Limited Genetic Structure of Gypsy Moth Populations Reflecting a Recent History in Europe
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pogue, M.G.; Schaefer, P.W. A Review of Selected Species of Lymantria Hübner [1819] (Lepidoptera: Noctuidae: Lymantriinae) from Subtropical and Temperate Regions of Asia, Including Descriptions of Three New Species, Some Potentially Invasive to North America; U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Washington, DC, USA, 2007; pp. 11–37.
- Schintlmeister, A. The taxonomy of the genus Lymantria Hübner, [1819] (Lepidoptera: Lymantriidae). Quadrifina 2004, 7, 1–248. [Google Scholar]
- Liebhold, A.M.; Mastro, V.C.; Schaefer, P.W. Learning from the legacy of Leopold Trouvelot. Bull. Entomol. Soc. Am. 1989, 35, 20–22. [Google Scholar] [CrossRef]
- McManus, M.L.; Csóka, G. History and Impact of Gypsy Moth in North America and Comparison to Recent Outbreaks in Europe. Acta Silv. Lignaria Hung. 2007, 3, 47–64. [Google Scholar]
- Ozumbekov, A.A.; Liebhold, A.M.; Ponomarev, V.I.; Tobin, P.C. Gypsy moth (Lepidoptera: Lymantriidae) in Central Asia. Am. Entomol. 2009, 55, 258–264. [Google Scholar] [CrossRef]
- Davidson Christopher, B.; Gottschalk Kurt, W.; Johnson James, E. European gypsy moth (Lymantria dispar L.) outbreaks: A review of the literature. In Gen Tech Rep NE-278; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2001; 15p. [Google Scholar]
- Cannon, R.J.C.; Koerper, D.; Ashby, S.; Baker, R.; Bartlett, P.W.; Brookes, G.; Burgess, R.; Cheek, S.; Evans, H.F.; Hammon, R.; et al. Gypsy moth, Lymantria dispar, outbreak in northeast London, 1995–2003. Int. J. Pest Manag. 2004, 50, 259–273. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Liebhold, A.M. Population dynamics of gypsy moth in North America. Annu. Rev. Entomol. 1990, 35, 571–596. [Google Scholar] [CrossRef]
- Tobin, P.C.; Blackburn, L.M. Long-distance dispersal of the gypsy moth (Lepidoptera: Lymantridae) facilitated its initial invasion of Wisconsin. Environ. Entomol. 2008, 37, 87–93. [Google Scholar] [CrossRef]
- Keena, M.A.; Côte, M.J.; Grinberg, P.S.; Wallner, W.E. World distribution of female flight and genetic variation in Lymantria dispar (Lepidoptera: Lymantriidae). Environ. Entomol. 2008, 37, 636–649. [Google Scholar] [CrossRef]
- Bogdanowicz, S.M.; Wallner, W.E.; Bell, J.; O’Dell, T.M.; Harrison, R.G. Asian gypsy moth (Lepidoptera: Lymantriidae) in North America: Evidence from molecular data. Ann. Entomol. Soc. Am. 1993, 86, 710–715. [Google Scholar] [CrossRef]
- Kang, T.H.; Sang, H.H.; Heung, S.L. Genetic structure and demographic history of Lymantria dispar (Linnaeus, 1758) (Lepidoptera: Erebidae) in its area of origin and adjacent areas. Ecol. Evol. 2017, 7, 9162–9178. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Molongoski, J.J.; Winograd, D.F.; Bogdanowicz, S.M.; Louyakis, A.S.; Lance, D.R.; Mastro, V.C.; Harrison, R.G. Genetic structure, admixture and invasion success in a Holarctic defoliator, the gypsy moth (Lymantria dispar, Lepidoptera: Erebidae). Mol. Ecol. 2015, 24, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- deWaard, J.R.; Mitchell, A.; Keena, M.A.; Gopurenko, D.; Boykin, L.M.; Armstrong, K.F.; Pogue, M.G.; Lima, J.; Floyd, R.; Hanner, R.H.; et al. Towards a global barcode library for Lymantria (Lepidoptera: Lymantriinae) tussock moths of biosecurity concern. PLoS ONE 2010. [Google Scholar] [CrossRef] [PubMed]
