Scatter Irradiation of Rat Brain Triggers Sex- and Brain Region-Specific Changes in the Expression of Non-Coding RNA Fragments
Abstract
1. Introduction
2. Results
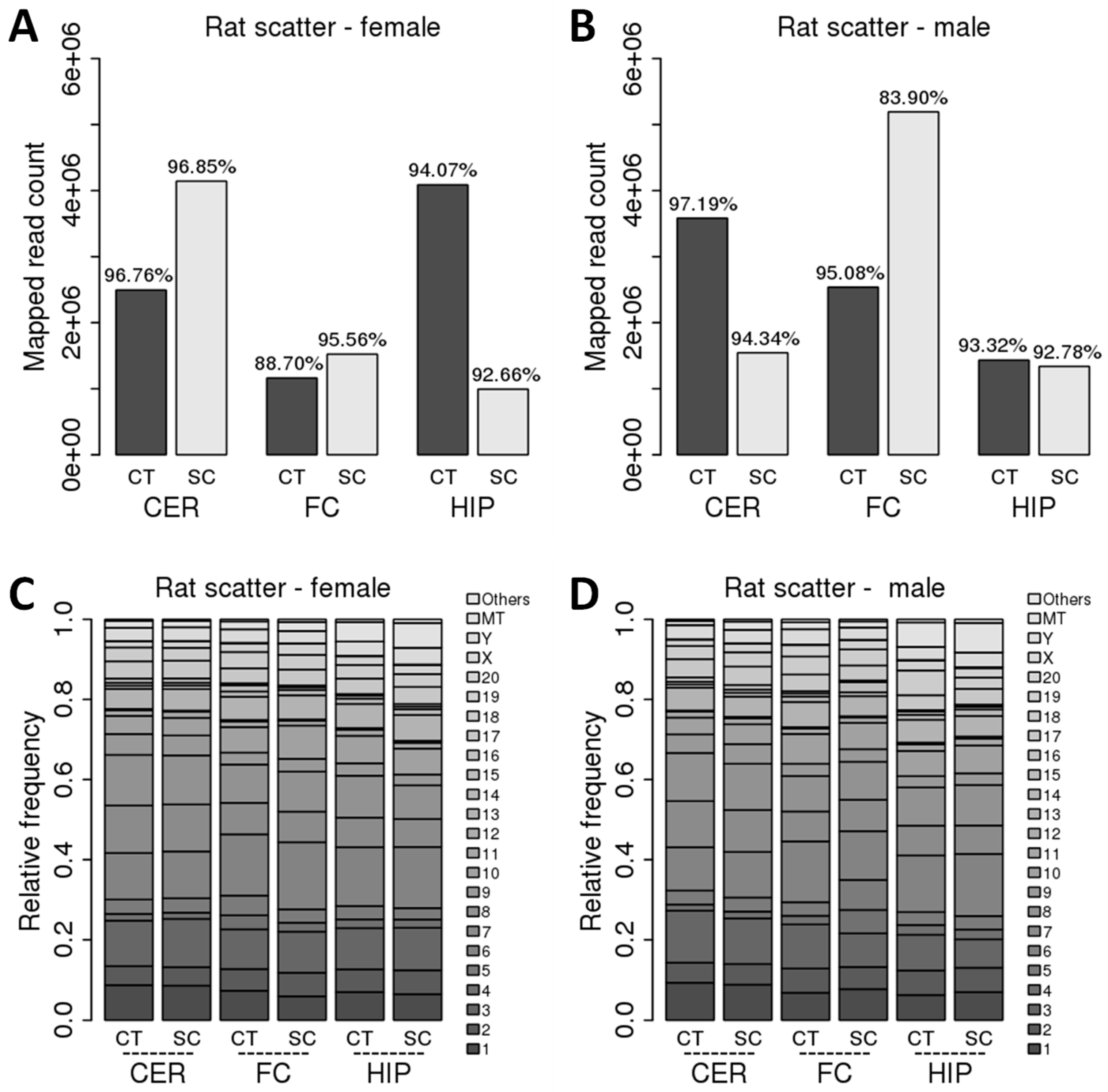
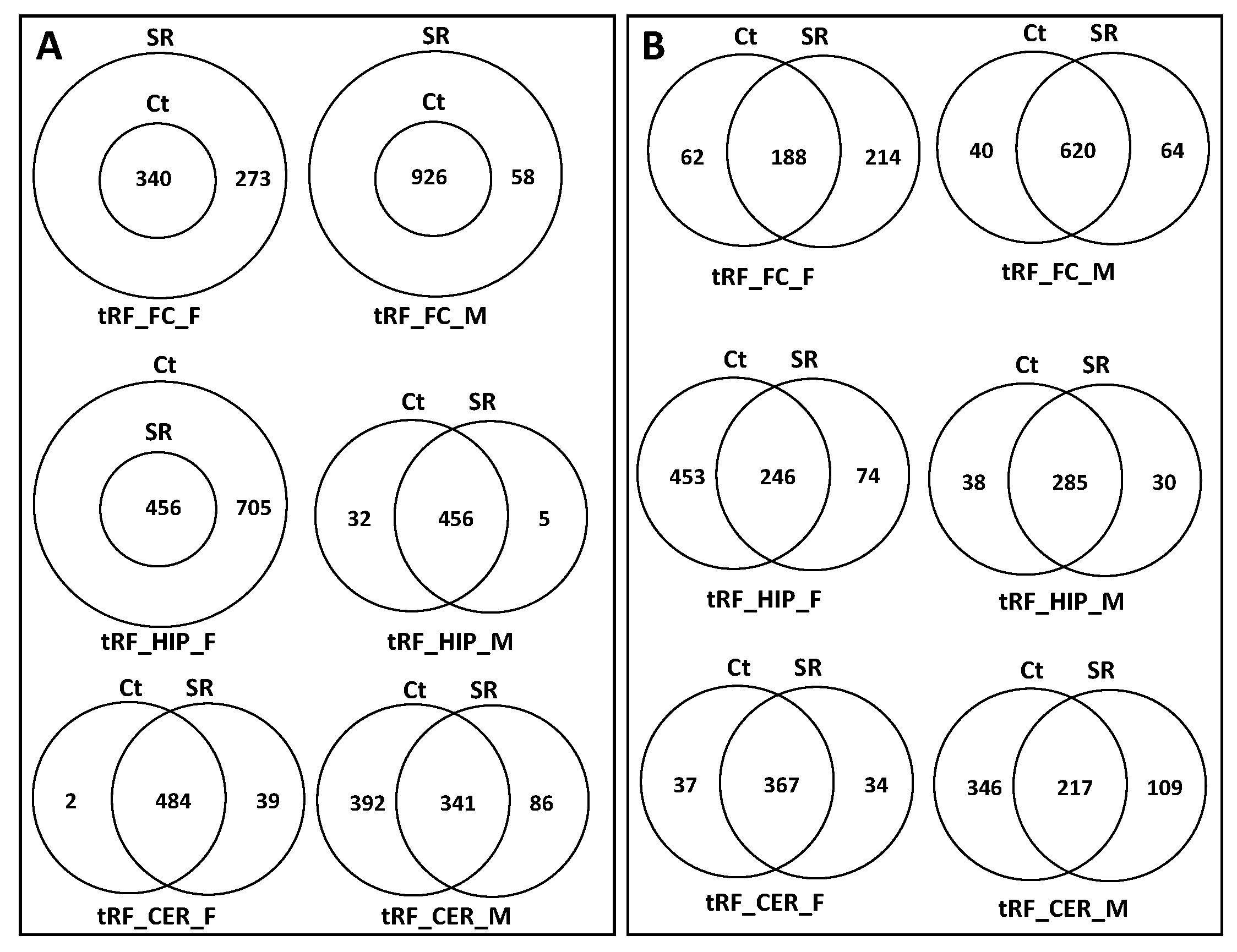
2.1. Comparison of ncRNA Reads between Different Brain Regions in Male and Female Rats in Control versus Scatter Radiation Exposure
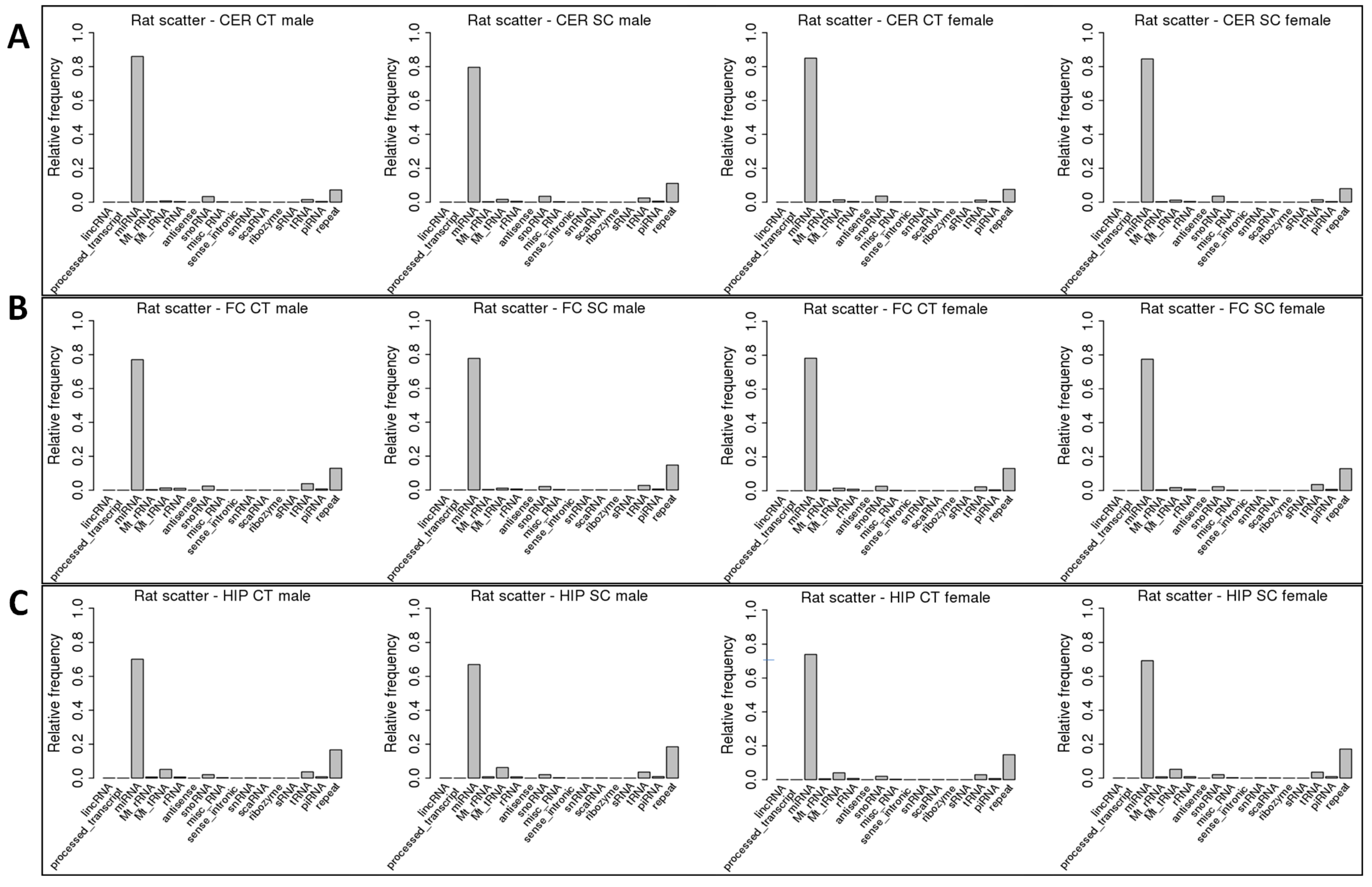
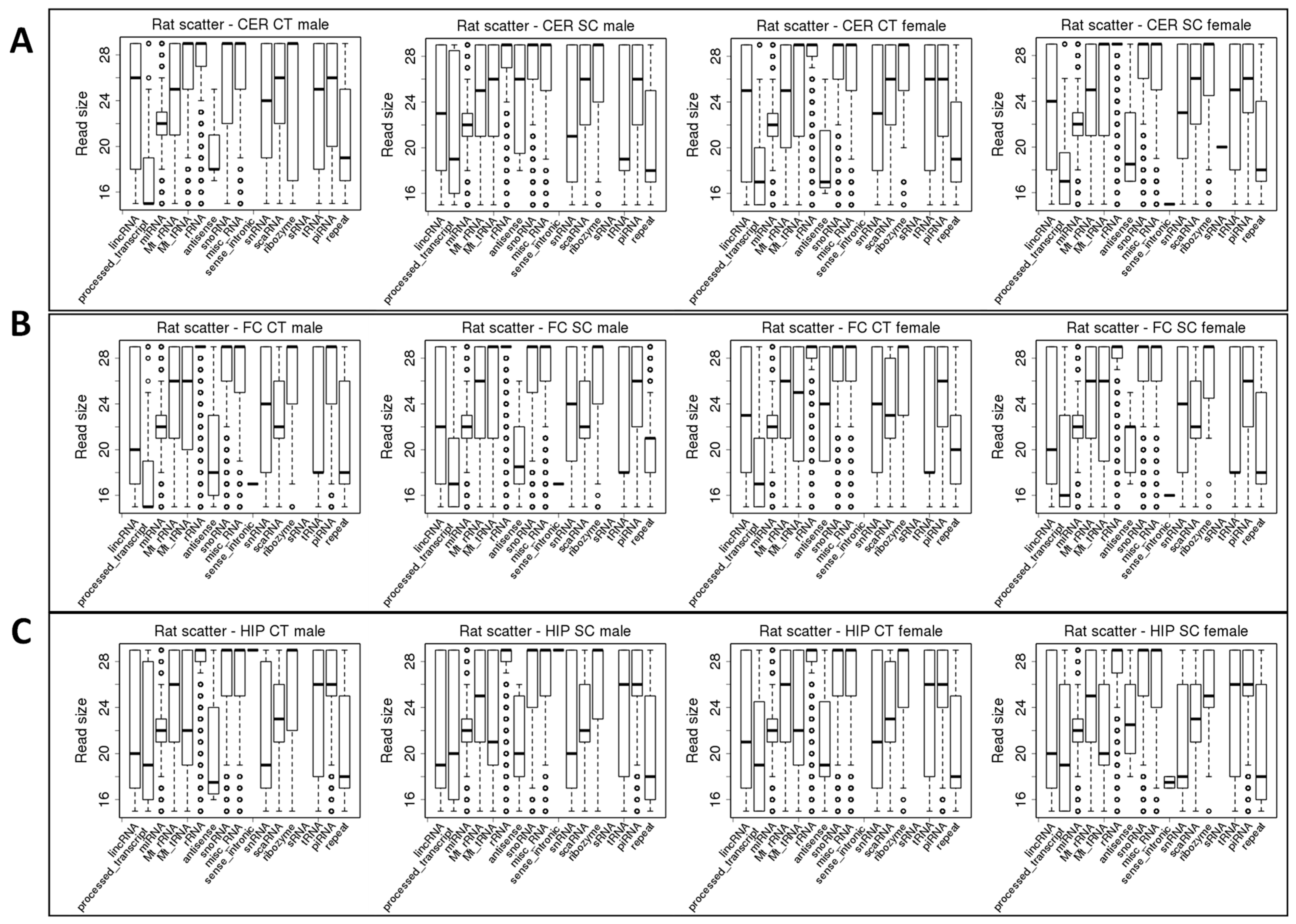
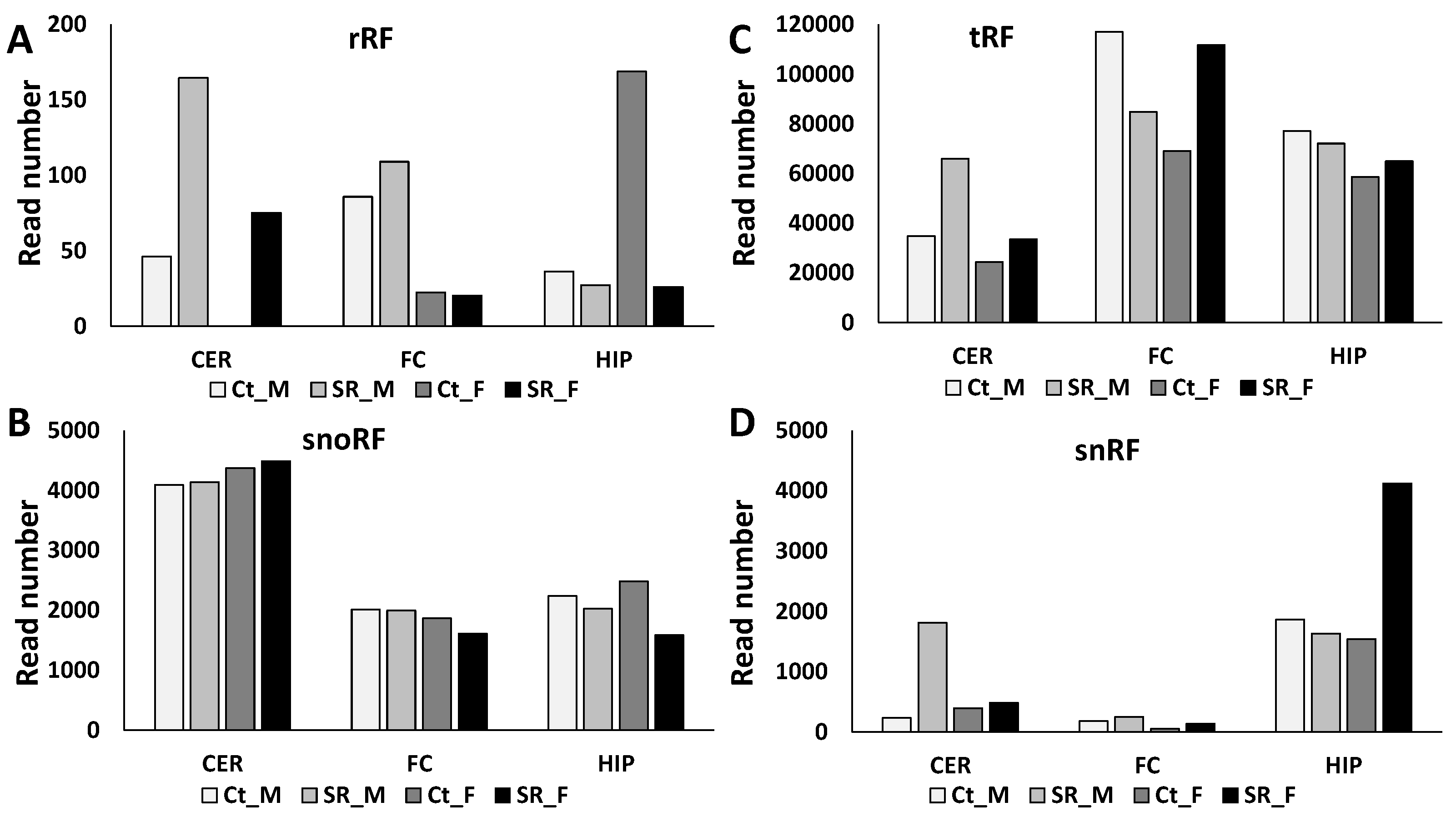
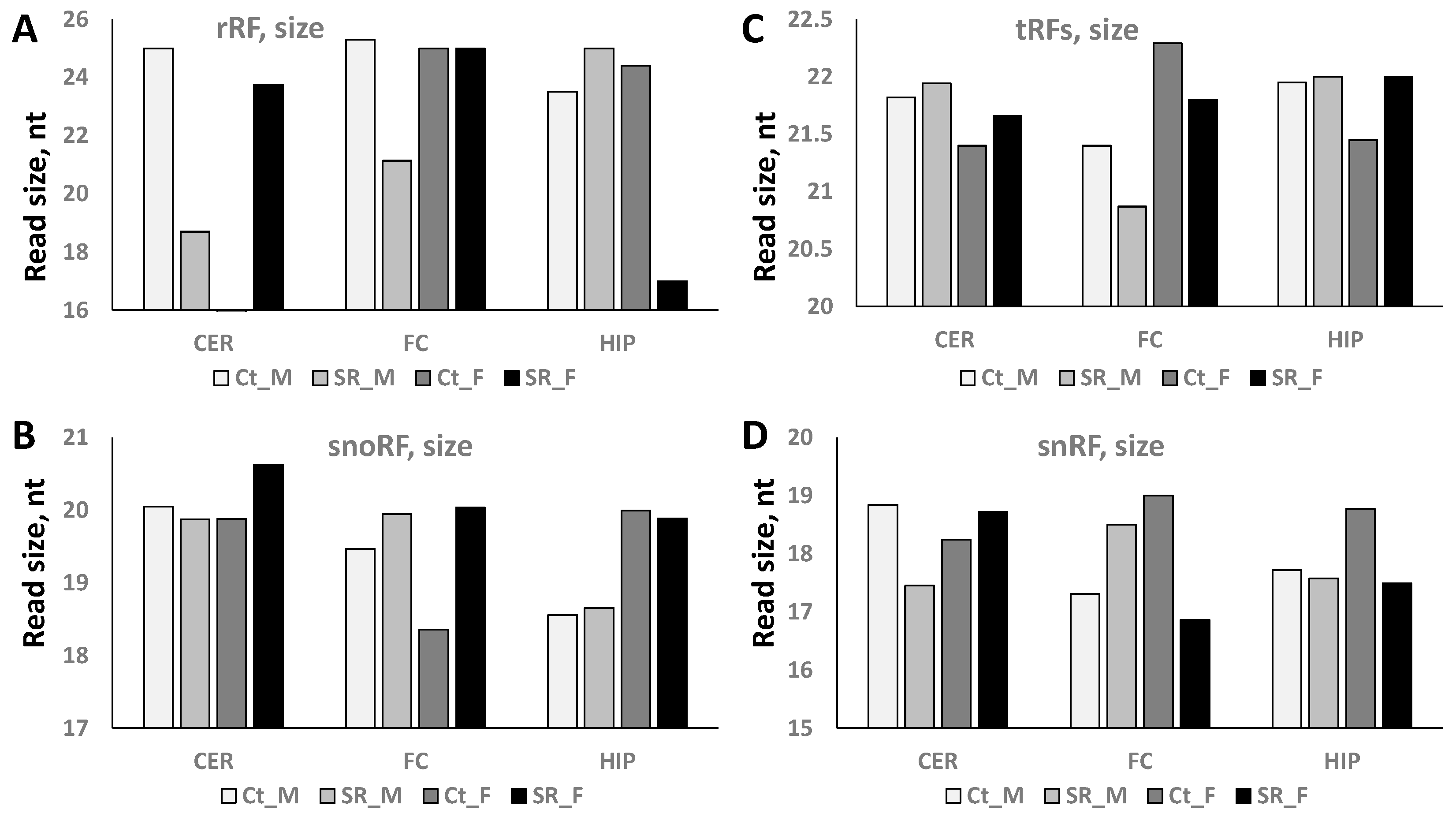
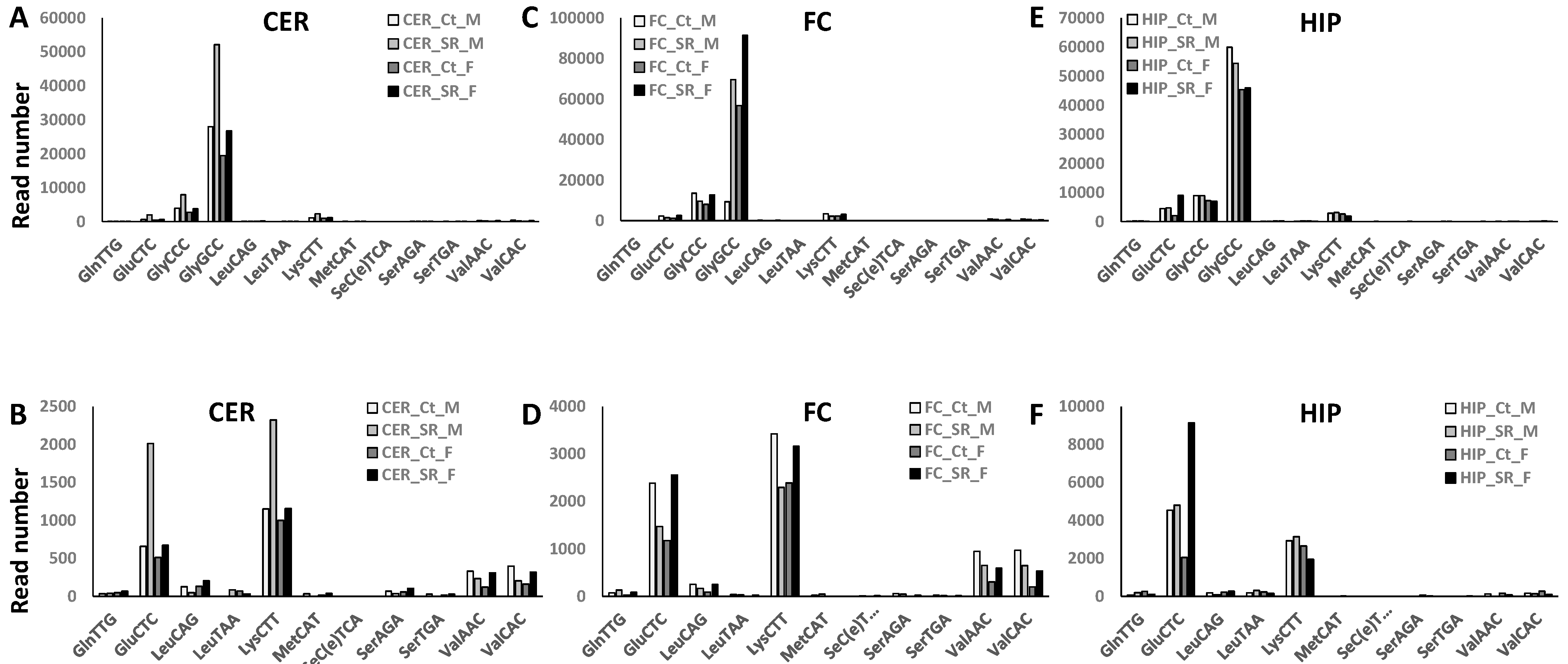
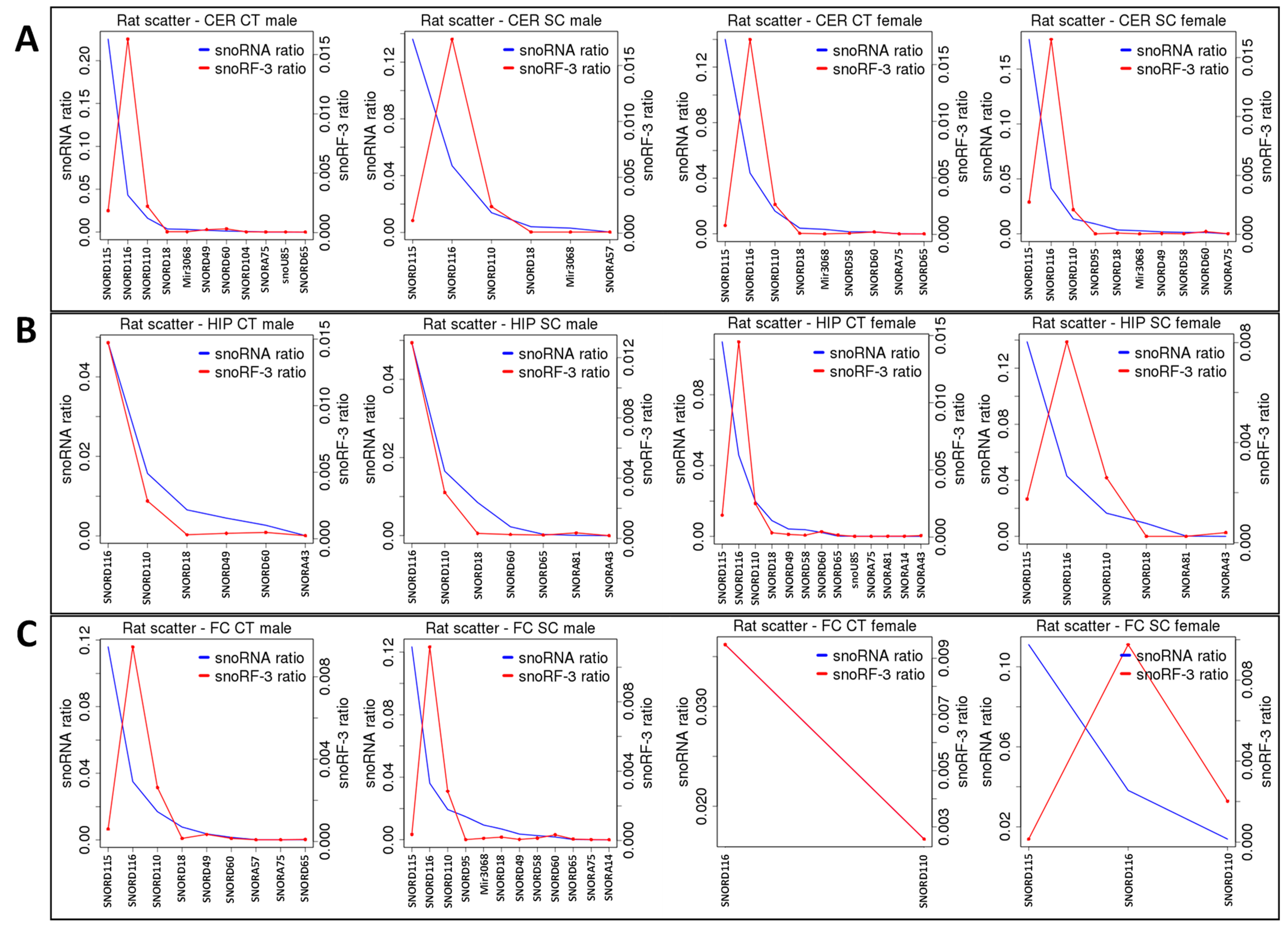
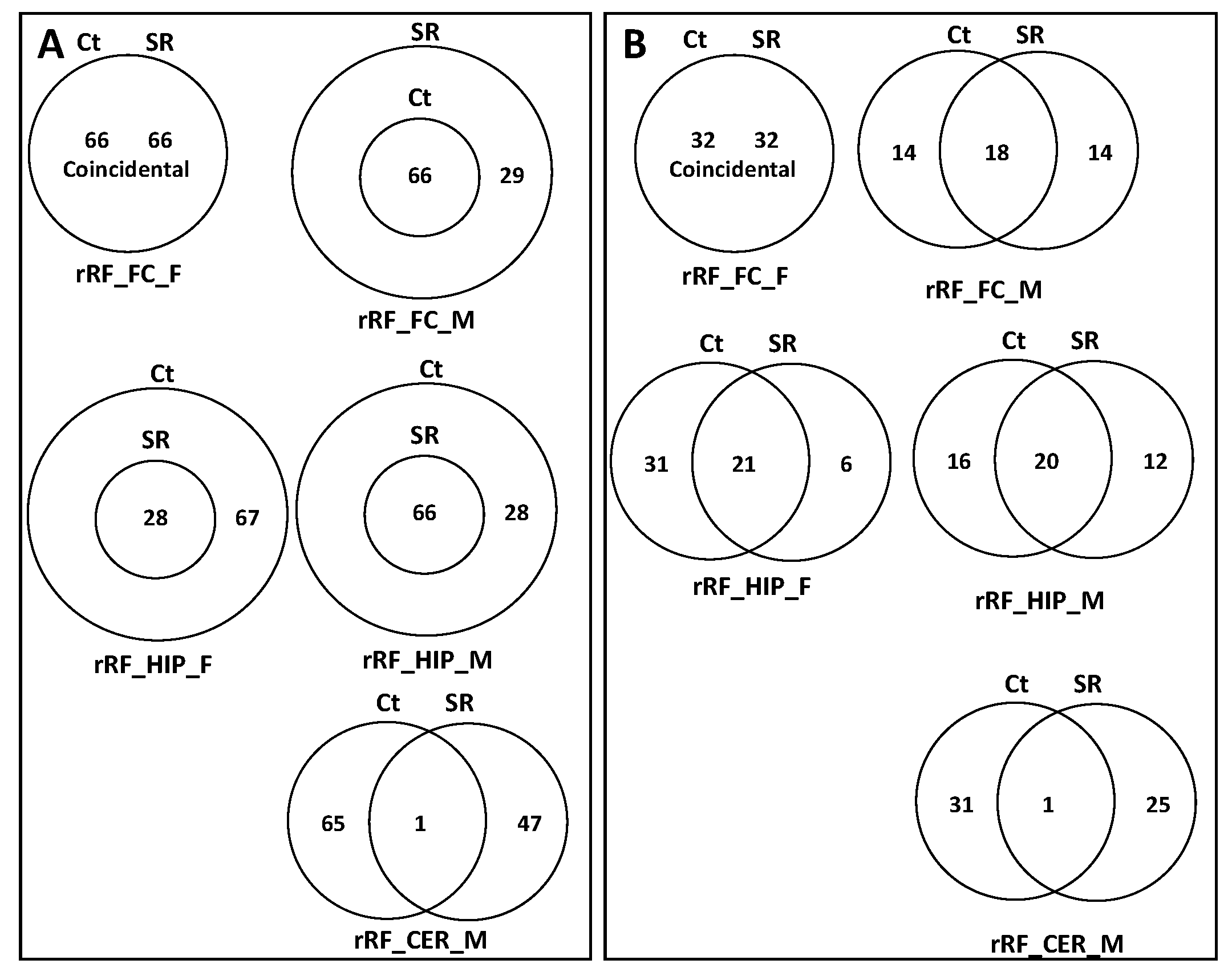
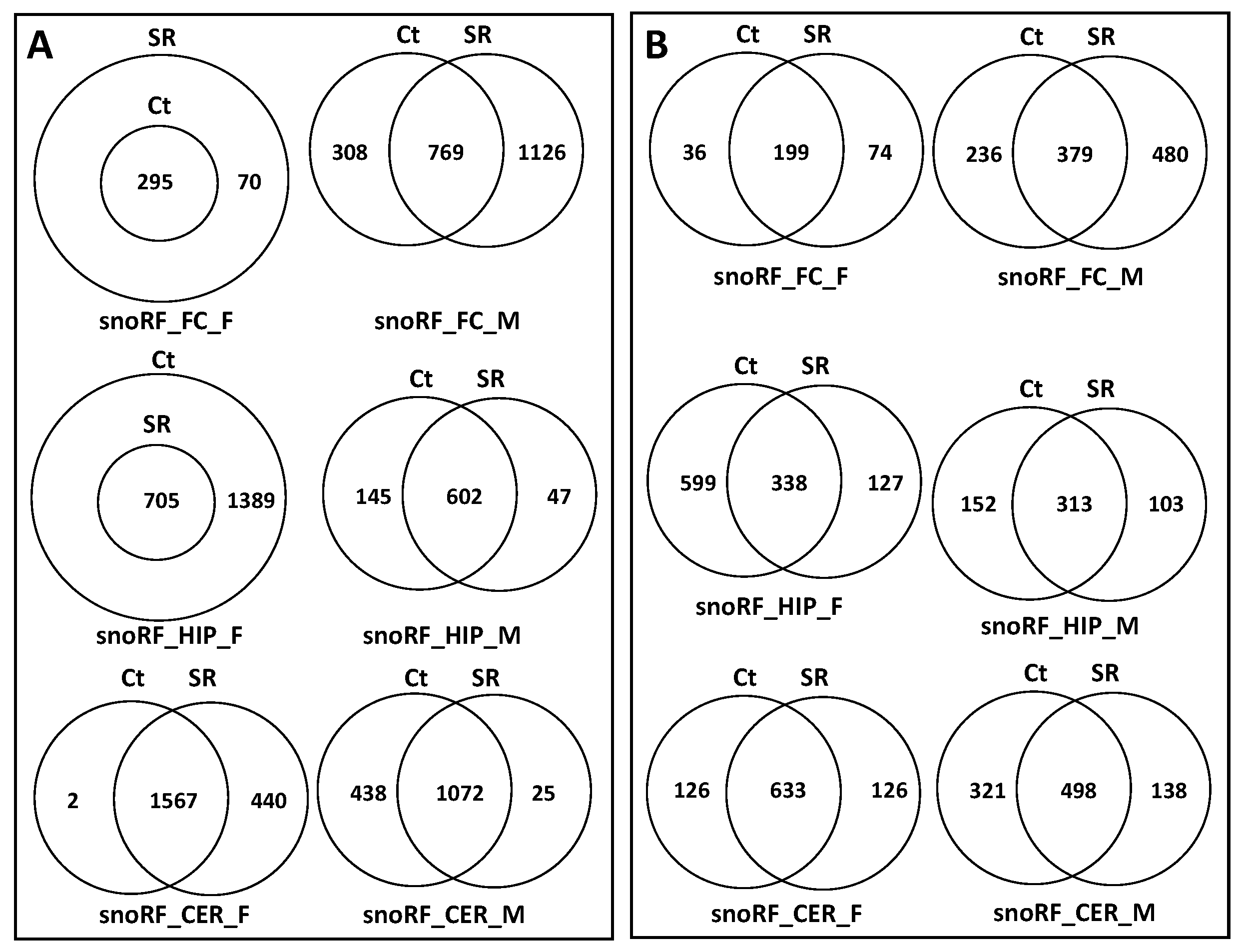
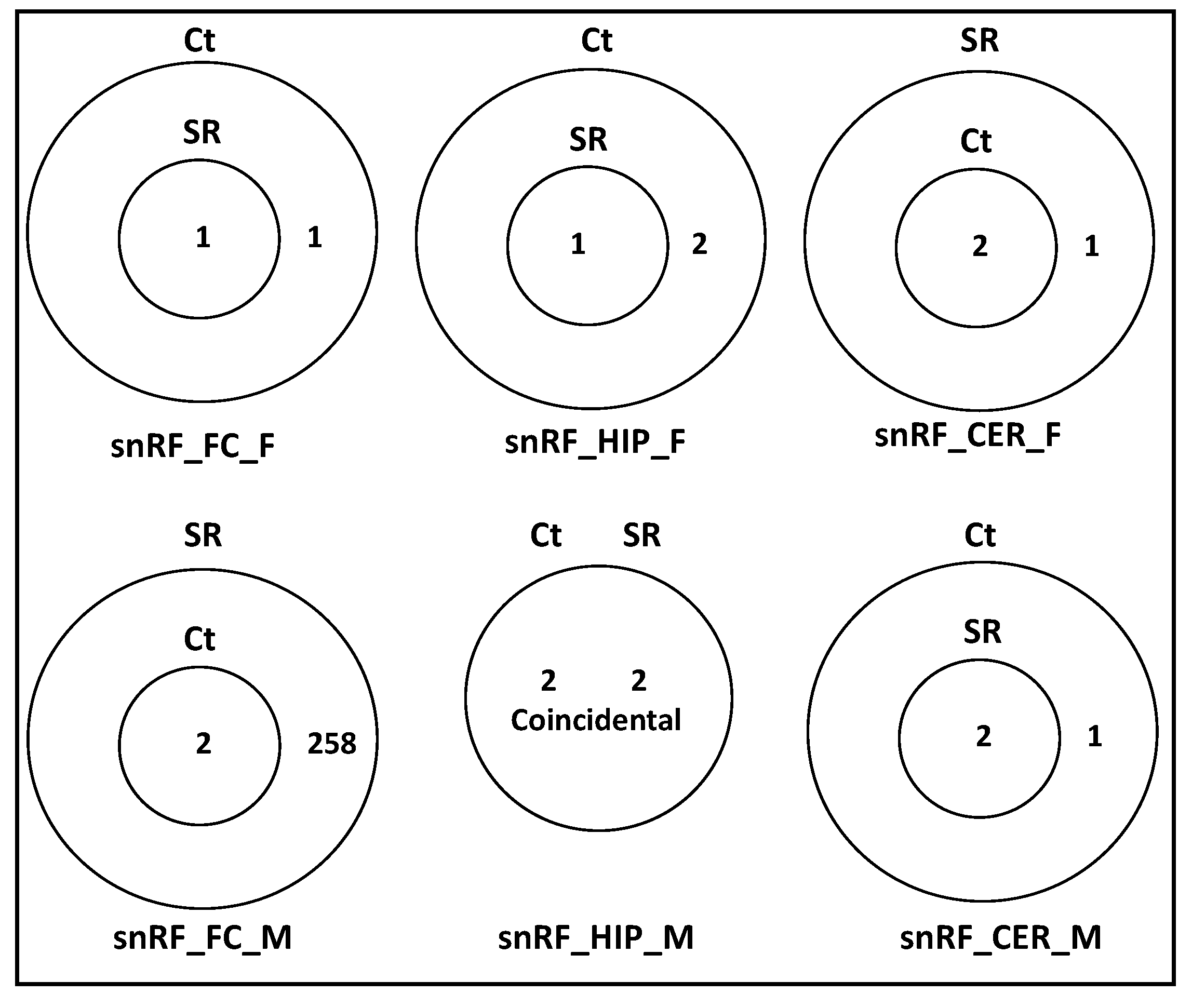
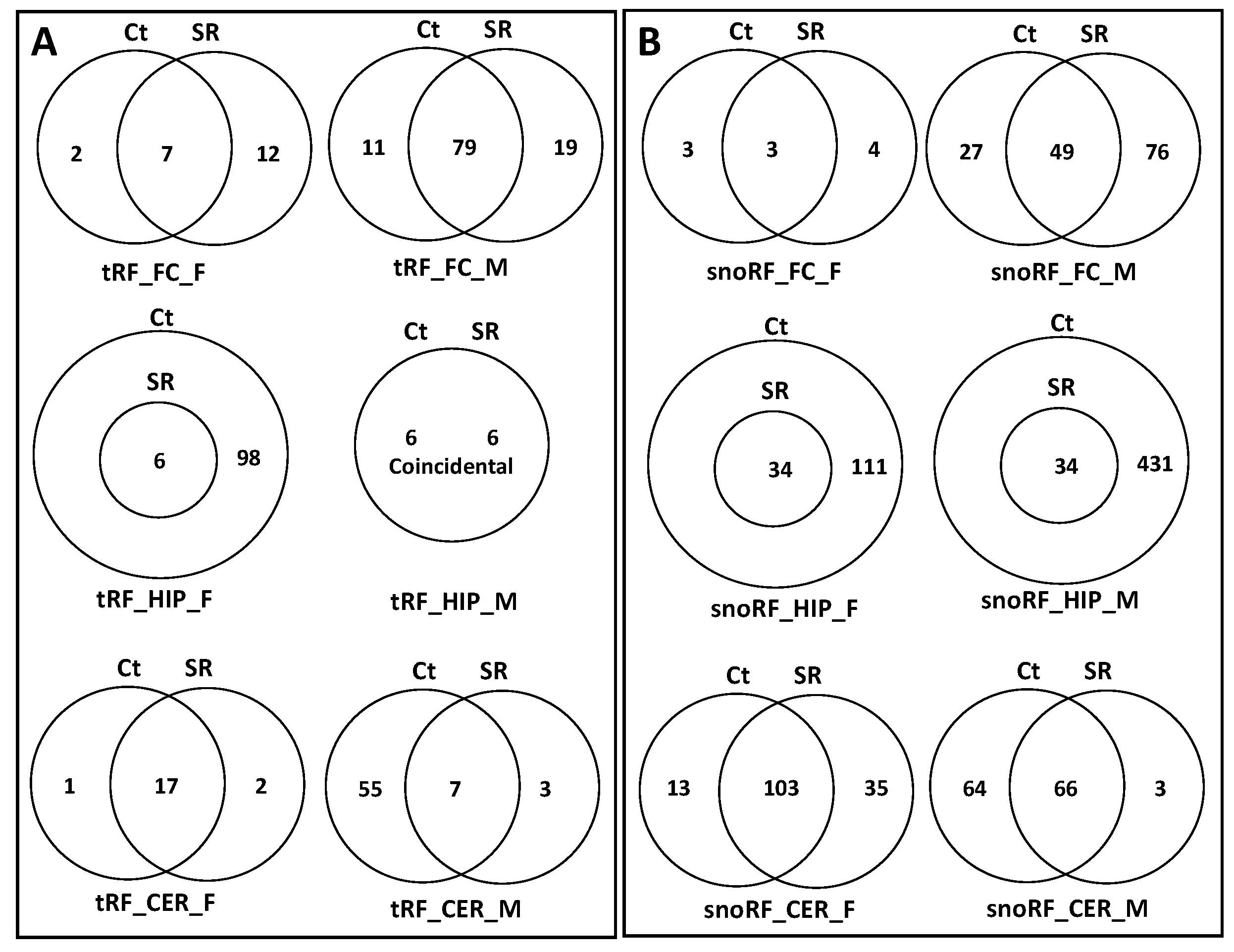
2.2. ncRF Analysis
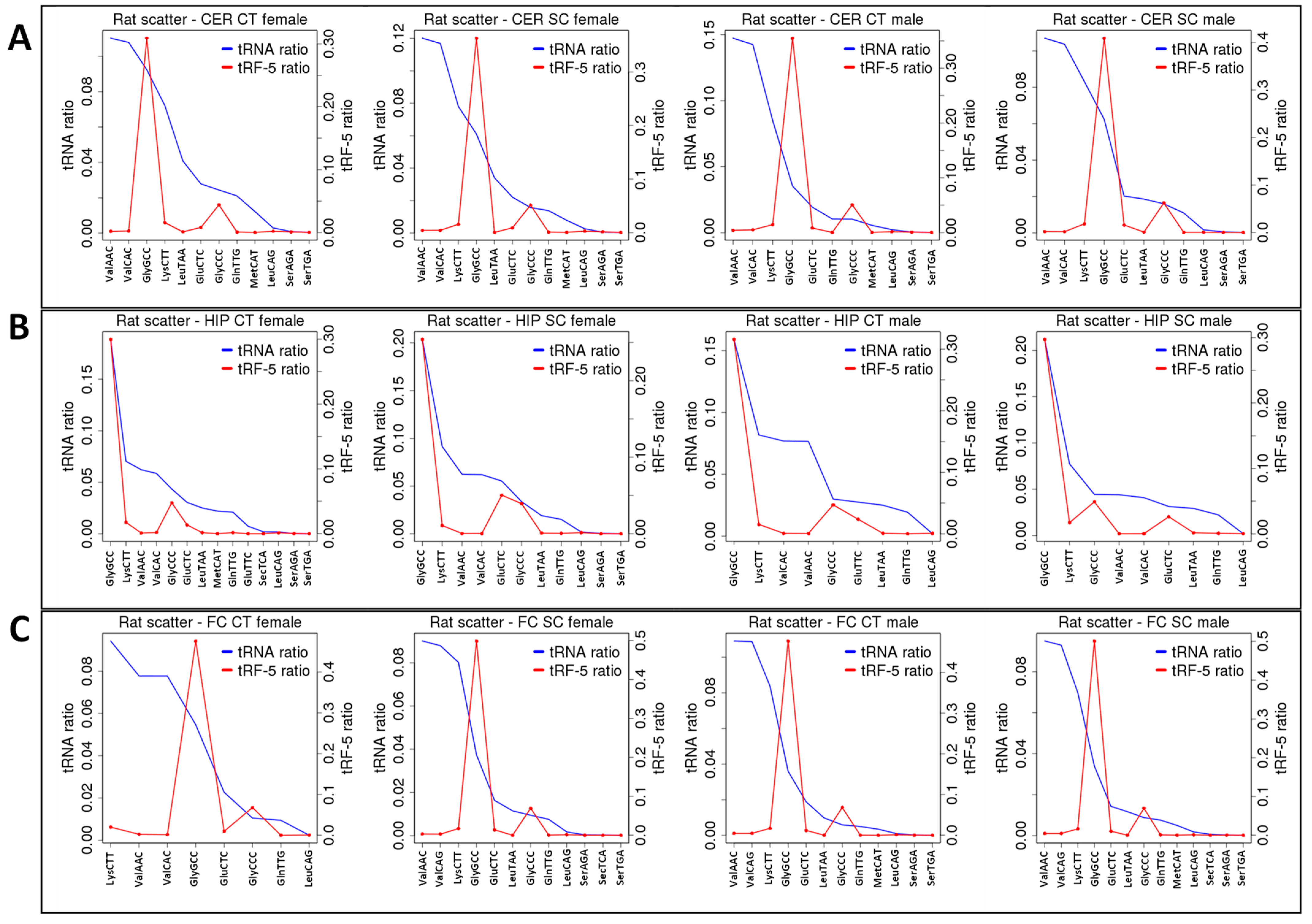
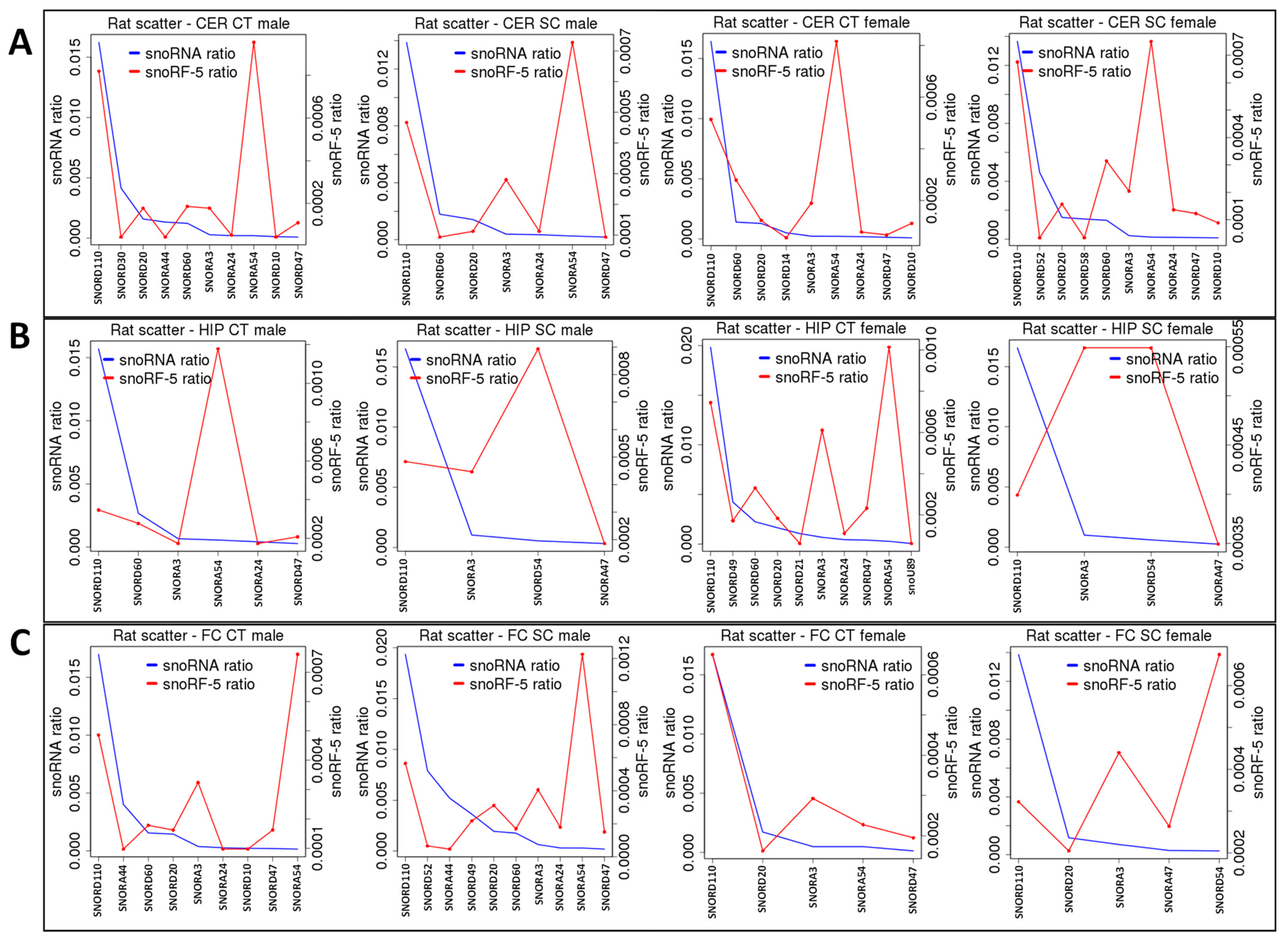
2.3. Correlation between ncRNA Reads and ncRF Reads
2.4. Prediction of Targets of ncRFs in Response to Radiation Using miRDB
2.5. Analysis of Overlapping and Unique Pathways targeted by ncRFs Using DAVID
3. Discussion
3.1. Brain- and Sex-Specific Differences in ncRNAs in Response to Scatter Radiation
3.2. Difference in the Processing of ncRFs in Response to Scatter Radiation
3.3. Predicted Targets
4. Materials and Methods
4.1. Animal Model Used in This Work
4.2. Sequencing
4.3. The Identification and Description of ncRFs
4.4. The Analysis of the Distribution of ncRF Reads across the Entire Length of Precursor ncRNAs
4.5. The Analysis of the Enrichment of ncRFs Relative to the Number of ncRNA Precursors
4.6. ncRFs Target Prediction Using miRDB
4.7. ncRFs Target Pathway Prediction Using DAVID
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Chen, G.; Zhu, S.; Li, S.; Wen, Z.; Li, B.; Zheng, Y.; Shi, L. Identification of Tissue-Specific Protein-Coding and Noncoding Transcripts across 14 Human Tissues Using RNA-seq. Sci. Rep. 2016, 6, 28400. [Google Scholar] [CrossRef]
- Onoguchi-Mizutani, R.; Kishi, Y.; Ogura, Y.; Nishimura, Y.; Imamachi, N.; Suzuki, Y.; Miyazaki, S.; Akimitsu, N. Identification of novel heat shock-induced long non-coding RNA in human cells. J. Biochem. 2021, 169, 497–505. [Google Scholar] [CrossRef]
- Yin, D.; Xu, F.; Lu, M.; Li, X. Long non-coding RNA RMST promotes oxygen-glucose deprivation-induced injury in brain microvascular endothelial cells by regulating miR-204-5p/VCAM1 axis. Life Sci. 2021, 284, 119244. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Liu, D. Small non-coding RNA and colorectal cancer. J. Cell. Mol. Med. 2019, 23, 3050–3057. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Fuentes, N.; Roy, A.; Mishra, V.; Cabello, N.; Silveyra, P. Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol. Sex Differ. 2018, 9, 18. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Chen, H.; Wang, Y.; Xu, Y.; Mao, H.; Li, J.; Mills, G.B.; Shu, Y.; Li, L.; et al. Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell 2016, 29, 711–722. [Google Scholar] [CrossRef]
