Bone Health, Body Composition and Physiological Demands in 70–85-Year-Old Lifelong Male Football Players
Abstract
:1. Introduction
2. Materials and Methods
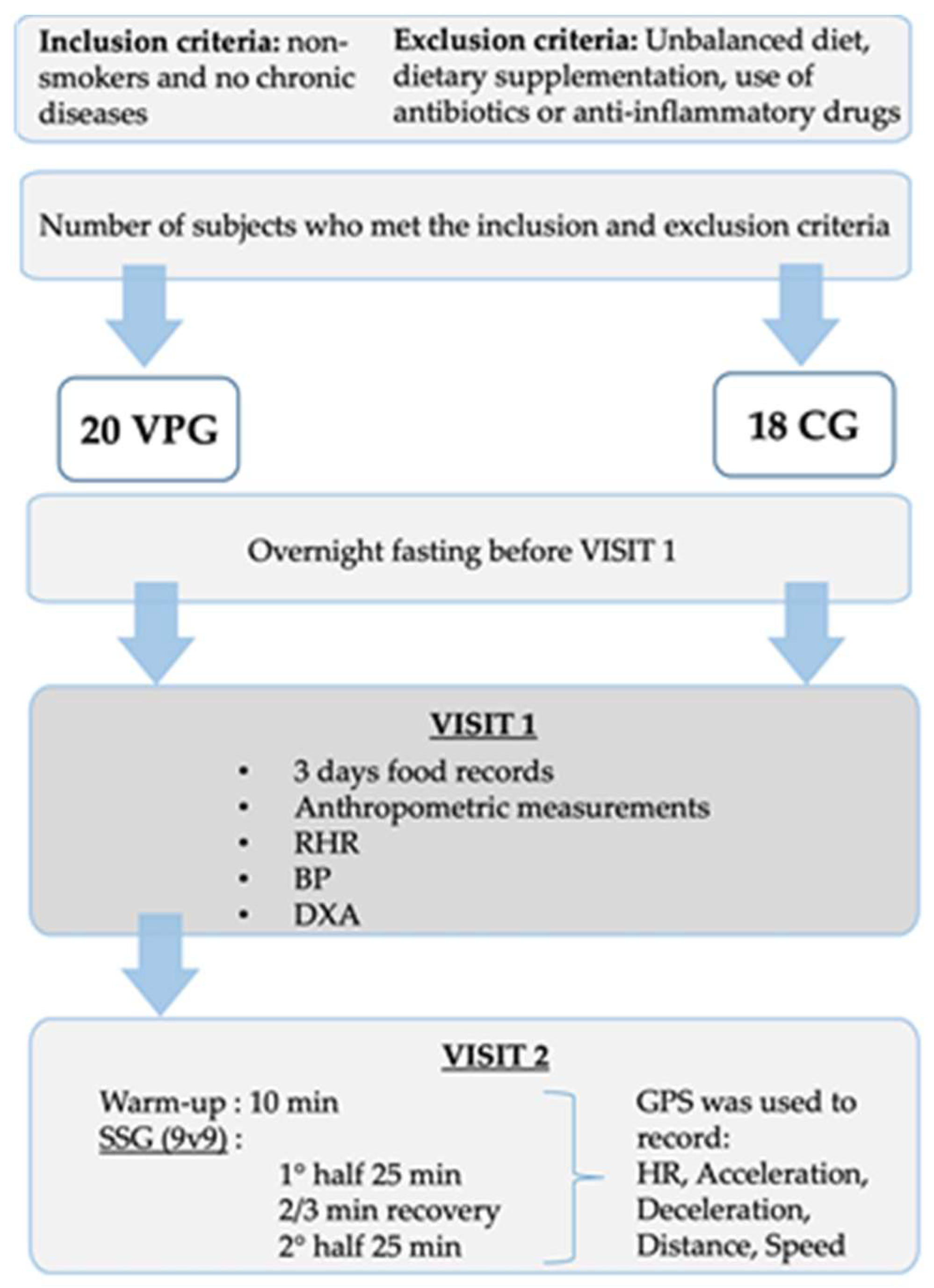
2.1. Participants
2.2. Study Design
2.3. Anthropometric Measurements
2.4. BP and RHR
2.5. Body Composition, BMD and BMC Assessment