- Quian, L.; Yulin, A.; Juncian, S.; Mei, X.; Jianlin, Y.; Cuiping, W.; Bin, L.; Dejun, H. COI gene geographic variation of gypsy moth (Lepidoptera: Lymantriidae) and a TaqMan PCR diagnostic assay. DNA Barcodes 2014, 2, 10–16. [Google Scholar] [CrossRef]
- Lacković, N.; Bertheau, C.; Stauffer, C.; Pernek, M.; Avtzis, D.N. Genetic split between coastal and continental populations of gypsy moth separated by Dinaric Alps. J. Appl. Entomol. 2016, 139, 721–726. [Google Scholar] [CrossRef]
- Lunt, D.H.; Zhang, D.X.; Szymura, J.M.; Hewitt, G.M. The insect cytochrome oxidase I gene: Evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol. Biol. 1996, 5, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acid. Res. 1999, 41, 95–98. [Google Scholar]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.J.; El Mousadik, A.; Pons, O. Identifying populations for conservation on the basis of genetic markers. Conserv. Biol. 1998, 12, 844–855. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Crandall, K.A.; Templeton, A.R. Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics 1993, 135, 959–969. [Google Scholar]
- Panchal, M. The automation of Nested Clade Phylogeographic Analysis. Bioinformatics 2007, 23, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Pons, O.; Petit, R.J. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 1996, 144, 1237–1245. [Google Scholar] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic interface and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Zahiri, R.; Djoumad, A.; Freschi, L.; Lamarche, J.; Holden, D.; Cervantes, S.; Ojeda, D.I.; Potvin, A.; Nisole, A.; et al. A Multi-Species TaqMan PCR Assay for the Identification of Asian Gypsy Moths (Lymantria spp.) and Other Invasive Lymantriines of Biosecurity Concern to North America. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Djoumad, A.; Nisole, A.; Zahiri, R. Comparative analysis of mitochondrial genomes of geographic variants of the gypsy moth, Lymantria dispar, reveals a previously undescribed genotypic entity. Sci. Rep. 2017, 7, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Vandergast, A.G.; Perry, W.M.; Lugo, R.V.; Hathaway, S.A. Genetic landscapes GIS Toolbox: Tools to map patterns of genetic divergence and diversity. Mol. Ecol. Resour. 2011, 11, 158–161. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2015. Available online: https://www.R-project.org/ (accessed on 20 September 2018).
- Oksanen, J.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.3-3. 2016. Available online: https://CRAN.Rproject.org/package=vegan (accessed on 20 September 2018).
- Phillips, J.D.; Gwiazdowski, R.A.; Ashlock, D.; Hanner, R. An exploration of sufficient sampling effort to describe intraspecific DNA barcode haplotype diversity: Examples from the ray-finned fishes (Chordata: Actinopterygii). DNA Barcodes 2015, 3, 66–73. [Google Scholar] [CrossRef]
- Biogeographic Regions in Europe. Available online: https://www.eea.europa.eu/data-and-maps/figures/biogeographical-regions-in-europe-2 (accessed on 19 January 2016).
