Population Dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a Village in a Region Targeted for Malaria Elimination in Panamá
Abstract
1. Introduction
2. Materials and Methods
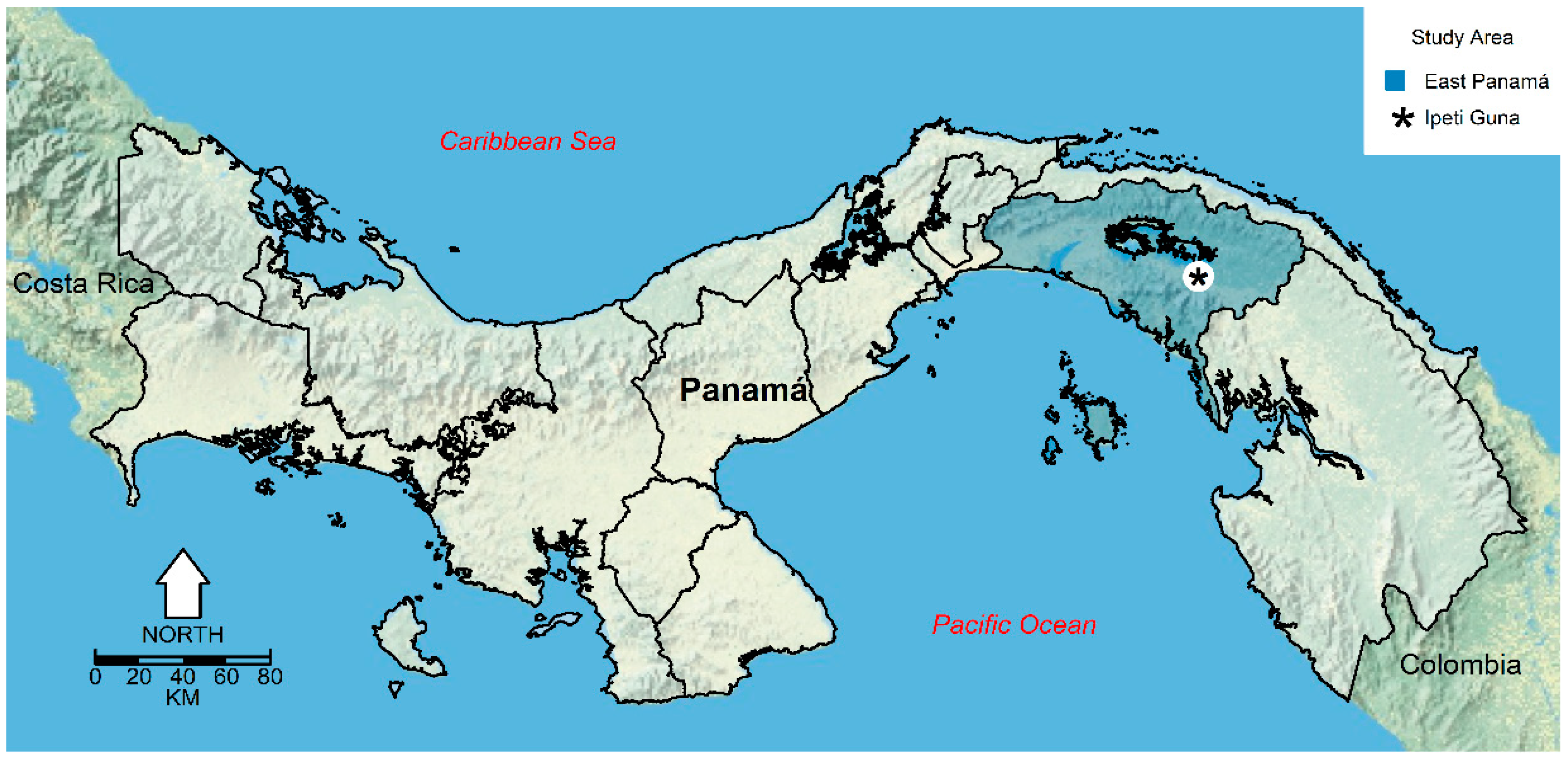
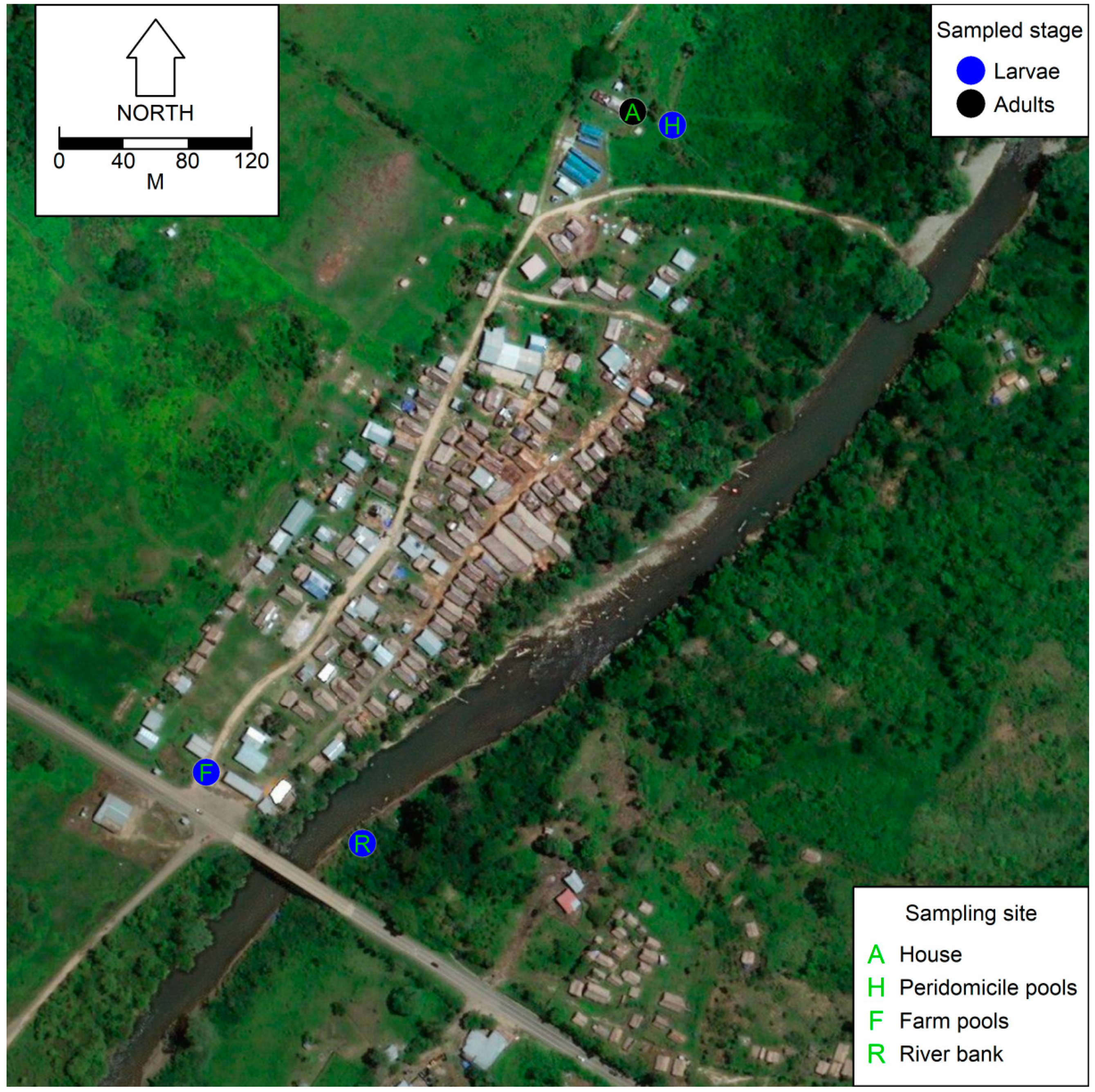
2.1. Study Site
2.2. Mosquito Sampling
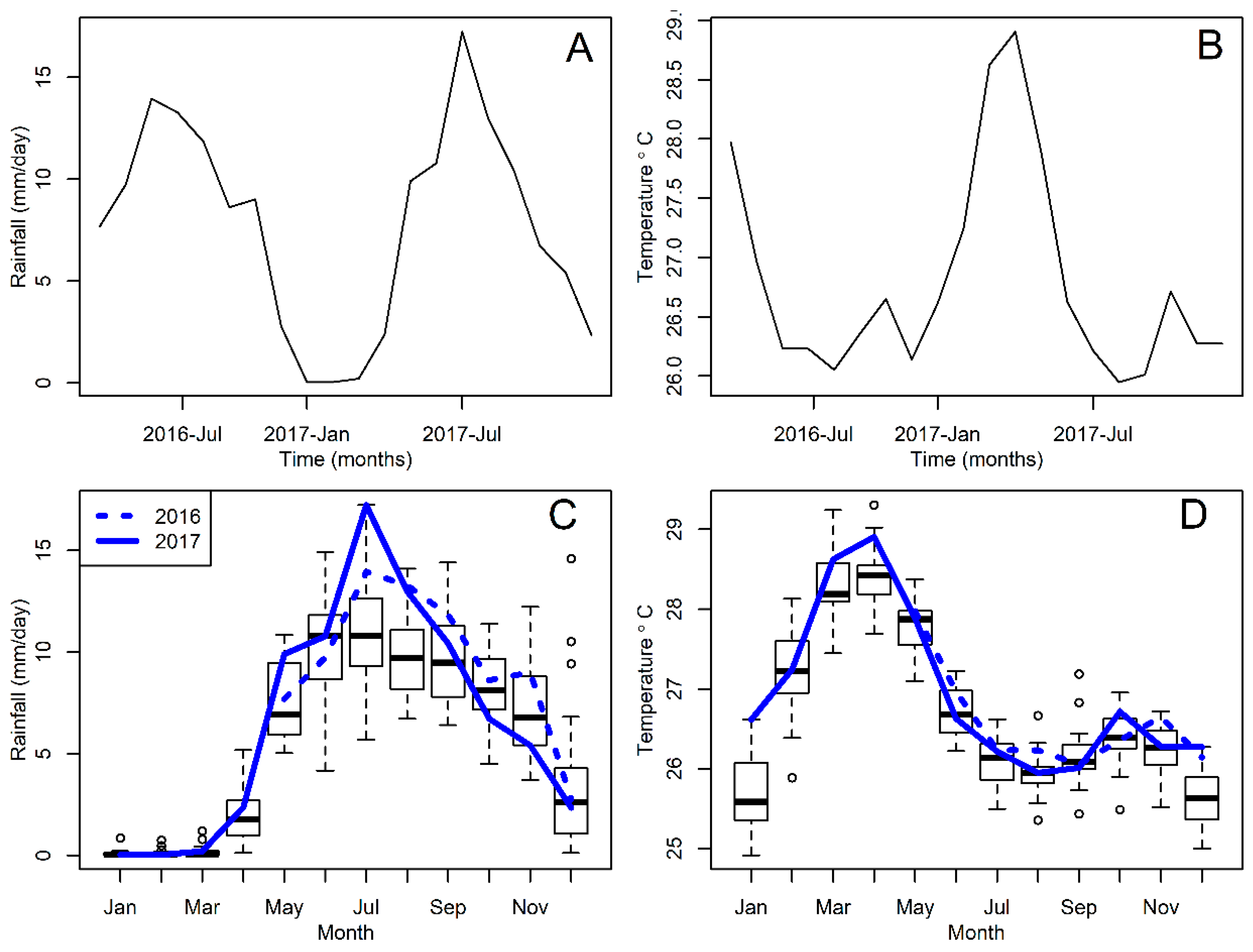
2.3. Weather Data