2.6. Heart Rate and Physical Demands during a Football Training Session
2.7. Statistical Analyses
3. Results
3.1. Whole-Body and Regional BMD and BMC
3.2. Anthropometrics, Body composition, Daily Caloric and Nutrient Intake, BP and RHR
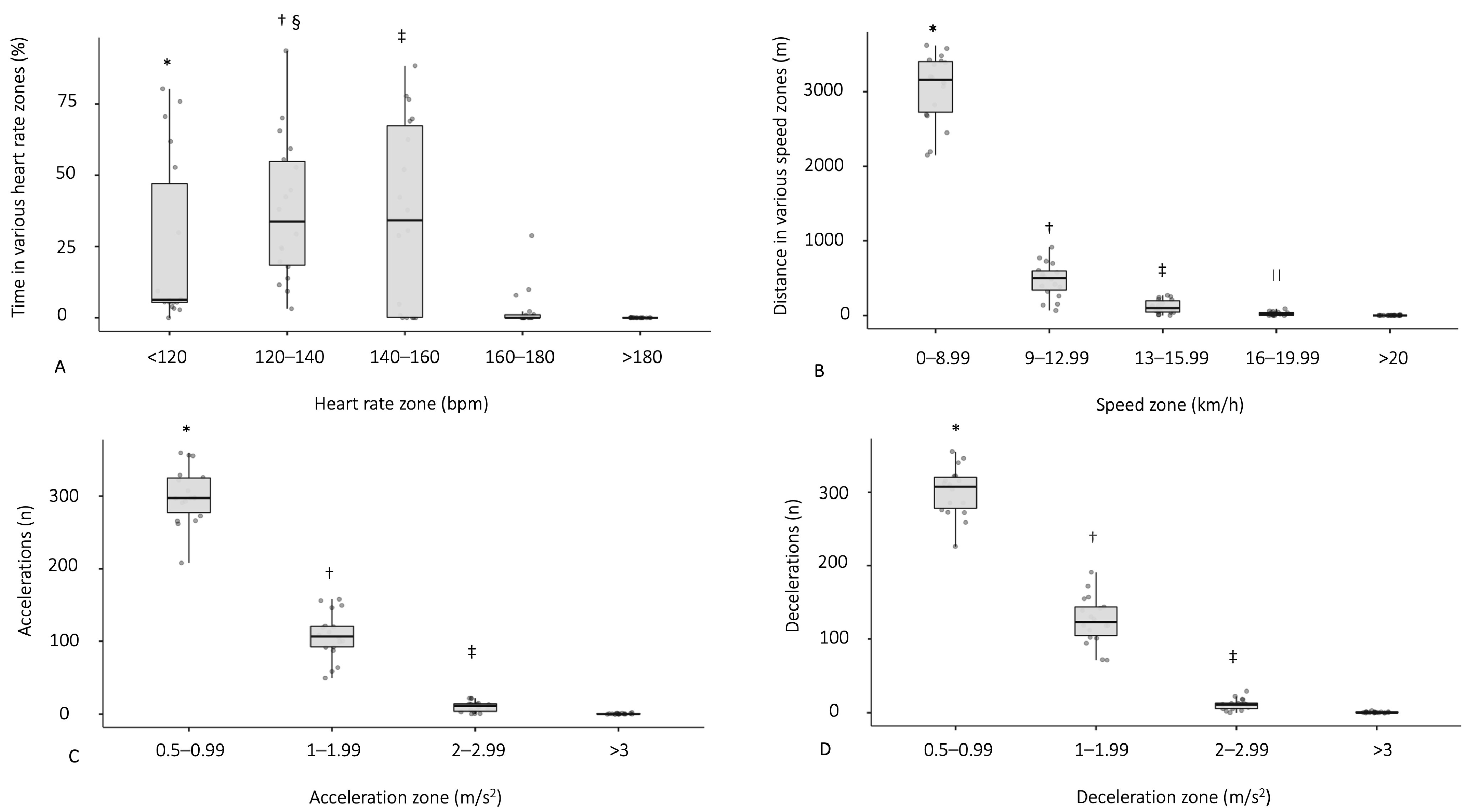
3.3. Physiological Demands during a Football Match
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 July 2023).
