Reliability of Near-Infrared Spectroscopy with and without Compression Tights during Exercise and Recovery Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
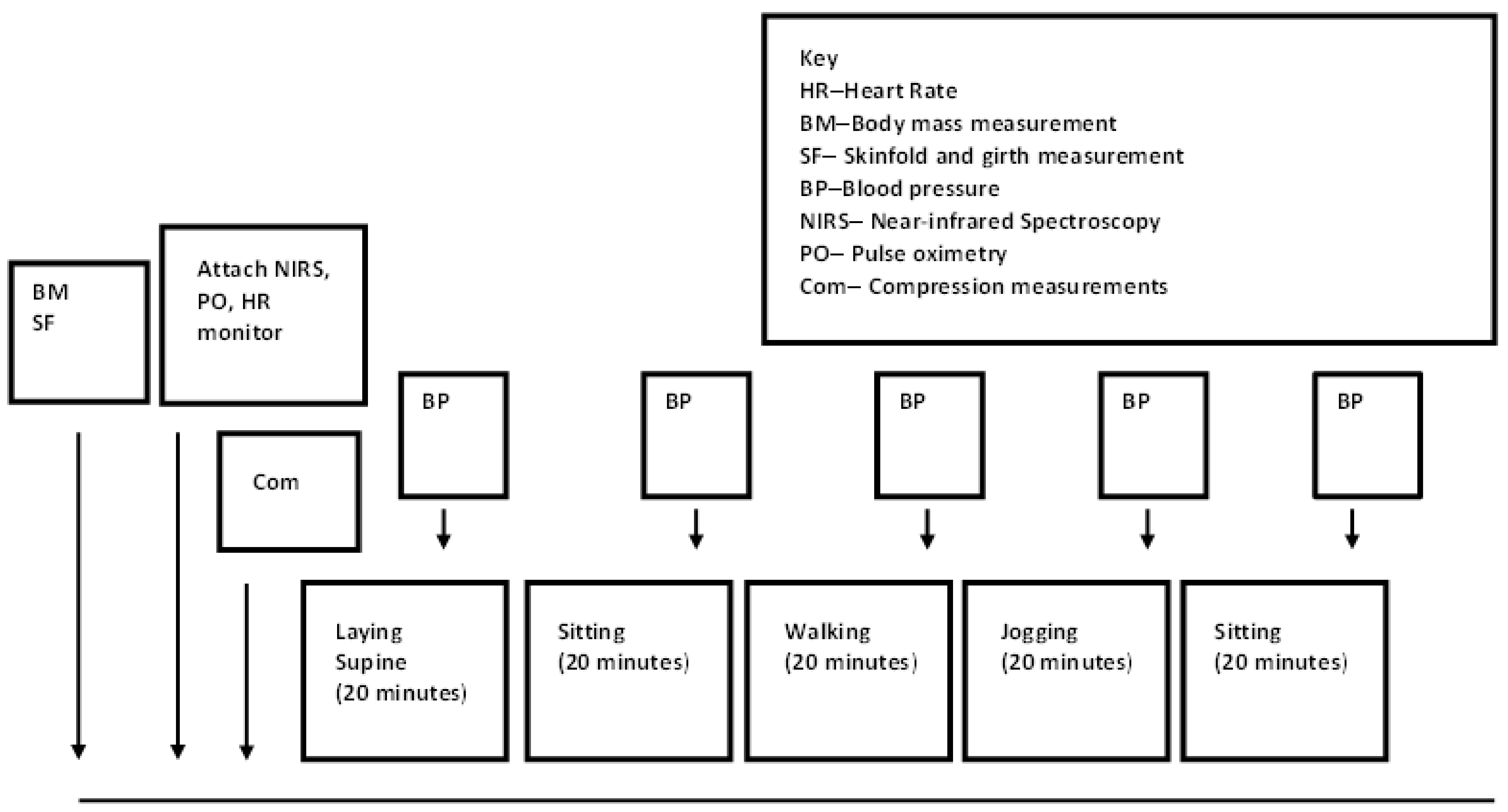
2.2. Experimental Trials
2.3. Compression Tights
2.4. Compression Measurements
2.5. Physiological and Perceptual Measurements
2.6. Near-Infrared Spectroscopy Measurements
2.7. Data Processing for NIRS
2.8. Statistical Analyses
3. Results
3.1. Compression Pressure
3.2. Anthropometric Measurements
3.3. Heart Rate, RPE, and Blood Pressure
3.4. Pulse Oximetry
3.5. Tissue Saturation Index
3.6. Muscle Oxygenation
3.7. Muscle Deoxygenation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, F.; Gissane, C.; Howatson, G.; van Someren, K.; Pedlar, C.; Hill, J. Compression Garments and Recovery from Exercise: A Meta-Analysis. Sports Med. 2017, 47, 2245–2267. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, M.; Martorelli, S.; Vilaca, J. Neuromuscular compression garments: Effects on neuromuscular strength and recovery. J. Hum. Kinet. 2011, 29A, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in soccer: Part ii-recovery strategies. Sports Med. 2013, 43, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Flanagan, S.D.; Comstock, B.A.; Fragala, M.S.; Earp, J.E.; Dunn-Lewis, C.; Ho, J.Y.; Thomas, G.A.; Solomon-Hill, G.; Penwell, Z.R.; et al. Effects of a whole body compression garment on markers of recovery after a heavy resistance workout in men and women. J. Strength Cond. Res. 2010, 24, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Beliard, S.; Chauveau, M.; Moscatiello, T.; Cros, F.; Ecarnot, F.; Becker, F. Compression garments and exercise: No influence of pressure applied. J. Sports Sci. Med. 2015, 14, 75–83. [Google Scholar]
- Bringard, A.; Denis, R.; Belluye, N.; Perrey, S. Effects of compression tights on calf muscle oxygenation and venous pooling during quiet resting in supine and standing positions. J. Sports Med. Phys. Fit. 2006, 46, 548–554. [Google Scholar]
- Berry, M.J.; McMurray, R.G. Effects of graduated compression stockings on blood lactate following an exhaustive bout of exercise. Am. J. Phys. Med. 1987, 66, 121–132. [Google Scholar] [CrossRef]
- Jakeman, J.R.; Byrne, C.; Eston, R.G. Lower limb compression garment improves recovery from exercise-induced muscle damage in young, active females. Eur. J. Appl. Physiol. 2010, 109, 1137–1144. [Google Scholar] [CrossRef]
- Sperlich, B.; Born, D.P.; Kaskinoro, K.; Kalliokoski, K.K.; Laaksonen, M.S. Squeezing the muscle: Compression clothing and muscle metabolism during recovery from high intensity exercise. PLoS ONE 2013, 8, e60923. [Google Scholar] [CrossRef]