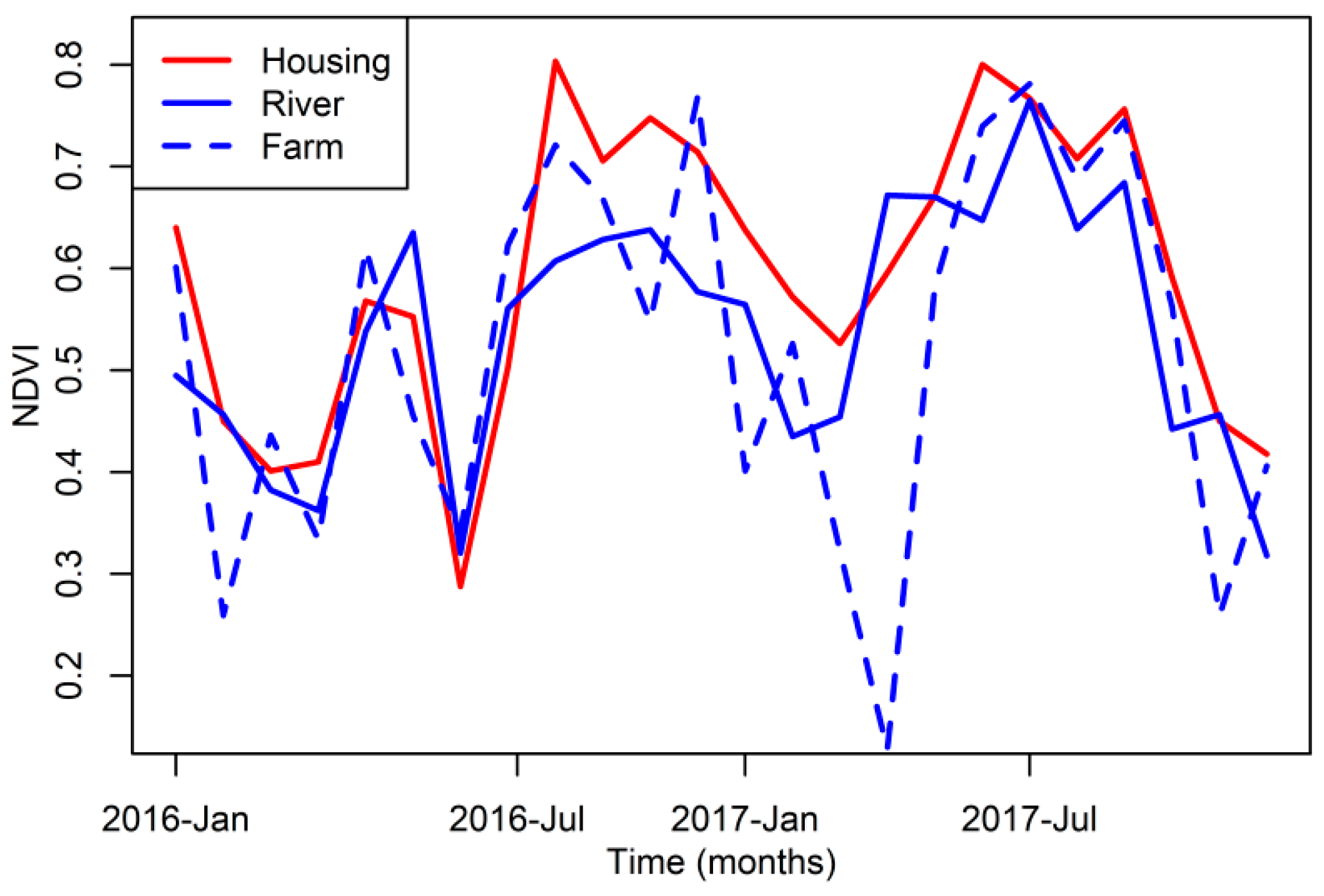
2.4. Vegetation Data
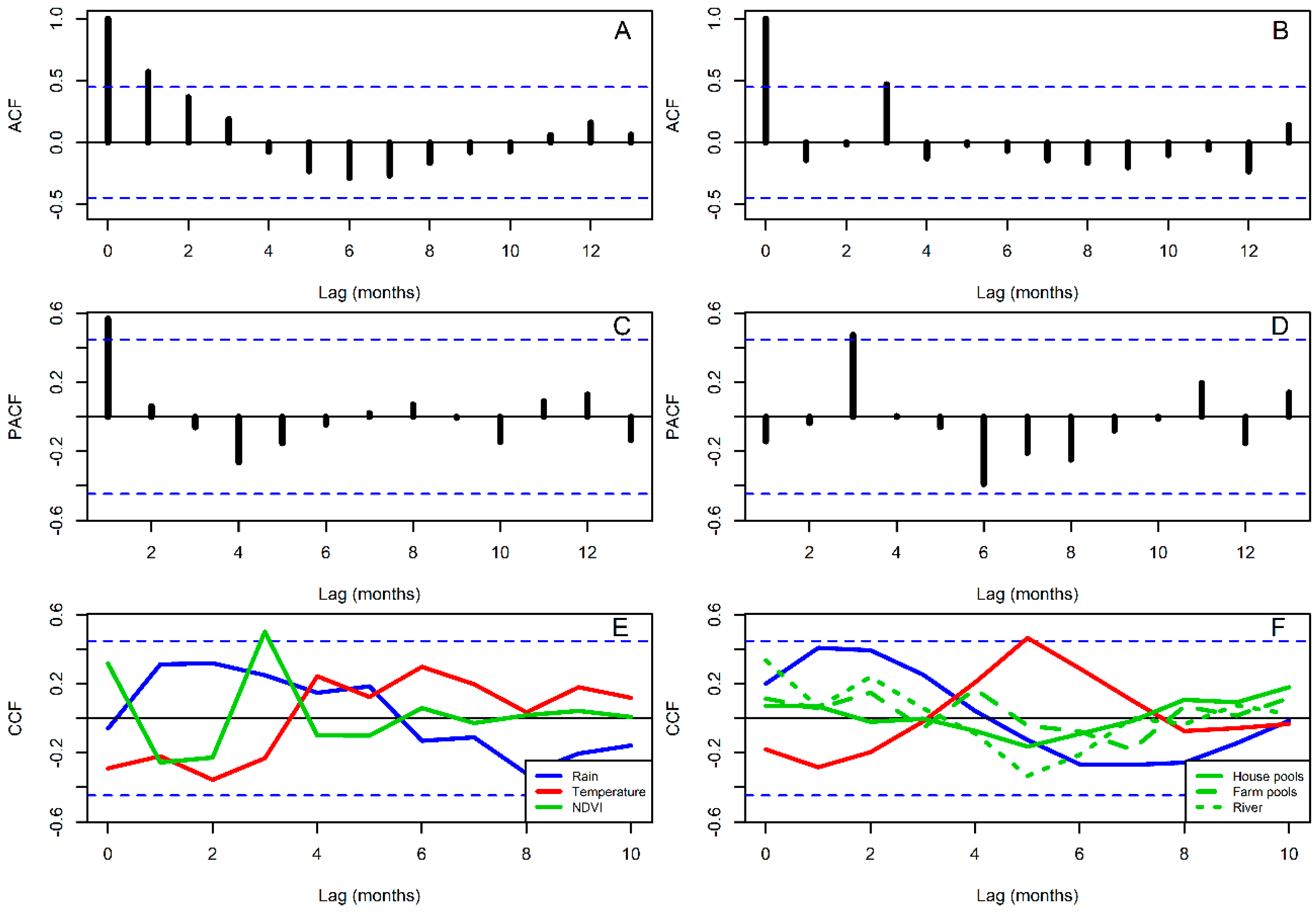
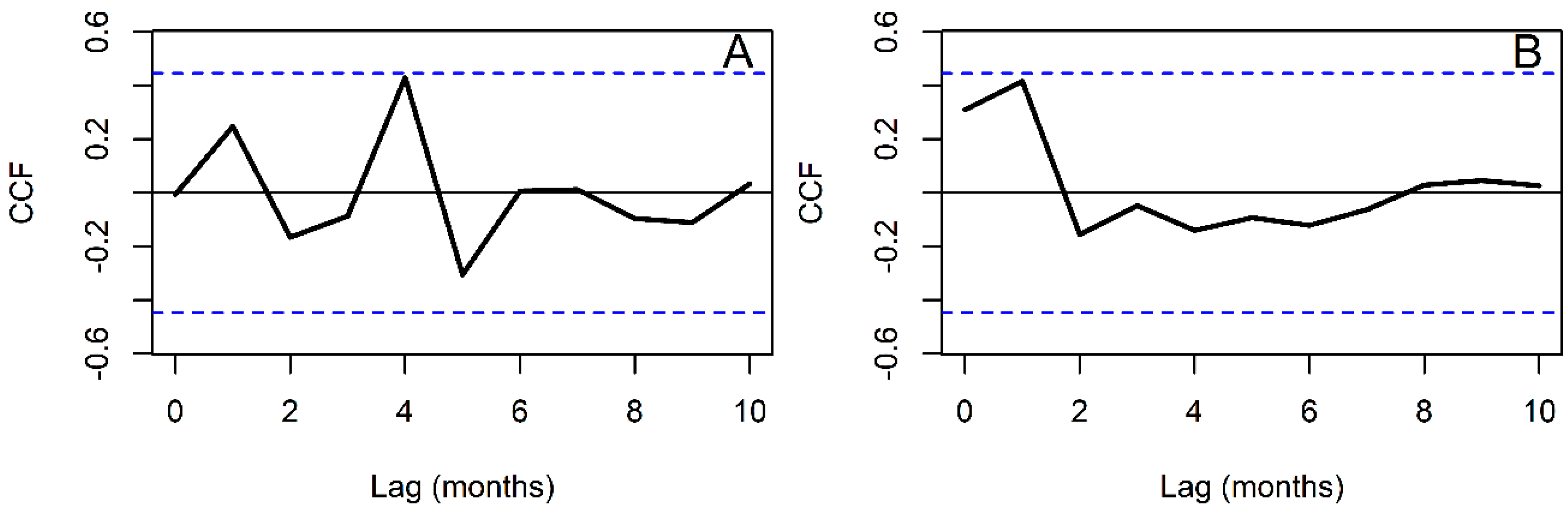
2.5. Time Series Analysis
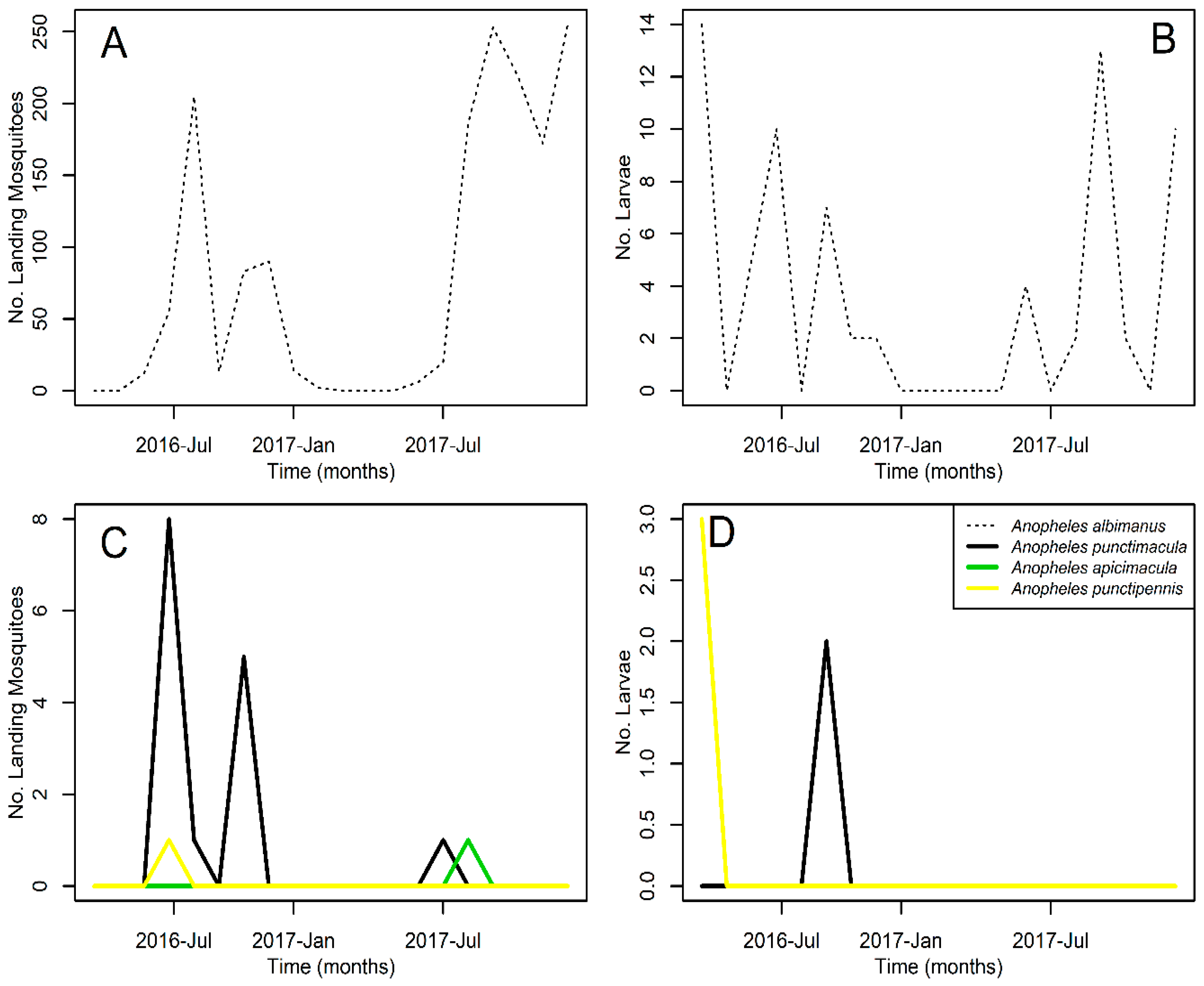
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Economist Intelligence Unit. Panama Country Report. Available online: http://country.eiu.com/panama (accessed on 8 May 2018).