- Haack, F.; Trakooljul, N.; Gley, K.; Murani, E.; Hadlich, F.; Wimmers, K.; Ponsuksili, S. Deep sequencing of small non-coding RNA highlights brain-specific expression patterns and RNA cleavage. RNA Biol. 2019, 16, 1764–1774. [Google Scholar] [CrossRef]
- Isakova, A.; Fehlmann, T.; Keller, A.; Quake, S.R. A mouse tissue atlas of small noncoding RNA. Proc. Natl. Acad. Sci. USA 2020, 117, 25634–25645. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Chen, D.; Zhang, B.; Tian, R.-R.; Wu, J.; Zhang, Y.; Xu, K.; Yang, L.-M.; Cheng, C.; et al. Annotation and cluster analysis of spatiotemporal- and sex-related lncRNA expression in rhesus macaque brain. Genome Res. 2017, 27, 1608–1620. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Wang, Z.-L.; Poon, M.-W.; Yang, J.-H. Spatial-temporal transcriptional dynamics of long non-coding RNAs in human brain. Hum. Mol. Genet. 2017, 26, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.; Gräfe, A.; Seiler, A.; Schumacher, S.; Nitsch, R.; Wulczyn, F.G. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005, 21, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Uhde, C.W.; Vives, J.; Jaeger, I.; Li, M. Rmst Is a Novel Marker for the Mouse Ventral Mesencephalic Floor Plate and the Anterior Dorsal Midline Cells. PLoS ONE 2010, 5, e8641. [Google Scholar] [CrossRef]
- Nie, J.-H.; Li, T.-X.; Zhang, X.-Q.; Liu, J. Roles of Non-Coding RNAs in Normal Human Brain Development, Brain Tumor, and Neuropsychiatric Disorders. Non-Coding RNA 2019, 5, 36. [Google Scholar] [CrossRef]
- Juhila, J.; Sipilä, T.; Icay, K.; Nicorici, D.; Ellonen, P.; Kallio, A.; Korpelainen, E.; Greco, D.; Hovatta, I. MicroRNA Expression Profiling Reveals MiRNA Families Regulating Specific Biological Pathways in Mouse Frontal Cortex and Hippocampus. PLoS ONE 2011, 6, e214950. [Google Scholar] [CrossRef]
- Yu, M.; Lu, B.; Zhang, J.; Ding, J.; Liu, P.; Lu, Y. tRNA-derived RNA fragments in cancer: Current status and future perspectives. J. Hematol. Oncol. 2020, 13, 121. [Google Scholar] [CrossRef]
- Gebetsberger, J.; Wyss, L.; Mleczko, A.M.; Reuther, J.; Polacek, N. A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 2017, 14, 1364–1373. [Google Scholar] [CrossRef]
- Pandey, K.K.; Madhry, D.; Ravikumar, Y.S.; Malvankar, S. Regulatory roles of tRNA-derived RNA fragments in human pathophysiology. Mol. Ther.-Nucleic Acids 2021, 26, 161–173. [Google Scholar] [CrossRef]
