Odor-Specific Daily Rhythms in the Olfactory Sensitivity and Behavior of Aedes aegypti Mosquitoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
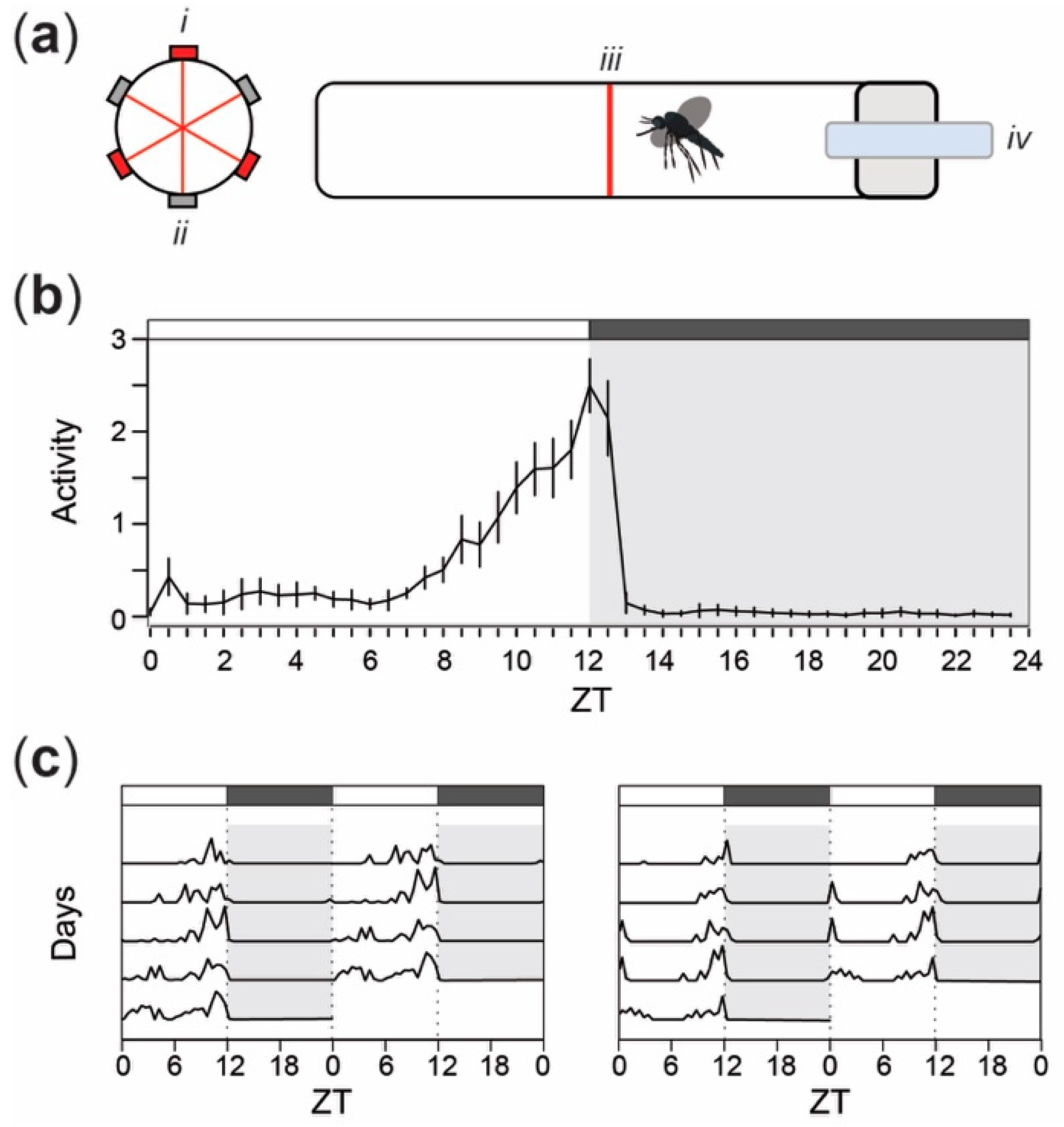
2.2. Activity Analysis
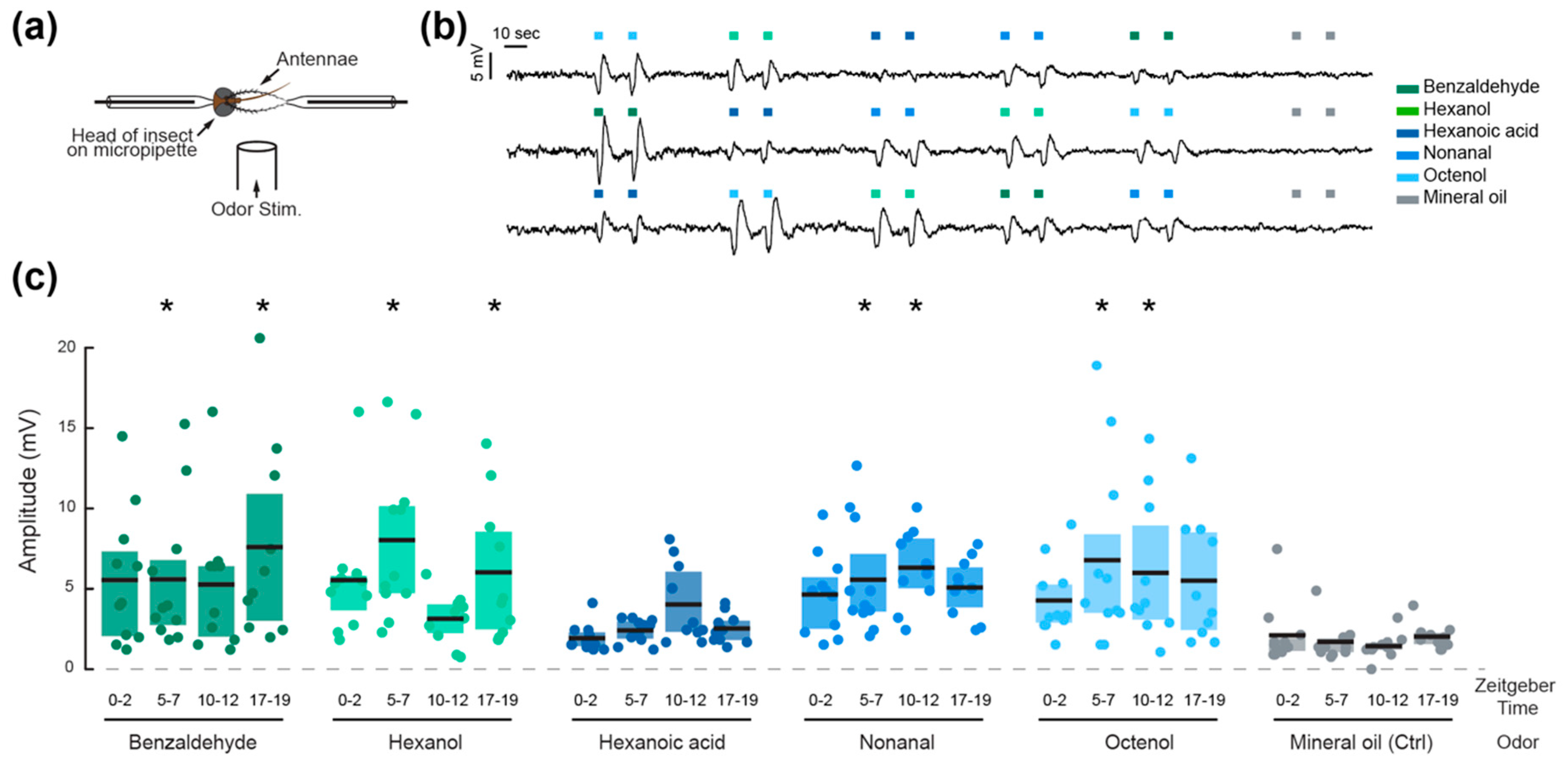
2.3. Olfactory Sensitivity
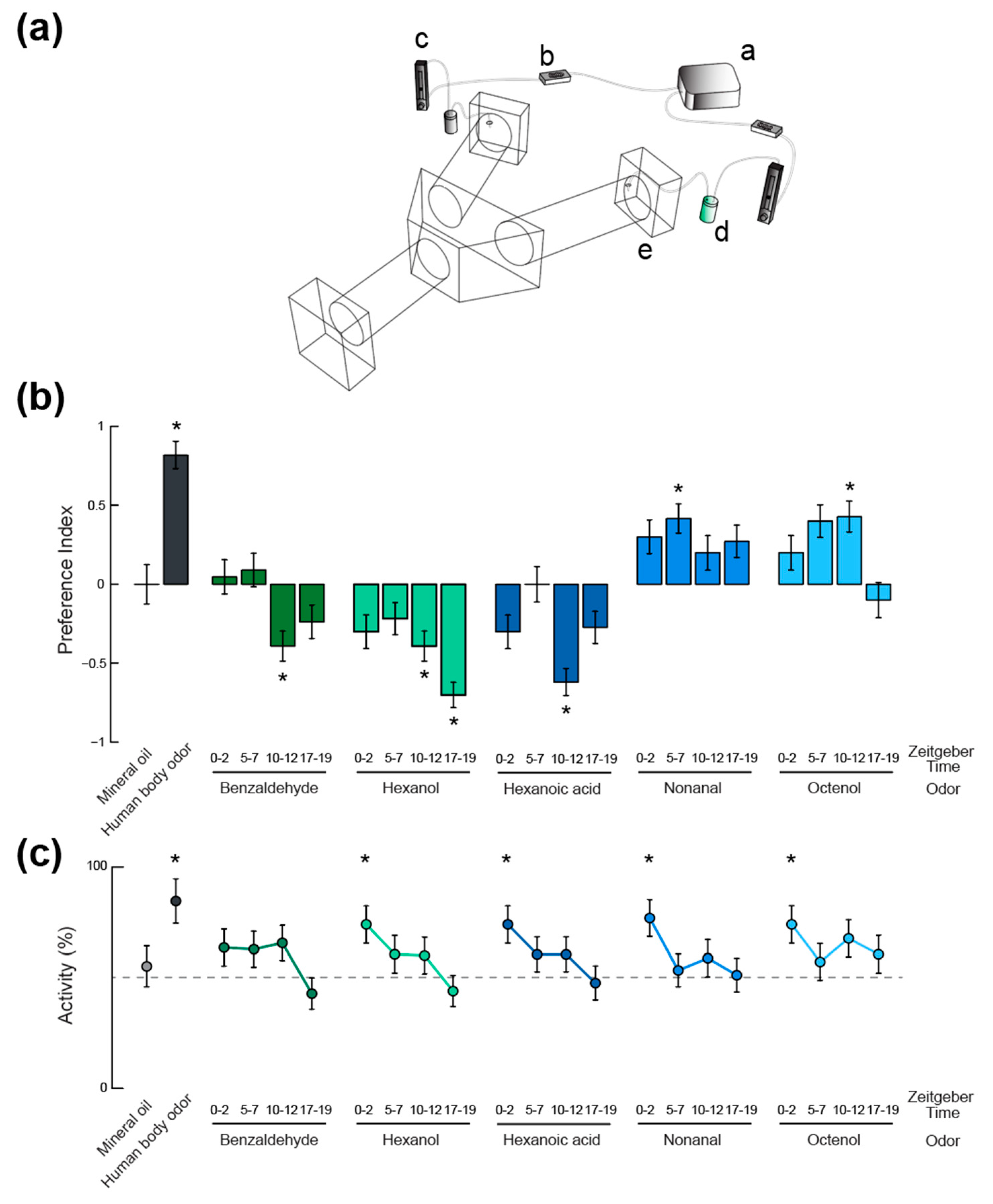
2.4. Olfactory Behavior
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, B.; Jones, M.D. The circadian rhythm of flight activity in the mosquito Aedes aegypti (L.). The phase-setting effects of light-on and light-off. J. Exp. Biol. 1969, 51, 59–70. [Google Scholar] [PubMed]
- Jones, M.D.R.; Hill, M.; Hope, A.M. The circadian flight activity of the mosquito Anopheles gambiae: Phase setting by the light regime. J. Exp. Biol. 1967, 47, 503–511. [Google Scholar] [PubMed]
- Rowland, M. Changes in the circadian flight activity of the mosquito Anopheles stephensi associated with insemination, blood-feeding, oviposition and nocturnal light intensity. Physiol. Entomol. 1989, 14, 77–84. [Google Scholar] [CrossRef]
- Peterson, E.L. Phase-resetting a mosquito circadian oscillator—I. Phase-resetting surface. J. Comp. Physiol. A 1980, 138, 201–211. [Google Scholar] [CrossRef]
- Sumba, L.A.; Okoth, K.; Deng, A.L.; Githure, J.; Knols, B.G.J.; Beier, J.C.; Hassanali, A. Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. J. Circadian Rhythms 2004, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.L.; Huang, J.; Walker, E.D.; Bayoh, M.N.; Vulule, J.; Miller, J.R. Ovipositional periodicity of caged Anopheles gambiae individuals. J. Circadian Rhythms 2008, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D.; Ritchie, S.A. Oviposition behaviour and parity rates of Aedes aegypti collected in sticky traps in Trinidad, West Indies. Acta Trop. 2010, 116, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.L.; Walker, E.D.; Yunker, A.J.; Dworkin, I. Daily blood feeding rhythms of laboratory-reared North American Culex pipiens. J. Circadian Rhythms 