- Karolewski, P.; Grzebyta, J.; Oleksyn, J.; Giertych, M.J. Effects of temperature on larval survival rate and duration of development in Lymantria monacha (L.) on needles of Pinus sylvestris (L.) and L. dispar (L.) on leaves of Quercus robur (L.). Pol. J. Ecol. 2007, 55, 595–600. [Google Scholar]
- Tobin, P.C.; Liebhold, A.M. Gypsy moth. In Encyclopedia of Biological Invasions; Simberloff, D., Rejmanek, M., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 298–304. [Google Scholar]
- Vanhanen, H.; Veteli, T.O.; Päivinen, S.; Kellomäki, S.; Niemelä, P. Climate Change and Range Shifts in Two Insect Defoliators: Gypsy Moth and Nun Moth—A Model Study. Silva Fenn. 2007, 41, 621–638. [Google Scholar] [CrossRef]
- Ochando, M.D.; Reyes, A.; Segura, D.; Callejas, D. Phylogeography: It’s Importance in Insect Pest Control; Nova Science Publishers Inc.: New York, NY, USA, 2011; p. 48. [Google Scholar]
- Taberlet, P.; Fumagalli, L.; Wust-Saucy, A.G.; Cosson, J.F. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 1998, 7, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 1999, 68, 87–112. [Google Scholar] [CrossRef]
- Hewitt, G.M. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Lister, A.M. Cryptic northern refugia and the origins of modern biota. Trends Ecol. Evol. 2001, 16, 608–613. [Google Scholar] [CrossRef]
- Excoffier, L.; Foll, M.; Petit, R.J. Genetic consequences of range expansions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 481–501. [Google Scholar] [CrossRef]
- Schmitt, T. Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. Front. Zool. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, P.J.; Kvifte, G.M.; Langguth, H.; Wagner, R. Glacial and postglacial species divergence and dispersal of European trickle midges (Diptera: Thaumaleidae). Arthropod Syst. Phyl. 2017, 75, 523–534. [Google Scholar]