- Rimaud, D.; Messonnier, L.; Castells, J.; Devillard, X.; Calmels, P. Effects of compression stockings during exercise and recovery on blood lactate kinetics. Eur. J. Appl. Physiol. 2010, 110, 425–433. [Google Scholar] [CrossRef]
- Ali, A.; Creasy, R.H.; Edge, J.A. Physiological effects of wearing graduated compression stockings during running. Eur. J. Appl. Physiol. 2010, 109, 1017–1025. [Google Scholar] [CrossRef]
- Trenell, M.I.; Rooney, K.B.; Sue, C.M.; Thomspon, C.H. Compression Garments and Recovery from Eccentric Exercise: A (31)P-MRS Study. J. Sports Sci. Med. 2006, 5, 106–114. [Google Scholar]
- Vercruyssen, F.; Easthope, C.; Bernard, T.; Hausswirth, C.; Bieuzen, F.; Gruet, M.; Brisswalter, J. The influence of wearing compression stockings on performance indicators and physiological responses following a prolonged trail running exercise. Eur. J. Sport Sci. 2014, 14, 144–150. [Google Scholar] [CrossRef]
- Engel, F.A.; Holmberg, H.C.; Sperlich, B. Is there evidence that runners can benefit from wearing compression clothing? Sports Med. 2016, 46, 1939–1952. [Google Scholar] [CrossRef]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef]
- Balas, J.; Kodejska, J.; Krupkova, D.; Hannsmann, J.; Fryer, S. Reliability of Near-Infrared Spectroscopy for Measuring Intermittent Handgrip Contractions in Sport Climbers. J. Strength Cond. Res. 2018, 32, 494–501. [Google Scholar] [CrossRef]
- Muthalib, M.; Jubeau, M.; Millet, G.Y.; Maffiuletti, N.A.; Ferrari, M.; Nosaka, K. Biceps brachii muscle oxygenation in electrical muscle stimulation. Clin. Physiol. Funct. Imaging 2010, 30, 360–368. [Google Scholar] [CrossRef]
- Celie, B.; Boone, J.; Van Coster, R.; Bourgois, J. Reliability of near infrared spectroscopy (NIRS) for measuring forearm oxygenation during incremental handgrip exercise. Eur. J. Appl. Physiol. 2012, 112, 2369–2374. [Google Scholar] [CrossRef]
- Crenshaw, A.G.; Elcadi, G.H.; Hellstrom, F.; Mathiassen, S.E. Reliability of near-infrared spectroscopy for measuring forearm and shoulder oxygenation in healthy males and females. Eur. J. Appl. Physiol. 2012, 112, 2703–2715. [Google Scholar] [CrossRef]
- Lucero, A.A.; Addae, G.; Lawrence, W.; Neway, B.; Credeur, D.P.; Faulkner, J.; Rowlands, D.; Stoner, L. Reliability of muscle blood flow and oxygen consumption response from exercise using near-infrared spectroscopy. Exp. Physiol. 2018, 103, 90–100. [Google Scholar] [CrossRef]
