Night-Time Heart Rate Variability during an Expedition to Mt Everest: A Case Report
Abstract
1. Introduction
2. Materials and Methods
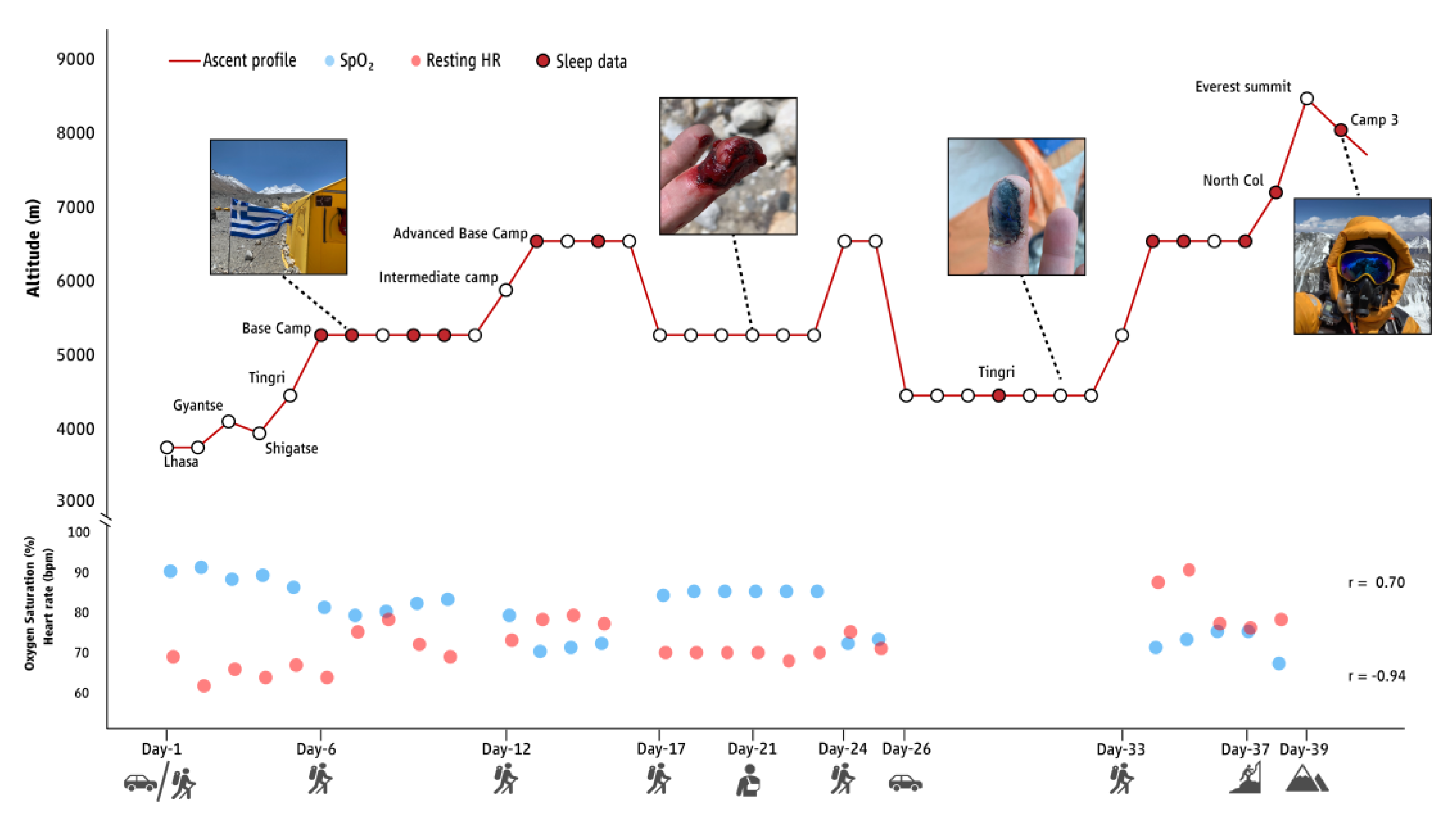
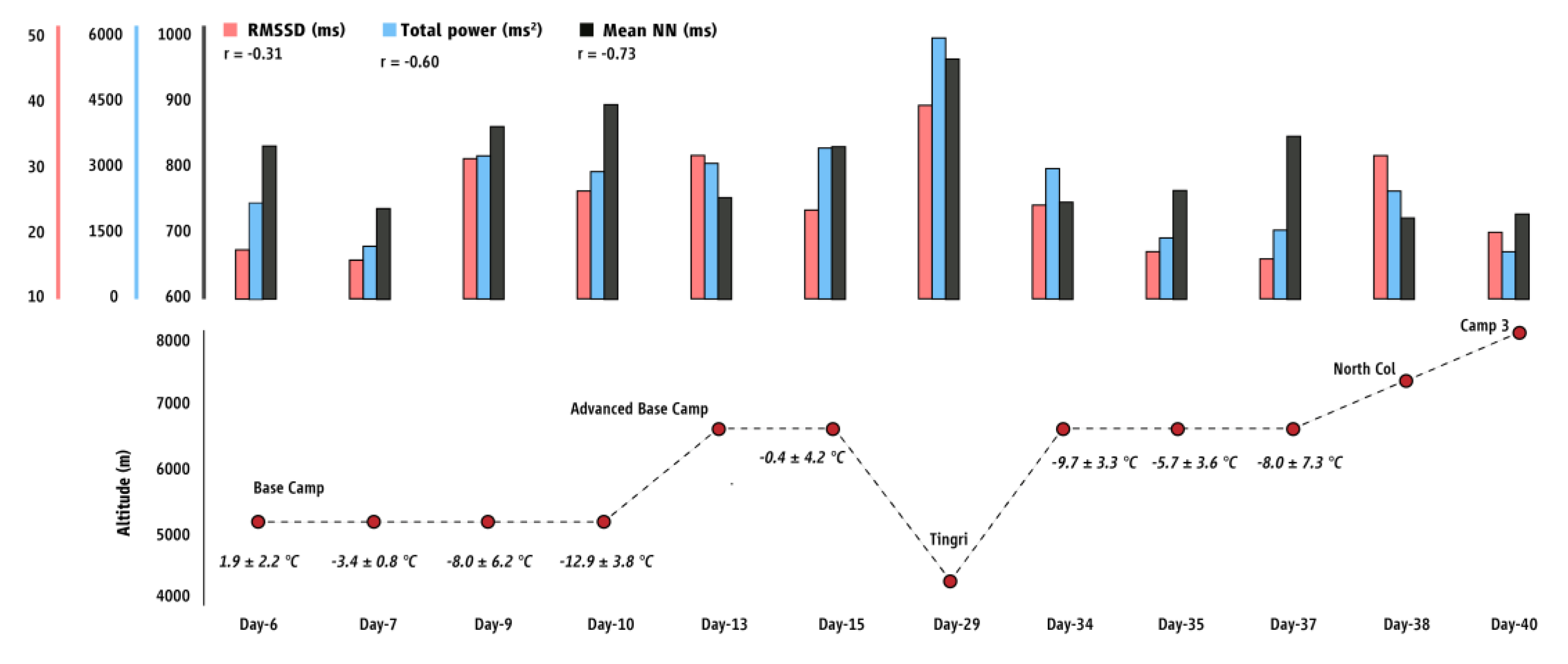
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letchford, A.; Paudel, R.; Thomas, O.D.; Booth, A.S.; Imray, C.H. Acute Mountain Sickness (AMS) Knowledge Among High Altitude Marathon Runners Competing in the Everest Marathon. Wilderness Environ. Med. 2016, 27, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Carroll, C.; Salisbury, R.; Wang, J.L. Mountaineers on Mount Everest: Effects of age, sex, experience, and crowding on rates of success and death. PLoS ONE 2020, 15, e0236919. [Google Scholar] [CrossRef] [PubMed]
- Maggiorini, M.; Bühler, B.; Walter, M.; Oelz, O. Prevalence of acute mountain sickness in the Swiss Alps. Br. Med. J. 1990, 301, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Nemethy, M.; Pressman, A.B.; Freer, L.; McIntosh, S.E. Mt Everest Base Camp Medical Clinic “Everest ER”: Epidemiology of medical events during the first 10 years of operation. Wilderness Environ. Med. 2015, 26, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Firth, P.G.; Zheng, H.; Windsor, J.S.; Sutherland, A.I.; Imray, C.H.; Moore, G.; Semple, J.L.; Roach, R.C.; Salisbury, R.A. Mortality on Mount Everest, 1921–2006: Descriptive study. Br. Med. J. 2008, 337, a2654. [Google Scholar] [CrossRef]
- Windsor, J.; Firth, P.; Grocott, M.; Rodway, G.W.; Montgomery, H. Mountain mortality: A review of deaths that occur during recreational activities in the mountains. Postgrad. Med. J. 2009, 85, 316–321. [Google Scholar] [CrossRef]
- Huey, R.B.; Eguskitza, X. Limits to human performance: Elevated risks on high mountains. J. Exp. Biol. 2001, 204, 3115–3119. [Google Scholar] [CrossRef]
- de Aquino Lemos, V.; Antunes, H.K.; dos Santos, R.V.; Lira, F.S.; Tufik, S.; de Mello, M.T. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 2012, 49, 1298–1306. [Google Scholar] [CrossRef]
- San, T.; Polat, S.; Cingi, C.; Eskiizmir, G.; Oghan, F.; Cakir, B. Effects of high altitude on sleep and respiratory system and theirs adaptations. Sci. World J. 2013, 2013, 241569. [Google Scholar] [CrossRef]