2014, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Meireles-Filho, A.C.A.; Kyriacou, C.P. Circadian rhythms in insect disease vectors. Mem. Inst. Oswaldo Cruz 2013, 108, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, R.B.; Schilman, P.E.; Minoli, S.A.; Lazzari, C.R. Daily Rhythms in Disease-Vector Insects. Biol. Rhythm Res. 2004, 35, 79–92. [Google Scholar] [CrossRef]
- Lazzari, C.R. Orientation Towards Hosts in Haematophagous Insects: An Integrative Perspective, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; Volume 37, ISBN 9780123748294. [Google Scholar]
- Krishnan, B.; Dryer, S.E.; Hardin, P.E. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 1999, 400, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Page, T.L.; Koelling, E. Circadian rhythm in olfactory response in the antennae controlled by the optic lobe in the cockroach. J. Insect Physiol. 2003, 49, 697–707. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, C.; Guo, A. Drosophila olfactory response rhythms require clock genes but not pigment dispersing factor or lateral neurons. J. Biol. Rhythms 2005, 20, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Rund, S.S.C.; Bonar, N.A.; Champion, M.M.; Ghazi, J.P.; Houk, C.M.; Leming, M.T.; Syed, Z.; Duffield, G.E. Daily rhythms in antennal protein and olfactory sensitivity in the malaria mosquito Anopheles gambiae. Sci. Rep. 2013, 3, 2494. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, R.B.; Minoli, S.A.; Lazzari, C.R. Circadian rhythm of behavioural responsiveness to carbon dioxide in the blood-sucking bug Triatoma infestans (Heteroptera: Reduviidae). J. Insect Physiol. 2004, 50, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Barrozo, R.B.; Couton, L.; Lazzari, C.R. Temporal modulation and adaptive control of the behavioural response to odours in Rhodnius prolixus. J. Insect Physiol. 