- Tóth, V.; Lakatos, F. Phylogeographic pattern of the plane leaf miner, Phyllonorycter platani (Staudinger, 1870) (Lepidoptera: Gracilariidae) in Europe. BMC Evol. Biol. 2018, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luo, Y.; Keena, M.A.; Wu, Y.; Shi, J. DNA barcoding of Chinese gypsy moths (Lepidoptera, Erebidae) reveals new haplotypes and divergence patterns within gypsy moth subspecies. J. Econ. Entomol. 2016, 109, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Feder, J.L.; Hunt, T.A.; Bush, L. The effects of climate, host plant phenology and host fidelity on the genetics of apple and hawthorn infesting races of Rhagoletis pomonella. Entomol. Exp. Appl. 1993, 69, 117–135. [Google Scholar] [CrossRef]
- Nosil, P.; Crespi, B.J. Ecological divergence promotes the evolution of cryptic reproductive isolation. P. Roy. Soc. B-Biol. Sci. 2006, 273, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Antwi, J.B.; Sword, G.A.; Medina, R.F. Host-associated differentiation in a highly polyphagous, sexually reproducing insect herbivore. Ecol. Evol. 2015, 5, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Peccoud, J.; Ollivier, A.; Plantegenest, M.; Simon, J.C. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl. Acad. Sci. USA 2009, 106, 7495–7500. [Google Scholar] [CrossRef] [PubMed]
- Via, S. Natural selection in action during speciation. Proc. Natl. Acad. Sci. USA 2009, 106, 9939–9946. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.F.; Wang, H.L.; Svensson, G.P.; Frago, E.; Anderbrant, O. Genetic divergence and evidence for sympatric host-races in the highly polyphagous brown tail moth, Euproctis chrysorrhoea (Lepidoptera: Erebidae). Evol. Ecol. 2014, 28, 829–848. [Google Scholar] [CrossRef]
