Landscape and Environmental Factors Influencing Stage Persistence and Abundance of the Bamboo Mosquito, Tripteroides bambusa (Diptera: Culicidae), across an Altitudinal Gradient
Abstract
1. Introduction
2. Methods
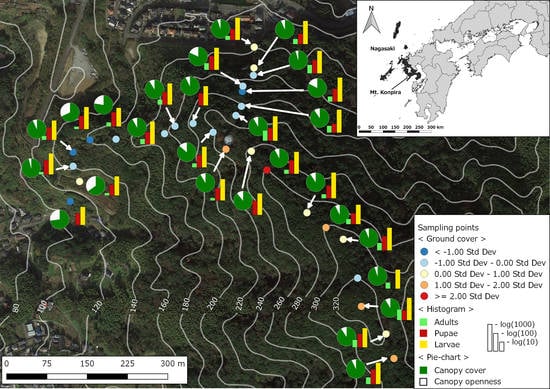
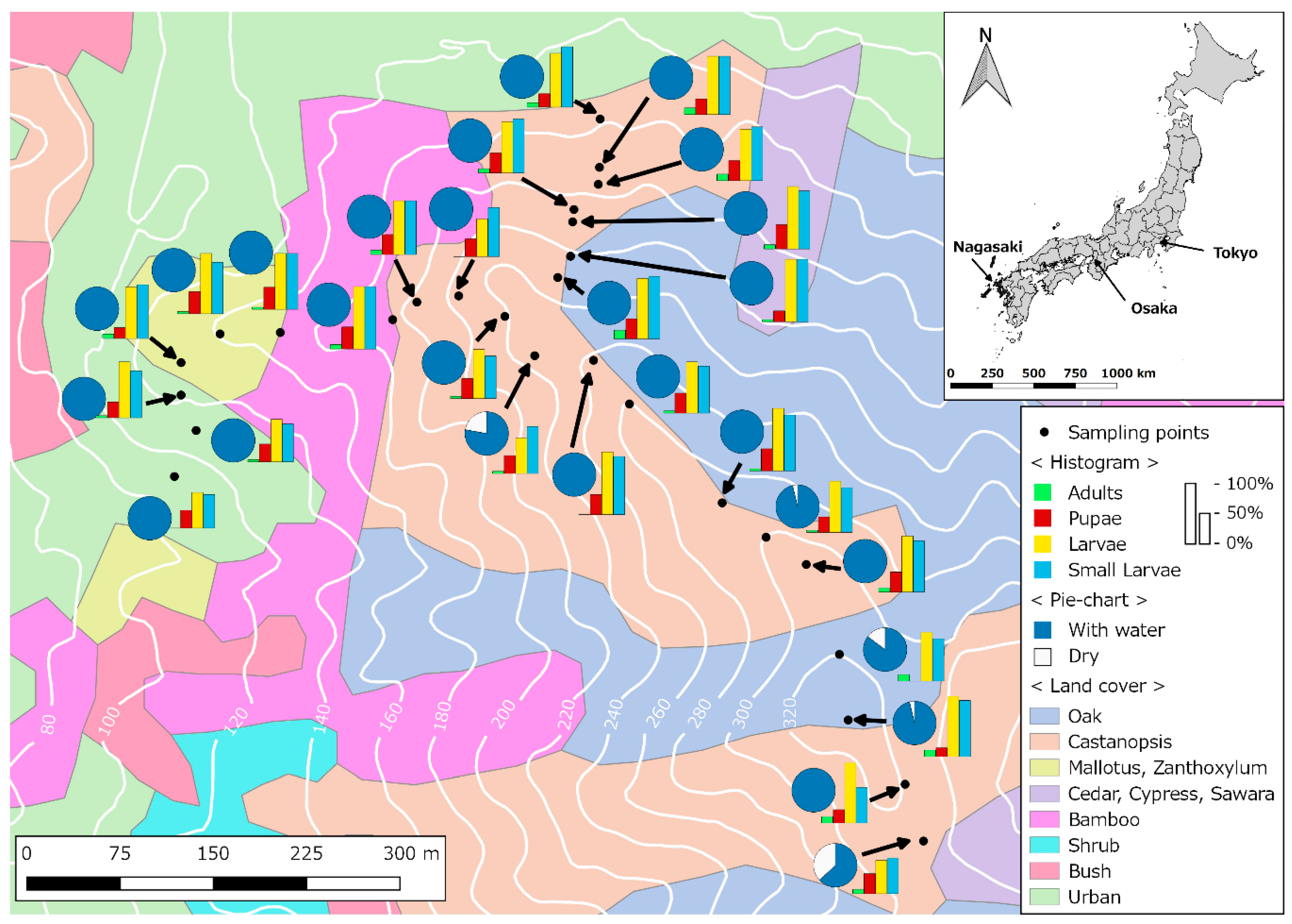
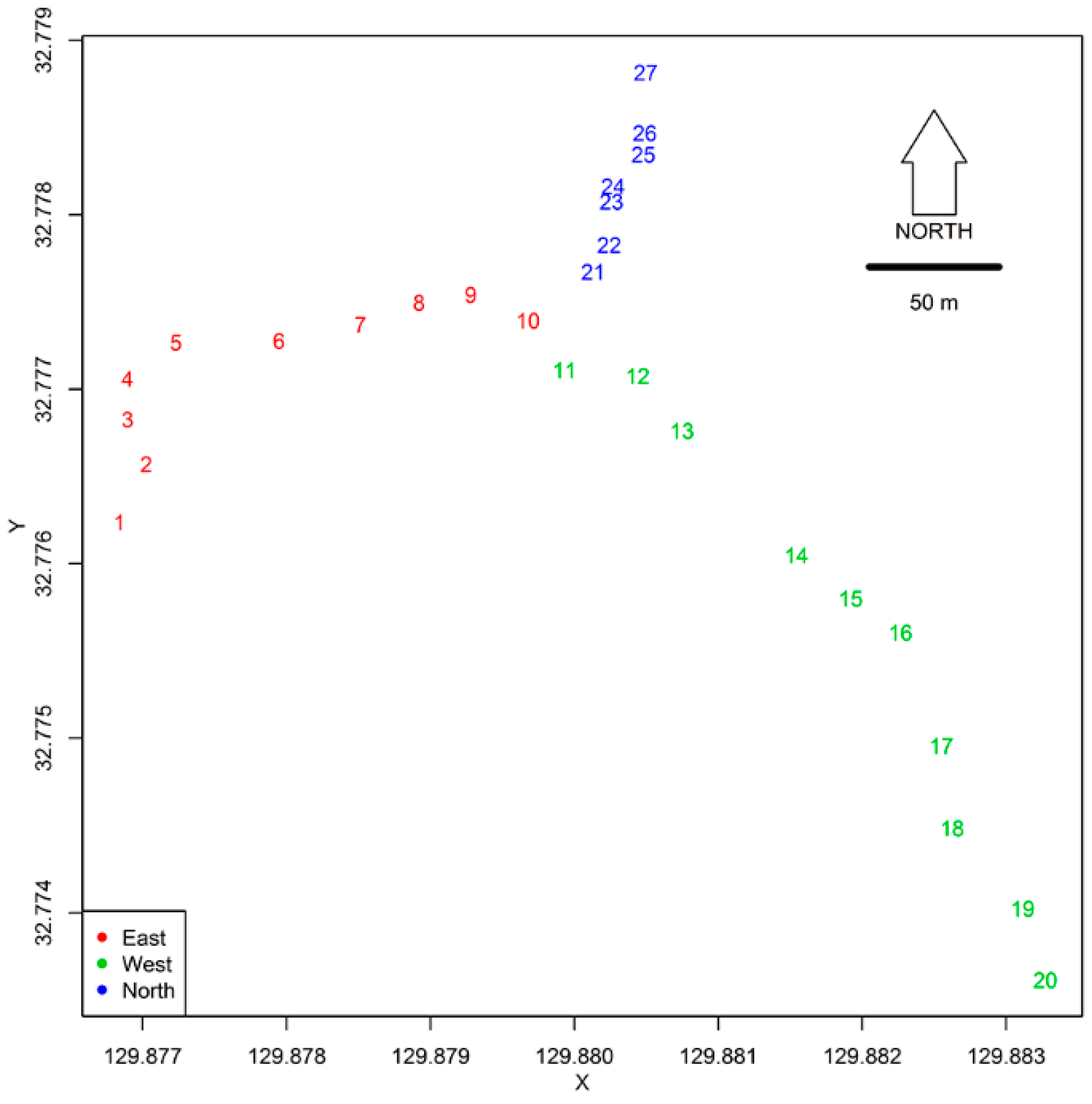
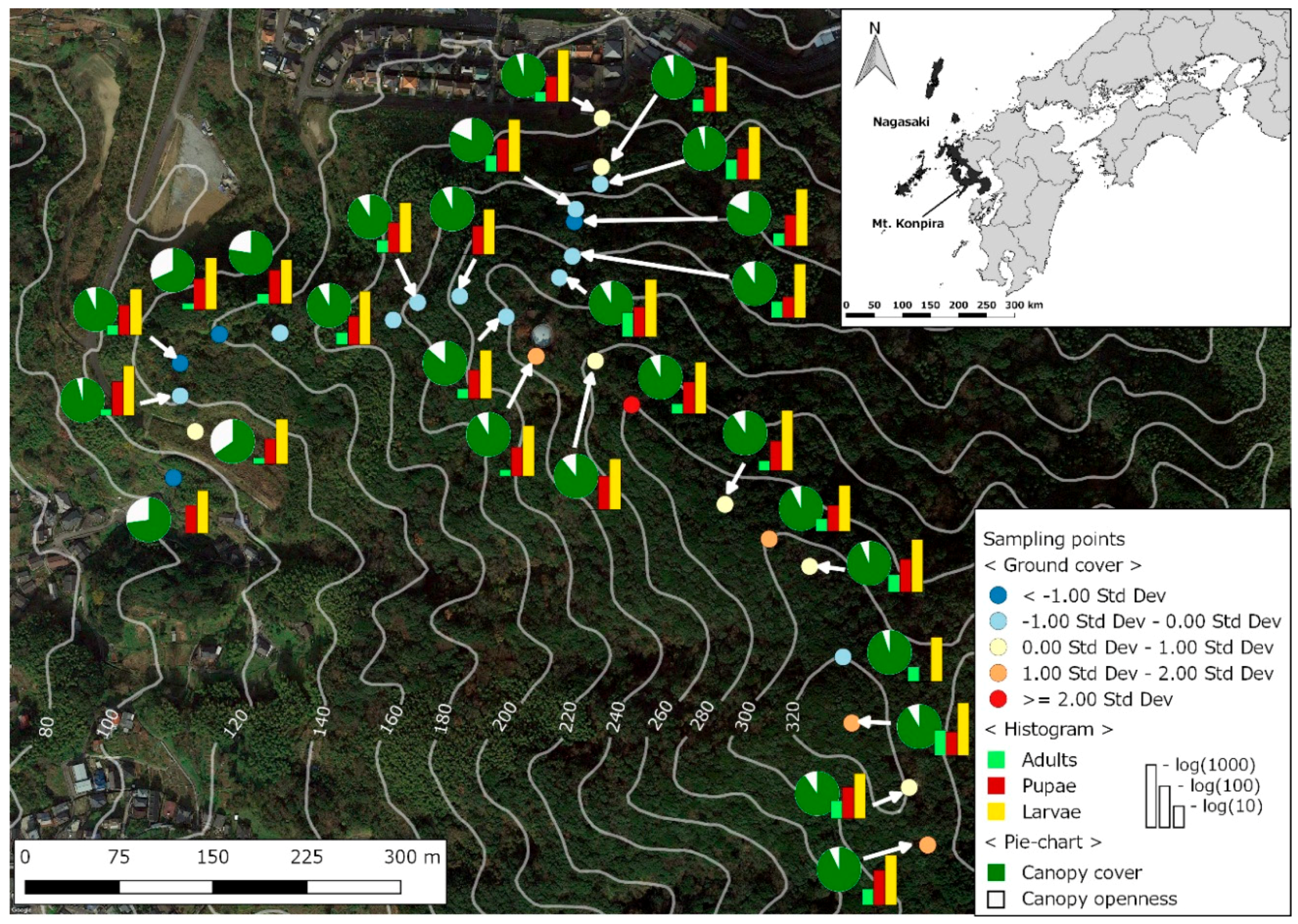
2.1. Study Site
2.2. Mosquito Sampling
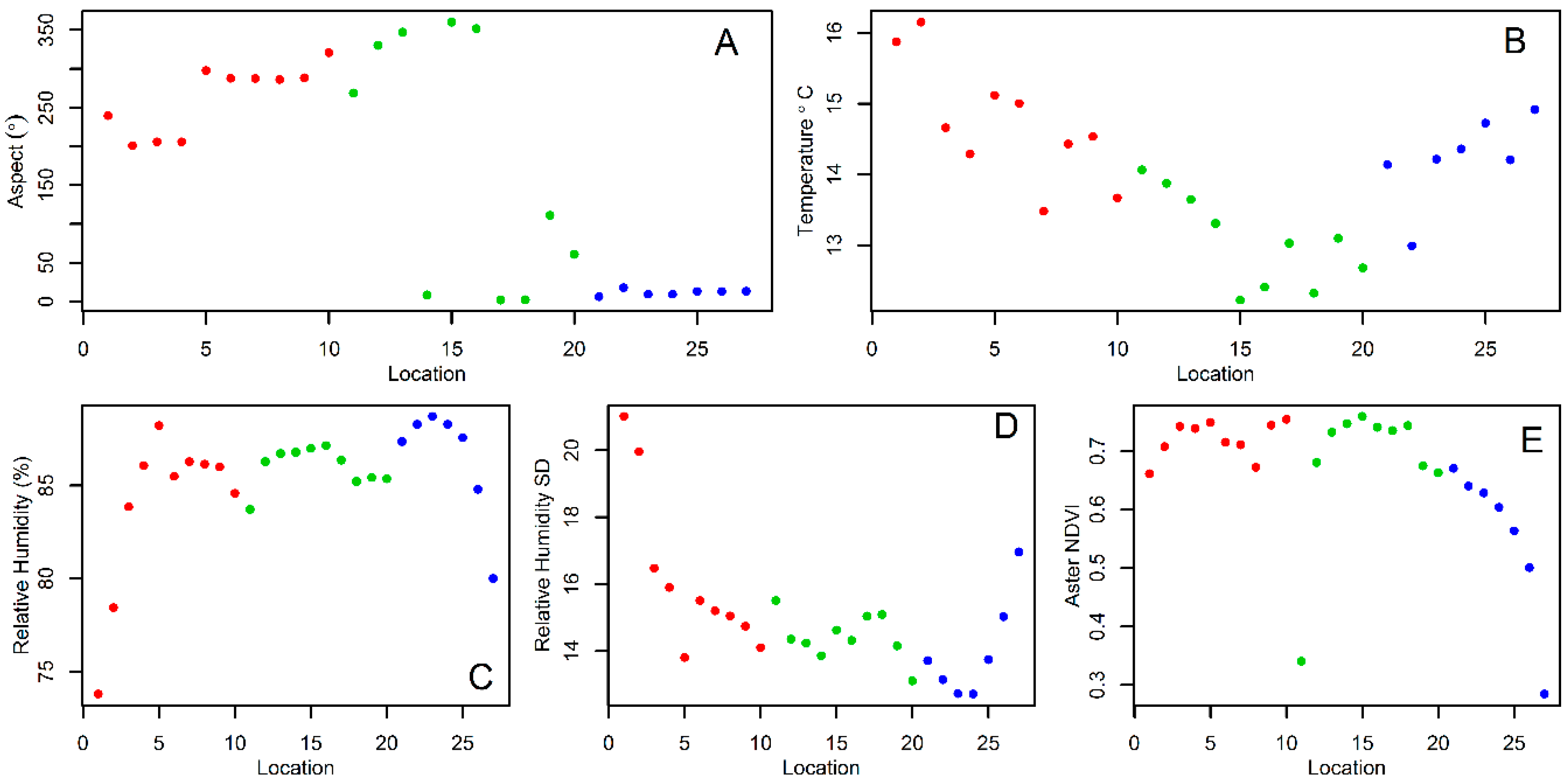
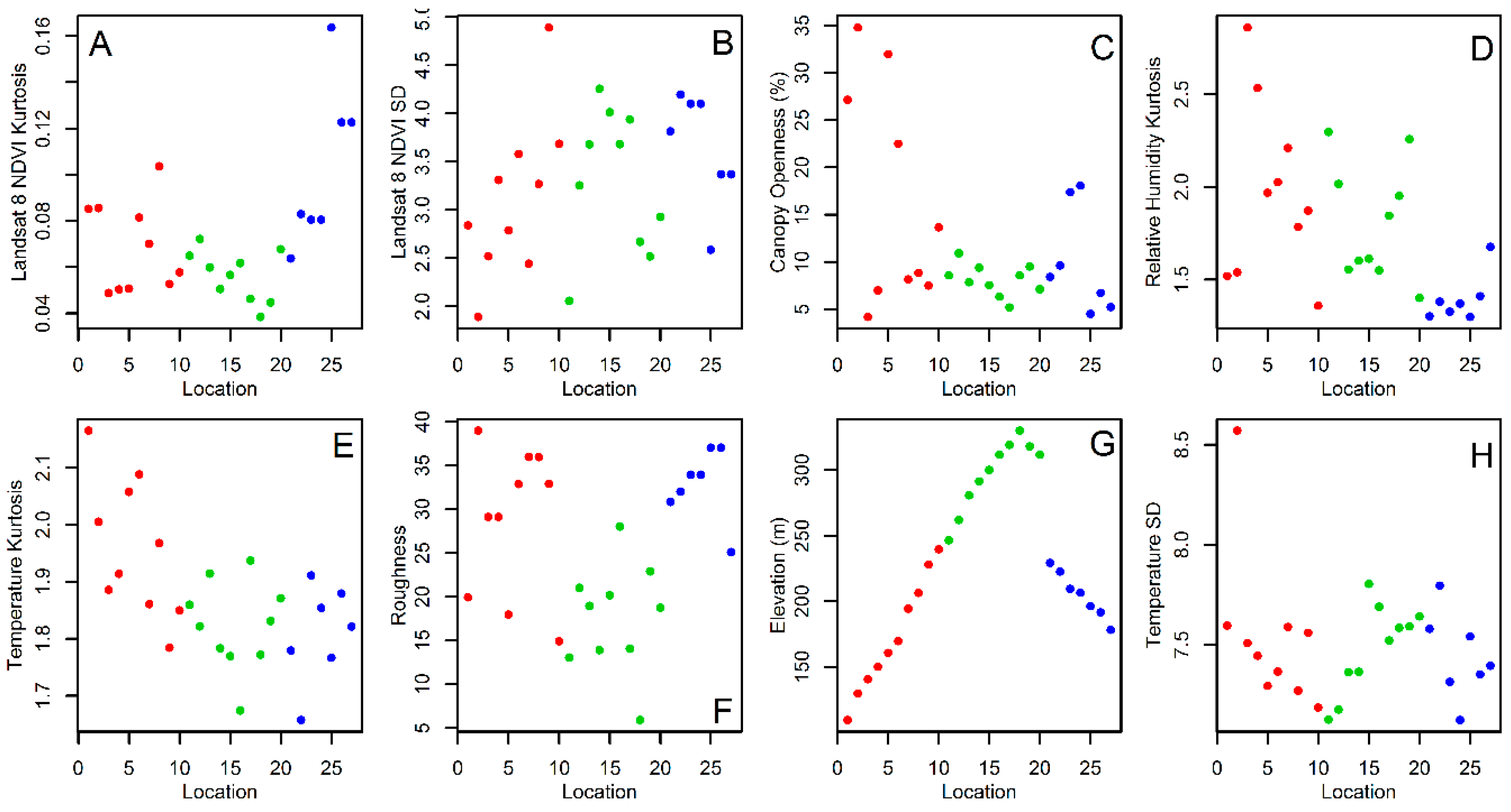
2.3. Environmental and Landscape Covariates
2.4. Statistical Modeling
2.5. Software
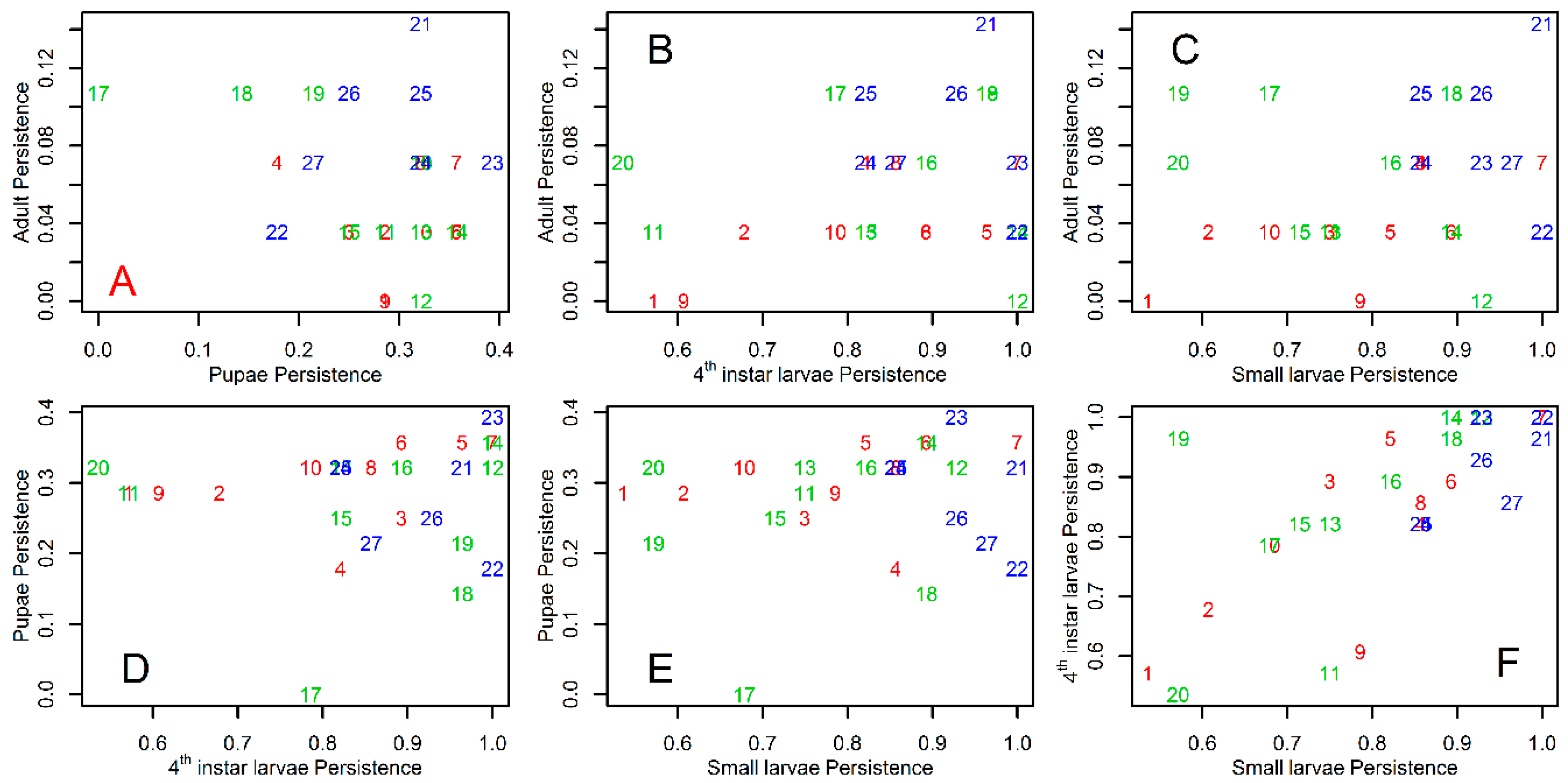
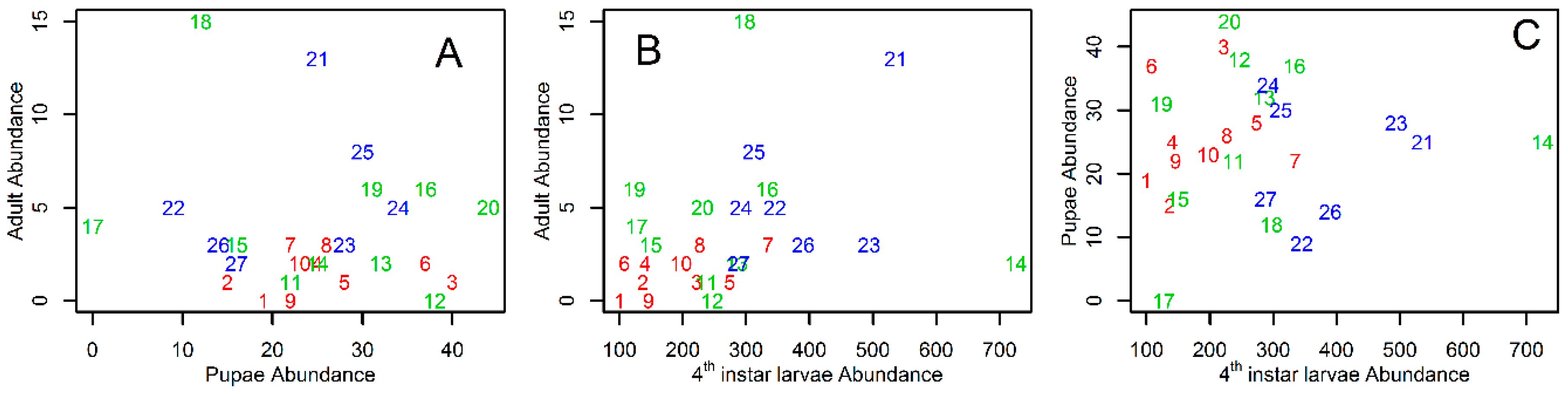
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silver, J.B. Mosquito Ecology: Field Sampling Methods, 3rd ed.; Springer: New York, NY, USA, 2008; p. 1498. [Google Scholar]
- Reisen, W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Perkins, T.A.; Reiner, J.R.C.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Le Menach, A.; Pulliam, J.R.C.; et al. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Drakeley, C.J.; Chiyaka, C.; Hay, S.I. A quantitative analysis of transmission efficiency versus intensity for malaria. Nat. Commun. 2010, 1, 108. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Palis, Y.; Curtis, C.F. Biting and resting behaviour of anophelines in Western Venezuela and implications for control of malaria transmission. Med. Vet. Entomol. 1992, 6, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-C.; Chaves, L.F.; Tsai, K.-H.; Chuang, T.-W. Increased adult Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) abundance in a dengue transmission hotspot, compared to a coldspot, within Kaohsiung city, Taiwan. Insects 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Calzada, J.E.; Valderama, A.; Saldaña, A. Cutaneous leishmaniasis and sand fly fluctuations are associated with El Niño in Panamá. PLoS Neglect. Trop. Dis. 2014, 8, e3210. [Google Scholar] [CrossRef] [PubMed]
- Kitron, U. Landscape ecology and epidemiology of vector-borne diseases: Tools for spatial analysis. J. Med. Entomol. 1998, 35, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K. Medical entomology—Back to the future? Infect. Genet. Evol. 2014, 28, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Royama, T. Analytical Population Dynamics; Chapman and Hall: London, UK, 1992. [Google Scholar]
- Turchin, P. Complex Population Dynamics; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Berryman, A.; Barbosa, P.; Schultz, J. The theory and classification of outbreaks. In Insect Outbreaks; Barbosa, P., Schultz, J.C., Eds.; Academic Press: San Diego, CA, USA, 1987; pp. 3–30. [Google Scholar]