2008, 54, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Meireles-Filho, A.C.; Britto, C.; Lima, J.B.P.; Valle, D.; Peixoto, A.A. Cloning and daily expression of the timeless gene in Aedes aegypti (Diptera:Culicidae). Insect Biochem. Mol. Biol. 2006, 36, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.J.; Rowley, W.A.; Christensen, B.M. Influence of developing Brugia pahangi on spontaneous flight activity of Aedes Aegypti (Diptera: Culicidae). J. Med. Entomol. 1986, 23, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Muir, L.E.; Thorne, M.J.; Kay, B.H. Aedes aegypti (Diptera: Culicidae) vision: Spectral sensitivity and other perceptual parameters of the female eye. J. Med. Entomol. 1992, 29, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W.; Masia, R. Membrane conductances of principal cells in Malpighian tubules of Aedes aegypti. J. Insect Physiol. 2002, 48, 375–386. [Google Scholar] [CrossRef]
- Witzgall, P.; Ansebo, L.; Yang, Z.; Angeli, G.; Sauphanor, B.; Bengtsson, M. Plant volatiles affect oviposition by codling moths. Chemoecology 2005, 15, 77–83. [Google Scholar] [CrossRef]
- Curran, A.M.; Rabin, S.I.; Prada, P.A.; Furton, K.G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 2005, 31, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Vinauger, C.; Lahondère, C.; Wolff, G.H.; Locke, L.T.; Liaw, J.E.; Parrish, J.Z.; Akbari, O.S.; Dickinson, M.H.; Riffell, J.A. Modulation of Host Learning in Aedes aegypti Mosquitoes. Curr. Biol. 2018, 28, 333–344.e8. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0. [Google Scholar]
- Haddow, A.J. Studies on the Biting Habits and Medical Importance of East African Mosquitos in the Genus Aëdes. I.—Subgenera Aëdimorphus, Banksinella and Dunnius. Bull. Entomol. Res. 1960, 50, 759–779. [Google Scholar] [CrossRef]
- Lima-Camara, T.N.; Bruno, R.V.; Luz, P.M.; Castro, M.G.; Lourenço-de-Oliveira, R.; Sorgine, M.H.F.; Peixoto, A.A. Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS ONE 2011, 6, e17690. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A. Tests of Significance in Harmonic Analysis. Proc. R. Soc. Lond. A 1929, 125, 54–59. [Google Scholar] [CrossRef]