- Schorn, A.J.; Gutbrod, M.J.; LeBlanc, C.; Martienssen, R. LTR-Retrotransposon Control by tRNA-Derived Small RNAs. Cell 2017, 170, 61–71.e11. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; Cabrera-Cabrera, F.; das Neves, R.F.C.; Souto-Padrón, T. Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: Relevance of tRNA-derived halves. BioMed Res. Int. 2014, 2014, 305239. [Google Scholar] [CrossRef]
- Chen, B.; Liu, S.; Wang, H.; Li, G.; Lu, X.; Xu, H. Differential Expression Profiles and Function Prediction of Transfer RNA-Derived Fragments in High-Grade Serous Ovarian Cancer. BioMed Res. Int. 2021, 2021, 5594081. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; O’Day, E.; Emara, M.M.; Wagner, G.; Lieberman, J.; Anderson, P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc. Natl. Acad. Sci. USA 2014, 111, 18201–18206. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, Y.; Luo, Y.; Xiong, X.; Wang, L.; Durante, K.; Li, J.; Zhou, F.; Guo, Y.; Chen, S.; et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: A multicenter prospective study. Mol. Cancer 2022, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Trebak, E.; Souza, L.A.C.; Shi, J.; Zhou, T.; Kehoe, P.G.; Chen, Q.; Earley, Y.F. Small RNA modifications in Alzheimer’s disease. Neurobiol. Dis. 2020, 145, 105058. [Google Scholar] [CrossRef]
- Li, W.-S.; Li, Y.-L.; Cao, R.; Ha, C.-F.; Sun, S.; Yu, L.; Li, J. Differential Expression and Bioinformatics Analysis of tRF/tiRNA in Endometriosis Patients. BioMed Res. Int. 2022, 2022, 9911472. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Zhang, X.; Xu, X.; Wang, P.; Yan, B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol. Immunol. 2021, 139, 106–114. [Google Scholar] [CrossRef]
- Yamasaki, S.; Ivanov, P.; Hu, G.-F.; Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009, 185, 35–42. [Google Scholar] [CrossRef]
- Fu, H.; Feng, J.; Liu, Q.; Sun, F.; Tie, Y.; Zhu, J.; Xing, R.; Sun, Z.; Zheng, X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2008, 583, 437–442. [Google Scholar] [CrossRef]
- Tao, E.-W.; Wang, H.L.; Cheng, W.Y.; Liu, Q.-Q. A specific tRNA half, 5′tiRNA-His-GTG, responds to hypoxia via the HIF1alpha/ANG axis and promotes colorectal cancer progression by regulating LATS2. J. Exp. Clin. Cancer Res. 2021, 40, 67. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Yan, Y.; Shen, Y.; Li, Z.; Li, X.; Zhang, Y.; Xue, Z.; Peng, C.; Chen, X.; et al. Differential Expression Profiles and Function Predictions for tRFs & tiRNAs in Skin Injury Induced by Ultraviolet Irradiation. Front. Cell Dev. Biol. 2021, 9, 707572. [Google Scholar]