- Berryman, A.A.; Stark, R.W. Assessing the risk of forest insect outbreaks. Z. Angew. Entomol. 1985, 99, 199–208. [Google Scholar] [CrossRef]
- Hoshi, T.; Imanishi, N.; Moji, K.; Chaves, L.F. Density dependence in a seasonal time series of the bamboo mosquito, Tripteroides bambusa (Diptera: Culicidae). Can. Entomol. 2017, 149, 338–344. [Google Scholar] [CrossRef]
- Hoshi, T.; Higa, Y.; Chaves, L.F. Uranotaenia novobscura ryukyuana (Diptera: Culicidae) population dynamics are denso-dependent and autonomous from weather fluctuations. Ann. Entomol. Soc. Am. 2014, 107, 136–142. [Google Scholar] [CrossRef]
- Benelli, G.; Jeffries, C.; Walker, T. Biological control of mosquito vectors: Past, present, and future. Insects 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Toma, T.; Miyagi, I. Notes on the mosquitoes collected at forest areas in the northern part of Okinawajima, Ryukyu islands, Japan. Jpn. J. Sanit. Zool. 1981, 32, 271–279. [Google Scholar] [CrossRef]
- Miyagi, I.; Toma, T. Studies on the mosquitoes in Yaeyama islands, Japan: 5. Notes on the mosquitoes collected in forest areas of Iriomotejima. Jpn. J. Sanitary Zool. 1980, 31, 81–91. [Google Scholar] [CrossRef]
- Chaves, L.F.; Hamer, G.L.; Walker, E.D.; Brown, W.M.; Ruiz, M.O.; Kitron, U.D. Climatic variability and landscape heterogeneity impact urban mosquito diversity and vector abundance and infection. Ecosphere 2011, 2, art70. [Google Scholar] [CrossRef]
- Hoshi, T.; Imanishi, N.; Higa, Y.; Chaves, L.F. Mosquito biodiversity patterns around urban environments in south-central Okinawa island, Japan. J. Am. Mosq. Control Assoc. 2014, 30, 260–267. [Google Scholar] [CrossRef]
- Washburn, J. Regulatory factors affecting larval mosquito populations in container and pool habitats: Implications for biological control. J. Am. Mosq. Control Assoc. 1995, 11, 279–283. [Google Scholar]
- Miyagi, I.; Toma, T.; Mogi, M. Biological control of container-breeding mosquitoes, Aedes albopictus and Culex quinquefasciatus, in a japanese island by release of Toxorhynchites splendens adults. Med. Vet. Entomol. 1992, 6, 290–300. [Google Scholar] [CrossRef]
- Chaves, L.F.; Imanishi, N.; Hoshi, T. Population dynamics of Armigeres subalbatus (Diptera: Culicidae) across a temperate altitudinal gradient. Bull. Entomol. Res. 2015, 105, 589–597. [Google Scholar] [CrossRef]
- Chaves, L.F. Mosquito species (Diptera: Culicidae) persistence and synchrony across an urban altitudinal gradient. J. Med. Entomol. 2017, 54, 329–339. [Google Scholar] [CrossRef]