- Flatt, T.; Partridge, L. Horizons in the evolution of aging. BMC Biol. 2018, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Søreide, K. Molecular and biological hallmarks of ageing. BJS 2016, 103, e29–e46. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022, 16, 391. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S. SHARE central coordination team. Data resource profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A. Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front. Endocrinol. 2019, 24, 255. [Google Scholar] [CrossRef]
- Boonen, S.; Laan, R.F.; Barton, I.P.; Watts, N.B. Effect of osteoporosis treatments on risk of non-vertebral fractures: Review and meta-analysis of intention-to-treat studies. Osteoporos. Int. 2005, 16, 1291–1298. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.B.; Gamble, G.D.; Reid, I.R. Effect of osteoporosis treatment on mortality: A meta-analysis. J. Clin. Endocrinol. Metab. 2010, 95, 1174–1181. [Google Scholar] [CrossRef]
- D’Onofrio, G.; Kirschner, J.; Prather, H.; Goldman, D.; Rozanski, A. Musculoskeletal exercise: Its role in promoting health and longevity. Prog. Cardiovasc. Dis. 2023, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical exercise and health: A focus on its protective role in neurodegenerative diseases. J. Funct. Morphol. Kinesiol. 2022, 29, 38. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise promotes healthy aging of skeletal muscle. Cell. Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Z.; Čović, N.; Helge, E.W.; Krustrup, P.; Mohr, M. Recreational football and bone health: A systematic review and meta-analysis. Sports Med. 2022, 52, 3021–3037. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Mowle, S.; Noon, M.; Eyre, E.; Clarke, N.D.; Hill, M.; Tallis, J.; Julin, M. The Effect of 12-weeks recreational football (soccer) for health intervention on functional movement in older adults. Int. J. Environ. Res. Public Health 2022, 20, 13625. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, N.; Stojanović, E.; Stojiljković, N.; Nikolić, D.; Scanlan, A.T.; Milanović, Z. Exercise training in overweight and obese children: Recreational football and high-intensity interval training provide similar benefits to physical fitness. Scand. J. Med. Sci. Sports 2018, 28 (Suppl. S1), 18–32. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, H.; Manuel Clemente, F.; Marques, A.; Milanovic, Z.; David Harper, L.; Figueiredo, A. Recreational football is medicine against non-communicable diseases: A systematic review. Scand. J. Med. Sci. Sports 2020, 30, 618–637. [Google Scholar] [CrossRef]
- Milanović, Z.; Pantelić, S.; Čović, N.; Sporiš, G.; Mohr, M.; Krustrup, P. Broad-spectrum physical fitness benefits of recreational football: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 926–939. [Google Scholar] [CrossRef]