- Jhumur, U.S.; Dötterl, S.; Jürgens, A. Floral odors of Silene otites: Their variability and attractiveness to mosquitoes. J. Chem. Ecol. 2008, 34, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Leal, W.S. Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc. Natl. Acad. Sci. USA 2009, 106, 18803–18808. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.R.; Beevor, P.S.; Cork, A.; Nesbitt, B.F.; Vale, G.A. 1-octen-3-ol: A potent olfactory stimulant and attractant. Insect Sci. Appl. 1984, 5, 335–339. [Google Scholar]
- Bowen, M.F. Patterns of sugar feeding on diapauseing and nondiapausing Culex pipiens (Diptera: Culicidae) females. J. Med. Entomol. 1992, 29, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Trpis, M.; McClelland, G.A.H.; Gillett, J.D.; Teesdale, C.; Rao, T.R. Diel periodicity in the landing of Aedes aegypti on man. Bull. World Health Organ. 1973, 48, 623–629. [Google Scholar] [PubMed]
- Chadee, D.D. Landing periodicity of the mosquito Aedes aegypti in Trinidad in relation to the timing of insecticidal space-spraying. Med. Vet. Entomol. 1988, 2, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.D.S.; Sciavico, C.J.D.; Eiras, Á.E. Periodicidade de oviposição de fêmeas de Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae) em laboratório e campo. Rev. Soc. Bras. Med. Trop. 2006, 39, 327–332. [Google Scholar] [CrossRef]
- Barrozo, R.B.; Lazzari, C.R. The response of the blood-sucking bug Triatoma infestans to carbon dioxide and other host odours. Chem. Senses 2004, 29, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zhukovskaya, M.I. Selective regulation of sensitivity to odours of different behavioural significance in the American cockroach, Periplaneta americana. Physiol. Entomol. 2008, 33, 162–166. [Google Scholar] [CrossRef]
- Schendzielorz, T.; Peters, W.; Boekhoff, I.; Stengl, M. Time of day changes in cyclic nucleotides are modified via octopamine and pheromone in antennae of the Madeira cockroach. J. Biol. Rhythms 2012, 27, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Schendzielorz, T.; Schirmer, K.; Stolte, P.; Stengl, M. Octopamine regulates antennal sensory neurons via daytime-dependent changes in cAMP and IP3levels in the hawkmoth Manduca sexta. PLoS ONE 2015, 10, e0121230. [Google Scholar] [CrossRef] [PubMed]