- Gorgas, W.C. Anti-mosquito work at Panama. Proc. R. Soc. Med. 1914, 31, 32–40. [Google Scholar]
- Gorgas, W.C. The conquest of the tropics for the white race. JAMA 1909, 52, 1967–1969. [Google Scholar] [CrossRef]
- Zetek, J. Behavior of Anopheles albimanus Wiede. and tarsimaculata Goeldi. Ann. Èntomol. Soc. Am. 1915, 8, 221–271. [Google Scholar] [CrossRef]
- Jennings, A.H. Some problems of mosquito control in the tropics. J. Econ. Èntomol. 1912, 5, 131–142. [Google Scholar] [CrossRef]
- Rozeboom, L.E. The The rôle of some common anopheline mosquitoes of Panama in the transmission of malaria. Am. J. Trop. Med. Hyg. 1938, s1-18, 289–302. [Google Scholar] [CrossRef]
- Dehné, E.J. Fifty years of malaria control in the Panama area. Am. J. Trop. Med. Hyg. 1955, 4, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Sutter, P.S. Nature’s agents or agents of empire? Entomological workers and environmental change during the construction of the Panama Canal. Isis 2007, 98, 724–754. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, L.; Calzada, J.E.; Gabster, A.; Young, J.; Márquez, R.; Torres, R.; Griffith, M. Social representations of malaria in the Guna indigenous population of Comarca Guna de Madungandi, Panama. Malar. J. 2017, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Béguin, A.; Hales, S.; Rocklöv, J.; Åström, C.; Louis, V.R.; Sauerborn, R. The opposing effects of climate change and socio-economic development on the global distribution of malaria. Glob. Environ. Chang. 2011, 21, 1209–1214. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2017; World Health Organization: Geneva, Switzerland, 2017; p. 196. [Google Scholar]
- Obaldia, N. Determinants of low socio-economic status and risk of Plasmodium vivax malaria infection in Panama (2009–2012): A case-control study. Malar. J. 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Samudio, F.; Santamaría, A.M.; Obaldía, N.; Pascale, J.M.; Bayard, V.; Calzada, J.E. Prevalence of Plasmodium falciparum mutations associated with antimalarial drug resistance during an epidemic in Kuna Yala, Panama, Central America. Am. J. Trop. Med. Hyg. 2005, 73, 839–841. [Google Scholar] [PubMed]
- Calzada, J.E.; Samudio, F.; Bayard, V.; Obaldia III, N.; de Mosca, I.B.; Pascale, J.M. Revising antimalarial drug policy in Central America: Experience in Panama. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Martínez Mauri, M. La autonomía indígena en Panamá: La Experiencia del Pueblo Kuna (Siglos XVI-XXI); Senacyt Panamá—Editorial Abya Yala: Quito, Ecuador, 2011. [Google Scholar]
- Ministerio de Economia y Finanzas. Indice de Pobreza Multidimensional de Panamá Año 2017; Ministerio de Economia y Finanzas: Panamá, República de Panamá, 2017. [Google Scholar]
- Contraloria General de la República de Panamá. Base de datos de Censos de Población. Available online: http://www.contraloria.gob.pa/inec/Redatam/index_censospma.htm (accessed on 8 May 2018).
- Hurtado, L.A.; Cáceres, L.; Chaves, L.F.; Calzada, J.E. When climate change couples social neglect: Malaria dynamics in Panamá. Emerg. Microbes Infect. 2014, 3, e27. [Google Scholar] [PubMed]
- Galindo, P.; Adames, A.; Peralta, P.; Johnson, C.; Read, R. Impacto de la hidroeléctrica de Bayano en la transmisión de arbovirus. Revista Médica de Panamá 1983, 8, 89–134. [Google Scholar] [PubMed]
- Hurtado, L.A.; Calzada, J.E.; Rigg, C.A.; Castillo, M.; Chaves, L.F. Climatic fluctuations and malaria transmission dynamics, prior to elimination, in Guna Yala, República de Panamá. Malar. J. 2018, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, J.R.; Bermingham, E.; Scott, M.E.; Rovira, J.R.; Conn, J.E. Species Composition and Distribution of Adult Anopheles (Diptera: Culicidae) in Panama. J. Med. Èntomol. 2008, 45, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Calzada, J.E.; Marquez, R.; Rigg, C.; Victoria, C.; De La Cruz, M.; Chaves, L.F.; Caceres, L. Characterization of a recent malaria outbreak in the autonomous indigenous region of Guna Yala, Panama. Malar. J. 2015, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, J.R.; Scott, M.E.; Bermingham, E.; Sanjur, O.I.; Wilkerson, R.; Rovira, J.; Gutiérrez, L.A.; Correa, M.M.; Grijalva, M.J.; Birnberg, L.; et al. Late Pleistocene environmental changes lead to unstable demography and population divergence of Anopheles albimanus in the northern Neotropics. Mol. Phylogenet. Evol. 2010, 57, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, J.R.; Scott, M.E.; Bermingham, E.; Sanjur, O.I.; Rovira, J.R.; Dutari, L.C.; Linton, Y.-M.; Bickersmith, S.; Conn, J.E. Novel genetic diversity within Anopheles punctimacula s.l: Phylogenetic discrepancy between the Barcode cytochrome c oxidase I (COI) gene and the rDNA second internal transcribed spacer (ITS2). Acta Trop. 2013, 128, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, L.; Rovira, J.; Torres, R.; García, A.; Calzada, J.; De La Cruz, M. Caracterización de la transmisión de la malaria por Plasmodium vivax en la región fronteriza de Panamá con Costa Rica en el municipio de Barú, Panamá. Biomédica 2012, 32, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, L.; Rovira, J.; García, A.; Torres, R. Determinación de la resistencia a insecticidas organofosforados, carbamatos y piretroides en tres poblaciones de Anopheles albimanus (Diptera: Culicidae) de Panamá. Biomédica 2011, 31, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Koenraadt, C.J.M. Climate Change and Highland Malaria: Fresh Air for a Hot Debate. Q. Rev. Boil. 2010, 85, 27–55. [Google Scholar] [CrossRef]
- Chaves, L.F. Climate change and the biology of insect vectors of human pathogens. In Invertebrates and Global Climate Change; Johnson, S., Jones, H., Eds.; Wiley: Chichester, UK, 2017; pp. 126–147. [Google Scholar]
- Minakawa, N.; Omukunda, E.; Zhou, G.; Githeko, A.; Yan, G. Malaria vector productivity in relation to the highland environment in Kenya. Am. J. Trop. Med. Hyg. 2006, 75, 448–453. [Google Scholar] [PubMed]
- Minakawa, N.; Sonye, G.; Yan, G. Relationships between occurrence of Anopheles gambiae s.l. (Diptera: Culicidae) and size and stability of larval habitats. J. Med. Èntomol. 2005, 42, 295–300. [Google Scholar] [PubMed]