- Austin, K.G.; Daigle, K.A.; Patterson, P.; Cowman, J.; Chelland, S.; Haymes, E.M. Reliability of near-infrared spectroscopy for determining muscle oxygen saturation during exercise. Res. Q. Exerc. Sport 2005, 76, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.C.; Nosaka, K.; Peiffer, J.J.; Ihsan, M.; Yeo, C.C.; Abbiss, C.R. Reliability of laser Doppler, near-infrared spectroscopy and Doppler ultrasound for peripheral blood flow measurements during and after exercise in the heat. J. Sports Sci. 2017, 35, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Slattery, K.M.; Sculley, D.V.; Lockie, R.G.; Dascombe, B.J. Reliability of telemetric electromyography and near-infrared spectroscopy during high-intensity resistance exercise. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2014, 24, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: Recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4591–4604. [Google Scholar] [CrossRef]
- Brophy-Williams, N.; Driller, M.W.; Shing, C.M.; Fell, J.W.; Halson, S.L. Confounding compression: The effects of posture, sizing and garment type on measured interface pressure in sports compression clothing. J. Sports Sci. 2015, 33, 1403–1410. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Duncan, A.; Meek, J.H.; Clemence, M.; Elwell, C.E.; Tyszczuk, L.; Cope, M.; Delpy, D.T. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys. Med. Biol. 1995, 40, 295–304. [Google Scholar] [CrossRef]
- Rodriguez, R.F.; Townsend, N.E.; Aughey, R.J.; Billaut, F. Influence of averaging method on muscle deoxygenation interpretation during repeated-sprint exercise. Scand. J. Med. Sci. Sports 2018, 28, 2263–2271. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Hopkins, W.G. Spreadsheets for analysis of validity and reliability. Sportscience 2015, 19, 36–42. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Buchheit, M.; Ufland, P.; Haydar, B.; Laursen, P.B.; Ahmaidi, S. Reproducibility and sensitivity of muscle reoxygenation and oxygen uptake recovery kinetics following running exercise in the field. Clin. Physiol. Funct. Imaging 2011, 31, 337–346. [Google Scholar] [CrossRef]
- Peseux, M.; Muzic, J.; Bouhaddi, M.; Menetrier, A. Changes in Tissue Oxygen Saturation with Well- and Tight-Fitted Compression Sleeves During an Incremental Exercise on Treadmill. Asian J. Sports Med. 2017, 8, e13949. [Google Scholar] [CrossRef]
- Menetrier, A.; Mourot, L.; Bouhaddi, M.; Regnard, J.; Tordi, N. Compression sleeves increase tissue oxygen saturation but not running performance. Int. J. Sports Med. 2011, 32, 864–868. [Google Scholar] [CrossRef]
- McManus, C.J.; Collison, J.; Cooper, C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018, 23, 015007. [Google Scholar] [CrossRef]
