, A.; Belenya, R.; Ranft, A.; Yakushev, I.; Utz, L.; Sundar, L. An energy costly architecture of neuromodulators for human brain evolution and cognition. Sci. Adv. 2023, 9, eadi7632. [Google Scholar] [CrossRef] [PubMed]
- Fedor, A.; Garcia, S.; Gunstad, J. The effects of a brief, water-based exercise intervention on cognitive function in older adults. Arch. Clin. Neuropsychol. 2015, 30, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Louras, P.; Savettiere, A.; McNerney, W.; Fairchild, K. Effects of High-Intensity Aquatic Exercise on Inflammation and Cognition in Older Adults with MCI. Innov. Aging 2023, 7 (Suppl. S1), 1025. [Google Scholar] [CrossRef]
- de Souza, R.F.; de Moraes, S.R.A.; Augusto, R.L.; de Freitas Zanona, A.; Matos, D.; Aidar, F.J.; da Silveira Andrade-da, B.L. Endurance training on rodent brain antioxidant capacity: A meta-analysis. Neurosci. Res. 2019, 145, 1–9. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Tucker, D.; Wang, R.; Ahmed, M.E.; Brann, D.; Zhang, Q. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 56, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- He, X.-F.; Liu, D.-X.; Zhang, Q.; Liang, F.-Y.; Dai, G.-Y.; Zeng, J.-S.; Pei, Z.; Xu, G.-Q.; Lan, Y. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Rabin, J.S.; Klein, H.; Kirn, D.R.; Schultz, A.P.; Yang, H.-S.; Hampton, O.; Jiang, S.; Buckley, R.F.; Viswanathan, A.; Hedden, T. Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 2019, 76, 1203–1210. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of different exercise modalities on oxidative stress: A systematic review. BioMed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Li, J.; Sun, Y.; Hasimu, H.; Liu, R.; Zhang, T. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1–40-induced toxicity. Acta Pharm. Sin. B 2015, 5, 47–54. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.-M.; Chen, L.; Luo, C.; Tang, A.; Tao, J. Acute exercise-induced nitric oxide production contributes to upregulation of circulating endothelial progenitor cells in healthy subjects. J. Hum. Hypertens. 2007, 21, 452–460. [Google Scholar] [CrossRef]
- Möbius-Winkler, S.; Hilberg, T.; Menzel, K.; Golla, E.; Burman, A.; Schuler, G.; Adams, V. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J. Appl. Physiol. 2009, 107, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Cubbon, R.M.; Murgatroyd, S.R.; Ferguson, C.; Bowen, T.S.; Rakobowchuk, M.; Baliga, V.; Cannon, D.; Rajwani, A.; Abbas, A.; Kahn, M. Human exercise-induced circulating progenitor cell mobilization is nitric oxide-dependent and is blunted in South Asian men. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 878–884. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Bruyndonckx, L.; Van Berckelaer, C.; Hoymans, V.Y.; Vrints, C.J.; Conraads, V.M. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur. J. Appl. Physiol. 2011, 111, 2375–2379. [Google Scholar] [CrossRef]
- Silva, J.F.R.d.; Rocha, N.G.; Nóbrega, A.C.L.d. Mobilization of endothelial progenitor cells with exercise in healthy individuals: A systematic review. Arq. Bras. Cardiol. 2012, 98, 182–191. [Google Scholar]
- Krüger, K.; Pilat, C.; Schild, M.; Lindner, N.; Frech, T.; Muders, K.; Mooren, F. Progenitor cell mobilization after exercise is related to systemic levels of G-CSF and muscle damage. Scand. J. Med. Sci. Sports 2015, 25, e283–e291. [Google Scholar] [CrossRef]
- Chang, E.; Paterno, J.; Duscher, D.; Maan, Z.N.; Chen, J.S.; Januszyk, M.; Rodrigues, M.; Rennert, R.C.; Bishop, S.; Whitmore, A.J. Exercise induces SDF-1 mediated release of endothelial progenitor cells with increased Vasculogenic function. Plast. Reconstr. Surg. 2015, 135, 340e. [Google Scholar] [CrossRef]
- Ribeiro, F.; Ribeiro, I.P.; Gonçalves, A.C.; Alves, A.J.; Melo, E.; Fernandes, R.; Costa, R.; Sarmento-Ribeiro, A.B.; Duarte, J.A.; Carreira, I.M. Effects of resistance exercise on endothelial progenitor cell mobilization in women. Sci. Rep. 2017, 7, 17880. [Google Scholar] [CrossRef]
