The Ecological Significance and Implications of Transovarial Transmission among the Vector-Borne Bunyaviruses: A Review
Abstract
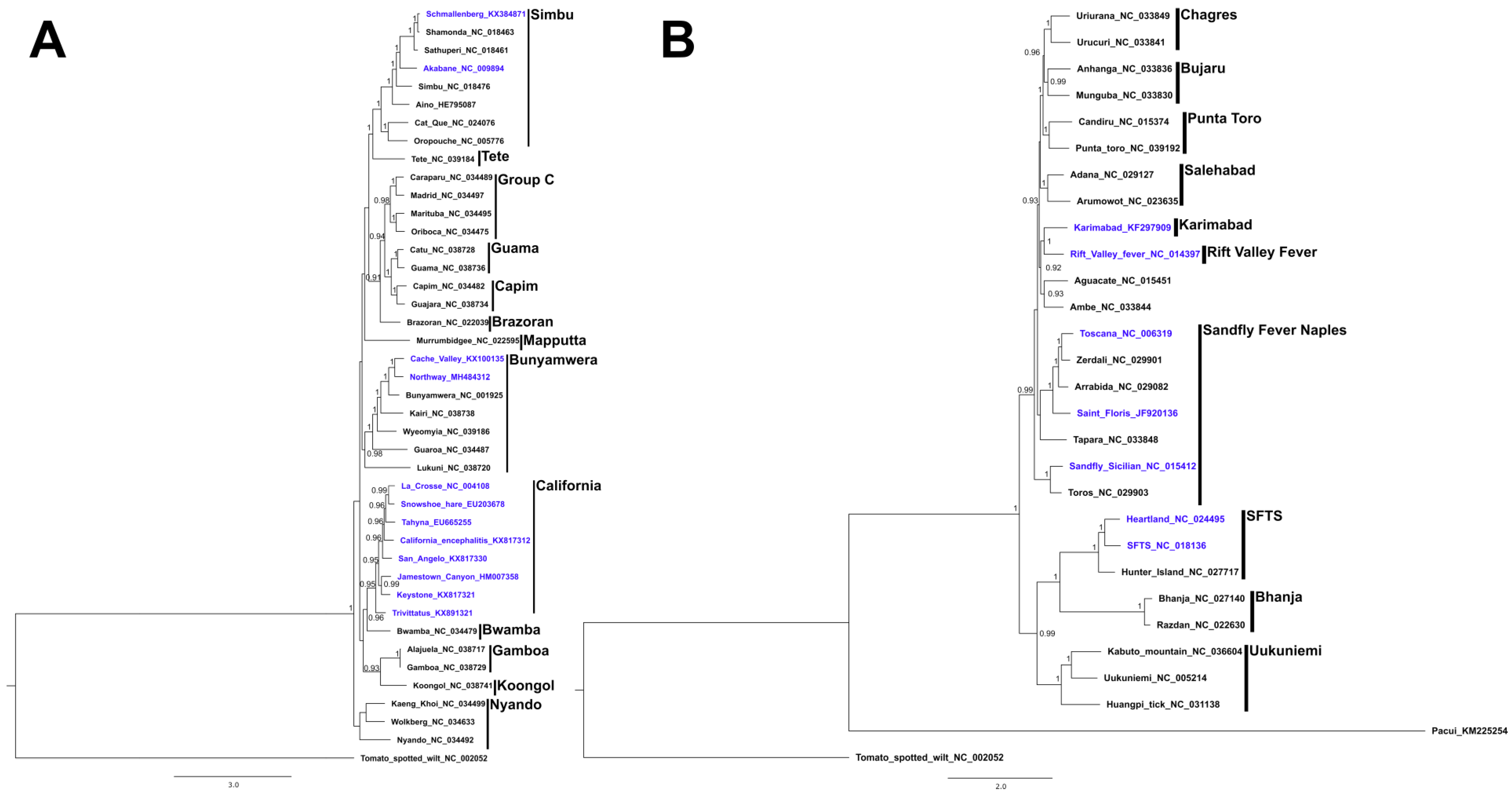
1. Introduction
2. Genus Orthobunyavirus
2.1. California Serogroup
2.1.1. La Crosse Virus
2.1.2. California Encephalitis Virus
2.1.3. Jamestown Canyon Virus
2.1.4. Trivittatus Virus
2.1.5. Snowshoe Hare Virus
2.1.6. San Angelo Virus
2.1.7. Tahyna Virus
2.1.8. Keystone Virus
2.2. Bunyamwera Serogroup
2.3. Simbu Serogroup
2.3.1. Akabane Virus
2.3.2. Schmallenberg Virus
3. Genus Phlebovirus
3.1. Rift Valley Fever Virus
3.2. Sand Fly-Borne Phleboviruses
3.3. Severe Fever with Thrombocytopenia Syndrome Virus
3.4. Heartland Virus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smithburn, K.C.; Haddow, A.J.; Gillett, J.D. Rift Valley fever. Isolation of the virus from wild mosquitoes. Br. J. Exp. Pathol. 1948, 29, 107–121. [Google Scholar] [PubMed]
- Yun, S.-M.; Lee, W.-G.; Ryou, J.; Yang, S.-C.; Park, S.-W.; Roh, J.Y.; Lee, Y.-J.; Park, C.; Han, M.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M.; Godsey, M.S.; Lambert, A.; Panella, N.A.; Burkhalter, K.L.; Harmon, J.R.; Lash, R.R.; Ashley, D.C.; Nicholson, W.L. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg. 2013, 89, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Beaty, B.J.; Blair, C.D.; Brault, A.C.; Dobler, G.; Drebot, M.A.; Haddow, A.D.; Kramer, L.D.; LaBeaud, A.D.; Monath, T.P.; et al. Bunyavirus taxonomy: Limitations and misconceptions associated with the current ICTV criteria used for species demarcation. Am. J. Trop. Med. Hyg. 2018, 99, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Saeed, O.; Afzal, M.R.; Ahrar, A.; Chughtai, M.; Hassan, A.; Ishfaq, M.F.; Lobanova, I.; Malik, M.I.; Malik, A.A.; Qureshi, M.A.; et al. Chapter 2—Mosquito-Borne Diseases. In Zika Virus Disease; Qureshi, A.I., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 27–45. [Google Scholar] [CrossRef]
- Moulton, D.W.; Thompson, W.H. California Group virus infections in small, forest-dwelling mammals of Wisconsin. Am. J. Trop. Med. Hyg. 1971, 20, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Pantuwatana, S.; Thompson, W.H.; Watts, D.M.; Hanson, R.P. Experimental infection of chipmunks and squirrels with La Crosse and Trivittatus viruses and biological transmission of La Crosse virus by Aedes triseriatus. Am. J. Trop. Med. Hyg. 1972, 21, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.H.; Anslow, R.O.; Hanson, R.P.; DeFoliart, G.R. La Crosse virus isolations from mosquitoes in Wisconsin, 1964–1968. Am. J. Trop. Med. Hyg. 1972, 21, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Sudia, W.D.; Newhouse, V.F.; Calisher, C.H.; Chamberlain, R.W. California Group arboviruses: Isolations from mosquitoes in North America. Mosq. News 1971, 31, 576–600. [Google Scholar]
- Wright, R.E.; DeFoliart, G.R. Associations of Wisconsin mosquitoes and woodland vertebrate hosts. Ann. Entomol. Soc. Am. 1970, 63, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Pantuwatana, S.; Thompson, W.H.; Watts, D.M.; Yuill, T.M.; Hanson, R.P. Isolation of La Crosse virus from field collected Aedes triseriatus larvae. Am. J. Trop. Med. Hyg. 1974, 23, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Pantuwatana, S.; DeFoliart, G.R.; Yuill, T.M.; Thompson, W.H. Transovarial transmission of La Crosse virus (California Encephalitis Group) in the mosquito, Aedes triseriatus. Science 1973, 182, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Patrican, L.A.; DeFoliart, G.R.; Yuill, T.M. La Crosse viremias in juvenile subadult and adult chipmunks following feeding by transovarially-infected Aedes triseriatus. Am. J. Trop. Med. Hyg. 1985, 34, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Thompson, W.H.; Yuill, T.M.; DeFoliart, G.R.; Hanson, R.P. Overwintering of La Crosse virus in Aedes triseriatus. Am. J. Trop. Med. Hyg. 1974, 23, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.J.; Thompson, W.H. Delineation of La Crosse virus in developmental stages of transovarially infected Aedes triseriatus. Am. J. Trop. Med. Hyg. 1976, 25, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; DeFoliart, G.R.; Yuill, T.M. Vertical transmission of La Crosse virus (California encephalitis group): Transovarial and filial infection rates in Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol. 1977, 14, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; DeFoliart, G.R.; Yuill, T.M. Aedes triseriatus and La Crosse virus lack of infection in eggs of the first ovarian cycle following oral infection of females. Am. J. Trop. Med. Hyg. 1979, 28, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.J.; Thompson, W.H. Tropisms of La Crosse virus in Aedes triseriatus following infective blood meals. J. Med. Entomol. 1978, 14, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Patrican, L.A.; DeFoliart, G.R. Lack of adverse effect of transovarially acquired La Crosse virus infection on the reproductive capacity of Aedes triseriatatus. J. Med. Entomol. 1985, 22, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.H. Higher venereal infection and transmission rates with La Crosse virus in Aedes triseriatus engorged before mating. Am. J. Trop. Med. Hyg. 1979, 1979, 5. [Google Scholar] [CrossRef]
- Zavortink, T.J. Mosquito Studies (Diptera, Culicidae) XXVIII: The New World Species Formerly Placed in Aedes (Finlaya); American Entomological Institute: Logan, UT, USA, 1972. [Google Scholar]
- Restifo, R.; Lanzaro, G. The occurrence of Aedes atropalpus (Coquillett) breeding in tires in Ohio and Indiana. Mosq. News 1980, 20, 292–294. [Google Scholar]
- Covell, C.V., Jr.; Bnowunu, A. Aedes atropalpus in abandoned tires in Jefferson County, Kentuky. Mosq. News 1979, 39, 142–145. [Google Scholar]
- White, D.J.; White, C. Aedes atropalpus breeding in artificial containers in Suffolk County, New York. Mosq. News 1980, 20, 106–107. [Google Scholar]
- Freier, J.E.; Beier, J.C. Oral and transovarial transmission of La Crosse virus by Aedes atropalpus. Am. J. Trop. Med. Hyg. 1984, 33, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Blair, C.D.; D’Anton, M.; Ewing, W.; Harborth, M.; Seiferth, R.; Xiang, J.; Lanciotti, R.S. La Crosse virus in Aedes albopictus mosquitoes, Texas, USA, 2009. Emerg. Infect. Dis. 2010, 16, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Gubler, D.J. Laboratory studies of transovarial transmission of La Crosse and other arboviruses by Aedes albopictus and Culex fatigans. Am. J. Trop. Med. Hyg. 1975, 24, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.T.; Gonzalez, J.A.; Reagan, K.L.; Blair, C.D.; Beaty, B.J. Comparative potential of Aedes triseriatus, Aedes albopictus, and Aedes aegypti to transovarially transmit La Crosse virus. J. Med. Entomol. 