- Gajdošík, J.; Baláš, J.í.; Krupková, D.; Psohlavec, L.; Draper, N. Effect of Climbing Speed on Pulmonary Oxygen Uptake and Muscle Oxygen Saturation Dynamics in the Finger Flexors. Int. J. Sports Physiol. Perform. 2021, 17, 176–184. [Google Scholar] [CrossRef]
- Soo, J.; Racinais, S.; Fairchild, T.J.; Ihsan, M.; Buchheit, M.; Girard, O. Effects of graded hypoxia during exhaustive intermittent cycling on subsequent exercise performance and neuromuscular responses. Eur. J. Appl. Physiol. 2021, 121, 3539–3549. [Google Scholar] [CrossRef]
- Fennell, C.R.J.; Hopker, J.G. The acute physiological and perceptual effects of recovery interval intensity during cycling-based high-intensity interval training. Eur. J. Appl. Physiol. 2021, 121, 425–434. [Google Scholar] [CrossRef]
- Gepner, Y.; Wells, A.J.; Gordon, J.A.; Arroyo, E.; Varanoske, A.N.; Coker, N.A.; Fukuda, D.H.; Stout, J.R.; Hoffman, J.R. Differences in muscle oxygenation between young and middle-aged recreationally active men during high-volume resistance exercise. Kinesiology 2019, 51, 3–11. [Google Scholar] [CrossRef]


| Gastrocnemius | Vastus Lateralis | |||
|---|---|---|---|---|
| Skinfold (mm) | Girth (cm) | Skinfold (mm) | Girth (cm) | |
| Control Trial 1 | 9.0 ± 2.4, | 39.5 ± 4.3 | 11.3 ± 5.1 | 57.0 ± 6.1 |
| Control Trial 2 | 11.3 ± 5.1 | 40.1 ± 6.1 | 11.6 ± 3.1 | 56.5 ± 5.2 |
| Compression Tights Trial 1 | 9.2 ± 4.0 | 40.5± 5.9 | 12.6 ± 5.2 | 54.7± 5.7 |
| Compression Tights Trial 2 | 9.5 ± 4.5 | 38.9 ± 4.7 | 11.3 ± 3.8 | 56.7 ± 5.3 |
| Control 1 (%) | Control 2 (%) | Systematic Bias (C) (%) | Bland and Altman 95% LoA | ICC | CV (%) | Compression Tights 1 (%) | Compression Tights 2 (%) | Systematic Bias (CT) (%) | Bland and Altman 95% LoA | ICC | CV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supine | 76.2. ± 3.5 | 76.6 ± 7.0 | 0.3 | −9.9, 10.6 | 0.56 | 3.8 | 73.9 ± 6.7 | 73.1 ± 4.3 | −0.7 | −9.0, 7.4 | 0.73 | 3.0 |
| Sitting | 73.4 ± 6.2 | 75.2 ± 6.9 | 1.7 | −3.2 6.7 | 0.92 | 1.9 | 73.2 ± 8.7 | 71.4 ± 6.7 | −1.7 | −13.4, 9.8 | 0.98 | 4.5 |
| Walking | 72.0 ± 7.2 | 71.6 ± 8.0 | −0.4 | −8.1, 7.2 | 0.87 | 4.0 | 70.9 ± 8.0 | 69.7 ± 5.6 | −1.1 | −13.2, 10.9 | 0.61 | 4.5 |
| Jogging | 67.7 ± 7.6 | 67.9 ± 7.6 | 0.2 | −5.8, 6.2 | 0.96 | 2.2 | 63.9 ± 6.3 | 67.5 ± 7.0 | 3.5 | −5.6, 12.8 | 0.80 | 4.5 |
| Sitting | 80.8 ± 4.6 | 81.3 ± 4.9 | 0.5 | −3.5, 4.4 | 0.91 | 1.4 | 80.5 ± 5.9 | 78.5 ± 6.0 | −1.9 | −11.5, 7.5 | 0.67 | 3.5 |
| Control 1 (%) | Control 2 (%) | Systematic Bias (C) (%) | Bland and Altman 95% LoA | ICC | CV (%) | Compression Tights 1 (%) | Compression Tights 2 (%) | Systematic Bias (CT) (%) | Bland and Altman 95% LoA | ICC | CV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supine | 73.3 ± 1.6 | 74.4 ± 3.0 | 1.0 | −4.3, 6.5 | 0.38 | 2.0 | 76.2 ± 3.5 | 76.2 ± 3.5 | 0.0 | −7.0, 7.0 | 0.47 | 2.6 |