- Minakawa, N.; Sonye, G.; Mogi, M.; Githeko, A.; Yan, G.Y. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J. Med. Èntomol. 2002, 39, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Gimnig, J.E.; Ombok, M.; Otieno, S.; Kaufman, M.G.; Vulule, J.M.; Walker, E.D. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J. Med. Èntomol. 2002, 39, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.L.; Lwetoijera, D.W.; Knols, B.G.J.; Takken, W.; Killeen, G.F.; Ferguson, H.M. Linking individual phenotype to density-dependent population growth: The influence of body size on the population dynamics of malaria vectors. Proc. R. Soc. B Biol. Sci. 2011, 278, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Cazelles, B.; Bouma, M.J.; Chaves, L.F.; Koelle, K. Shifting patterns: Malaria dynamics and rainfall variability in an African highland. Proc. R. Soc. B Biol. Sci. 2008, 275, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Ahumada, J.A.; Chaves, L.F.; Rodo, X.; Bouma, M. Malaria resurgence in the East African highlands: Temperature trends revisited. Proc. Natl. Acad. Sci. USA 2006, 103, 5829–5834. [Google Scholar] [CrossRef] [PubMed]
- Rejmankova, E.; Roberts, D.; Harbach, R.; Pecor, J.; Peyton, E.; Manguin, S.; Krieg, R.; Polanco, J.; Legters, L. Environmental and regional determinants of Anopheles (Diptera: Culicidae) larval distribution in Belize, Central America. Environ. Èntomol. 1993, 22, 978–992. [Google Scholar] [CrossRef]
- Rejmankova, E.; Savage, H.; Rodriguez, M.; Roberts, D.; Rejmanek, M. Aquatic vegetation as a basis for classification of Anopheles albimanus Weideman (Diptera: Culicidae) larval habitats. Environ. Èntomol. 1992, 21, 598–603. [Google Scholar] [CrossRef]
- Rejmankova, E.; Savage, H.M.; Rejmanek, M.; Arredondo-Jimenez, J.I.; Roberts, D.R. Multivariate Analysis of Relationships between Habitats, Environmental Factors and Occurrence of Anopheline Mosquito Larvae Anopheles albimanus and A. pseudopunctipennis in Southern Chiapas, Mexico. J. Appl. Ecol. 1991, 28, 827–841. [Google Scholar]
- Berti, J.; González, J.; Navarro-Bueno, E.; Zoppi, E.; Gordon, E.; Delgado, L. Larval seasonality of the mosquito Anopheles aquasalis (Diptera: Culicidae) and other insects associated to its habitat in Sucre, Venezuela. Rev. Boil. Trop. 2010, 58, 777–787. [Google Scholar]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- USNPS. Data Sources & Accuracy for National Park Service Maps. Available online: https://www.nps.gov/hfc/carto/data-sources.cfm (accessed on 8 May 2018).
- Rubio-Palis, Y.; Curtis, C.F. Evaluation of different methods of catching anopheline mosquitoes in western Venezuela. J. Am. Mosq. Control Assoc. 1992, 8, 261–267. [Google Scholar] [PubMed]
- World Health Organization. Técnicas Entomológicas de Campo Para la Lucha Antipalúdica; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Wilkerson, R.C.; Strickman, D.; Fernández Salas, I. Clave Ilustrada Para la Identificación de Las Hembras de Mosquitos Anofelinos de México y Centroamérica; Universidad Autónoma de Nuevo León: San Nicolás de Los Garza, México, 1993. [Google Scholar]
- Stojanovich, C.; Gorham, J.; Scott, H. Clave Ilustrada Para los Mosquitos Anofelinos de America Central y Panama; Training Branch, Communicable Disease Center: Atlanta, GA, USA, 1966; p. 37. [Google Scholar]
- Patterson, T.C. Google Earth as a (not just) geography education tool. J. Geogr. 2007, 106, 145–152. [Google Scholar] [CrossRef]
- KMNI. KMNI Climate Explorer. Available online: http://climexp.knmi.nl (accessed on 8 May 2018).
- NOAA. GPCP Version 2.3 Combined Precipitation Data Set. Available online: https://www.esrl.noaa.gov/psd/data/gridded/data.gpcp.html (accessed on 8 May 2018).
- NOAA. GHCN_CAMS Gridded 2m Temperature (Land). Available online: https://www.esrl.noaa.gov/psd/data/gridded/data.ghcncams.html (accessed on 8 May 2018).
- NASALPDAAC. NASA Land Processes Distributed Active Archive Center. Available online: https://lpdaac.usgs.gov (accessed on 8 May 2018).
- Busetto, L.; Ranghetti, L. MODIStsp: An R package for automatic preprocessing of MODIS Land Products time series. Comput. Geosci. 2016, 97, 40–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Shumway, R.H.; Stoffer, D.S. Time Series Analysis and Its Applications, 3rd ed.; Springer: New York, NY, USA, 2011; p. 572. [Google Scholar]
- Pan American Health Organization (PAHO). Report on the Situation of Malaria in the Americas, 2014; Pan American Health Organization: Washington, DC, USA, 2016. [Google Scholar]
- Pan American Health Organization (PAHO). Report on the Situation of Malaria in the Americas, 2000–2015; Pan American Health Organization: Washington, DC, USA, 2016. [Google Scholar]
- Obaldia, N.; Baro, N.K.; Calzada, J.E.; Santamaria, A.M.; Daniels, R.; Wong, W.; Chang, H.-H.; Hamilton, E.J.; Arevalo-Herrera, M.; Herrera, S.; et al. Clonal Outbreak of Plasmodium falciparum Infection in Eastern Panama. J. Infect. Dis. 2015, 211, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Hashizume, M.; Satake, A.; Minakawa, N. Regime shifts and heterogeneous trends in malaria time series from Western Kenya Highlands. Parasitology 2012, 139, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Satake, A.; Hashizume, M.; Minakawa, N. Indian Ocean Dipole and Rainfall Drive a Moran Effect in East Africa Malaria Transmission. J. Infect. Dis. 2012, 205, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Bouma, M.J.; Poveda, G.; Rojas, W.; Chavasse, D.; Quiñones, M.; Cox, J.; Patz, J. Predicting high-risk years for malaria in Colombia using parameters of El Niño Southern Oscillation. Trop. Med. Int. Health 1997, 2, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Grillet, M.-E.; El Souki, M.; Laguna, F.; León, J.R. The periodicity of Plasmodium vivax and Plasmodium falciparum in Venezuela. Acta Trop. 2014, 129, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Kaneko, A.; Taleo, G.; Pascual, M.; Wilson, M.L. Malaria transmission pattern resilience to climatic variability is mediated by insecticide-treated nets. Malar. J. 2008, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.J.; Ameh, D.; Bottomley, C.; Green, C.; Jawara, M.; Milligan, P.J.; Snell, P.C.; Conway, D.J.; Lindsay, S.W. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: A randomised controlled trial. Lancet 2009, 374, 998–1009. [Google Scholar] [CrossRef]