- Hereward, J.P.; Walter, G.H.; DeBarro, P.J.; Lowe, A.J.; Riginos, C. Gene flow in the green mirid, Creontiades dilutus (Hemiptera: Miridae), across arid and agricultural environments with different host plant species. Ecol. Evol. 2013, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Bertheau, C.; Bankhead-Dronnet, S.; Martin, C.; Lieutier, F.; Roux-Morabito, G. Lack of genetic differentiation after host range extension argues for the generalist nature of Pityogenes chalcographus (Curculionidae: Scolytinae). Ann. For. Sci. 2012, 69, 313–323. [Google Scholar] [CrossRef]
- Dasmahapatra, K.K.; Walters, J.R.; Briscoe, A.D.; Davey, J.W.; Whibley, A.; Nadeau, N.J.; Zimin, A.V.; Hughes, D.S.T.; Ferguson, L.C.; Martin, S.H.; et al. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 2012, 487, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, N.J.; Martin, S.H.; Kozak, K.M.; Salazar, C.; Dasmahapatra, K.K.; Davey, J.W.; Baxter, S.W.; Blaxter, M.L.; Mallet, J.; Jiggins, C.D. Genome-wide patterns of divergence and gene flow across a butterfly radiation. Mol. Ecol. 2013, 22, 814–826. [Google Scholar] [CrossRef] [PubMed]
- McFadden, M.W.; McManus, M.L. An Insect Out of Control? The potential for spread and establishment of the gypsy moth in new forest areas in the United States. In Forest Insect Guilds: Patterns of Interaction with Host Trees; Baranchikov, Y.N., Mattson, W.J., Hain, F.P., Payne, T.L., Eds.; U.S. Department of Agriculture, Forest Service: Radnor, PA, USA, 1991; pp. 172–186. [Google Scholar]