- Dey, S.; Joshi, A. Stability via asynchrony in Drosophila metapopulations with low migration rates. Science 2006, 312, 434–436. [Google Scholar] [CrossRef]
- Yang, G.-J.; Brook, B.W.; Whelan, P.I.; Cleland, S.; Bradshaw, C.J.A. Endogenous and exogenous factors controlling temporal abundance patterns of tropical mosquitoes. Ecol. Appl. 2008, 18, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Scott, T.W.; Morrison, A.C.; Takada, T. Hot temperatures can force delayed mosquito outbreaks via sequential changes in Aedes aegypti demographic parameters in autocorrelated environments. Acta Trop. 2014, 129, 15–24. [Google Scholar] [CrossRef]
- Mori, A.; Wada, Y. The seasonal abundance of Aedes albopictus in Nagasaki. Trop. Med. 1978, 20, 29–37. [Google Scholar]
- Reisen, W.K.; Milby, M.M.; Meyer, R.P.; Reeves, W.C. Population ecology of Culex tarsalis (Diptera: Culicidae) in a foothill environment in Kern county, California: Temporal changes in male relative abundance and swarming behavior. Ann. Entomol. Soc. Am. 1983, 76, 809–815. [Google Scholar] [CrossRef]
- Reisen, W.K.; Milby, M.M.; Reeves, W.C.; Meyer, R.P.; Bock, M.E. Population ecology of Culex tarsalis (Diptera: Culicidae) in a foothill environment in Kern county, California: Temporal changes in female relative abundance, reproductive status, and survivorship. Ann. Entomol. Soc. Am. 1983, 76, 800–808. [Google Scholar] [CrossRef]
- Day, J.F.; Ramsey, A.M.; Zhang, J.T. Environmentally mediated seasonal variation in mosquito body size. Environ. Entomol. 1990, 19, 469–473. [Google Scholar] [CrossRef]
- Scott, T.W.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Zhou, H.; Edman, J.D. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Population dynamics. J. Med. Entomol. 2000, 37, 77–88. [Google Scholar] [CrossRef]
- Yang, G.-J.; Bradshaw, C.; Whelan, P.; Brook, B. Importance of endogenous feedback controlling the long-term abundance of tropical mosquito species. Popul. Ecol. 2008, 50, 293–305. [Google Scholar] [CrossRef]
- Reisen, W.K.; Lothrop, H.D. Effects of sampling design on the estimation of adult mosquito abundance. J. Am. Mosq. Control Assoc. 1999, 15, 105–114. [Google Scholar] [PubMed]
- Chaves, L.F.; Morrison, A.C.; Kitron, U.D.; Scott, T.W. Nonlinear impacts of climatic variability on the density-dependent regulation of an insect vector of disease. Glob. Chang. Biol. 2012, 18, 457–468. [Google Scholar] [CrossRef]