| Sitting | 70.7 ± 4.1 | 71.5 ± 4.1 | 0.8 | −4.3, 6.0 | 0.80 | 1.9 | 73.3 ± 5.0 | 73.9 ± 4.5 | 0.6 | −8.7, 9.9 | 0.51 | 3.4 |
| Walking | 72.9 ± 4.1 | 73.7 ± 4.9 | 0.8 | −5.3, 6.9 | 0.77 | 2.2 | 74.4 ± 5.2 | 74.7 ± 5.5 | 0.2 | −7.2, 7.7 | 0.75 | 2.7 |
| Jogging | 71.6 ± 5.1 | 69.1 ± 5.2 | −2.4 | −8.8, 3.9 | 0.80 | 2.4 | 65.4 ± 5.9 | 65.0 ± 6.8 | −0.8 | −9.2, 7.6 | 0.78 | 3.1 |
| Sitting | 72.4 ± 5.3 | 70.9 ± 5.8 | −0.4 | −7.2, 6.5 | 0.79 | 2.6 | 75.3 ± 3.8 | 75.6 ± 5.3 | 0.3 | −9.2, 9.9 | 0.46 | 3.5 |
| Control 1 (ΔµM) | Control 2 (ΔµM) | Systematic Bias (C) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | Compression Tights 1 (ΔµM) | Compression Tights 2 (ΔµM) | Systematic Bias (CT) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supine | −2.1 ± 6.5 | 0.5 ± 5.5 | 2.6 | −6.2, 9.6 | 0.68 | 3.5 | 0.9 ± 9.4 | −0.7 ± 5.7 | −1.6 | −12.8, 10.0 | 0.62 | 1.0 |
| Sitting | 13.1 ± 8.4 | 13.2 ± 6.6 | 0.1 | −7.8, 7.9 | 0.79 | 3.6 | 1.7 ± 12.1 | 4.7 ± .8.0 | 2.4 | −8.7, 12.6 | 0.83 | 1.2 |
| Walking | 1.3 ± 5.8 | 0.5 ± 5.0 | −0.9 | −5.6, 5.1 | 0.67 | 3.3 | 0.5 ± 9.7 | −0.6 ± 19.1 | −0.1 | −17.9, 17.8 | 0.73 | 8.2 |
| Jogging | −0.9 ± 8.4 | −0.6 ± 9.7 | 0.2 | −12.7, 13.0 | 0.68 | 6.2 | −5.6 ± 9.9 | −3.6 ± 19.3 | 2.0 | −19.2, 22.4 | 0.70 | 8.8 |
| Sitting | 21.9 ± 9.6 | 26.5 ± 5.1 | 4.5 | −8.1, 12.2 | 0.59 | 2.7 | 18.3 ± 16.9 | 7.4 ± 18.9 | −11.0 | −26.4, 11.9 | 0.84 | 7.5 |
| Control 1 (ΔµM) | Control 2 (ΔµM) | Systematic Bias (C) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | Compression Tights 1 (ΔµM) | Compression Tights 2 (ΔµM) | Systematic Bias (CT) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supine | −2.4± 4.3 | −3.5± 4.5 | −1.1 | −8.1, 6.5 | 0.5 | 0.6 | −3.0 ± 8.2 | −1.0 ± 3.3 | 2.1 | −8.2, 10.6 | 0.50 | 4.5 |
| Sitting | −0.8 ± −3.5 | −1.0 ± 7.6 | −0.1 | −8.6, 8.3 | 0.5 | 3.1 | 3.3 ± 10.3 | 5.2 ± 12.7 | 2.0 | −7.8, 10.4 | 0.89 | 4.5 |
| Walking | 5.2 ± 5.9 | −5.7 ±3.5 | −10.8 | −15.5, 7.4 | 0.64 | 3.0 | 1.2 ± 3.8 | −0.1 ± 5.9 | −1.4 | −8.1, −6.9 | 0.35 | 4.1 |
| Jogging | 1.9± 6.4 | −2.6 ±5.1 | −4.61 | −11.6, 7.0 | 0.52 | 3.8 | 1.3 ± 4.8 | −1.3 ± 7.9 | −2.6 | −11.7, −8.3 | 0.55 | 4.4 |
| Sitting | 5.7 ± 5.5 | −0.1± 8.4 | −5.50 | −17.8, 6.8 | 0.61 | 4.0 | 1.8 ± 7.0 | 3.4 ± 5.5 | 1.7 | −7.8, 9.8 | 0.61 | 4.0 |
| Control 1 (ΔµM) | Control 2 (ΔµM) | Systematic Bias (C) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | Compression Tights 1 (ΔµM) | Compression Tights 2 (ΔµM) | Systematic Bias (CT) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supine | −9.3 ± 4.4 | −8.1 ± 2.7 | 1.1 | −5.7, 7.1 | 0.43 | 2.1 | −11.5 ± 8.6 | −11.0 ± 9.4 | 0.3 | −12.5, 14.3 | 0.82 | 4.0 |