2006, 43, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Woodring, J.; Chandler, L.J.; Oray, C.T.; McGaw, M.M.; Blair, C.D.; Beaty, B.J. Short Report: Diapause, transovarial transmission, and filial infection rates in geographic strains of La Crosse virus infected Aedes triseriatus. Am. J. Trop. Med. Hyg. 1998, 58, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Holmes, J.; Higgs, S.; Beaty, B.; Black, W. Selection of refractory and permissive strains of Aedes triseriatus (Diptera: Culicidae) for transovarial transmission of La Crosse virus. J. Med. Entomol. 1999, 36, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.H.; Holmes, J.L.; Beaty, B.J.; Black IV, W.C. Quantitative trait loci conditioning transovarial transmission of La Crosse virus in the eastern treehold mosquito, Ochlerotatus triseriatus. Insect Mol. Boil. 2003, 12, 307–318. [Google Scholar] [CrossRef]
- Hammon, W.M.; Reeves, W.C. California Encephalitis virus—A newly described agent. Calif. Med. 1952, 77, 303–309. [Google Scholar] [PubMed]
- LeDuc, J.W. Review Article 1: The ecology of California group viruses. J. Med. Entomol. 1979, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Crane, G.T.; Elbel, R.E.; Calisher, C.H. Transovarial transmission of California encephalitis virus in the mosquito Aedes dorsalis at Blue Lake, Utah. Mosq. News 1977, 37, 479–482. [Google Scholar]
- Reisen, W.; Hardy, J.; Reeves, W.; Presser, S.; Milby, M.; Meyer, R. Persistence of mosquito-borne viruses in Kern County, California, 1983–1988. Am. J. Trop. Med. Hyg. 1990, 43, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Reeves, W.C.; Hardy, J.L. Transovarial and Transstadial Transmission of California Encephalitis Virus in Aedes dorsalis and Aedes melanimon. Am. J. Trop. Med. Hyg. 1982, 31, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Reeves, W.C.; Hardy, J.L. Evaluation of the efficiency of transovarial transmission of California encephalitis strains in Aedes dorsalis and Aedes melanimon. Am. J. Trop. Med. Hyg. 1982, 1982, 2. [Google Scholar] [CrossRef]
- Kramer, L.D.; Reeves, W.C.; Hardy, J.L.; Presser, S.B.; Eldridge, B.F.; Bowen, M.D. Vector competence of California mosquitoes for California encephalitis and California encephalitis-like viruses. Am. J. Trop. Med. Hyg. 1992, 47, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Hardy, J.L.; Reeves, W.C. Stabilized infection of Caliornia encephalitis virus in Aedes dorsalis and its implications for viral maintenance in nature. Am. J. Trop. Med. Hyg. 1982, 31, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.M.; Mossel, E.C.; Beaty, M.K.; Beck, E.T.; Geske, D.; Blair, C.D.; Beaty, B.J.; Black, W.C. Identification of super-infected Aedes triseriatus mosquitoes collected as eggs from the field and partial characterization of the infecting La Crosse viruses. Virol. J. 2010, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, P. California group virus disease. In The Arboviruses: Epidemiology and Ecology; Monath, T., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 2, pp. 99–136. [Google Scholar]
- Berry, R.; Weigert, B.L.; Calisher, C.; Parsons, M.; Bear, G. Evidence for transovarial transmission of Jamestown Canyon virus in Ohio. Mosq. News 1977, 37, 494–496. [Google Scholar]
- Boromisa, R.D.; Grimstad, P.R. Virus-vector-host relationships of Aedes stimulans and Jamestown Canyon virus in a northern Indiana enzootic focus. Am. J. Trop. Med. Hyg. 1986, 35, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Heard, P.B.; Zhang, M.; Grimstad, P. Isolation of Jamestown Canyon virus (California serogroup) from Aedes mosquitoes in an enzootic focus in Michigan. J. Am. Mosq. Control Assoc. 1990, 6, 1–468. [Google Scholar]