- Celli, A. Remarks on the Epidemiology and prophylaxis of malaria in the light of recent researches. Br. Med. J. 1900, 1, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F. Casas Muertas and Oficina No. 1: Internal migrations and malaria trends in Venezuela 1905–1945. Parasitol. Res. 2007, 101, 19–23. [Google Scholar] [CrossRef] [PubMed]
- De Zulueta, J. Changes in the geographical distribution of malaria throughout history. Parassitologia 1987, 29, 193–205. [Google Scholar] [PubMed]
- De Zulueta, J. Malaria and Mediterranean history. Parassitologia 1973, 15, 1–15. [Google Scholar] [PubMed]
- Kitron, U.; Spielman, A. Suppression of transmission of malaria through source reduction: Antianopheline measures applied in Israel, the United States, and Italy. Rev. Infectious Dis. 1989, 11, 391–406. [Google Scholar] [CrossRef]
- Kitron, U. Malaria, agriculture, and development: Lessons from past campaigns. Int. J. Health Serv. 1987, 17, 295–326. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, M. Malaria: Poverty, Race, and Public Health in the United States; Johns Hopkins University Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Kendall, A.I. Malarial infection in certain native villages of the canal zone. JAMA 1906, 46, 1266–1273. [Google Scholar] [CrossRef]
- Kendall, A.I. Malarial infection in certain native villages of the canal zone. JAMA 1906, 46, 1151–1154. [Google Scholar] [CrossRef]
- Mukabana, W.R.; Kannady, K.; Kiama, G.M.; Ijumba, J.N.; Mathenge, E.M.; Kiche, I.; Nkwengulila, G.; Mboera, L.; Mtasiwa, D.; Yamagata, Y.; et al. Ecologists can enable communities to implement malaria vector control in Africa. Malar. J. 2006, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.; Kitron, U.; Pollack, R.J. Time Limitation and the Role of Research in the Worldwide Attempt to Eradicate Malaria. J. Med. Èntomol. 1993, 30, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Palis, Y.; Bevilacqua, M.; Medina, D.A.; Moreno, J.E.; Cárdenas, L.; Sánchez, V.; Estrada, Y.; Anaya, W.; Martínez, Á. Malaria entomological risk factors in relation to land cover in the Lower Caura River Basin, Venezuela. Memórias Inst. Oswaldo Cruz 2013, 108, 220–228. [Google Scholar] [CrossRef]
- Rubio-Palis, Y.; Moreno, J.E.; Bevilacqua, M.; Medina, D.; Martínez, Á.; Cardenas, L.; Guzmán, H.; González, J. Caracterización ecológica de los anofelinos y otros culícidos en territorio indígena del Bajo Caura, Estado Bolívar, Venezuela. Boletín Malariol. Salud Ambient. 2010, 50, 95–107. [Google Scholar]
- Lindsay, S.; Joyce, A. Climate Change and the Disappearance of Malaria from England. Glob. Chang. Hum. Health 2000, 1, 184–187. [Google Scholar] [CrossRef]
- Rubio-Palis, Y.; Curtis, C.F. Biting and resting behaviour of anophelines in western Venezuela and implications for control of malaria transmission. Med. Vet. Èntomol. 1992, 6, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.E.; Rubio-Palis, Y.; Sánchez, V.; Martínez, Á. Characterization of anophelines larval habitats in the municipality of Sifontes, Bolívar State, Venezuela. Boletín Malariol. Salud Ambient. 2015, 55, 117–131. [Google Scholar]
- Kaneko, A.; Taleo, G.; Kalkoa, M.; Yamar, S.; Kobayakawa, T.; Björkman, A. Malaria eradication on islands. Lancet 2000, 356, 1560–1564. [Google Scholar] [CrossRef]
- Kaneko, A. A community-directed strategy for sustainable malaria elimination on islands: Short-term MDA integrated with ITNs and robust surveillance. Acta Trop. 2010, 114, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Poirot, E.; Skarbinski, J.; Sinclair, D.; Kachur, S.P.; Slutsker, L.; Hwang, J. Mass drug administration for malaria. Cochrane Database Syst. Rev. 2013, 12, CD008846. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.; Rubio-Palis, Y.; Medina, D.A.; Cárdenas, L. Malaria Control in Amerindian Communities of Venezuela. EcoHealth 2015, 12, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Apgar, M.J.; Allen, W.; Moore, K.; Ataria, J. Understanding adaptation and transformation through indigenous practice: The case of the Guna of Panama. Ecol. Soc. 2015, 20, 45. [Google Scholar] [CrossRef]
- Smith, D.L.; Drakeley, C.J.; Chiyaka, C.; Hay, S.I. A quantitative analysis of transmission efficiency versus intensity for malaria. Nat. Commun. 2010, 1, 108. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Morrison, A.C.; Kitron, U.D.; Scott, T.W. Nonlinear impacts of climatic variability on the density-dependent regulation of an insect vector of disease. Glob. Chang. Biol. 2012, 18, 457–468. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Gurney, W.S.C. Population dynamics in a periodically varying environment. J. Theor. Biol. 1976, 56, 459–475. [Google Scholar] [CrossRef]
- Tuljapurkar, S. Population dynamics in variable environments. VI. Cyclical environments. Theor. Popul. Biol. 1985, 28, 1–17. [Google Scholar] [CrossRef]
- Tuljapurkar, S.D. Population dynamics in variable environments. II. Correlated environments, sensitivity analysis and dynamics. Theor. Popul. Biol. 1982, 21, 114–140. [Google Scholar] [CrossRef]
- Chaves, L.F.; Scott, T.W.; Morrison, A.C.; Takada, T. Hot temperatures can force delayed mosquito outbreaks via sequential changes in Aedes aegypti demographic parameters in autocorrelated environments. Acta Trop. 2014, 129, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Martin Banda, P.; Foster Pemba, D.; Sunahara, T.; Minakawa, N. Beyond buzzing: Mosquito watching stimulates malaria bednet use a household-based cluster-randomized controlled assessor blind educational trial. Emerg. Microbes Infect 2013, 2, e67. [Google Scholar] [CrossRef] [PubMed]