- Chaves, L.F.; Higa, Y.; Lee, S.H.; Jeong, J.Y.; Heo, S.T.; Kim, M.; Minakawa, N.; Lee, K.H. Environmental forcing shapes regional house mosquito synchrony in a warming temperate island. Environ. Entomol. 2013, 42, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Hayashi, T. Results of mosquito surveillance using dry-ice traps from 2003 to 2013 at the national institute of infectious diseases, Tokyo, Japan. Med. Entomol. Zool. 2014, 65, 131–137. [Google Scholar] [CrossRef]
- Chaves, L.F. Globally invasive, withdrawing at home: Aedes albopictus and Aedes japonicus facing the rise of Aedes flavopictus. Int. J. Biometeorol. 2016, 60, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; Trapido, H.; Carpenter, S.J.; Blanton, F.S. The abundance cycles of arboreal mosquitoes during six years at a sylvan yellow fever locality in Panama. Ann. Entomol. Soc. Am. 1956, 49, 543–547. [Google Scholar] [CrossRef]
- Sun, W.K.C. The seasonal succession of mosquitoes in Taiwan. J. Med. Entomol. 1964, 1, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J. Seasonal changes in population structure of Culex pipiens quinquefasciatus Say (Diptera: Culicidae): Study of an isolated population. J. Med. Entomol. 1975, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Hsi, B.P. Interrelationships between selected meteorologic phenomena and immature stages of Culex pipiens quinquefasciatus Say: Study of an isolated population. J. Med. Entomol. 1975, 12, 299–308. [Google Scholar] [CrossRef]
- Hayes, J.; Downs, T.D. Seasonal changes in an isolated population of Culex pipiens quinquefasciatus (Diptera: Culicidae): A time series analysis. J. Med. Entomol. 1980, 17, 63–69. [Google Scholar] [CrossRef]
- Toma, T.; Sakamoto, S.; Miyagi, I. The seasonal appearance of Aedes albopictus in Okinawajima, the Ryukyu archipelago, Japan. Mosq. News 1982, 42, 179–183. [Google Scholar]
- Makiya, K. Population dynamics of mosquitoes in Nagoya district b. Larval and imaginal populations of Aedes albopictus (Skuse) in a cemetery of Nagoya city. Jpn. J. Sanitary Zool. 1974, 25, 41–49. [Google Scholar] [CrossRef]
- Takagi, M.; Tsuda, Y.; Suwonkerd, W.; Sugiyama, A.; Prajakwong, S.; Wada, Y. Vector mosquitoes of japanese encephalitis (Diptera: Culicidae) in northern Thailand: Seasonal changes in larval community structure. Appl. Entomol. Zool. 1997, 32, 333–340. [Google Scholar] [CrossRef]
- Tsuda, Y.; Takagi, M.; Wada, Y. Ecological study on mosquito communities in tree holes in Nagasaki, Japan, with special reference to Aedes albopictus (Diptera: Culicidae). Jpn. J. Sanitary Zool. 1994, 45, 103–111. [Google Scholar] [CrossRef]
- Suwonkerd, W.; Tsuda, Y.; Takagi, M.; Wada, Y. Seasonal ocurrence of Aedes aegypti and Ae. albopictus in used tires in 1992-1994, Chiangmai, Thailand. Trop. Med. 1996, 38, 101–105. [Google Scholar]