- Tao, E.-W.; Cheng, W.Y.; Li, W.; Yu, J.; Gao, Q. tiRNAs: A novel class of small noncoding RNAs that helps cells respond to stressors and plays roles in cancer progression. J. Cell. Physiol. 2019, 235, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Gebetsberger, J.; Zywicki, M.; Kunzi, A.; Polacek, N. tRNA-derived fragments target the ribosome and function as regulatory non-coding RNA in Haloferax volcanii. Archaea 2012, 2012, 260909. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.; Jobava, R.; Parisien, M.; Putnam, A. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol. Cell. Biol. 2014, 34, 2450–2463. [Google Scholar] [CrossRef] [PubMed]
- Durdevic, Z.; Mobin, M.B.; Hanna, K.; Lyko, F.; Schaefer, M. The RNA Methyltransferase Dnmt2 Is Required for Efficient Dicer-2-Dependent siRNA Pathway Activity in Drosophila. Cell Rep. 2013, 4, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.; Hatzoglou, M. The Many Virtues of tRNA-derived Stress-induced RNAs (tiRNAs): Discovering Novel Mechanisms of Stress Response and Effect on Human Health. J. Biol. Chem. 2015, 290, 29761–29768. [Google Scholar] [CrossRef]
- Fiselier, A.; Byeon, B.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. Sex-Specific Expression of Non-Coding RNA Fragments in Frontal Cortex, Hippocampus and Cerebellum of Rats. Epigenomes 2022, 6, 11. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.-Z.; Lin, H.; Zuo, Y.-C. The effect of regions flanking target site on siRNA potency. Genomics 2013, 102, 215–222. [Google Scholar] [CrossRef]
- Selitsky, S.R.; Baran-Gale, J.; Honda, M. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci. Rep. 2015, 5, 7675. [Google Scholar] [CrossRef]
- Mothersill, C.; Moran, G.; McNeil, F.; Gow, M.D.; Denbeigh, J.; Prestwich, W.; Seymour, C.B. A role for bioelectric effects in the induction of bystander signals by ionizing radiation? Dose-Response 2007, 5, 214–229. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Kolb, B. Low dose radiation effects on the brain–from mechanisms and behavioral outcomes to mitigation strategies. Cell Cycle 2017, 16, 1266–1270. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Mychasiuk, R.; Muhammad, A.; Hossain, S.; Ilnytskyy, S.; Ghose, A.; Kirkby, C.; Ghasroddashti, E.; Kovalchuk, O.; Kolb, B. Liver irradiation causes distal bystander effects in the rat brain and affects animal behaviour. Oncotarget 2016, 7, 4385–4398. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, A.; Mychasiuk, R.; Muhammad, A.; Hossain, S.; Ilnytskyy, Y.; Ghose, A.; Kirkby, C.; Ghasroddashti, E.; Kolb, B.; Kovalchuk, O. Profound and Sexually Dimorphic Effects of Clinically-Relevant Low Dose Scatter Irradiation on the Brain and Behavior. Front. Behav. Neurosci. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Ding, N.; Hu, W.; Zhang, X.; Wang, B.; Hua, J.; Wei, W.; Zhu, Q. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015, 12, 1355–1363. [Google Scholar] [CrossRef]