- Mohr, M.; Fatouros, I.G.; Asghar, M.; Buono, P.; Nassis, G.P.; Krustrup, P. Football training as a non-pharmacological treatment of the global aging population-A topical review. Front. Aging 2023, 9, 1146058. [Google Scholar] [CrossRef]
- Maimone, V.M.; Yasseri, T. Football is becoming more predictable; network analysis of 88 thousand matches in 11 major leagues. R. Soc. Open Sci. 2021, 15, 210617. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.F.; Andersen, T.R.; Andersen, L.J.; Randers, M.B.; Hornstrup, T.; Hansen, P.R.; Bangsbo, J.; Krustrup, P. Cardiovascular function is better in veteran football players than age-matched untrained elderly healthy men. Scand. J. Med. Sci. Sports 2015, 25, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Randers, M.B.; Nybo, L.; Petersen, J.; Nielsen, J.J.; Christiansen, L.; Bendiksen, M.; Brito, J.; Bangsbo, J.; Krustrup, P. Activity profile and physiological response to football training for untrained males and females, elderly and youngsters: Influence of the number of players. Scand. J. Med. Sci. Sports 2010, 20, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Meeusen, R.; Tiberi, M.; Corti, C.; Pagano, R.; Capranica, L. Aerobic and anaerobic profiles, heart rate and match analysis in older soccer players. Ergonomics 2006, 48, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Hagman, M.; Helge, E.W.; Hornstrup, T.; Fristrup, B.; Nielsen, J.J.; Jørgensen, N.R.; Andersen, J.L.; Helge, J.W.; Krustrup, P. Bone mineral density in lifelong trained male football players compared with young and elderly untrained men. J. Sport Health Sci. 2018, 7, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.D.; Field, A.; Corr, L.D.; Naughton, R.J. The physiological, physical, and biomechanical demands of walking football: Implications for exercise prescription and future research in older Adults. J. Aging Phys. Act. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Caspers, A.; Godhe, M.; Helge, T.; Eriksen, J.; Fransson, D.; Börjesson, M.; Ekblom-Bak, E. Walking football for Health—Physiological response to playing and characteristics of the players. Sci. Med. Footb. 2023, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Vitucci, D.; Labruna, G.; Imperlini, E.; Randers, M.B.; Schmidt, J.F.; Hagman, M.; Andersen, T.R.; Russo, R.; Orrù, S.; et al. Effect of lifelong football training on the expression of muscle molecular markers involved in healthy longevity. Eur. J Appl. Physiol. 2017, 117, 721–730. [Google Scholar] [CrossRef]
- Mancini, A.; Vitucci, D.; Randers, M.B.; Schmidt, J.F.; Hagman, M.; Andersen, T.R.; Imperlini, E.; Mandola, A.; Orrù, S.; Krustrup, P.; et al. Lifelong Football Training: Effects on Autophagy and Healthy Longevity Promotion. Front. Physiol. 2019, 19, 132. [Google Scholar] [CrossRef]