- Chaves, L.F.; Moji, K. Density dependence, landscape, and weather impacts on aquatic Aedes japonicus japonicus (Diptera: Culicidae) abundance along an urban altitudinal gradient. J. Med. Entomol. 2018, 55, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Meyer, R.P.; Shields, J.; Arbolante, C. Population ecology of preimaginal Culex tarsalis (Diptera: Culicidae) in Kern county, California. J. Med. Entomol. 1989, 26, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Milby, M.M.; Meyer, R.P. Population dynamics of adult Culex mosquitoes (Diptera: Culicidae) along the Kern river, Kern county, California, in 1990. J. Med. Entomol. 1992, 29, 531–543. [Google Scholar] [CrossRef]
- Barker, C.M.; Eldridge, B.F.; Reisen, W.K. Seasonal abundance of Culex tarsalis and Culex pipiens complex mosquitoes (Diptera: Culicidae) in California. J. Med. Entomol. 2010, 47, 759–768. [Google Scholar] [CrossRef]
- Reisen, W.K.; Thiemann, T.; Barker, C.M.; Lu, H.L.; Carroll, B.; Fang, Y.; Lothrop, H.D. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J. Med. Entomol. 2010, 47, 230–237. [Google Scholar] [CrossRef]
- Chaves, L.F. Climate change and the biology of insect vectors of human pathogens. In Invertebrates and Global Climate Change; Johnson, S., Jones, H., Eds.; Wiley: Chichester, UK, 2017; pp. 126–147. [Google Scholar]
- Eisen, L.; Bolling, B.G.; Blair, C.D.; Beaty, B.J.; Moore, C.G. Mosquito species richness, composition, and abundance along habitat-climate-elevation gradients in the northern Colorado front range. J. Med. Entomol. 2008, 45, 800–811. [Google Scholar] [CrossRef]
- Mogi, M. Overwintering strategies of mosquitoes (Diptera: Culicidae) on warmer islands may predict impact of global warming on Kyushu, Japan. J. Med. Entomol. 1996, 33, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Couret, J. Meta-analysis of factors affecting ontogenetic development rate in the Culex pipiens (Diptera: Culicidae) complex. Environ. Entomol. 2013, 42, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Couret, J.; Benedict, M.Q. A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae). BMC Ecol. 2014, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Rejmankova, E.; Savage, H.; Rejmanek, M.; Arredondo-Jimenez, J.; Roberts, D. Multivariate analysis of relationships between habitats, environmental factors and occurrence of anopheline mosquito larvae Anopheles albimanus and A. pseudopunctipennis in southern Chiapas, Mexico. J. Appl. Ecol. 1991, 28, 827–841. [Google Scholar] [CrossRef]