- Flecke, C.; Dolzer, J.; Krannich, S.; Stengl, M. Perfusion with cGMP analogue adapts the action potential response of pheromone-sensitive sensilla trichoidea of the hawkmoth Manduca sexta in a daytime-dependent manner. J. Exp. Biol. 2006, 209, 3898–3912. [Google Scholar] [CrossRef] [PubMed]
- Rosén, W.Q.; Han, G.B.; Löfstedt, C. The circadian rhythm of the sex-pheromone-mediated behavioral response in the turnip moth, Agrotis segetum, is not controlled at the peripheral level. J. Biol. Rhythms 2003, 18, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Merlin, C.; Lucas, P.; Rochat, D.; François, M.C.; Maïbèche-Coisne, M.; Jacquin-Joly, E. An antennal circadian clock and circadian rhythms in peripheral pheromone reception in the moth Spodoptera littoralis. J. Biol. Rhythms 2007, 22, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, A.S.M.; Page, T.L. Circadian regulation of olfactory receptor neurons in the cockroach antenna. J. Biol. Rhythms 2009, 24, 144–152. [Google Scholar] [CrossRef]
- Schuckel, J.; Siwicki, K.K.; Stengl, M. Putative circadian pacemaker cells in the antenna of the hawkmoth Manduca sexta. Cell Tissue Res. 2007, 330, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, J.D.; Durand, N.F.; Vinyard, B.T.; Dickens, J.C. Functional development of the octenol response in Aedes aegypti. Front. Physiol. 2013, 4, 39. [Google Scholar] [CrossRef]
- Grant, A.J.; Dickens, J.C. Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. PLoS ONE 2011, 6, e21785. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Qiu, Y.T.; Wang, G.; Kwon, J.Y.; Rutzler, M.; Kwon, H.W.; Pitts, R.J.; van Loon, J.J.A.; Takken, W.; Carlson, J.R.; et al. Odor Coding in the Maxillary Palp of the Malaria Vector Mosquito Anopheles gambiae. Curr. Biol. 2007, 17, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Cork, A.; Park, K.C. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med. Vet. Entomol. 1996, 10, 269–276. [Google Scholar] [CrossRef]