- Sokolov, M.; Neumann, R. Changes in gene expression as one of the key mechanisms involved in radiation-induced bystander effect (Review). Biomed. Rep. 2018, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Bylicky, M.; Chopra, S.; Coleman, C.N.; Aryankalayil, M.J. Long and short non-coding RNA and radiation response: A review. Transl. Res. 2021, 233, 162–179. [Google Scholar] [CrossRef]
- Koturbash, I.; Zemp, F.; Kolb, B.; Kovalchuk, O. Sex-specific radiation-induced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 722, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.; Li, J.; Liu, F.; Benashski, S.E.; McCullough, L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. USA 2011, 108, 11662–11667. [Google Scholar] [CrossRef]
- Hale, M.A.; Johnson, N.E.; Berglund, J.A. Repeat-associated RNA structure and aberrant splicing. Biochim. Et Biophys. Acta Gene Regul. Mech. 2019, 1862, 194405. [Google Scholar] [CrossRef]
- Cleary, J.D.; Pattamatta, A.; Ranum, L.P.W. Repeat-associated non-ATG (RAN) translation. J. Biol. Chem. 2018, 293, 16127–16141. [Google Scholar] [CrossRef]
- Bernstein, L.J.; Edelstein, K.; Sharma, A.; Alain, C. Chemo-brain: An activation likelihood estimation meta-analysis of functional magnetic resonance imaging studies. Neurosci. Biobehav. Rev. 2021, 130, 314–325. [Google Scholar] [CrossRef]
- Beltran, M.; de Herreros, G.A. Antisense non-coding RNAs and regulation of gene transcription. Transcription 2016, 7, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Pozzoli, U.; Menozzi, G.; Fumagalli, M.; Cereda, M.; Comi, G.P.; Cagliani, R.; Bresolin, N.; Sironi, M. Both selective and neutral processes drive GC content evolution in the human genome. BMC Evol. Biol. 2008, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kuo, C.C.; Chen, L. GC content around splice sites affects splicing through pre-mRNA secondary structures. BMC Genom. 2011, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Trabzuni, D.; Ramasamy, A.; Imran, S.; Walker, R.; Smith, C.; Weale, M.; Hardy, J.; Ryten, M.; North American Brain Expression Consortium. Widespread sex differences in gene expression and splicing in the adult human brain. Nat. Commun. 2013, 4, 2771. [Google Scholar] [CrossRef] [PubMed]