- Souza, L.V.; De Meneck, F.; Fernandes, T.; Oliveira, E.M.; Franco, M.d.C. Physical activity intervention improved the number and functionality of endothelial progenitor cells in low birth weight children. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 60–70. [Google Scholar] [CrossRef]
- Souza, L.V.; De Meneck, F.; Oliveira, V.; Higa, E.M.; Akamine, E.H.; do Carmo Franco, M. Beneficial impact of moderate to vigorous physical activity program on circulating number and functional capacity of endothelial progenitor cells in children: The crucial role of nitric oxide and VEGF-A. Pediatr. Exerc. Sci. 2019, 31, 322–329. [Google Scholar] [CrossRef]
- Sylviana, N.; Goenawan, H.; Susanti, Y.; Lesmana, R.; Megantara, I. Effect of different intensities aerobic exercise to cardiac angiogenesis regulation on Wistar rats. Pol. J. Vet. Sci. 2022, 25, 119–128. [Google Scholar]
- Addington, C.; Dharmawaj, S.; Heffernan, J.; Sirianni, R.; Stabenfeldt, S. Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol. 2017, 60, 206–216. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, F.; Han, X.; Huang, X. Repetitive transcranial magnetic stimulation increases neurological function and endogenous neural stem cell migration via the SDF-1α/CXCR4 axis after cerebral infarction in rats. Exp. Ther. Med. 2021, 22, 1037. [Google Scholar] [CrossRef]
- Brewster, J. Applications and Challenges of Neural Stem Cell Therapy. Senior Honors Thesis, Liberty University, Lynchburg, VA, USA, 2022. [Google Scholar]
- Liu, W.; Xu, B.; Xue, W.; Yang, B.; Fan, Y.; Chen, B.; Xiao, Z.; Xue, X.; Sun, Z.; Shu, M. A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair. Biomaterials 2020, 243, 119941. [Google Scholar] [CrossRef]
- Wong, R.S.; Cheong, S.-K. Therapeutic potentials of neural stem cells in Alzheimer’s disease. Malays. J. Pathol. 2020, 42, 157–170. [Google Scholar]
- Fu, C.-H.; Iascone, D.M.; Petrof, I.; Hazra, A.; Zhang, X.; Pyfer, M.S.; Tosi, U.; Corbett, B.F.; Cai, J.; Lee, J. Early seizure activity accelerates depletion of hippocampal neural stem cells and impairs spatial discrimination in an Alzheimer’s disease model. Cell Rep. 2019, 27, 3741–3751.e4. [Google Scholar] [CrossRef]
- Scandella, V.; Knobloch, M. Sensing the environment: Extracellular lactate levels control adult neurogenesis. Cell Stem Cell 2019, 25, 729–731. [Google Scholar] [CrossRef]
- Komaki, A.; Shahidi, S.; Hashemi-Firouzi, N.; Rafat, Z.; Keymoradzadeh, A.; Golipoor, Z. Combined Effect of Co-administration of Stromal Cell-Derived Factor-1 and Granulocyte-Colony Stimulating Factor on Rat Model of Alzheimer’s Disease. Front. Behav. Neurosci. 2022, 16, 796230. [Google Scholar] [CrossRef]
- Farahzadi, R.; Fathi, E.; Vietor, I. Mesenchymal stem cells could be considered as a candidate for further studies in cell-based therapy of Alzheimer’s disease via targeting the signaling pathways. ACS Chem. Neurosci. 2020, 11, 1424–1435. [Google Scholar] [CrossRef]
- Emmons, R.; Niemiro, G.M.; Owolabi, O.; De Lisio, M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J. Appl. Physiol. 2016, 120, 624–632. [Google Scholar] [CrossRef]
- Marędziak, M.; Śmieszek, A.; Chrząstek, K.; Basinska, K.; Marycz, K. Physical activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells Int. 2015, 2015, 379093. [Google Scholar] [CrossRef]
- Barha, C.K.; Falck, R.S.; Best, J.R.; Nagamatsu, L.S.; Hsiung, G.-Y.R.; Sheel, A.W.; Hsu, C.L.; Kramer, A.F.; Voss, M.W.; Erickson, K.I. Reshaping the path of mild cognitive impairment by refining exercise prescription: A study protocol of a randomized controlled trial to understand the “what,” “for whom,” and “how” of exercise to promote cognitive function. Trials 2022, 23, 766. [Google Scholar] [CrossRef]