- Kramer, L.D.; Bowen, M.D.; Hardy, J.L.; Reeves, W.C.; Presser, S.B.; Eldridge, B.F. Vector competence of alpine, Central Valley, and costal mosquitoes from California for Jamestown Canyon virus. J. Med. Entomol. 1993, 30, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Eklund, C. Trivittatus virus. In International Catalogue of Arboviruses Including Certain Other Virus of Vertebrates; Karabatsos, N., Ed.; American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985; pp. 43–68. [Google Scholar]
- Monath, T.P.C.; Nuckolls, J.G.; Berall, J.; Bauer, H.; Chappell, W.A.; Coleman, P.H. Studies on Caliornia encephalitis in Minnesota. Am. J. Epidemiol. 1970, 92, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Andrews, W.N.; Rowley, W.A.; Wong, Y.W.; Dorsey, D.C.; Hausler, J.W.J. Isolation of Trivittatus Virus from Larvae and Adults Reared from Field-Collected Larvae of Aedes Trivittatus (Diptera: Gulicidae). J. Med. Entomol. 1977, 13, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.M.; Rowley, W.A.; Wong, Y.W.; Dorsey, D.C.; Hausler, W.J., Jr. Laboratory studies of transovarial transmission of trivittatus virus by Aedes trivittatus. Am. J. Trop. Med. Hyg. 1978, 27, 184–186. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.M.; Bergman, S.K.A.; Gould, A.P.; Grass, P.N.; Miller, M.A.; Spratt, E.E. California encephalitis virus prevalence throughout the Yukon Territory, 1971–1974. Am. J. Trop. Med. Hyg. 1975, 24, 676–684. [Google Scholar] [CrossRef] [PubMed]
- McLintock, J.; Curry, P.; Wagner, R.; Leung, M.; Iversen, J. Isolation of snowshoe hare virus from Aedes implicatus larvae in Saskatchewan. Mosq. News 1976, 36, 233–237. [Google Scholar]
- Schopen, S.; Labuda, M.; Beaty, B. Vertical and venereal transmission of California group viruses by Aedes triseriatus and Culiseta inornata mosquitoes. Acta Virol. 1991, 35, 373–382. [Google Scholar] [PubMed]
- Kading, R.C.; Crabtree, M.B.; Bird, B.H.; Nichol, S.T.; Erickson, B.R.; Horiuchi, K.; Biggerstaff, B.J.; Miller, B.R. Deletion of the NSm virulence gene of Rift Valley fever virus inhibits virus replication in and dissemination from the midgut of Aedes aegypti mosquitoes. PLoS Negl. Trop. Dis. 2014, 8, e2670. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.E.; Garza, E.H.; Irons, J.V. San Angelo virus. In Proceedings of the 11th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Atlanta, GA, USA, 31 October–3 November 1993. [Google Scholar]
- Tesh, R.B. Experimental studies on the transovarial transmission of Kunjin and San Angelo viruses in mosquitoes. Am. J. Trop. Med. Hyg. 1980, 29, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Shroyer, D.A. The mechanism of arbovirus transovarial transmission in mosquitoes San Angelo virus in Aedes albopictus. Am. J. Trop. Med. Hyg. 1980, 29, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Cornet, M. The location of San Angelo virus in developing ovaries of transovarially infected Aedes albopictus mosquitoes as revealed by fluorescent antibody technique. Am. J. Trop. Med. Hyg. 1981, 30, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Koch, A. Chapter 1—Insects and Their Endosymbionts. In Symbiosis; Academic Press: Cambridge, MA, USA, 1967; pp. 1–106. [Google Scholar]
- Lanham, U.N. The Blochmann Bodies: Hereditary Intracellular Symbionts of Insects. Boil. Rev. 1968, 43, 269–286. [Google Scholar] [CrossRef]