- Honjo, K.; Chaves, L.F.; Satake, A.; Kaneko, A.; Minakawa, N. When they don’t bite, we smell money: Understanding malaria bednet misuse. Parasitology 2013, 140, 580–586. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, W.P.; Mangeni, J.N.; Steketee, R.; Greenwood, B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect. Dis. 2010, 10, 545–555. [Google Scholar] [CrossRef]
- Lines, J.; Lengeler, C.; Cham, K.; de Savigny, D.; Chimumbwa, J.; Langi, P.; Carroll, D.; Mills, A.; Hanson, K.; Webster, J.; et al. Scaling-up and sustaining insecticide-treated net coverage. Lancet Infect. Dis. 2003, 3, 465–466. [Google Scholar] [CrossRef]
- Magris, M.; Rubio-Palis, Y.; Alexander, N.; Ruiz, B.; Galván, N.; Frias, D.; Blanco, M.; Lines, J. Community-randomized trial of lambdacyhalothrin-treated hammock nets for malaria control in Yanomami communities in the Amazon region of Venezuela. Trop. Med. Int. Health 2007, 12, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Peeters Grietens, K.; Nguyen Xuan, X.; Muela Ribera, J.; Ngo Duc, T.; van Bortel, W.; Truong Ba, N.; Van, K.P.; Le Xuan, H.; D’Alessandro, U.; Erhart, A. Social Determinants of Long Lasting Insecticidal Hammock-Use Among the Ra-Glai Ethnic Minority in Vietnam: Implications for Forest Malaria Control. PLoS ONE 2012, 7, e29991. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.F.; Kitchen, S.F. The Comparative Susceptibility of Anopheles quadrimaculatus, Say, and Anopheles punctipennis, Say, to Plasmodium vivax, Grassi and Feletti, and Plasmodium falciparum, Welch. Am. J. Trop. Med. Hyg. 1936, s1-16, 67–71. [Google Scholar] [CrossRef]
- Simmons, J.S. Observations on the Importance of Anopheles punctimacula as a Malaria Vector in Panama, and Report of Experimental Infections in A. neomaculipalpus, A. apicimacula, and A. eiseni. Am. J. Trop. Med. Hyg. 1937, s1-17, 191–212. [Google Scholar]
- Simmons, J.S. Anopheles (Anopheles) punctimacula Naturally Infected with Malaria Plasmodia. Am. J. Trop. Med. Hyg. 1936, s1-16, 105–108. [Google Scholar] [CrossRef]
- Iwashita, H.; Dida, O.; Sonye, G.O.; Sunahara, T.; Futami, K.; Njenga, S.M.; Chaves, L.F.; Minakawa, N. Push by a net, pull by a cow: Can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control. Parasites Vectors 2014, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, U.; Sombroek, H.; Majambere, S.; van Loon, E.; Takken, W.; Lindsay, S. Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malar. J. 2009, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, U.; Sonye, G.; Killeen, G.F.; Knols, B.G.J.; Becker, N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: Operational observations from a rural town in western Kenya. Trop. Med. Int. Health 2004, 9, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Palis, Y.; Moreno, J.E.; Sánchez, V.; Estrada, Y.; Anaya, W.; Bevilacqua, M.; Cárdenas, L.; Martínez, Á.; Medina, D. Can Mosquito Magnet® substitute for human-landing catches to sample anopheline populations? Memórias Inst. Oswaldo Cruz 2012, 107, 546–549. [Google Scholar] [CrossRef]
- Chaves, L.F.; Jian, J.-Y.; Moji, K. Overwintering in the Bamboo Mosquito Tripteroides bambusa (Diptera: Culicidae) During a Warm, But Unpredictably Changing, Winter. Environ. Èntomol. 2018, 47, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Predescu, M.; Levins, R.; Awerbuch-Friedlander, T. Analysis of a nonlinear system for community intervention in mosquito control. Discret. Contin. Dyn. Syst.-Ser. B 2006, 6, 605–622. [Google Scholar]
- Predescu, M.; Sirbu, G.; Levins, R.; Awerbuch-Friedlander, T. On the dynamics of a deterministic and stochastic model for mosquito control. Appl. Math. Lett. 2007, 20, 919–925. [Google Scholar] [CrossRef]
- Displacement Solutions. Cambio Climático y desplazamiento en la región autónoma de Guna Yala, Panamá; Displacement Solutions: Geneva, Switzerland, 2014; p. 103. [Google Scholar]
| Adults | Larvae | |||||
|---|---|---|---|---|---|---|
| Species | Mean ± SD | Total | Persistence (%) | Mean ± SD | Total | Persistence (%) |
| Anopheles albimanus | 79.25 ± 96.40 | 1585 | 75 | 3.55 ± 4.70 | 71 | 60 |
| Anopheles punctimacula | 0.75 ± 2.05 | 15 | 15 | 0.10 ± 0.45 | 2 | 5 |
| Anopheles apicimacula | 0.05 ± 0.22 | 1 | 5 | — | — | — |
| Anopheles punctipennis | 0.05 ± 0.22 | 1 | 5 | 0.15 ± 0.67 | 3 | 5 |
| Variables [Lag in Months] | AIC |
|---|---|
| AR[1] | 234.51 |
| AR[1], NDVI[3] | 227.60 |
| Parameter [Lag in Months] | Estimate ± S.D. |
|---|---|
| Intercept () | 83.60 ± 39.79 * |
| AR[1]) | 0.71 ± 0.16 * |
| NDVI[3] | 3.85 ± 1.15 * |
| ) | 3320 |
| Variables [Lag in Months] | AIC |
|---|---|
| SAR[3] | 108.60 |
| SAR[3], Adults[1] | 107.40 |
| SAR[3], Temperature[5] | 102.20 |
| SAR[3], Temperature[5], Adults[1] | 101.87 |
| Parameter [Lag in Months] | Estimate ± S.D. |
|---|---|
| Intercept () | 2.59 ± 1.53 * |
| SAR[3]) | 0.67 ± 0.19 * |
| Temperature[5] | 1.35 ± 0.44 * |
| Adults[1] | 0.010 ± 0.006 |
| ) | 6.69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtado, L.A.; Rigg, C.A.; Calzada, J.E.; Dutary, S.; Bernal, D.; Koo, S.I.; Chaves, L.F. Population Dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a Village in a Region Targeted for Malaria Elimination in Panamá. Insects 2018, 9, 164. https://doi.org/10.3390/insects9040164
Hurtado LA, Rigg CA, Calzada JE, Dutary S, Bernal D, Koo SI, Chaves LF. Population Dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a Village in a Region Targeted for Malaria Elimination in Panamá. Insects. 2018; 9(4):164. https://doi.org/10.3390/insects9040164
Chicago/Turabian StyleHurtado, Lisbeth Amarilis, Chystrie A. Rigg, José E. Calzada, Sahir Dutary, Damaris Bernal, Susana Isabel Koo, and Luis Fernando Chaves. 2018. "Population Dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a Village in a Region Targeted for Malaria Elimination in Panamá" Insects 9, no. 4: 164. https://doi.org/10.3390/insects9040164
APA StyleHurtado, L. A., Rigg, C. A., Calzada, J. E., Dutary, S., Bernal, D., Koo, S. I., & Chaves, L. F. (2018). Population Dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a Village in a Region Targeted for Malaria Elimination in Panamá. Insects, 9(4), 164. https://doi.org/10.3390/insects9040164