- Louro, R.; Nakaya, H.I.; Amaral, P.P.; Festa, F.; Sogayar, M.C.; da Silva, A.M.; Verjovski-Almeida, S.; Reis, E.M. Androgen responsive intronic non-coding RNAs. BMC Biol. 2007, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Louro, R.; Smirnova, A.S.; Verjovski-Almeida, S. Long intronic noncoding RNA transcription: Expression noise or expression choice? Genomics 2009, 93, 291–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, F.; Liu, J.; Chang, H.; Liu, L.; Yang, A.; Liu, X. Transfer RNA-derived fragments as potential exosome tRNA-derived fragment biomarkers for osteoporosis. Int. J. Rheum. Dis. 2018, 21, 1659–1669. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Lanzón, M.P.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.M.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef]
- Jehn, J.; Treml, J.; Wulsch, S.; Ottum, B.; Erb, V.; Hewel, C.; Kooijmans, R.N.; Wester, L.; Fast, I.; Rosenkranz, D. 5′ tRNA halves are highly expressed in the primate hippocampus and might sequence-specifically regulate gene expression. RNA 2020, 26, 694–707. [Google Scholar] [CrossRef]
- Han, L.; Lai, H.; Yanf, Y.; Hu, J.; Li, Z.; Ma, B.; Xu, W.; Liu, W.; Wei, W.; Li, D.; et al. A 5′-tRNA halve, tiRNA-Gly promotes cell proliferation and migration via binding to RBM17 and inducing alternative splicing in papillary thyroid cancer. J. Exp. Clin. Cancer Res. 2021, 40, 222. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, H.; Ding, Q.; Zou, Y.; Tang, C.; Lu, Y.; Xu, X. tiRNA-Val promotes angiogenesis via Sirt1-Hif-1alpha axis in mice with diabetic retinopathy. Biol. Res. 2022, 55, 14. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Feng, H.; Zhang, C.; Zhang, X.; Liu, Y.; Yang, D.-G.; Du, L.-J.; Sun, Y.-C.; Yang, M.-L.; Gao, F.; et al. Differential Expression Profiles and Functional Prediction of tRNA-Derived Small RNAs in Rats After Traumatic Spinal Cord Injury. Front. Mol. Neurosci. 2019, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Jokar, S.; Marques, I.A.; Khazaei, S.; Martins-Marques, T.; Girao, H.; Laranjo, M.; Botelho, M.F. The Footprint of Exosomes in the Radiation-Induced Bystander Effects. Bioengineering 2022, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Polster, A.; Dittmer, P.J.; Perni, S.; Bichraoui, H.; Sather, W.A.; Beam, K.G. Stac Proteins Suppress Ca2+-Dependent Inactivation of Neuronal l-type Ca2+ Channels. J. Neurosci. 2018, 38, 9215–9227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Young, T.L. Evaluation of Lipin 2 as a candidate gene for autosomal dominant 1 high-grade myopia. Gene 2005, 352, 10–19. [Google Scholar] [CrossRef]
- Donkor, J.; Zhang, P.; Wong, S.; O’Loughlin, L.; Dewald, J.; Kok, B.P.; Brindley, D.N.; Reue, K. A Conserved Serine Residue Is Required for the Phosphatidate Phosphatase Activity but Not the Transcriptional Coactivator Functions of Lipin-1 and Lipin-2. J. Biol. Chem. 2009, 284, 29968–29978. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, X.; Hao, Y.; Zhao, Y.; Du, L.; Huang, Y.; Liu, Z.; Wang, Y.; Wang, N.; Zhang, P. NR6A1 regulates lipid metabolism through mammalian target of rapamycin complex 1 in HepG2 cells. Cell Commun. Signal. 2019, 17, 77. [Google Scholar] [CrossRef]