- Bardos, V.; Ryba, J.; Hubalek, Z. Isolation of Tahyna virus from Culiseta annulata (Schrk.) larvae collected in natural surroundings. In Proceedings of the 12th Annual Meeting of the Czechoslovak Society for Microbiology, Kosice, Czechoslovakia, 9–12 April 1993; p. 246. [Google Scholar]
- Moreau, J.P.; Bihan-Faou, P.; Sinegre, G. Tahyna virus transovarial transmission, trials in Aedes caspius. Med. Trop. 1976, 36, 441–442. [Google Scholar]
- Danielova, V.; Ryba, J. Laboratory demonstration of transovarial transmission of Tahyna virus in Aedes vexans and the role of this mechanism in overwintering of this arbovirus [laboratory animals]. Folia Parasitol. (Czechoslov.) 1979, 26, 361–368. [Google Scholar]
- Labuda, M.; Ciampor, F.; Kozuch, O. Experimental model of transovarial transmission of Tahyna virus in Aedes aegypti mosquitoes. Acta Virol. 1983, 27, 245–250. [Google Scholar] [PubMed]
- Le Duc, J.W.; Suyemoto, W.; Eldridge, B.F.; Russell, P.K.; Barr, A.R. Ecology of California encephalitis viruses on the Del Mar Va Peninsula II. Demonstration of transovarial transmission. Am. J. Trop. Med. Hyg. 1975, 24, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Corner, L.C.; Robertson, A.K.; Hayles, L.B.; Iversen, J.O. Cache Valley virus: Experimental infection in Culiseta inornata. Can. J. Microbiol. 1980, 26, 287–290. [Google Scholar] [CrossRef]
- Kramer, L.D.; Hardy, J.L.; Reeves, W.C.; Presser, S.B.; Bowen, M.D.; Eldridge, B.F. Vector competence of selected mosquito species (Diptera: Culicidae) for california strains of Northway virus (Bunyaviridae: Bunyavirus). J. Med. Entomol. 1993, 30, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Oya, A.; Okuno, T.; Ogata, T.; Kobayashi, I.; Matsuyama, T. Akabane, a new arbor virus isolated in Japan. Jpn. J. Med Sci. Boil. 1961, 14, 101–108. [Google Scholar] [CrossRef]
- Kurogi, H.; Inaba, Y.; Takahashi, E.; Sato, K.; Omori, T.; Miura, Y.; Goto, Y.; Fujiwara, Y.; Hatano, Y.; Kodama, K.; et al. Epizootic congenital arthrogryposis-hydranencephaly syndrome in cattle: Isolation of Akabane virus from affected fetuses. Arch. Virol. 1976, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.; Mellor, P.S. Culicoides: Biological vectors of akabane virus. Vet. Microbiol. 1989, 21, 125–131. [Google Scholar] [CrossRef]
- Allingham, P.G.; Standfast, H.A. An investigation of transovarial transmission of Akabane virus in Culicoides brevitarsis. Aust. Vet. J. 1990, 67, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Scheuch, M.; Hoper, D.; Jungblut, R.; Holsteg, M.; Schirrmeier, H.; Eschbaumer, M.; Goller, K.V.; Wernike, K.; Fischer, M.; et al. Novel orthobunyavirus in Cattle, Europe, 2011. Emerg. Infect. Dis. 2012, 18, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.D.; Kristensen, B.; Kirkeby, C.; Rasmussen, T.B.; Belsham, G.J.; Bodker, R.; Botner, A. Culicoids as vectors of Schmallenberg virus. Emerg. Infect. Dis. 2012, 18, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Larska, M.; Lechowski, L.; Grochowska, M.; Zmudzinski, J.F. Detection of the Schmallenberg virus in nulliparous Culicoides obsoletus/scoticus complex and C. punctatus-The possibility of transovarial virus transmission in the midge population and of a new vector. Vet. Microbiol. 2013, 166, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Nanyingi, M.O.; Munyua, P.; Kiama, S.G.; Muchemi, G.M.; Thumbi, S.M.; Bitek, A.O.; Bett, B.; Muriithi, R.M.; Njenga, M.K. A systematic review of Rift Valley Fever epidemiology 1931–2014. Infect. Ecol. Epidemiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Meegan, J.M.; Bailey, C.L. Rift Valley Fever. In The Arboviruses: Epidemiology and Ecology; Monath, T.P.C., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 4, pp. 51–76. [Google Scholar]