- Fox, J.L.; O’Grady, C.J.; Scanlan, A.T.; Sargent, C.; Stanton, R. Validity of the polar team pro sensor for measuring speed and distance indoors. J. Sci. Med. Sport 2019, 22, 1260–1265. [Google Scholar] [CrossRef]
- Reinhardt, L.; Schwesig, R.; Lauenroth, A.; Schulze, S.; Kurz, E. Enhanced sprint performance analysis in soccer: New insights from a GPS-based tracking system. PLoS ONE 2019, 31, e0217782. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Skoradal, M.B.; Helge, E.W.; Jørgensen, N.R.; Mortensen, J.; Weihe, P.; Krustrup, P.; Mohr, M. Osteogenic impact of football training in 55- to 70-year-old women and men with prediabetes. Scand. J. Med. Sci. Sports 2018, 28, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Helge, E.W.; Aagaard, P.; Jakobsen, M.D.; Sundstrup, E.; Randers, M.B.; Karlsson, M.K.; Krustrup, P. Recreational football training decreases risk factors for bone fractures in untrained premenopausal women. Scand. J Med. Sci. Sports 2010, 20, 31–39. [Google Scholar] [CrossRef]
- Mohr, M.; Helge, E.W.; Petersen, L.F.; Lindenskov, A.; Weihe, P.; Mortensen, J.; Jørgensen, N.R.; Krustrup, P. Effects of soccer vs swim training on bone formation in sedentary middle-aged women. Eur. J. Appl. Physiol. 2015, 115, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Kapoor, N.; Bondu, J.D.; Thomas, N.; Paul, T.V. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 2016, 20, 846–852. [Google Scholar]
- Alghadir, A.H.; Aly, F.A.; Gabr, S.A. Effect of moderate aerobic training on bone metabolism indices among adult humans. Pak. J. Med. Sci. 2014, 30, 840–844. [Google Scholar] [CrossRef]
- Stunes, A.K.; Brobakken, C.L.; Sujan, M.A.J.; Aagård, N.; Brevig, M.S.; Wang, E.; Syversen, U.; Mosti, M.P. Acute effects of strength and endurance training on bone turnover markers in young adults and elderly men. Front. Endocrinol. 2022, 30, 91524. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2009, 2, 693–724. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 4, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Bilezikian, J.P.; Vanderschueren, D. Osteoporosis in Men: The Effects of Gender on Skeletal Health, 2nd ed.; Elsevier Inc.: London, UK, 2009; pp. 207–219. [Google Scholar]
- Mourier, A.; Gautier, J.F.; De, K.E.; Bigard, A.X.; Villette, J.M.; Garnier, J.P.; Duvallet, A.; Guezennec, C.Y.; Cathelineau, G. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Effects of branched-chain amino acid supplements. Diabetes Care 1997, 20, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Davis, C.K.; Brock, D.W.; Weltman, J.Y.; Swift, D.; Barrett, E.J.; Gaesser, G.A.; Weltman, A. Effect of exercise training intensity on abdominal visceral fat and body composition. Med. Sci. Sports Exerc. 