- Rinker, D.C.; Pitts, R.J.; Zhou, X.; Suh, E.; Rokas, A.; Zwiebel, L.J. Blood meal-induced changes to antennal transcriptome profiles reveal shifts in odor sensitivities in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2013, 110, 8260–8265. [Google Scholar] [CrossRef] [PubMed]
- Takken, W.; Dekker, T.; Wijnholds, Y.G. Odor-Mediated Behavior of Anopheles gambiae Giles Sensu Stricto and An. stephensi Liston in Response to CO2, Acetone, and 1-Octen-3-ol (Diptera: Culicidae). J. Insect Behav. 1997, 10, 395–407. [Google Scholar] [CrossRef]
| Df | Sum Sq | Mean Sq | F Value | Pr(>F) | |
|---|---|---|---|---|---|
| Odor | 6 | 1419 | 236.46 | 24.711 | <2 × 10−16 |
| Time of day | 3 | 78 | 26.05 | 2.722 | 0.0437 |
| Odor:Time of day | 18 | 435 | 24.15 | 2.524 | 0.0005 |
| Residuals | 557 | 5330 | 9.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eilerts, D.F.; VanderGiessen, M.; Bose, E.A.; Broxton, K.; Vinauger, C. Odor-Specific Daily Rhythms in the Olfactory Sensitivity and Behavior of Aedes aegypti Mosquitoes. Insects 2018, 9, 147. https://doi.org/10.3390/insects9040147
Eilerts DF, VanderGiessen M, Bose EA, Broxton K, Vinauger C. Odor-Specific Daily Rhythms in the Olfactory Sensitivity and Behavior of Aedes aegypti Mosquitoes. Insects. 2018; 9(4):147. https://doi.org/10.3390/insects9040147
Chicago/Turabian StyleEilerts, Diane F., Morgen VanderGiessen, Elizabeth A. Bose, Kyera Broxton, and Clément Vinauger. 2018. "Odor-Specific Daily Rhythms in the Olfactory Sensitivity and Behavior of Aedes aegypti Mosquitoes" Insects 9, no. 4: 147. https://doi.org/10.3390/insects9040147
APA StyleEilerts, D. F., VanderGiessen, M., Bose, E. A., Broxton, K., & Vinauger, C. (2018). Odor-Specific Daily Rhythms in the Olfactory Sensitivity and Behavior of Aedes aegypti Mosquitoes. Insects, 9(4), 147. https://doi.org/10.3390/insects9040147