- Davies, F.G.; Linthicum, K.J.; James, A.D. Rainfall and epizootic Rift Valley fever. Bull. World Health Organ. 1985, 63, 941–943. [Google Scholar] [PubMed]
- Linthicum, K.J.; Anyamba, A.; Tucker, C.J.; Kelley, P.W.; Myers, M.F.; Peters, C.J. Climate and Satellite Indicators to Forecast Rift Valley Fever Epidemics in Kenya. Science 1999, 285, 397. [Google Scholar] [CrossRef] [PubMed]
- Linthicum, K.J.; Davies, F.G.; Kairo, A. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an inter-epizootic peiod in Kenya. J. Hyg. 1985, 95, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.H.; McElroy, A.K. Rift Valley fever virus: Unanswered questions. Antivir. Res. 2016, 132, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.; Bouloy, M.; Bird, B.H.; Kemp, A.; Paweska, J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010, 41, 61. [Google Scholar] [CrossRef] [PubMed]
- Linthicum, K.J.; Britch, S.C.; Anyamba, A. Rift Valley fever: An emerging mosquito-borne disease. Annu. Rev. Entomol. 2016, 61, 395–415. [Google Scholar] [CrossRef] [PubMed]
- Gargan, T.P., II; Clark, G.G.; Dohm, D.J.; Turell, M.J.; Bailey, C.L. Vector potential of selected North American mosquito species for Rift Valley fever virus. Am. J. Trop. Med. Hyg. 1988, 38, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Britch, S.C.; Aldridge, R.L.; Kline, D.L.; Boohene, C.; Linthicum, K.J. Potential for mosquitoes (Diptera: Culicidae) from Florida to transmit Rift Valley fever virus. J. Med. Entomol. 2013, 50, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Wilson, W.C.; Bennett, K.E. Potential for North American mosquitoes (Diptera: Culicidae) to transmit Rift Valley fever virus. J. Med. Entomol. 2010, 47, 884. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Byrd, B.D.; Harrison, B.A. Potential for populations of Aedes j. japonicus to transmit Rift Valley fever virus in the USA. J. Am. Mosq. Control Assoc. 2013, 29, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Linthicum, K.J.; Beaman, J.R. Transmission of Rift Valley fever virus by adult mosquitoes after ingestion of virus as larvae. Am. J. Trop. Med. Hyg. 1990, 43, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Mores, C.N.; Turell, M.J.; Dyer, J.; Rossi, C.A. Phylogenetic relationships among Orthobunyaviruses isolated from mosquitoes captured in Peru. Vector-Borne Zoonotic Dis. 2008, 9, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mochkovski, S.D.; Diomina, N.A.; Nossina, V.D.; Pavlova, E.A.; Livchitz, J.L.; Pels, H.J.; Roubtzova, V.P. Researches on sandfly fever. Part VIII. Transmission of sandfly fever virus by sandflies hatched from eggs laid by infected females. Meditsinskaya Parazitologiya I Parazit. Bolezn. 1937, 6, 922–937. [Google Scholar]
- Petrischeva, P.; Alymov, A. On transovarial transmission of virus of pappataci fever by sandflies. Arch. Biol. Sci. 1938, 53, 138–144. [Google Scholar]
- Whittingham, H.E. The etiology of phlebotomus fever. Public Health 1924, 38, 56–60. [Google Scholar] [CrossRef]
- Theodor, O. On the relation of Phlebotomus papatasii to the temperature and humidity of the environment. Bull. Entomol. Res. 1936, 27, 653–671. [Google Scholar]