2008, 40, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Schjerve, I.E.; Tyldum, G.A.; Tjonna, A.E.; Stolen, T.; Loennechen, J.P.; Hansen, H.E.; Haram, P.M.; Heinrich, G.; Bye, A.; Najjar, S.M.; et al. Both aerobic endurance and strength training programs improve cardiovascular health in obese adults. Clin. Sci. 2008, 115, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Norregaard, L.; Thorso, F. Activity profile of competition soccer. Can. J. Sport Sci. 1991, 6, 110–116. [Google Scholar]
- Rienzi, E.; Drust, B.; Reilly, T.; Carter, J.E.; Martin, A. Investigation of anthropometric and work-rate profiles of elite South American international soccer players. J. Sports Med. Phys. Fit 2000, 40, 162–169. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [PubMed]
- Velluzzi, F.; Cossu, G.; Fosci, M.; Montisci, R.; Zaccheddu, R.; Minerba, L.; Musu, M.; Pintus, E.; Fortin, D.; Romano, F.; et al. Effect of a low-moderate exercise program on dysmetabolism in older adults: Results of a randomized controlled trial. Nutrients 2022, 15, 3337. [Google Scholar] [CrossRef]
- Pescatello, L.S.; MacDonald, H.V.; Lamberti, L.; Johnson, B.T. Exercise for hypertension: A prescription update integrating existing recommendations with emerging research. Curr. Hypertens. Rep. 2015, 17, 87. [Google Scholar] [CrossRef]
- Collins, J.; Maughan, R.J.; Gleeson, M.; Bilsborough, J.; Jeukendrup, A.; Morton, J.P.; Phillips, S.M.; Armstrong, L.; Burke, L.M.; Close, G.L.; et al. UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research. Br. J. Sports Med. 2021, 55, 416. [Google Scholar] [CrossRef]
- Beato, M.; Impellizzeri, F.M.; Coratella, G.; Schena, F. Quantification of energy expenditure of recreational football. J. Sports Sci. 2016, 34, 2185–2188. [Google Scholar] [CrossRef]
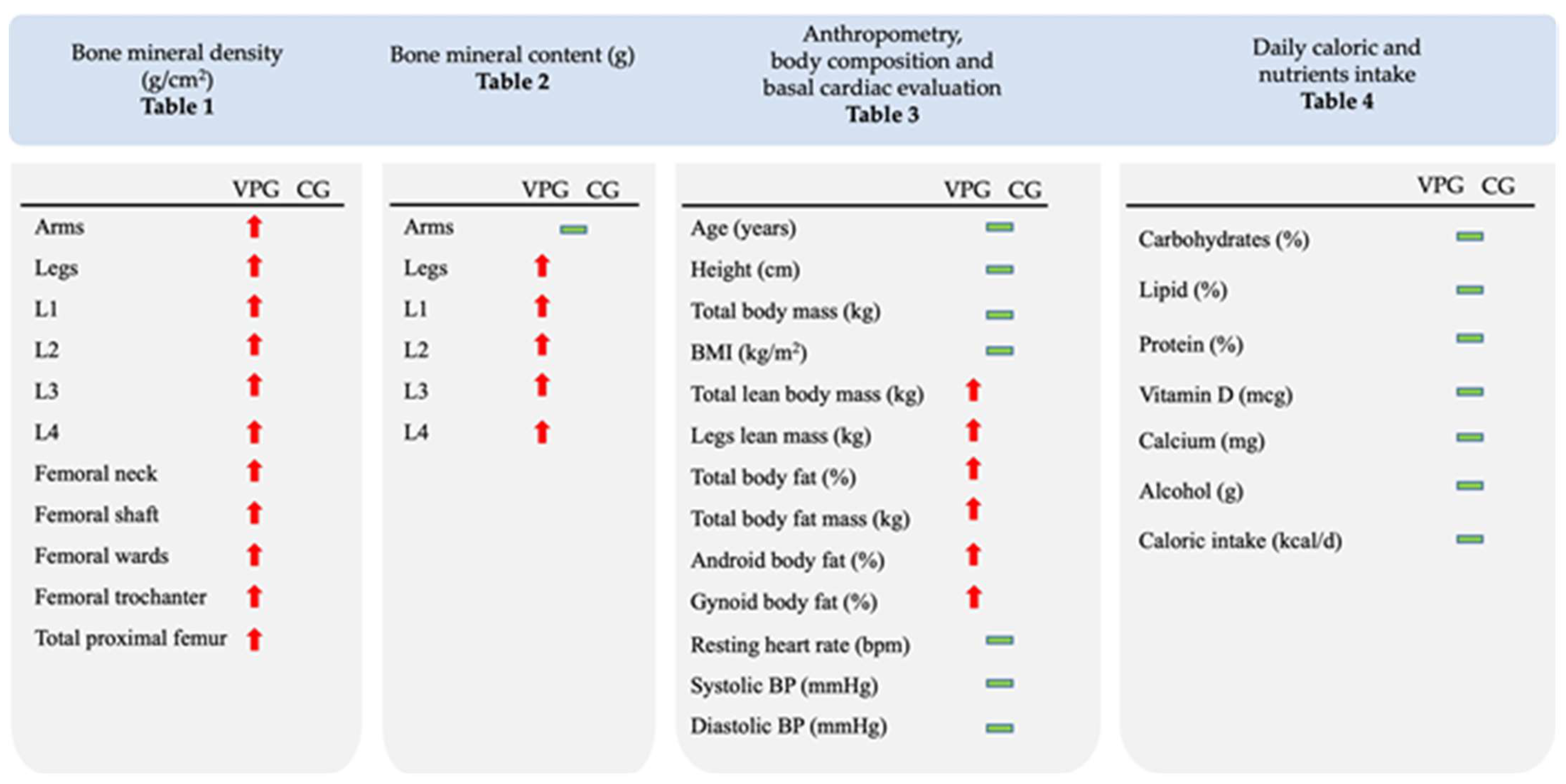
| VPG (n = 20) | CG (n = 18) | Delta (%) | p-Value | ES | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Whole-body | 1.344 ± 0.131 | 1.187 ± 0.072 | 13.2 | <0.001 | 1.660 | 0.820 | 2.470 |
| Arms | 1.047 ± 0.096 | 0.972 ± 0.108 | 7.7 | 0.030 | 0.734 | 0.048 | 1.400 |
| Legs | 1.423 ± 0.165 | 1.218 ± 0.089 | 16.8 | <0.001 | 1.520 | 0.708 | 2.300 |
| L1 | 1.196 ± 0.156 | 1.053 ± 0.131 | 13.6 | 0.005 | 0.986 | 0.257 | 1.690 |
| L2 | 1.365 ± 0.299 | 1.187 ± 0.155 | 15.0 | 0.009 | 0.898 | 0.191 | 1.590 |
| L3 | 1.460 ± 0.265 | 1.223 ± 0.184 | 19.4 | 0.003 | 1.040 | 0.311 | 1.740 |
| L4 | 1.523 ± 0.241 | 1.252 ± 0.219 | 21.6 | <0.001 | 1.290 | 0.520 | 2.030 |
| Femoral neck | |||||||
| Right | 0.916 ± 0.135 | 0.847 ± 0.092 | 8.1 | 0.048 | 0.585 | 0.094 | 1.256 |
| Left | 0.950 ± 0.154 | 0.827 ± 0.102 | 14.9 | 0.010 | 0.919 | 0.190 | 1.634 |