- Keena, M.A.; Grinberg, P.S.; Wallner, W.E. Inheritance of female flight in Lymantria dispar (Lepidoptera: Lymantriidae). Environ. Entomol. 2007, 36, 484–494. [Google Scholar] [CrossRef] [PubMed]
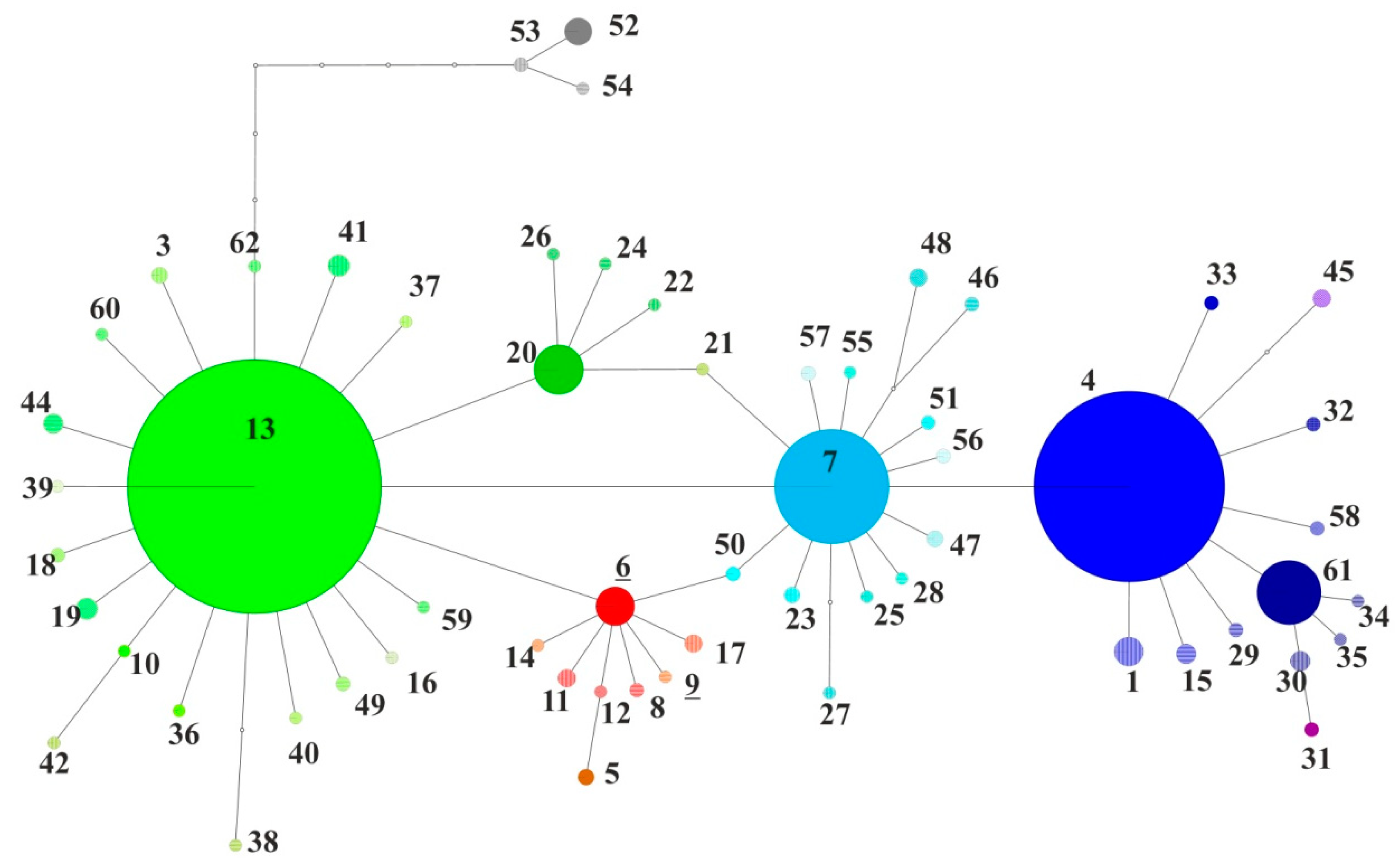
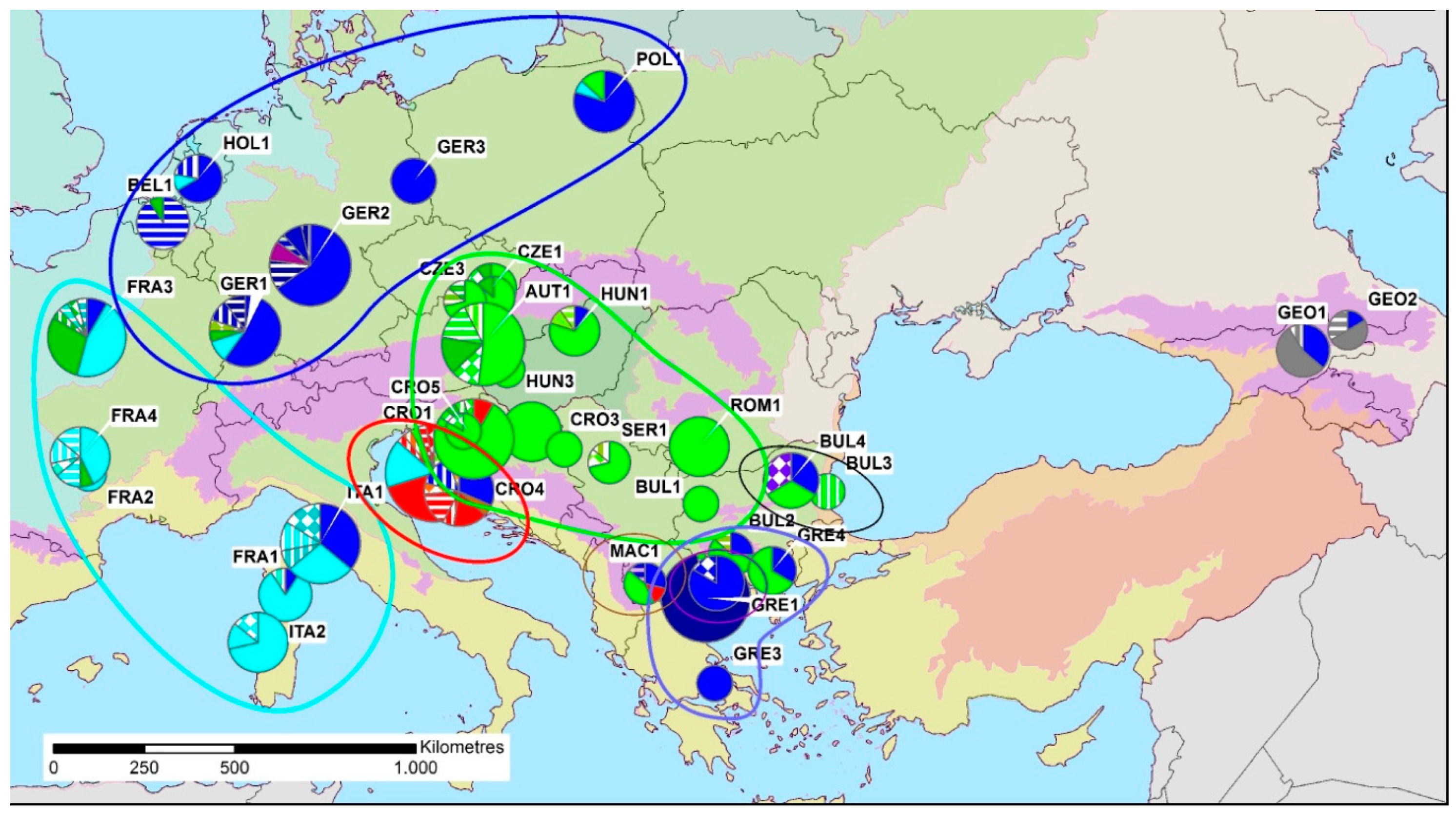
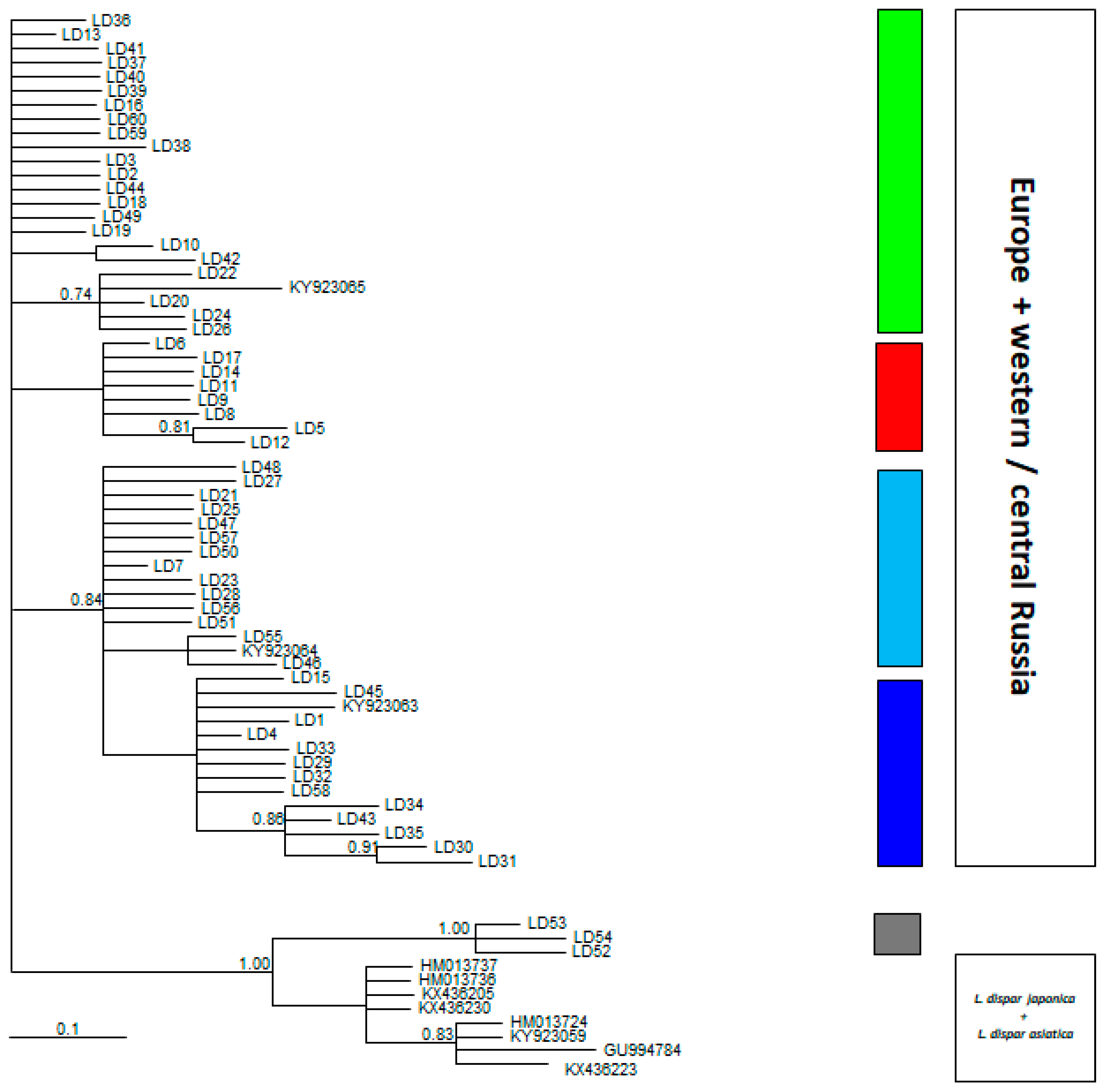
| Country | Abbrev. | Host | Lat. | Long. | N | HT | Hd ± SD | π ± SD | MNPD ± SD | r (5) |
|---|---|---|---|---|---|---|---|---|---|---|
| Austria | AUT1 | QE | 47.75 | 16.54 | 27 | 5 | 0.695 ± 0.078 | 0.001 ± 0.001 | 0.871 ± 0.632 | 1.966 |
| Belgium | BEL1 | FS | 50.82 | 4.41 | 11 | 2 | 0.181 ± 0.143 | 0.001 ± 0.000 | 0.727 ± 0.584 | 0.455 |
| Bulgaria | BUL1 | QR | 43.09 | 23.46 | 5 | 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.000 |
| BUL2 | QR | 41.71 | 24.16 | 8 | 3 | 0.607 ± 0.164 | 0.002 ± 0.001 | 1.357 ± 0.933 | 1.518 | |
| BUL3 | QR | 42.74 | 27.74 | 5 | 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.000 | |
| BUL4 | QR | 43.21 | 26.62 | 12 | 3 | 0.727 ± 0.058 | 0.002 ± 0.001 | 1.939 ± 1.181 | 1.788 | |
| Croatia | CRO1 | QI | 44.65 | 14.47 | 37 | 10 | 0.818 ± 0.038 | 0.003 ± 0.002 | 2.087 ± 1.193 | 2.555 |
| CRO2 | QR | 45.47 | 17.99 | 14 | 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.000 | |
| CRO3 | QR | 44.95 | 19.07 | 5 | 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.000 | |
| CRO4 | QP | 44.15 | 15.3 | 19 | 8 | 0.859 ± 0.052 | 0.004 ± 0.002 | 2.666 ± 1.485 | 2.815 | |
| CRO5 | VA1 | 45.22 | 16.28 | 25 | 6 | 0.426 ± 0.121 | 0.000 ± 0.000 | 0.473 ± 0.425 | 1.167 | |
| CRO6 | QR | 45.65 | 15.59 | 6 | 2 | 0.333 ± 0.215 | 0.000 ± 0.000 | 0.333 ± 0.380 | 0.833 | |
| Czech Republic | CZE1 | QE | 49.11 | 17.04 | 10 | 4 | 0.533 ± 0.180 | 0.001 ± 0.001 | 0.600 ± 0.519 | 1.500 |
| CZE2 | QR | 48.88 | 17.1 | 7 | 2 | 0.285 ± 0.196 | 0.000 ± 0.000 | 0.285 ± 0.340 | 0.714 | |