- Rejmankova, E.; Savage, H.; Rodriguez, M.; Roberts, D.; Rejmanek, M. Aquatic vegetation as a basis for classification of Anopheles albimanus Weideman (Diptera: Culicidae) larval habitats. Environ. Entomol. 1992, 21, 598–603. [Google Scholar] [CrossRef]
- Hurtado, L.A.; Rigg, C.A.; Calzada, J.E.; Dutary, S.; Bernal, D.; Koo, S.I.; Chaves, L.F. Population dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a village in a region targeted for malaria elimination in Panamá. Insects 2018, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.O.; Chaves, L.F.; Hamer, G.L.; Sun, T.; Brown, W.M.; Walker, E.D.; Haramis, L.; Goldberg, T.L.; Kitron, U.D. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast illinois, USA. Parasites Vectors 2010, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Shand, L.; Brown, W.M.; Chaves, L.F.; Goldberg, T.L.; Hamer, G.L.; Haramis, L.; Kitron, U.; Walker, E.D.; Ruiz, M.O. Predicting West Nile virus infection risk from the synergistic effects of rainfall and temperature. J. Med. Entomol. 2016, 53, 935–944. [Google Scholar] [CrossRef]
- Zani, P.A.; Swanson, S.E.T.; Corbin, D.; Cohnstaedt, L.W.; Agotsch, M.D.; Bradshaw, W.E.; Holzapfel, C.M. Geographic variation in tolerance of transient thermal stress in the mosquito Wyeomyia smithii. Ecology 2005, 86, 1206–1211. [Google Scholar] [CrossRef]
- Tanaka, K.; Mizusawa, K.; Saugstad, E.S. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu archipelago and the Ogasawara islands) and Korea (Diptera: Culicidae). Contrib. Am. Entomol. Inst. 1979, 16, 1–987. [Google Scholar]
- Kamimura, K. The distribution and habit of medically important mosquitoes of Japan. Jpn. J. Sanitary Zool. 1968, 19, 15–34. [Google Scholar] [CrossRef]
- Maekawa, Y.; Tsuda, Y.; Sawabe, K. A nationwide survey on distribution of mosquitoes in Japan. Med. Entomol. Zool. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- Kurihara, Y. Synecological analysis of the larval association of dipterous insect in the bamboo container. Jpn. J. Ecol. 1958, 8, 113–117. [Google Scholar]
- Moriya, K. Seasonal trends of field population of mosquitoes with ovitrap in Kanagawa prefecture: 1) comparison of the populations of four residental areas in Kamakura city in 1971. Jpn. J. Sanitary Zool. 1974, 25, 237–244. [Google Scholar] [CrossRef]
- Sunahara, T.; Mogi, M. Can the tortoise beat the hare? A possible mechanism for the coexistence of competing mosquitoes in bamboo groves. Ecol. Res. 1997, 12, 63–70. [Google Scholar] [CrossRef]