| Femoral shaft | |||||||
| Right | 1.167 ± 0.162 | 1.048 ± 0.159 | 11.4 | 0.030 | 0.745 | 0.047 | 1.420 |
| Left | 1.192 ± 0.155 | 1.057 ± 0.162 | 12.8 | 0.016 | 0.854 | 0.133 | 1.554 |
| Femoral wards | |||||||
| Right | 0.755 ± 0.137 | 0.616 ± 0.071 | 22.6 | <0.001 | 1.245 | 0.476 | 1.993 |
| Left | 0.760 ± 0.148 | 0.631 ± 0.080 | 20.4 | <0.001 | 1.055 | 0.306 | 1.780 |
| Femoral trocanter | |||||||
| Right | 0.935 ± 0.142 | 0.810 ± 0.111 | 15.4 | 0.006 | 0.966 | 0.240 | 1.675 |
| Left | 0.937 ± 0.120 | 0.814 ± 0.103 | 15.1 | 0.003 | 1.090 | 0.336 | 1.824 |
| Total proximal femur | |||||||
| Right | 1.030 ± 0.130 | 0.916 ± 0.115 | 12.4 | 0.009 | 0.917 | 0.198 | 1.620 |
| Left | 1.047 ± 0.129 | 0.918 ± 0.115 | 14.1 | 0.004 | 1.039 | 0.293 | 1.764 |
| VPG (n = 20) | CG (n = 18) | Delta (%) | p-Value | ES | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Whole-body | 3248.0 ± 411.0 | 2869.2 ± 264.4 | 13.2 | 0.002 | 1.085 | 148.67 | 608.80 |
| Arms | 446.1 ± 63.9 | 419.6 ± 41.78 | 6.3 | 0.145 | 0.484 | 9.530 | 62.41 |
| Legs | 1297.4 ± 196.7 | 1100.1 ± 121.4 | 17.9 | <0.001 | 1.192 | 88.21 | 306.37 |
| L1 | 19.4 ± 3.71 | 16.0 ± 3.22 | 21.3 | 0.006 | 0.964 | 1.030 | 5.71 |
| L2 | 22.5 ± 4.94 | 18.6 ± 4.11 | 21.0 | 0.014 | 0.843 | 0.84 | 6.86 |
| L3 | 26.7 ± 6.54 | 20.8 ± 4.34 | 28.4 | 0.003 | 1.056 | 2.22 | 9.61 |
| L4 | 30.5 ± 5.72 | 22.9 ± 4.63 | 33.2 | <0.001 | 1.468 | 4.23 | 11.12 |
| Variable (Units) | VPG (n = 20) | CG (n = 18) | Delta(%) | p-Value | ES | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age (years) | 73.4 ± 3.7 | 75.6 ± 4.2 | 2.9 | 0.060 | 0.554 | 0.113 | 1.214 |
| Height (cm) | 174.1 ± 4.8 | 176.4 ± 5.2 | 1.3 | 0.930 | 0.464 | 0.197 | 1.114 |
| Total body mass (kg) | 81.9 ± 8.3 | 81.6 ± 11.3 | 0.4 | 0.160 | 0.027 | −0.610 | 0.668 |
| BMI (kg/m2) | 27.0 ± 2.7 | 26.2 ±3.6 | 3.0 | 0.450 | 0.248 | −0.409 | 0.882 |
| Total lean body mass (kg) | 57.3 ± 4.4 | 53.6 ± 7.1 | 6.9 | 0.043 | 0.673 | 0.002 | 1.347 |
| Leg lean mass (kg) | 19.2 ± 1.8 | 17.6 ± 2.7 | 9.1 | 0.019 | 0.447 | −0.003 | 1.339 |
| Total body fat (%) | 25.4 ± 5.7 | 30.1 ± 3.8 | 4.7 | 0.005 | 0.977 | 0.250 | 1.662 |
| Total body fat mass (kg) | 21.0 ± 6.6 | 24.5 ± 5.4 | 14.3 | 0.047 | 0.057 | −1.228 | 0.095 |
| Android body fat (%) | 33.3 ± 7.8 | 40.0 ± 7.1 | 6.7 | 0.009 | 0.896 | −0.189 | 1.583 |
| Gynoid body fat (%) | 23.8 ± 5.4 | 29.3 ± 3.8 | 5.5 | 0.001 | 1.169 | −0.411 | 1.880 |
| Resting heart rate (bpm) | 60.7 ± 10.1 | 62.6 ± 11.9 | 3.0 | 0.595 | 0.174 | −0.811 | 0.467 |
| Systolic BP (mmHg) | 137.8 ± 14.5 | 135.3 ± 15.1 | 1.8 | 0.598 | 0.173 | −0.468 | 0.810 |
| Diastolic BP (mmHg) | 80.3 ± 8.4 | 78.4 ± 9.0 | 2.4 | 0.508 | 0.217 | −0.426 | 0.855 |
| Variable (Units) | VPG (n = 19) | CG (n = 9) | p-Value |
|---|---|---|---|