- Quigley, A.; MacKay-Lyons, M.; Eskes, G. Effects of exercise on cognitive performance in older adults: A narrative review of the evidence, possible biological mechanisms, and recommendations for exercise prescription. J. Aging Res. 2020, 2020, 1407896. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Golka, K.; Hengstler, J.G.; Kadhum, T.; Digutsch, J.; Genç, E.; Wascher, E.; Getzmann, S. Does physical fitness affect cognitive functions differently across adulthood? An advantage of being older. Front. Psychol. 2023, 14, 1134770. [Google Scholar] [CrossRef]
| Exerkines | Effects on Cognitive Impairments |
|---|---|
| Interleukin-6 (IL-6) | Stimulates the growth of new neurons in the hippocampus |
| Cathepsin B (CTSB) | Neuroprotective effects, especially in hippocampal neurogenesis and neuronal migration |
| Brain-derived neurotrophic factor (BDNF) | Neuronal differentiation and survival, synaptic integrity, brain plasticity, memory, and cognitive flexibility |
| Glial-derived neurotrophic factor (GDNF) | Gliogenesis |
| Irisin | Cell proliferation and BDNF expression in brain tissue, contributing to the generation of new neurons, protection to neurons against the toxic effects of Aβ |
| Fractalkine (FNDC5/irisin) | Activates (cAMP/PKA/CREB) in brain, promotion of hippocampal neurogenesis, improve cognitive function |
| Apelin | Neuroprotective |
| Clusterin | Facilitating Aβ clearance across the BBB |
| C-X3-C Motif Chemokine Ligand 1 (CX3CL1) | Implicated in memory-associated synaptic plasticity in the rat hippocampus |
| Fibroblast growth factor 2 (FGF2) | Promote the growth of new neurons and blood vessels in the brain |
| Fibroblast growth factor 21 (FGF21) | Hindered the formation of amyloid plaques, neurofibrillary tangles, and overall neurodegeneration |
| Insulin-like Growth Factor-1 (IGF-1) | Neurogenesis |
| Leukemia-inhibitory factor (LIF) | Growth and development of neurons |
| Vascular endothelial growth factor (VEGF) | Neurogenesis in the dentate gyrus of the hippocampus, angiogenesis, improved results in a short-term memory assessment |
| 3-Hydroxybutyrate (3OHB) | Rise in BDNF can be attributed to the presence of 3OHB subsequent to physical activity, enhancing cognitive function and memorization by the effect of the autophagy-dependent nature of β-hydroxybutyrate |
| Lactate | Fuel source of brain, impact on the functionality and performance of transporters and enzymes responsible for the astrocyte-neuron lactate shuttle, brain plasticity |
| Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α | Neuroprotective |
| Anti -inflammatory cytokines | IL-6 triggers the production of IL-1 receptor antagonist (IL-1ra) and IL-10, which exert anti-inflammatory effects. IL-1ra blocks inflammation mediated by IL-1, while IL-10 inhibits the production of several pro-inflammatory cytokines, including IL-1α, IL-1β, IL-8, TNF, and macrophage inflammatory protein-α. The regulation of cellular metabolism in macrophages is another vital role played by IL-10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saheli, M.; Moshrefi, M.; Baghalishahi, M.; Mohkami, A.; Firouzi, Y.; Suzuki, K.; Khoramipour, K. Cognitive Fitness: Harnessing the Strength of Exerkines for Aging and Metabolic Challenges. Sports 2024, 12, 57. https://doi.org/10.3390/sports12020057
Saheli M, Moshrefi M, Baghalishahi M, Mohkami A, Firouzi Y, Suzuki K, Khoramipour K. Cognitive Fitness: Harnessing the Strength of Exerkines for Aging and Metabolic Challenges. Sports. 2024; 12(2):57. https://doi.org/10.3390/sports12020057
Chicago/Turabian StyleSaheli, Mona, Mandana Moshrefi, Masoumeh Baghalishahi, Amirhossein Mohkami, Yaser Firouzi, Katsuhiko Suzuki, and Kayvan Khoramipour. 2024. "Cognitive Fitness: Harnessing the Strength of Exerkines for Aging and Metabolic Challenges" Sports 12, no. 2: 57. https://doi.org/10.3390/sports12020057
APA StyleSaheli, M., Moshrefi, M., Baghalishahi, M., Mohkami, A., Firouzi, Y., Suzuki, K., & Khoramipour, K. (2024). Cognitive Fitness: Harnessing the Strength of Exerkines for Aging and Metabolic Challenges. Sports, 12(2), 57. https://doi.org/10.3390/sports12020057