- Craven, R.; Eliason, D.; Francy, D.; Reiter, P.; Campos, E.; Jakob, W.; Smith, G.; Bozzi, C.; Moore, C.; Maupin, G. Importation of Aedes albopictus and other exotic mosquito species into the united states in used tires from asia. J. Am. Mosq. Control Assoc. 1988, 4, 138–142. [Google Scholar] [PubMed]
- Laird, M.; Calder, L.; Thornton, R.C.; Syme, R.; Holder, P.W.; Mogi, M. Japanese Aedes albopictus among 4 mosquito species reaching New Zealand in used tires. J. Am. Mosq. Control Assoc. 1994, 10, 14–23. [Google Scholar] [PubMed]
- Chaves, L.F.; Harrington, L.C.; Keogh, C.L.; Nguyen, A.M.; Kitron, U.D. Blood feeding patterns of mosquitoes: Random or structured? Front. Zool. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, I. Feeding habits of some japanese mosquitoes on cold-blooded animals in laboratory. Trop. Med. 1972, 14, 203–217. [Google Scholar]
- Miyagi, I. Colonizations of Culex (Lophoceraomyia) infantulus edwards and Tripteroides (Tripteroides) bambusa (Yamada) in laboratory. Trop. Med. 1973, 15, 196–203. [Google Scholar]
- Mori, A.; Ueda, M.; Kurokawa, K. Observations on the overwintering of Tripteroides bambusa (Diptera: Culicidae) in Nagasaki. Trans. Nagasaki Biol. Soc. 1985, 29, 55–60. [Google Scholar]
- Chaves, L.F.; Jian, J.-Y.; Moji, K. Overwintering in the bamboo mosquito Tripteroides bambusa (Diptera: Culicidae) during a warm, but unpredictably changing, winter. Environ. Entomol. 2018, 47, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Zea Iriarte, W.L.; Tsuda, Y.; Wada, Y.; Takagi, M. Distribution of mosquitoes on a hill of Nagasaki city, with emphasis to the distance from human dwellings. Trop. Med. 1991, 33, 55–60. [Google Scholar]
- Abrams, M. The advanced spaceborne thermal emission and reflection radiometer (ASTER): Data products for the high spatial resolution imager on nasa’s terra platform. Int. J. Remote Sens. 2000, 21, 847–859. [Google Scholar] [CrossRef]
- Roy, D.P.; Wulder, M.A.; Loveland, T.R.; Woodcock, C.E.; Allen, R.G.; Anderson, M.C.; Helder, D.; Irons, J.R.; Johnson, D.M.; Kennedy, R.; et al. Landsat-8: Science and product vision for terrestrial global change research. Remote Sens. Environ. 2014, 145, 154–172. [Google Scholar] [CrossRef]
- Chavez, P.S. Image-based atmospheric corrections-revisited and improved. Photogramm. Eng. Remote Sens. 1996, 62, 1025–1035. [Google Scholar]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- NASALPDAAC. NASA Land Processes Distributed Active Archive Center. Available online: https://lpdaac.usgs.gov (accessed on 8 October 2018).
- JAXA. Digital Elevation Model. Available online: https://fgd.gsi.go.jp/download/mapGis.php?tab=dem (accessed on 18 November 2018).