| CZE3 | QE | 48.85 | 16.01 | 7 | 3 | 0.523 ± 0.208 | 0.001 ± 0.001 | 0.857 ± 0.681 | 1.429 | |
| France | FRA1 | QS | 41.71 | 9.33 | 11 | 3 | 0.345 ± 0.172 | 0.000 ± 0.000 | 0.363 ± 0.377 | 0.909 |
| FRA2 | QP | 44.47 | 2.47 | 5 | 2 | 0.400 ± 0.237 | 0.000 ± 0.000 | 0.400 ± 0.435 | 1.000 | |
| FRA3 | QE | 47.82 | 1.92 | 24 | 7 | 0.721 ± 0.070 | 0.002 ± 0.001 | 1.554 ± 0.961 | 2.038 | |
| FRA4 | QE | 44.85 | 2.12 | 14 | 6 | 0.802 ± 0.090 | 0.001 ± 0.001 | 1.219 ± 0.824 | 2.500 | |
| Georgia | GEO1 | MA | 41.94 | 44.48 | 11 | 3 | 0.618 ± 0.103 | 0.008 ± 0.005 | 5.818 ± 3.013 | 1.407 |
| GEO2 | MA | 41.88 | 46.13 | 6 | 3 | 0.733 ± 0.155 | 0.005 ± 0.003 | 3.933 ± 2.290 | 1.833 | |
| Germany | GER1 | VA2 | 48.26 | 7.76 | 20 | 6 | 0.636 ± 0.115 | 0.001 ± 0.001 | 1.194 ± 0.797 | 1.838 |
| GER2 | VA3 | 49.91 | 10.19 | 26 | 6 | 0.566 ± 0.108 | 0.001 ± 0.001 | 1.141 ± 0.764 | 1.579 | |
| GER3 | QE | 51.91 | 14.35 | 8 | 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.000 | |
| Greece | GRE1 | QO | 40.77 | 23.11 | 31 | 3 | 0.127 ± 0.079 | 0.000 ± 0.000 | 0.193 ± 0.250 | 0.323 |
| GRE2 | QO | 41.08 | 23.54 | 12 | 2 | 0.303 ± 0.147 | 0.000 ± 0.000 | 0.303 ± 0.336 | 0.682 | |
| GRE3 | QO | 38.63 | 22.97 | 5 | 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.000 | |
| GRE4 | QO | 41.12 | 25.41 | 9 | 2 | 0.500 ± 0.128 | 0.001 ± 0.001 | 1.000 ± 0.739 | 0.952 | |
| Holland | HOL1 | QR | 51.98 | 5.68 | 9 | 3 | 0.555 ± 0.165 | 0.000 ± 0.000 | 0.611 ± 0.530 | 1.389 |
| Hungary | HUN1 | VA4 | 47.83 | 19.96 | 10 | 4 | 0.533 ± 0.180 | 0.001 ± 0.001 | 0.800 ± 0.628 | 1.500 |
| HUN2 | VA5 | 47.71 | 17.12 | 11 | 4 | 0.709 ± 0.099 | 0.001 ± 0.001 | 0.872 ± 0.661 | 1.851 | |