| Sitting | 2.3 ± 4.6 | 1.8 ± 4.8 | −0.5 | −7.5, 6.8 | 0.57 | 2.9 | −2.2 ± 9.7 | −2.1 ± 9.4 | 0.1 | −12.2, 12.5 | 0.71 | 5.3 |
| Walking | −5.1 ± 2.6 | −5.6 ± 3.5 | −0.3 | −4.6, 4.1 | 0.57 | 1.1 | 3.6 ± 9.0 | −2.0 ± 7.8 | −1.9 | −15.0, 8.7 | 0.65 | 5.1 |
| Jogging | 0.1 ± 7.4 | 1.7 ± 6.1 | 1.6 | −9.7, 11.7 | 0.52 | 1.6 | 10.3 ± 12.2 | 5.5 ± 12.0 | −4.8 | −16.0, 10.6 | 0.76 | 6.2 |
| Sitting | −2.7 ± 4.0 | −2.9 ± 3.4 | −0.3 | −5.0, 4.8 | 0.61 | 5.4 | 0.0 ± 16.7 | −2.0 ± 9.9 | −2.1 | −25.0, −21.9 | 0.53 | 9.9 |
| Control 1 (ΔµM) | Control 2 (ΔµM) | Systematic Bias (C) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | Compression Tights 1 (ΔµM) | Compression Tights 2 (ΔµM) | Systematic Bias (CT) (ΔµM) | Bland and Altman 95% LoA (ΔµM) | ICC | CV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supine | −3.3 ± 2.0 | −4.4 ± 2.9 | −1.1 | −5.2, 3.5 | 0.50 | 1.8 | −1.3 ± 7.3 | −0.8 ± 7.2 | 0.6 | −4.4, 5.0 | 0.93 | 0.6 |
| Sitting | −1.3 ± 1.7 | −1.3 ± 4.6 | 0.0 | −5.2, 5.3 | 0.42 | 2.7 | −1.8 ± 3.7 | −0.7 ± 2.9 | 0.9 | −3.0, 4.7 | 0.79 | 1.5 |
| Walking | −2.1 ± 2.0 | −2.6 ± 5.9 | −0.5 | −7.4, 6.9 | 0.40 | 3.5 | −3.3 ± 3.5 | −1.8 ± 2.9 | 1.5 | −4.2, 5.1 | 0.82 | 1.6 |
| Jogging | 2.2 ± 5.5 | −1.9 ± 7.0 | −4.1 | −10.6, 4.3 | 0.81 | 2.8 | −1.6 ± 5.4 | 0.0 ± 4.5 | 1.4 | −2.7, 5.0 | 0.89 | 1.6 |
| Sitting | −3.5 ± 2.8 | −3.7 ± 4.6 | −0.1 | −6.0, 5.8 | 0.56 | 2.5 | −3.1 ± 5.4 | −2.8 ± 3.8 | 0.2 | −7.5, 7.9 | 0.53 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biddulph, B.; Morris, J.G.; Lewis, M.; Hunter, K.; Sunderland, C. Reliability of Near-Infrared Spectroscopy with and without Compression Tights during Exercise and Recovery Activities. Sports 2023, 11, 23. https://doi.org/10.3390/sports11020023
Biddulph B, Morris JG, Lewis M, Hunter K, Sunderland C. Reliability of Near-Infrared Spectroscopy with and without Compression Tights during Exercise and Recovery Activities. Sports. 2023; 11(2):23. https://doi.org/10.3390/sports11020023
Chicago/Turabian StyleBiddulph, Brett, John G Morris, Martin Lewis, Kirsty Hunter, and Caroline Sunderland. 2023. "Reliability of Near-Infrared Spectroscopy with and without Compression Tights during Exercise and Recovery Activities" Sports 11, no. 2: 23. https://doi.org/10.3390/sports11020023
APA StyleBiddulph, B., Morris, J. G., Lewis, M., Hunter, K., & Sunderland, C. (2023). Reliability of Near-Infrared Spectroscopy with and without Compression Tights during Exercise and Recovery Activities. Sports, 11(2), 23. https://doi.org/10.3390/sports11020023