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ross, S.M. Introduction to Probability Models; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: San Francisco, CA, USA, 1998. [Google Scholar]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer: New York, NY, USA, 2013; p. 600. [Google Scholar]
- Brunsdon, C.; Comber, L. An Introduction to R for Spatial Analysis and Mapping; Sage Publications Ltd.: London, UK, 2015; p. 343. [Google Scholar]
- Hanski, I. Metapopulation Ecology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Ranta, E.; Lundberg, P.; Kaitala, V. Ecology of Populations; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Levins, R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull. Entomol. Soc. Am. 1969, 15, 237–240. [Google Scholar] [CrossRef]
- Edman, J.D. Disease control through manipulation of vector-host interaction: Some historical and evolutionary perspectives. In Proceedings of a Symposium: The Role of Vector-Host Interactions in Disease Tranmission; Scott, T.W., Grumstrup-Scott, J., Eds.; Entomological Society of America: Washington, DC, USA, 1988; pp. 43–50. [Google Scholar]
- Perfecto, I.; Vandermeer, J. Biodiversity conservation in tropical agroecosystems—A new conservation paradigm. In Annals of the New York Academy of Sciences; Blackwell Publishing: Hoboken, NJ, USA, 2008; Volume 1134, pp. 173–200. [Google Scholar]
- Ebenman, B. Evolution in organisms that change their niches during the life-cycle. Am. Nat. 1992, 139, 990–1021. [Google Scholar] [CrossRef]
- Mutuku, F.M.; Bayoh, M.N.; Gimnig, J.E.; Vulule, J.M.; Kamau, L.; Walker, E.D.; Kabiru, E.; Hawley, W.A. Pupal habitat productivity of Anopheles gambiae complex mosquitoes in a rural village in Western Kenya. Am. J. Trop. Med. Hyg. 2006, 74, 54–61. [Google Scholar] [CrossRef]
- Burke, R.; Barrera, R.; Lewis, M.; Kluchinsky, T.; Claborn, D. Septic tanks as larval habitats for the mosquitoes Aedes aegypti and Culex quinquefasciatus in Playa Playita, Puerto Rico. Med. Vet. Entomol. 2010, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (mpb-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Egizi, A.; Fefferman, N.H.; Fonseca, D.M. Evidence that implicit assumptions of ‘no evolution’ of disease vectors in changing environments can be violated on a rapid timescale. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140136. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140135. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Koenraadt, C.J.M. Climate change and highland malaria: Fresh air for a hot debate. Q. Rev. Biol. 2010, 85, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Levins, R. Evolution in Changing Environments. Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968; p. 120. [Google Scholar]
| Stage | Parameter | Estimate | Std. Error | z Value | Pr(>|z|) |
|---|---|---|---|---|---|
| Adult | (Intercept) | 0.9154 | 0.1992 | 4.59 | <0.0000 * |
| Aspect | −0.0029 | 0.0011 | −2.5 | 0.0123 * | |
| Moran’s I | −0.036944 | --- | --- | 0.495 | |
| Residual deviance: 13.619 on 25 degrees of freedom | |||||
| Pupae | (Intercept) | 8.8265 | 6.6304 | 1.33 | 0.1831 |
| Aspect | 0.0011 | 0.0005 | 2.07 | 0.0389 * | |
| Mean water temperature | 0.1706 | 0.0891 | 1.91 | 0.0557 | |
| Mean relative humidity | −0.1014 | 0.0608 | −1.67 | 0.0954 | |
| SD relative humidity | −0.2659 | 0.1128 | −2.36 | 0.0184 * | |
| Moran’s I | = 0.064435 | --- | --- | 0.320 | |
| Residual deviance: 17.450 on 22 degrees of freedom | |||||
| Fourth | (Intercept) | −2.5509 | 1.1521 | −2.21 | 0.0268 * |
| Instar | Mean relative humidity | 0.0287 | 0.0134 | 2.13 | 0.0331 * |
| Larvae | Moran’s I | −0.036247 | --- | --- | 0.478 |
| Residual deviance: 7.4042 on 25 degrees of freedom | |||||
| Small | (Intercept) | −2.5876 | 1.1851 | −2.18 | 0.0290 * |
| Larvae | Mean relative humidity | 0.034 | 0.0144 | 2.36 | 0.0182 * |
| Mean ASTER NDVI | −0.7009 | 0.3545 | −1.98 | 0.0480 * | |
| Moran’s I | −0.17784 | --- | --- | 0.762 | |
| Residual deviance: 7.0688 on 24 degrees of freedom | |||||
| Parameter | Estimate | Std. Error | z Value | Pr(>|z|) |
|---|---|---|---|---|
| (Intercept) | −28.0156 | 11.6329 | −2.41 | 0.0160 * |
| Kurtosis Landsat 8 NDVI | −1.0094 | 0.2321 | −4.35 | <0.0001 * |
| SD Landsat 8 NDVI | −17.7669 | 4.4391 | −4.00 | 0.0001 * |
| Mean canopy openness | −0.0551 | 0.0192 | −2.88 | 0.0040 * |
| Mean relative humidity | 0.3799 | 0.1189 | 3.19 | 0.0014 * |
| SD elative humidity | 0.3775 | 0.1816 | 2.08 | 0.0377 * |
| Kurtosis relative humidity | −1.8969 | 0.5093 | −3.72 | 0.0002 * |
| Aspect | −0.003 | 0.0008 | −3.49 | 0.0005 * |
| Moran’s I | −0.2094 | 0.796 |
| Parameter | Estimate | Std. Error | z Value | Pr(>|z|) |
|---|---|---|---|---|
| (Intercept) | 36.9328 | 8.8753 | 4.16 | <0.0001 |
| Kurtosis Landsat 8 NDVI | −0.2112 | 0.1038 | −2.04 | 0.0418 * |
| Mean relative humidity | −0.2989 | 0.0765 | −3.91 | 0.0001 * |
| SD relative humidity | −0.6585 | 0.1376 | −4.79 | <0.0001 * |
| Kurtosis Temperature | −0.6957 | 0.3039 | −2.29 | 0.0221 * |
| Aspect | 0.0017 | 0.0005 | 3.16 | 0.0016 * |
| Roughness | 0.0212 | 0.0085 | 2.49 | 0.0129 * |
| Overdispersion Theta | 14.03 | 6.53 | --- | --- |
| Moran’s I | −0.21259 | --- | --- | 0.804 |
| Parameter | Estimate | Std. Error | z Value | Pr(>|z|) |
|---|---|---|---|---|
| (Intercept) | 23.4876 | 4.6154 | 5.09 | <0.0001 * |
| Kurtosis Landsat 8 NDVI | −0.4094 | 0.1237 | −3.31 | 0.0009 * |
| SD Landsat 8 NDVI | −11.868 | 3.9843 | −2.98 | 0.0029 * |
| Elevation | −0.0048 | 0.0017 | −2.88 | 0.0040 * |
| Kurtosis relative humidity | −0.8272 | 0.2269 | −3.65 | 0.0003 * |
| Mean temperature | −0.6078 | 0.1192 | −5.1 | <0.0001 * |
| SD temperature | −0.7598 | 0.3113 | −2.44 | 0.0147 * |
| Aspect | −0.0012 | 0.0004 | −2.85 | 0.0043 * |
| Overdispersion Theta | 12.78 | 3.63 | --- | --- |
| Moran’s I | −0.46048 | --- | --- | 0.981 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, L.F.; Friberg, M.D.; Jian, J.-Y.; Moji, K. Landscape and Environmental Factors Influencing Stage Persistence and Abundance of the Bamboo Mosquito, Tripteroides bambusa (Diptera: Culicidae), across an Altitudinal Gradient. Insects 2019, 10, 41. https://doi.org/10.3390/insects10020041
Chaves LF, Friberg MD, Jian J-Y, Moji K. Landscape and Environmental Factors Influencing Stage Persistence and Abundance of the Bamboo Mosquito, Tripteroides bambusa (Diptera: Culicidae), across an Altitudinal Gradient. Insects. 2019; 10(2):41. https://doi.org/10.3390/insects10020041
Chicago/Turabian StyleChaves, Luis Fernando, Mariel D. Friberg, Jiun-Yu Jian, and Kazuhiko Moji. 2019. "Landscape and Environmental Factors Influencing Stage Persistence and Abundance of the Bamboo Mosquito, Tripteroides bambusa (Diptera: Culicidae), across an Altitudinal Gradient" Insects 10, no. 2: 41. https://doi.org/10.3390/insects10020041
APA StyleChaves, L. F., Friberg, M. D., Jian, J.-Y., & Moji, K. (2019). Landscape and Environmental Factors Influencing Stage Persistence and Abundance of the Bamboo Mosquito, Tripteroides bambusa (Diptera: Culicidae), across an Altitudinal Gradient. Insects, 10(2), 41. https://doi.org/10.3390/insects10020041