- Tan, P.; Xue, T.; Wang, Y.; Hu, Z.; Su, J.; Yang, R.; Ji, J.; Ye, M.; Chen, Z.; Huang, C.; et al. Hippocampal NR6A1 impairs CREB-BDNF signaling and leads to the development of depression-like behaviors in mice. Neuropharmacology 2022, 209, 108990. [Google Scholar] [CrossRef]
- Rusch, H.L.; Robinson, J.; Yun, S.; Osier, N.D.; Martin, C.; Brewin, C.R.; Gill, J.M. Gene expression differences in PTSD are uniquely related to the intrusion symptom cluster: A transcriptome-wide analysis in military service members. Brain Behav. Immun. 2019, 80, 904–908. [Google Scholar] [CrossRef]
- Wong, N.; Wang, X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–D152. [Google Scholar] [CrossRef]
| Brain Region | tRF | snoRF | ||
|---|---|---|---|---|
| Sex | Ct | SR | Ct | SR |
| HIP_M | axon guidance, axon, axonogenesis, chemical synaptic transmission, cocaine addiction, dendrite cytoplasm, excitatory postsynaptic potential, glutamatergic synapse, morphine addiction, neuron differentiation, positive regulation of dendrite development, postsynaptic cell membrane, postsynaptic density, regulation of neuro-transmitter secretion, regulation of synaptic transmission, response to cocaine, retrograde endocannabinoid signaling | |||
| HIP_F | axon guidance, glutamatergic synapse, neuron projection, neurotrophin signaling, postsynaptic density | axonogenesis, axon guidance, dendrite morphogenesis, neurogenesis | ||
| CER_M | axon guidance, brain development, dendritic spine, neuron projection, neurotrophin signaling | learning | amphetamine addiction, axonal growth cone, regulation of neuron projection development, positive regulation of neuron projection | |
| CER_F | axonogenesis, dendrite | |||
| FC_M | glutamatergic synapse | brain development, neuronal cell body | neuron projection, postsynaptic membrane, synapse | axonogenesis, dendrite cytoplasm, dopaminergic synapse, glutamatergic synapse |
| FC_F | presynaptic membrane, synapse | cAMP signaling pathway, cell junction, glutamatergic synapse | axon guidance, growth cone, positive regulation of neuron projection development | |
| Brain Region | HIP | CER | FC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEX | Male | Female | Male | Female | Male | Female | ||||||
| Ct/SC | Ct | SC | Ct | SC | Ct | SC | Ct | SC | Ct | SC | Ct | SC |
| Distribution of ncRNA reads | ||||||||||||
| miRNA | 70% | 66% | 74% | 69% | 85% | 79% | ||||||
| ra-ncRNA reads | 16% | 19% | 15% | 18% | 6% | 10% | ||||||
| mtRNA reads | 3% | 5% | 3% | 6% | ||||||||