| HUN3 | VA6 | 47.07 | 17.34 | 5 | 2 | 0.600 ± 0.175 | 0.000 ± 0.000 | 0.600 ± 0.562 | 1.000 | |
| Italy | ITA1 | QI | 42.99 | 10.5 | 25 | 5 | 0.776 ± 0.046 | 0.002 ± 0.001 | 1.513 ± 0.941 | 2.245 |
| ITA2 | QS | 40.51 | 8.46 | 14 | 3 | 0.483 ± 0.142 | 0.000 ± 0.000 | 0.527 ± 0.467 | 1.209 | |
| FYROM | MAC1 | N/A | 41.36 | 21.21 | 7 | 4 | 0.809 ± 0.129 | 0.002 ± 0.001 | 1.714 ± 1.131 | 2.381 |
| Poland | POL1 | VA5 | 53.29 | 22.61 | 15 | 3 | 0.361 ± 0.144 | 0.001 ± 0.001 | 0.590 ± 0.500 | 0.905 |
| Romania | ROM1 | N/A | 44.5 | 23.74 | 14 | 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.000 |
| Serbia | SER1 | QE | 44.47 | 5.57 | 7 | 3 | 0.523 ± 0.208 | 0.001 ± 0.001 | 0.857 ± 0.681 | 1.429 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacković, N.; Pernek, M.; Bertheau, C.; Franjević, D.; Stauffer, C.; Avtzis, D.N. Limited Genetic Structure of Gypsy Moth Populations Reflecting a Recent History in Europe. Insects 2018, 9, 143. https://doi.org/10.3390/insects9040143
Lacković N, Pernek M, Bertheau C, Franjević D, Stauffer C, Avtzis DN. Limited Genetic Structure of Gypsy Moth Populations Reflecting a Recent History in Europe. Insects. 2018; 9(4):143. https://doi.org/10.3390/insects9040143
Chicago/Turabian StyleLacković, Nikola, Milan Pernek, Coralie Bertheau, Damjan Franjević, Christian Stauffer, and Dimitrios N. Avtzis. 2018. "Limited Genetic Structure of Gypsy Moth Populations Reflecting a Recent History in Europe" Insects 9, no. 4: 143. https://doi.org/10.3390/insects9040143
APA StyleLacković, N., Pernek, M., Bertheau, C., Franjević, D., Stauffer, C., & Avtzis, D. N. (2018). Limited Genetic Structure of Gypsy Moth Populations Reflecting a Recent History in Europe. Insects, 9(4), 143. https://doi.org/10.3390/insects9040143