- Tesh, R.B.; Chaniotis, B.N. Transovarial transmission of viruses by phlebotomine sandflies. Ann. N. Y. Acad. Sci. 1975, 266, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bartelloni, P.J.; Tesh, R.B. Clinical and serologic responses of volunteers infected with phlebotomus fever virus (Sicilian type). Am. J. Trop. Med. Hyg. 1976, 25, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Modi, G.B. Maintenance of toscana virus in Phlebotomus perniciosus by vertical transmission. Am. J. Trop. Med. Hyg. 1987, 36, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Chaniotis, B.N.; Peralta, P.H.; Johnson, K.M. Ecology of viruses isolated from Panamanian phlebotomine sandflies. Am. J. Trop. Med. Hyg. 1974, 23, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.; Saidi, S.; Javadian, E.; Nadim, A. Studies on the epidemiology of sandfly fever in Iran. I. Virus isolates obtained from Phlebotomus. Am. J. Trop. Med. Hyg. 1977, 26, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Schmidt, M.; Said, M.I. Phlebotomus fever in Egypt. Isolation of phlebotomus fever viruses from Phlebotomus papatasi. Am. J. Trop. Med. Hyg. 1960, 9, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Aitken, T.; Woodall, J.P.; de Andrade, A.; Bensabath, G.; Shope, R.E. Pacui virus, phlebotomine flies, and small mammals in Brazil: An epidemiological study. Am. J. Trop. Med. Hyg. 1975, 24, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Modi, G.B. Studies on the biology of Phleboviruses in sand flies (Diptera: Psychodidae) I. experimental infection of the vector. Am. J. Trop. Med. Hyg. 1984, 33, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Boshell, J.S.; Young, D.G.; Morales, A.A.; Corredor, A.A.; Modi, G.B.; de Carrasquilla, C.F.; de Rodriquez, C.; Gaitan, M.O. Biology of arboledas virus a new phlebotomus fever serogroup virus (Bunyaviridae: Phlebovirus) isolated from sand flies in Colombia. Am. J. Trop. Med. Hyg. 1986, 35, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Endris, R.G.; Tesh, R.B.; Young, D.G. Transovarial Transmission of Rio Grande Virus (Bunyaviridae: Phlebovirus) by the Sand Fly, Lutzomyia Anthophora. Am. J. Trop. Med. Hyg. 1983, 32, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.-D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel Bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; He, Y.-W.; Dai, Y.-A.; Xiong, Y.; Zheng, H.; Zhou, D.-J.; Li, J.; Sun, Q.; Luo, X.-L.; Cheng, Y.-L.; et al. Hemorrhagic fever caused by a novel Bunyavirus in China: Pathogenesis and correlates of fatal outcome. Clin. Infect. Dis. 2012, 54, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Yi, J.; Kim, G.; Choi, S.J.; Jun, K.I.; Kim, N.-H.; Choe, P.G.; Kim, N.-J.; Lee, J.-K.; Oh, M.-D. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg. Infect. Dis. J. 2013, 19, 1892. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T.; et al. The first identification and retrospective study of severe fever with thrombocytopenia dyndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, J.; Liang, M.; Jiang, X.; Jiang, M.; Yin, H.; Wang, Z.; Li, C.; Zhang, Q.; Jin, C.; et al. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg. Infect. Dis. J. 2013, 19, 756. [Google Scholar] [CrossRef] [PubMed]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 2018, 55, 757–759. [Google Scholar] [CrossRef] [PubMed]
- ProMED-mail. Invasive tick—USA (09): (New York). 2018. Available online: http://httwww.promedmail.org/post/20180719.5915226 (accessed on 19 July 2018).
- ProMED-mail. Invasive tick—USA (11): (Pennsylvania). 2018. Available online: http://www.promedmail.org/post/20180801.5942213 (accessed on 1 August 2018).