| GC, antisense | 57% | 49% | 44% | 53% | 33% | 43% | ||||||
| ncRNA read size, nt | ||||||||||||
| lncRNA | 26 | 23 | ||||||||||
| Processed | 15 | 19 | ||||||||||
| antisense | 17 | 20 | 18–19 | 22–23 | 18 | 26 | ||||||
| Sense-intronic | 18 | X | X | |||||||||
| sRNAs | X | |||||||||||
| ncRF read number | ||||||||||||
| tRF reads | ↓ | ↑ | ↑ | ↑ | ||||||||
| rRF reads | ↓ | ↑ | ↑ | ↑ | ||||||||
| snoRF reads | ↓ | |||||||||||
| snRF reads | ↑ | ↑ | ↑ | |||||||||
| rRF5′ | X | |||||||||||
| rRF3′ | X | X | X | X | ||||||||
| ncRF read size | ||||||||||||
| tRF read size | ↑ | ↑ | ↑ | ↓ | ↓ | |||||||
| rRF read size | ↓ | ↓ | ↓ | |||||||||
| snoRF read size | ↑ | ↑ | ||||||||||
| snRF read size | ↓ | ↓ | ↑ | ↓ | ||||||||
| tRF abundance | ||||||||||||
| tRF-GlyCCC | ↑ | ↑ | ↑ | ↑ | ||||||||
| tRF-GlyGCC | ↓ | ↑ | ↑ | |||||||||
| tRF-GluCTC | ↑ | ↑↑ | ↑ | ↓ | ↑ | |||||||
| tRF-LysCTT | ↓ | ↑↑ | ↑ | ↓ | ↑ | |||||||
| tRF-LeuCAG | ↓ | ↑ | ↓ | ↑ | ||||||||
| tRF-ValAAC | ↓ | ↑ | ↓ | ↑ | ||||||||
| tRF-ValCAC | ↓ | ↑ | ↓ | ↑ | ||||||||
| ncRF processing | ||||||||||||
| tRF-GlyGCC | = | = | − | ++ | = | ↓ | ||||||
| tRF-GlyCCC | = | ++ | − | ++ | = | = | ||||||
| snoRD20-5′ | − | + | ||||||||||
| snoRD60-5′ | − | |||||||||||
| snoRA24-5′ | + | + | ||||||||||
| snoRD10-5′ | + | − | ||||||||||
| snoRD47-5′ | + | |||||||||||
| snoRA3-5′ | ++ | |||||||||||
| snoRA54-5′ | ++ | |||||||||||
| snoRA81-3′ | + | |||||||||||
| snoRD110-3′ | + | |||||||||||
| rRF-5′ | ++ | |||||||||||
| snRF-U1-3′ | + | + | ++ | + | + | + | ||||||
| snRF-U5-3′ | + | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiselier, A.; Byeon, B.; Ilnytskyy, Y.; Kovalchuk, O.; Kovalchuk, I. Scatter Irradiation of Rat Brain Triggers Sex- and Brain Region-Specific Changes in the Expression of Non-Coding RNA Fragments. Epigenomes 2022, 6, 35. https://doi.org/10.3390/epigenomes6040035
Fiselier A, Byeon B, Ilnytskyy Y, Kovalchuk O, Kovalchuk I. Scatter Irradiation of Rat Brain Triggers Sex- and Brain Region-Specific Changes in the Expression of Non-Coding RNA Fragments. Epigenomes. 2022; 6(4):35. https://doi.org/10.3390/epigenomes6040035
Chicago/Turabian StyleFiselier, Anna, Boseon Byeon, Yaroslav Ilnytskyy, Olga Kovalchuk, and Igor Kovalchuk. 2022. "Scatter Irradiation of Rat Brain Triggers Sex- and Brain Region-Specific Changes in the Expression of Non-Coding RNA Fragments" Epigenomes 6, no. 4: 35. https://doi.org/10.3390/epigenomes6040035
APA StyleFiselier, A., Byeon, B., Ilnytskyy, Y., Kovalchuk, O., & Kovalchuk, I. (2022). Scatter Irradiation of Rat Brain Triggers Sex- and Brain Region-Specific Changes in the Expression of Non-Coding RNA Fragments. Epigenomes, 6(4), 35. https://doi.org/10.3390/epigenomes6040035