| Carbohydrates (%) | 40.0 ± 8.6 | 39.8 ± 3.9 | 0.947 |
| Lipid (%) | 39.5 ± 7.9 | 40.0 ± 4.9 | 0.859 |
| Protein (%) | 17.2 ± 3.7 | 18.6 ± 3.8 | 0.362 |
| Vitamin D (mcg) | 2.8 ± 3.2 | 2.4 ± 1.8 | 0.885 |
| Calcium (mg) | 526.1 ± 276.8 | 603.5 ± 170.0 | 0.449 |
| Alcohol (g) | 19.6 ± 17.2 | 23.3 ± 20.0 | 0.977 |
| Caloric intake (kcal/d) | 1878.7 ± 491.1 | 1823.8 ± 493.7 | 0.785 |
| Variable (Units) | Mean ± SD (Min–Max) |
|---|---|
| Mean HR (bpm) | 131 ± 15 (108–157) |
| Peak HR (bpm) | 155 ± 18 (126–191) |
| ThHR max (bpm) | 157 ± 2 (155–159) |
| Mean HR of ThHR max (%) | 83.9 ± 8.6 (72.0–98.7) |
| Peak of ThHRmax (%) | 98.6 ± 10.2 (83.7–120.7) |
| Total distance (m) | 3666 ± 721 (2260–4539) |
| Relative distance (m/min) | 61 ± 12 (38–76) |
| Maximal speed (km/h) | 18.6 ± 3.0 (12.8–26.9) |
| Average speed (km/h) | 3.9 ± 0.7 (2.6–4.8) |
| Number of sprints > 18 km/h (n) | 0.4 ± 0.6 (0.0–2.0) |
| Total number of accelerations (n) | 419 ± 61 (294–529) |
| Total number of accelerations by zone (n): | |
| 0.5–0.99 m/s2 | 300 ± 38 (208–360) |
| 1–1.99 m/s2 | 108 ± 32 (49–158) |
| 2–2.99 m/s2 | 10 ± 7 (0–22) |
| >3 m/s2 | 0 ± 1 (0–2) |
| Total number of decelerations (n) | 428 ± 65 (286–552) |
| Total number of decelerations by zone (n): | |
| 0.5–0.99 m/s2 | 300 ± 33 (226–355) |
| 1–1.99 m/s2 | 126 ± 32 (71–191) |
| 2–2.99 m/s2 | 11 ± 7 (0–28) |
| >3 m/s2 | 1 ± 1 (0–3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martone, D.; Vitucci, D.; Mancini, A.; Ermidis, G.; Panduro, J.; Cosco, L.F.; Randers, M.B.; Larsen, M.N.; Mohr, M.; Buono, P.; et al. Bone Health, Body Composition and Physiological Demands in 70–85-Year-Old Lifelong Male Football Players. Sports 2023, 11, 205. https://doi.org/10.3390/sports11100205
Martone D, Vitucci D, Mancini A, Ermidis G, Panduro J, Cosco LF, Randers MB, Larsen MN, Mohr M, Buono P, et al. Bone Health, Body Composition and Physiological Demands in 70–85-Year-Old Lifelong Male Football Players. Sports. 2023; 11(10):205. https://doi.org/10.3390/sports11100205
Chicago/Turabian StyleMartone, Domenico, Daniela Vitucci, Annamaria Mancini, Georgios Ermidis, Jeppe Panduro, Loretta Francesca Cosco, Morten Bredsgaard Randers, Malte Nejst Larsen, Magni Mohr, Pasqualina Buono, and et al. 2023. "Bone Health, Body Composition and Physiological Demands in 70–85-Year-Old Lifelong Male Football Players" Sports 11, no. 10: 205. https://doi.org/10.3390/sports11100205
APA StyleMartone, D., Vitucci, D., Mancini, A., Ermidis, G., Panduro, J., Cosco, L. F., Randers, M. B., Larsen, M. N., Mohr, M., Buono, P., & Krustrup, P. (2023). Bone Health, Body Composition and Physiological Demands in 70–85-Year-Old Lifelong Male Football Players. Sports, 11(10), 205. https://doi.org/10.3390/sports11100205