- Porcelli, M.J.; Gugelchuk, G.M. A trek to the top: A review of acute mountain sickness. J. Am. Osteopath. Assoc. 1995, 95, 718–720. [Google Scholar] [CrossRef]
- Hainsworth, R.; Drinkhill, M.J.; Rivera-Chira, M. The autonomic nervous system at high altitude. Clin. Auton. Res. 2007, 17, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zuzewicz, K.; Biernat, B.; Kempa, G.; Kwarecki, K. Heart rate variability in exposure to high altitude hypoxia of short duration. Int. J. Occup. Saf. Ergon. 1999, 5, 337–346. [Google Scholar] [CrossRef]
- Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [CrossRef]
- Jackowska, M.; Dockray, S.; Endrighi, R.; Hendrickx, H.; Steptoe, A. Sleep problems and heart rate variability over the working day. J. Sleep Res. 2012, 21, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Meerlo, P.; Sgoifo, A.; Suchecki, D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med. Rev. 2008, 12, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, R.; Algarin, C.; Rojas, O.; Garrido, M.; Duran-Aguero, S.; Causa, L.; Held, C.; Lozoff, B.; Ferri, R.; Peirano, P. Night-time cardiac autonomic modulation as a function of sleep-wake stages is modified in otherwise healthy overweight adolescents. Sleep Med. 2019, 64, 30–36. [Google Scholar] [CrossRef]
- Insalaco, G.; Salvaggio, A.; Pomidori, L.; Cogo, A.; Romano, S. Heart rate variability during sleep at high altitude: Effect of periodic breathing. Sleep Breath 2016, 20, 197–204. [Google Scholar] [CrossRef]
- Brown, J.P.; Grocott, M.P. Humans at altitude: Physiology and pathophysiology. Contin. Educ. Anaesth. Crit. Care Pain 2012, 13, 17–22. [Google Scholar] [CrossRef]
- Magalhães, J.; Ascensão, A.; Viscor, G.; Soares, J.; Oliveira, J.; Marques, F.; Duarte, J. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat. Space Environ. Med. 2004, 75, 16–22. [Google Scholar]
- Ioannou, L.G.; Tsoutsoubi, L.; Nybo, L.; Tsianos, G.I.; Flouris, A.D. Habitual heat exposure and acclimatization associated with athletic performance in the multistage marathon des sables. J. Hum. Perform. Extreme Environ. 2018, 14, 9. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Castiglioni, P.; Rizzo, F.; Faini, A.; Mazzoleni, P.; Lombardi, C.; Meriggi, P.; Parati, G.; investigators, H. Linear and fractal heart rate dynamics during sleep at high altitude. Investigation with textile technology. Methods Inf. Med. 2010, 49, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Poirier, M.P.; Bravi, A.; Wright-Beatty, H.E.; Herry, C.; Seely, A.J.; Kenny, G.P. Changes in heart rate variability during the induction and decay of heat acclimation. Eur. J. Appl. Physiol. 2014, 114, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Kaltsatou, A.; Flouris, A.D.; Herry, C.L.; Notley, S.R.; Macartney, M.J.; Seely, A.J.E.; Kenny, G.P. Heart rate variability in older workers during work under the Threshold Limit Values for heat exposure. Am. J. Ind. Med. 2020, 63, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Hoddes, E.; Zarcone, V.; Dement, W. Stanford sleepiness scale. In Enzyklopädie der Schlafmedizin; Springer: Berlin/Heidelberg, Germany, 1972; p. 1184. [Google Scholar]
- Karinen, H.M.; Uusitalo, A.; Vaha-Ypya, H.; Kahonen, M.; Peltonen, J.E.; Stein, P.K.; Viik, J.; Tikkanen, H.O. Heart rate variability changes at 2400 m altitude predicts acute mountain sickness on further ascent at 3000-4300 m altitudes. Front. Physiol. 2012, 3, 336. [Google Scholar] [CrossRef]
- Calbet, J.A.; Lundby, C. Air to muscle O2 delivery during exercise at altitude. High Alt. Med. Biol. 2009, 10, 123–134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzios, K.; Pappas, A.; Tsianos, G.-I.; Flouris, A.D. Night-Time Heart Rate Variability during an Expedition to Mt Everest: A Case Report. Sports 2023, 11, 48. https://doi.org/10.3390/sports11020048
Mantzios K, Pappas A, Tsianos G-I, Flouris AD. Night-Time Heart Rate Variability during an Expedition to Mt Everest: A Case Report. Sports. 2023; 11(2):48. https://doi.org/10.3390/sports11020048
Chicago/Turabian StyleMantzios, Konstantinos, Aggelos Pappas, Georgios-Ioannis Tsianos, and Andreas D. Flouris. 2023. "Night-Time Heart Rate Variability during an Expedition to Mt Everest: A Case Report" Sports 11, no. 2: 48. https://doi.org/10.3390/sports11020048
APA StyleMantzios, K., Pappas, A., Tsianos, G.-I., & Flouris, A. D. (2023). Night-Time Heart Rate Variability during an Expedition to Mt Everest: A Case Report. Sports, 11(2), 48. https://doi.org/10.3390/sports11020048