- Zhuang, L.; Sun, Y.; Cui, X.-M.; Tang, F.; Hu, J.-G.; Wang, L.-Y.; Cui, N.; Yang, Z.-D.; Huang, D.-D.; Zhang, X.-A.; et al. Transmission of severe fever with thrombocytopenia syndrome virus by Haemaphysalis longicornis Ticks, China. Emerg. Infect. Dis. 2018, 24, 868–871. [Google Scholar] [CrossRef] [PubMed]
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albariño, C.G.; Zaki, S.R.; Rollin, P.E.; et al. A new phlebovirus sssociated with severe febrile illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Pastula, D.M.; Turabelidze, G.; Yates, K.F.; Jones, T.F.; Lambert, A.J.; Panella, A.J.; Kosoy, O.I.; Velez, J.O.; Fischer, M.; Staples, J.E. Heartland virus disease—United States, 2012–2013. Mmwr. Morb. Mortal. Wkly. Rep. 2014, 63, 270–271. [Google Scholar] [PubMed]
- Muehlenbachs, A.; Fata, C.R.; Lambert, A.J.; Paddock, C.D.; Velez, J.O.; Blau, D.M.; Staples, J.E.; Karlekar, M.B.; Bhatnagar, J.; Nasci, R.S.; et al. Heartland virus associated death in Tennessee. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Godsey, J.M.S.; Savage, H.M.; Burkhalter, K.L.; Bosco-Lauth, A.M.; Delorey, M.J. Transmission of Heartland Virus (Bunyaviridae: Phlebovirus) by experimentally infected Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2016, 53, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Boil. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Raikhel, A.S. Vitellogenesis in Mosquitoes. In Advances in Disease Vector Research; Harris, K.F., Ed.; Springer: New York, NY, USA, 1992; pp. 1–39. [Google Scholar]
| Virus | Vector Species | Percent of F1 Progeny Infected | Reference |
|---|---|---|---|
| Arbia virus | Phlebotomus perniciosus | 20.7 | [100] |
| Karimabad virus | Phlebotomus papatasi | 60.0 | [100] |
| Pacui virus | Lutzomyia longipalpis | 32.9 | [100] |
| Saint Floris virus | Phlebotomus papatasi | 6.3 | [100] |
| Sicilian | Phlebotomus papatasi | 1.5 | [100] |
| Toscana | Phlebotomus perniciosus | 30.1 | [100] |
| Arboledas virus | Lutzomyia gomezi | 80.0 | [101] |
| Rio Grande virus | Lutzomyia anthophora | 54.8 | [102] |
| Driver | Virus | Vector | Reference |
|---|---|---|---|
| Vector influences on TOT | |||
| Gonotrophic cycle | SAV | Ae. albopictus | [56] |
| CEV | Ae. melanimon Ae. dorsalis | [38] | |
| Venereal transmission | LACV | Ae. triseriatus | [16,21] |
| Survival and development time | CEV | Ae. melanimon Ae. dorsalis | [37] |
| SAV | Ae. albopictus | [56] | |
| Transmission barriers; vector competence | CEV | Ae. dorsalis | [40] |
| LACV | Ae. triseriatus | [41] | |
| RVFV | Cx. pipiens Ae. circumluteolus Ae. mcintoshi | [87] | |
| SFTSV | H. longicornis (tick) | [111] | |
| HRTV | A. americanum (tick) | [115] | |
| SAV | Ae. albopictus | [58] | |
| LACV | Ae triseriatus | [16] | |
| CEV | Ae. melanimon | [38] | |
| Quantitative trait loci | LACV | Ae. triseriatus | [31,32] |
| Maternal inheritance | SAV | Ae. albopictus | [57] |
| Viral influences on TOT | |||
| M segment critical for TOT | LACV | Ae. triseriatus | [53] |
| NSm deletion | RVFV | Ae. aegypti | [54] |
| Amino acid residues in NSm | LACV | Ae. triseriatus | [41] |
| Environmental influences on TOT | |||
| Persistence through interepidemic periods | RVFV | Ae. mcintoshi | [79] |
| Water temperature | JCV | Ae. squamiger | [46] |
| SAV | Ae. albopictus | [56] | |
| Climate patterns/El Nino | RVFV | Ae. mcintoshi | [78] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergren, N.A.; Kading, R.C. The Ecological Significance and Implications of Transovarial Transmission among the Vector-Borne Bunyaviruses: A Review. Insects 2018, 9, 173. https://doi.org/10.3390/insects9040173
Bergren NA, Kading RC. The Ecological Significance and Implications of Transovarial Transmission among the Vector-Borne Bunyaviruses: A Review. Insects. 2018; 9(4):173. https://doi.org/10.3390/insects9040173
Chicago/Turabian StyleBergren, Nicholas A., and Rebekah C. Kading. 2018. "The Ecological Significance and Implications of Transovarial Transmission among the Vector-Borne Bunyaviruses: A Review" Insects 9, no. 4: 173. https://doi.org/10.3390/insects9040173
APA StyleBergren, N. A., & Kading, R. C. (2018). The Ecological Significance and Implications of Transovarial Transmission among the Vector-Borne Bunyaviruses: A Review. Insects, 9(4), 173. https://doi.org/10.3390/insects9040173






